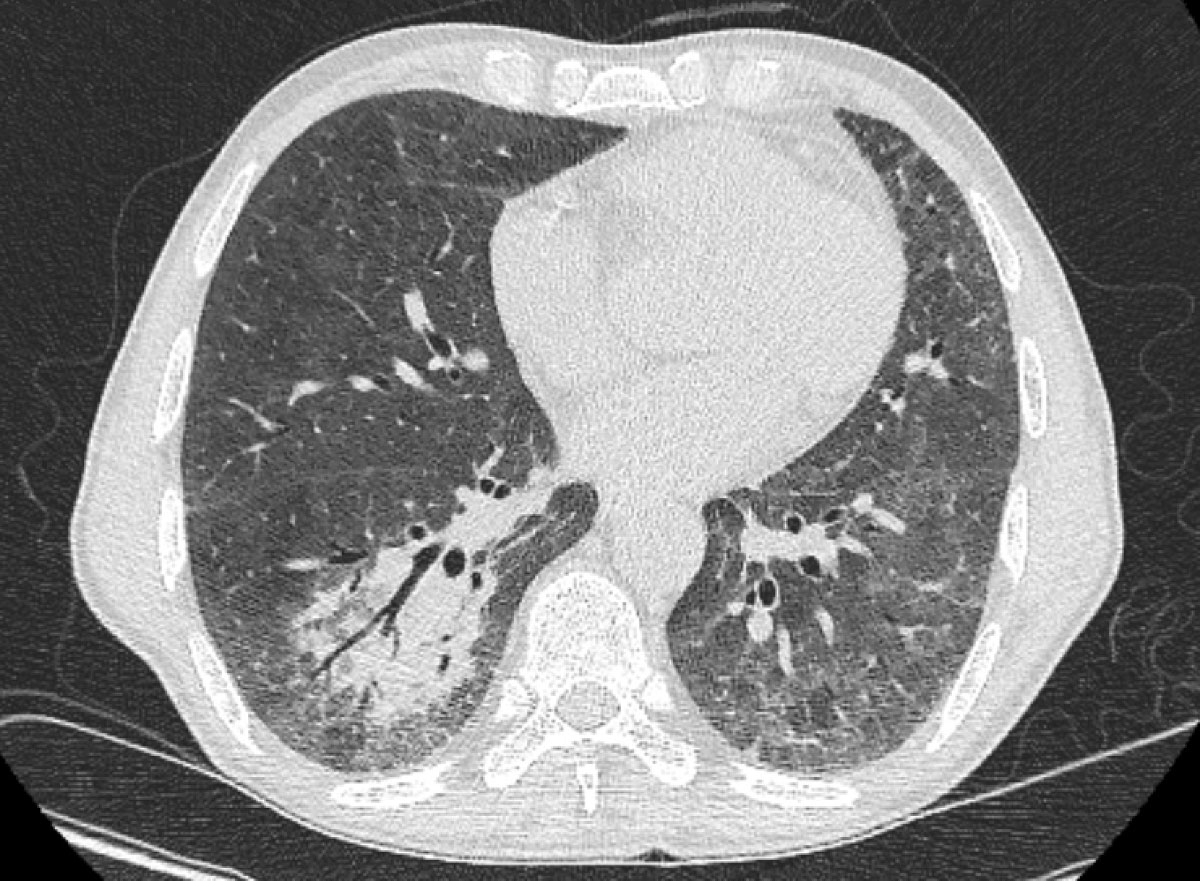
Figure 1 Chest computed tomography on day 3 of admission showing a right lower lobe consolidation with air bronchogram.
DOI: https://doi.org/10.4414/smw.2020.20306
Cryptococcosis is the third most common invasive fungal infection in solid organ transplant recipients, usually presenting late post-transplant [1, 2]. In a retrospective cohort of 158 solid organ transplant patients in the United States, Cryptococcus-associated infections were observed at a median of 464 days after the transplant, presenting mainly with central nervous system (CNS) infection (45%) followed by pulmonary involvement (25%) and, less frequently, with septicaemia, skin and soft tissue or surgical site infection [3]. In the last decade Cryptococcus gattii has emerged as a significant pathogen affecting normal and immunocompromised hosts [4]. We report the first documented case of a C. gattii infection in Switzerland in a kidney-pancreas transplant recipient. In addition to its unique epidemiology, this case further illustrates the challenges associated with the diagnosis of cryptococcal community-acquired pneumonia, and the management of this infection in the context of other opportunistic coinfections and/or immune reconstitution syndrome.
A 54-year-old male kidney-pancreas transplant recipient (9 years previously) with chronic kidney failure (G4A2) presented with a 2-week history of fever and productive cough. The patient was on maintenance immunosuppression including prednisone 5 mg/day, tacrolimus 3 mg/day and mycophenolate mofetil 2000 mg/day. He had never travelled outside of Switzerland and Portugal. Before admission, he had spent 4 weeks in rural Portugal working on the reconstruction of a farmhouse, with exposures to chickens, goats, dogs, pigeons and pigs. During the last week, he started experiencing high fever and a dry cough. Because of symptom progression, he presented to the emergency department, where a chest X-ray showed a right lower lobe infiltrate. He was admitted with a diagnosis of community-acquired pneumonia and treatment with oral levofloxacin was initiated. His CD4 lymphocyte count was 139 cells/mm3. Admission blood and sputum cultures, polymerase chain-reaction (PCR) testing for Mycoplasma and Chlamydia, urine antigen for Legionella and pneumococcus were negative. Owing to persistence of his symptoms, chest computed tomography (CT) was performed on post-admission day 3, revealing a left upper lobe pleura-based nodular lesion and bilateral consolidations (fig. 1). Because of a lack of treatment response and suspicious CT findings, a serum cryptococcal antigen test was requested, and came back positive at a titre of 1:1280 (CrAg® LFA, Immy Diagnostics, Norman, Oklahoma, USA) on post-admission day 3. A bronchoscopy was performed on post-admission day 4. A bronchoalveolar lavage (BAL) gram-stain demonstrated large cellular round forms (fig. 2 ). Grocott and periodic acid-Schiff stains were positive for yeasts, and mucicarmine staining identified a cryptococcal infection (fig. 3). The BAL fluid culture turned positive for Cryptococcus sp. 2 days post-incubation, subsequently identified as Cryptococcus gattii by matrix assisted laser desorption ionisations time of flight mass spectrometry (MALDI TOF MS) (Bruker Daltonik GmbH, Bremen, Germany) with a confidence score of 2.17. The softwares used for MALDI TOF analysis were Flex Control 3.4 and MALDI Biotyper Compass 4.1.80 (Bruker Daltonik GmbH, Bremen, Germany). Susceptibility testing performed through microdilution (YeastOne®, Sensititre, TREK Diagnostic System Inc., Cleveland, Ohio, USA) showed the following minimal inhibitory concentrations: flucytosine 0.5 mg/l, posaconazole 0.06 mg/l, voriconazole 0.03 mg/l, itraconazole 0.015 mg/l, fluconazole 2 mg/l and amphotericin B 0.12 mg/l. BAL PCR was positive for Pneumocystis jirovecii (cycle threshold 30), with a negative Pneumocystis modified toluidine blue stain. Serum galactomannan enzyme immunoassay was negative whereas β-d-glucan on post-admission day 4 was positive at a titre of >500 pg/ml. A brain CT scan and lumbar puncture were negative for CNS involvement. A diagnosis of a community-acquired multilobar pneumonia due to C. gattii was established, immunosuppression was tapered and antifungal therapy with intravenous liposomal amphotericin B 3 mg/kg once daily and oral flucytosine 25mg/kg twice daily was initiated on post-admission day 4. Concomitantly, treatment with oral atovaquone 1500 mg once daily was started for a diagnosis of probable Pneumocystis jirovecii pneumonia, based on the positive P. jirovecii PCR and β-d-glucan results.

Figure 1 Chest computed tomography on day 3 of admission showing a right lower lobe consolidation with air bronchogram.

Figure 2 Direct gram stain of broncho-alveolar lavage fluid (magnification 63×) showing large cellular round forms (white arrows).
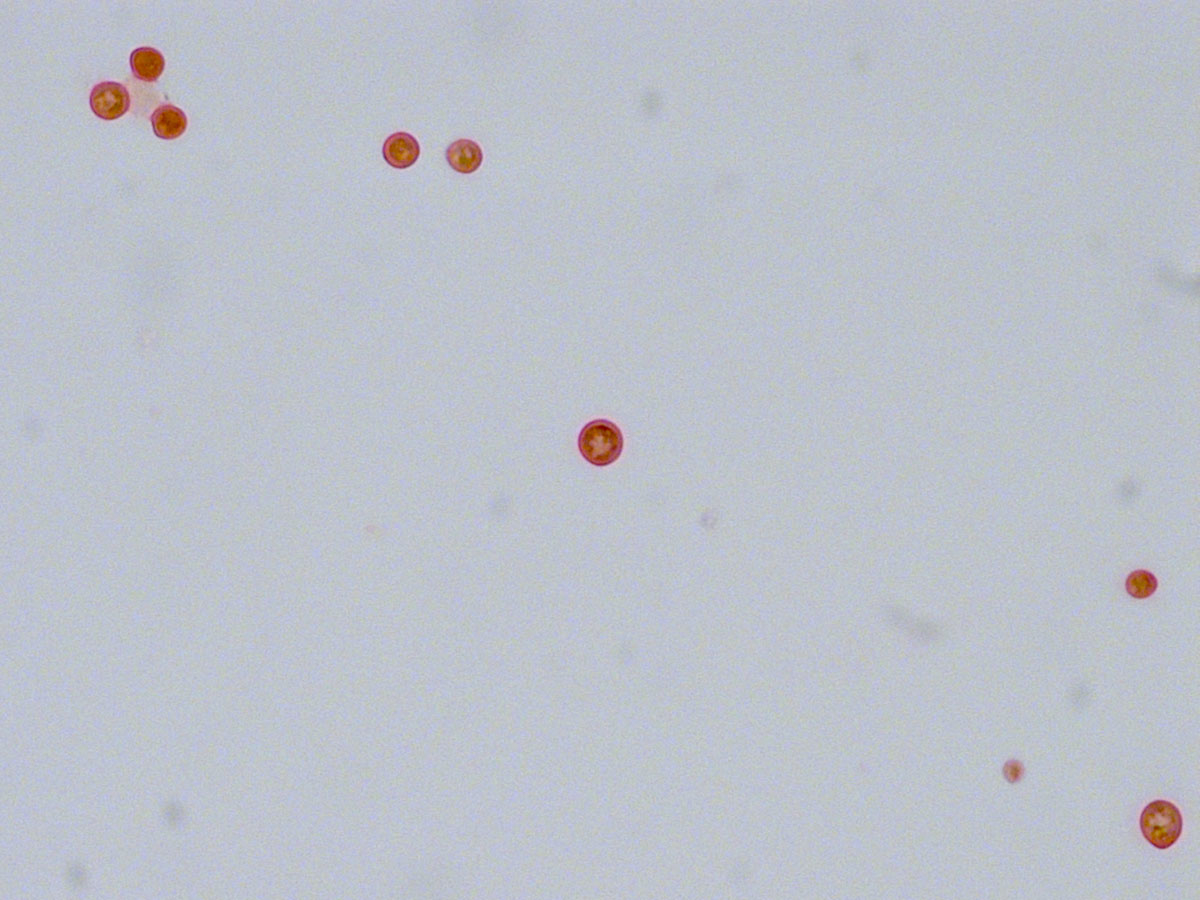
Figure 3 Mucicarmine stain of broncho-alveolar lavage fluid (magnification 63×) confirmed a cryptococcal infection, by highlighting the yeast’s characteristic large polysaccharide.
Three days after treatment initiation and after an initial satisfactory clinical response, the patient developed high fever and acute onset respiratory distress, and was eventually intubated on post-admission day 7. A chest CT (post-admission day 7) revealed bilateral diffuse ground-glass opacities and consolidations (fig. 4). Despite the early presentation, an immune reconstitution syndrome was suspected and empirical treatment with intravenous methylprednisolone 1 mg/kg/day was started. On the basis of the radiographic presentation, it was not feasible to make a definitive diagnosis of P. jirovecii pneumonia versus immune reconstitution syndrome. However, based on the timing,a positive Pneumocystis PCR titre and high β-d-glucan, a diagnosis of probable P. jirovecii pneumonia was retained. Because of deteriorating renal function, atovaquone was switched to intravenous clindamycin 600 mg thrice daily and oral primaquine 30 mg once daily on post-admission day 9. The patient had a good clinical response and was eventually extubated on post-admission day 10. Owing to bone marrow suppression, flucytosine treatment was discontinued and replaced by oral fluconazole 200 mg once daily (renal dose) on post-admission day 9, which was co-administered with liposomal amphotericin B for a total of 30 days. Maintenance treatment was initiated on post-admission day 31 with oral fluconazole 200 mg once daily for a total of 1 year. Treatment for P. jirovecii pneumonia was continued for 21 days, followed by secondary prophylaxis with oral trimethoprim-sulfamethoxazole three times weekly on post-admission day 30. Glucocorticoids were tapered down during the next 4 weeks to 10 mg of prednisone orally once daily. The patient tolerated the treatment well, followed an inpatient pulmonary rehabilitation programme and was discharged on post-admission day +38. At his last follow-up 3 months from treatment initiation, the patient was alive with complete resolution of his symptoms. A check CT showed almost complete resolution of his lesions, with a persistent ground-glass opacity in the lower right lobe and posterobasal subpleural reticulations (fig. 5).
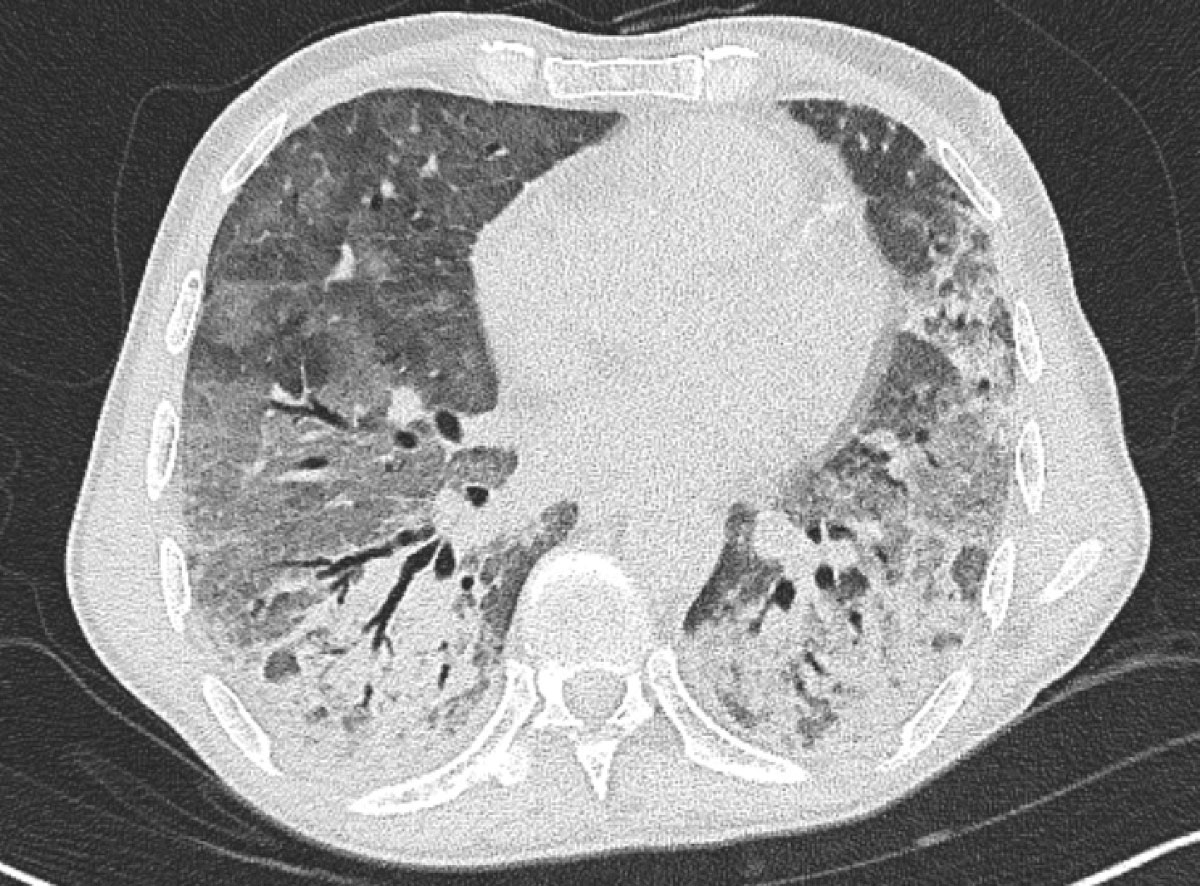
Figure 4 Chest computed tomography on day 7 showing diffuse bilateral infiltrates and ground-glass opacities.
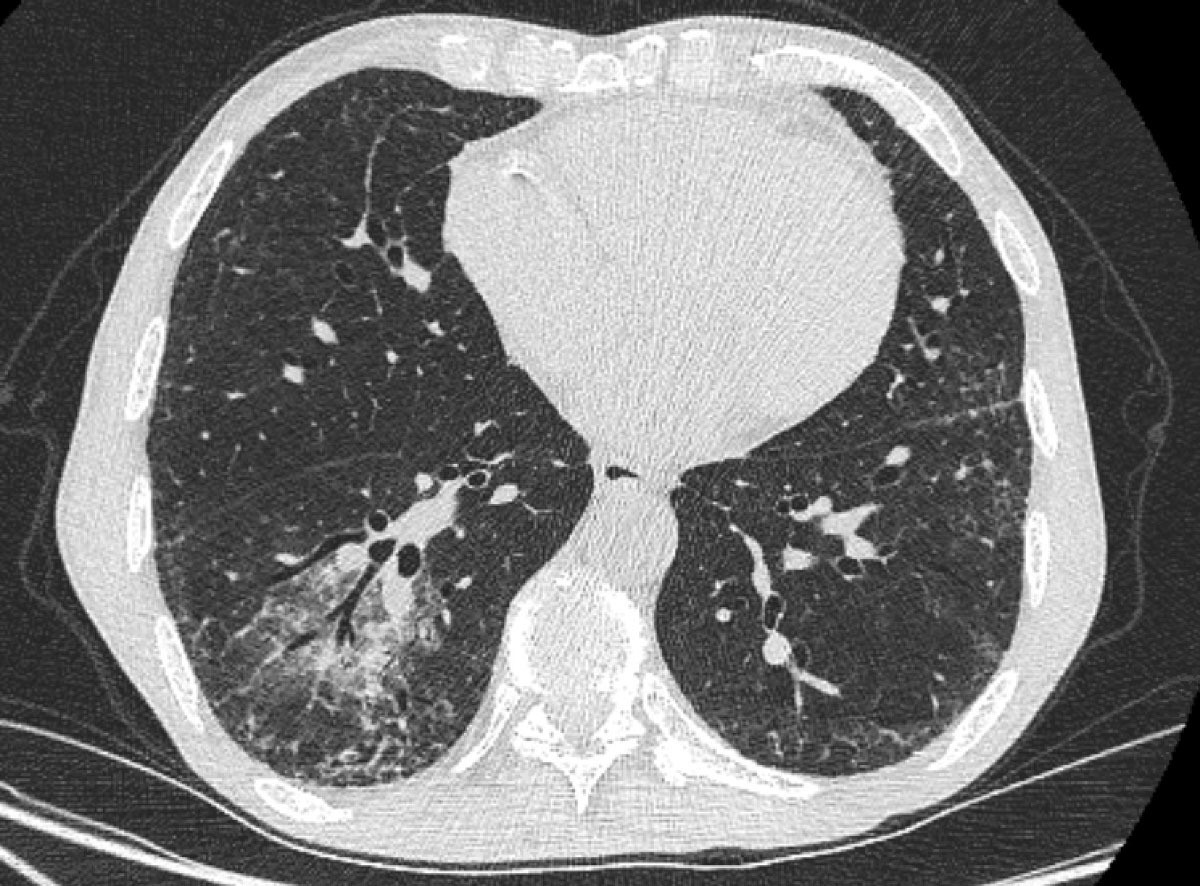
Figure 5 Chest computed tomography 3 months later showed global improvement, with a persistent ground glass opacity in the lower right lobe and posterobasal subpleural reticulations.
This is the first case of C. gattii infection in Switzerland. In contrast to its initial sequestered distribution in Australia and British Columbia, C. gattii now has a global distribution, mainly associated with eucalyptus trees, but also with animals. A recent study by Cogliati et al. first investigated the distribution of Cryptococcus spp. in the Mediterranean basin, confirming that both C. neoformans and C. gattii are present, with carob and olive trees representing important niches for these yeasts [5]. More precisely, C. gattii is emerging in Europe and has been identified in Spain, Italy and Greece [6]. Furthermore, the presence in Europe of an autochthonous strain (AFLP4) belonging to the VGI molecular type and responsible of human infection has been reported [6]. Our patient had never travelled outside Switzerland or Portugal. Considering that his symptoms started while in Portugal, we postulate that this is an imported, rather than autochthonous, case in Switzerland.
This case report illustrates the importance of C. gattii as a significant pathogen in immunocompromised hosts presenting with a community-acquired pneumonia. Considering the protean presentation of this clinical entity, late onset after transplantation, and non-specific clinical and radiological presentation, a diagnosis of cryptococcal community-acquired pneumonia in solid organ transplant recipients may be delayed, leading to potential lethal outcomes. Radiology findings may vary from single or multiple pulmonary nodules, segmental or lobar consolidation to a reticulonodular pattern [7]. Although diagnosis can be easily made with a serum cryptococcal antigen test, its sensitivity – if requested at all – may be suboptimal in solid organ transplant recipients, particularly lung transplant recipients [8]. In this case, the initial diagnosis was based on a positive serum cryptococcal antigen test, requested on post-admission day 3 because of high clinical suspicion. Surrogate noninvasive diagnostic tests, such as measurement of serum cryptococcal antigen, are of major importance for the diagnosis of pulmonary infections due to a number of bacterial and fungal pathogens. However, noninvasive surrogate tests do not allow recovery of the pathogen and thus additional analyses. For instance, BAL allowed us to further confirm the diagnosis, identify the pathogen as C. gattii and perform additional susceptibility analyses. Sanger sequencing of the PCR-amplified intergenic spacer region IGS1 of the rRNA operon [9], a multilocus sequence typing marker with high allelic diversity [10], revealed 100% identity to allele 7. This allele has been associated with two different sequence types (STs) in the C. gattii multilocus sequence typing database, each represented by a single strain: CBS8742 (ST212, isolated in India) and IHEM11800 (ST218 isolated in Congo). Both of these strains have been recovered from human cerebrospinal fluid.
Furthermore, the detection of P. jirovecii was feasible following a bronchoscopy. P. jirovecii pneumonia is a well-known complication in solid organ transplant recipients, with an overall incidence rate of 0.01/1000 person-days in our region, according to the Swiss Transplant Cohort [11]. The diagnosisof P. jirovecii pneumonia remains challenging, although several identification methods are available. Molecular testing has improved the detection of P. jirovecii DNA; however, differentiation between infection and colonisation remains laborious [12]. In a study by Fauchier et al, a Pneumocystis PCR cycle threshold lower than 31 may exclude Pneumocystis colonisation in immunocompromised patients not infected with human immunodeficiency virus (HIV) with a specificity of 80% [13]. More data are required to define the level of PCR positivity that differentiates between Pneumocystis colonisation and infection. In this case, based on a cycle threshold value of 30, suggestive radiology findings and a high β-d-glucan titre, a probable diagnosis of P. jirovecii pneumonia was established.
The presence of a high level of β-d-glucan in the patient’s serum merits special discussion. The high-level β-d-glucan was attributed to a probable P. jirovecii pneumonia, as Cryptococcus spp. are commonly considered, along with the Mucorales, fungal pathogens associated with negative β-d-glucan [14]. Notably, β-d-glucan has been detected in the serum of patients with cryptococcal meningitis, but with a lower sensitivity and sensibility than in the cerebrospinal fluid [15]. Although it remains possible that severe cryptococcal infections may lead to higher blood concentrations of β-d-glucan, more data are required from carefully designed studies to definitively answer this question. Notably, there were no other potential confounders, such as cellulose filters, etc., that could have contributed to a false positive β-d-glucan in this case.
Immune reconstitution syndrome has been described in solid organ transplant recipients treated for cryptococcal infection, due to the initiation of an effective antifungal therapy in conjunction with rapid tapering of immunosuppression [16]. In a cohort of 89 transplant recipients with cryptococcosis, immune reconstitution syndrome developed in 13 patients at a median of 45 days (15–76 days) after initiation of antifungal therapy and was more likely to develop in patients with CNS disease or disseminated infection [17]. Immune reconstitution occurs during the first 2–5 weeks of antiretroviral treatment initiation in HIV patients treated for cryptococcal meningitis [18]. In fact, early initiation of antiretroviral treatment has been associated with higher early and long-term mortality in such patients [19]. It is likely that our patient might have also developed an immune reconstitution syndrome after antifungal treatment initiation and immunosuppression tapering, which responded to treatment with high-dose corticosteroids administered for the treatment of P. jirovecii pneumonia. However, the early timing and an alternative diagnosis of probable P. jirovecii pneumonia make it less likely.
In conclusion, this case illustrates that the differential diagnosis of community-acquired pneumonia in solid organ transplant recipients should include less frequent pathogens based on the epidemiology and possible exposures, with Cryptococcus gattii emerging as an important pathogen in Switzerland and Europe. The management of this infection could be challenging and the eventuality of coinfection or immune reconstitution syndrome need to be considered.
Written informed consent for publication was obtained from the patient.
We are grateful to Vincent Braunersreuther for assistance with mucicarmine stain.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Forrest GN , Bhalla P , DeBess EE , Winthrop KL , Lockhart SR , Mohammadi J , et al. Cryptococcus gattii infection in solid organ transplant recipients: description of Oregon outbreak cases. Transpl Infect Dis. 2015;17(3):467–76. doi:.https://doi.org/10.1111/tid.12370
2 Neofytos D , Horn D , Anaissie E , Steinbach W , Olyaei A , Fishman J , et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48(3):265–73. doi:.https://doi.org/10.1086/595846
3 George IA , Santos CAQ , Olsen MA , Powderly WG . Epidemiology of Cryptococcosis and Cryptococcal Meningitis in a Large Retrospective Cohort of Patients After Solid Organ Transplantation. Open Forum Infect Dis. 2017;4(1):ofx004. doi:.https://doi.org/10.1093/ofid/ofx004
4 Maziarz EK , Perfect JR . Cryptococcosis. Infect Dis Clin North Am. 2016;30(1):179–206. doi:.https://doi.org/10.1016/j.idc.2015.10.006
5 Cogliati M , D’Amicis R , Zani A , Montagna MT , Caggiano G , De Giglio O , et al. Environmental distribution of Cryptococcus neoformans and C. gattii around the Mediterranean basin. FEMS Yeast Res. 2016;16(4):fow045. doi:.https://doi.org/10.1093/femsyr/fow045
6 Hagen F , Colom MF , Swinne D , Tintelnot K , Iatta R , Montagna MT , et al. Autochthonous and dormant Cryptococcus gattii infections in Europe. Emerg Infect Dis. 2012;18(10):1618–24. doi:.https://doi.org/10.3201/eid1810.120068
7 Hatcher CR, Jr , Sehdeva J , Waters WC, 3rd , Schulze V , Logan WD, Jr , Symbas P , et al. Primary pulmonary cryptococcosis. J Thorac Cardiovasc Surg. 1971;61(1):39–49. doi:.https://doi.org/10.1016/S0022-5223(19)42273-3
8 Singh N , Alexander BD , Lortholary O , Dromer F , Gupta KL , John GT , et al. Pulmonary cryptococcosis in solid organ transplant recipients: clinical relevance of serum cryptococcal antigen. Clin Infect Dis. 2008;46(2):e12–8. doi:.https://doi.org/10.1086/524738
9 Litvintseva AP , Thakur R , Vilgalys R , Mitchell TG . Multilocus sequence typing reveals three genetic subpopulations of Cryptococcus neoformans var. grubii (serotype A), including a unique population in Botswana. Genetics. 2006;172(4):2223–38. doi:.https://doi.org/10.1534/genetics.105.046672
10 Meyer W , Aanensen DM , Boekhout T , Cogliati M , Diaz MR , Esposto MC , et al. Consensus multi-locus sequence typing scheme for Cryptococcus neoformans and Cryptococcus gattii. Med Mycol. 2009;47(6):561–70. doi:.https://doi.org/10.1080/13693780902953886
11 Neofytos D , Hirzel C , Boely E , Lecompte T , Khanna N , Mueller NJ , et al.; Swiss Transplant Cohort Study. Pneumocystis jirovecii pneumonia in solid organ transplant recipients: a descriptive analysis for the Swiss Transplant Cohort. Transpl Infect Dis. 2018;20(6):e12984. doi:.https://doi.org/10.1111/tid.12984
12 Fujisawa T , Suda T , Matsuda H , Inui N , Nakamura Y , Sato J , et al. Real-time PCR is more specific than conventional PCR for induced sputum diagnosis of Pneumocystis pneumonia in immunocompromised patients without HIV infection. Respirology. 2009;14(2):203–9. doi:.https://doi.org/10.1111/j.1440-1843.2008.01457.x
13 Fauchier T , Hasseine L , Gari-Toussaint M , Casanova V , Marty PM , Pomares C . Detection of Pneumocystis jirovecii by Quantitative PCR To Differentiate Colonization and Pneumonia in Immunocompromised HIV-Positive and HIV-Negative Patients. J Clin Microbiol. 2016;54(6):1487–95. doi:.https://doi.org/10.1128/JCM.03174-15
14 Obayashi T , Kawai T , Yoshida M , Mori T , Goto H , Yasuoka A , et al. Plasma (1-->3)-beta-D-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet. 1995;345(8941):17–20. doi:.https://doi.org/10.1016/S0140-6736(95)91152-9
15 Rhein J , Bahr NC , Morawski BM , Schutz C , Zhang Y , Finkelman M , et al. Detection of High Cerebrospinal Fluid Levels of (1→3)-β-d-Glucan in Cryptococcal Meningitis. Open Forum Infect Dis. 2014;1(3):ofu105. doi:.https://doi.org/10.1093/ofid/ofu105
16 Baddley JW , Forrest GN ; AST Infectious Diseases Community of Practice. Cryptococcosis in solid organ transplantation. Am J Transplant. 2013;13(s4, Suppl 4):242–9. doi:.https://doi.org/10.1111/ajt.12116
17 Sun HY , Alexander BD , Huprikar S , Forrest GN , Bruno D , Lyon GM , et al. Predictors of immune reconstitution syndrome in organ transplant recipients with cryptococcosis: implications for the management of immunosuppression. Clin Infect Dis. 2015;60(1):36–44. doi:.https://doi.org/10.1093/cid/ciu711
18 Boulware DR , Meya DB , Muzoora C , Rolfes MA , Huppler Hullsiek K , Musubire A , et al.; COAT Trial Team. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014;370(26):2487–98. doi:.https://doi.org/10.1056/NEJMoa1312884
19 Makadzange AT , Ndhlovu CE , Takarinda K , Reid M , Kurangwa M , Gona P , et al. Early versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub-saharan Africa. Clin Infect Dis. 2010;50(11):1532–8. doi:.https://doi.org/10.1086/652652
No financial support and no other potential conflict of interest relevant to this article was reported.