Examinations and assessments in patients with a newly acquired spinal cord injury – retrospective chart analysis as part of a quality improvement project
DOI: https://doi.org/10.4414/smw.2020.20291
Anke
Scheel-Sailerab*, Clara Odilia
Sailercd*, Patricia
Lamparta, Michael
Baumbergera, Markus
Bergera, Gabi
Muellera, Diana
Sigrist-Nixa, Klaus
Schmitta, Timo
Siepmannde, Jürgen
Pannekaf
aSwiss Paraplegic Centre (SPC), Nottwil,
bDepartment of Health Sciences and Medicine,
cDepartment of Endocrinology, Diabetology and Metabolism, University Hospital Basel, Department of Clinical Research,
dDresden International University, Division of Health Care Sciences,
eDepartment of Neurology, University Hospital Carl Gustav Carus,
fDepartment of Urology, Inselspital, Bern University Hospital,
Examinations and assessments in patients with a newly acquired spinal cord injury – retrospective chart analysis as part of a quality improvement project
Summary
AIMS OF THE STUDY
Examinations and assessments can be used to ensure good quality rehabilitation. Within the framework of a quality improvement project, the aims of the current analysis were: first, to analyse the time points of selected examinations and assessments in the rehabilitation process of patients with a newly acquired spinal cord injury. Second, to identify differences between the subgroups with different aetiologies, levels and completeness of spinal cord injuries. And third, to compare the examinations and assessments performed with the guideline recommendations and to use discrepancies as a starting point for a quality improvement project.
METHODS
In this retrospective chart analysis, adult patients with a newly acquired spinal cord injury who were admitted to a single specialised acute care and rehabilitation clinic for their first rehabilitation between December 2013 and December 2014 were included and assessed until discharge. The main objective was to assess the time to examinations or assessments after injury or hospital admission in comparison to the respective recommendations. Analyses were done using time-to-event analysis and represented graphically using Kaplan-Meier plots.
RESULTS
Of the 105 patients included in this study (median age 58 years, 29% female), 61% had a traumatic and 39% a non-traumatic spinal cord injury; 39% were paraplegic and 61% were quadriplegic; and 59% had a motor complete and 41% a sensor-motor incomplete spinal cord injury. The percentage of patients for whom the respective assessment or examination was performed and the percentage of these patients for whom it performed within the recommended time were: 90% and 71% for magnetic resonance imaging; 85% and 90% for computed tomography; 87% and 79% for the manual muscle test; 95% and 59% for the International Standards for Neurological Classification of Spinal Cord (ISNCSCI); 84% and 50% for electrophysiological assessment; 73% and 90% for urodynamic testing; and 49% and 53% for lung function testing.
CONCLUSIONS
Our data suggest a relevant gap between recommendations and clinical routine for time to some assessments after spinal cord injury. Within the framework of a quality improvement project, the next steps should be to build a national and international consensus on specific time frames for examinations and assessments in patients with a newly acquired spinal cord injury and thereafter, to develop an institutional implementation strategy.
Introduction
Quality management in rehabilitation is defined in the Rehab Cycle® and consists of treatment processes, patient health outcomes, the structural quality of the rehabilitation institution and principles of continuous improvement in all these areas [1, 2]. In the rehabilitation process, the impaired functioning due to a patient’s injury or disease is described within the biopsychosocial model of the International Classification of Functioning, Disability and Health (ICF) [3]. Based on the impaired functioning, specific health interventions (e.g., self-care training and mobility training) are selected, and these guide goal setting which respects the patient’s individual preferences [4, 5]. Examinations (e.g., urodynamic testing, magnetic resonance imaging (MRI)) and assessments (e.g., SCIM-III, ISNCSCI) are used to describe the patient’s lesion and functioning and to inform the patient’s expected outcome, i.e. neurological recovery and the resulting improvement in functioning [5]. The expected outcomes in relation to the patient’s diagnosis are disease specific, comparable and clearly defined [6, 7]. Ultimately, these data describing the expected outcome can be used to improve the quality of the health intervention through the evaluation of examinations, assessments, intervention planning and patient outcomes.
A spinal cord injury exemplifies a complex condition with impaired functioning in various areas of the biopsychosocial model [8]. The initial acute care requires knowledge from the fields of traumatology, neurology, urology and pneumology. Different examinations and assessments are used to initiate treatment processes and prevent complications during both acute care and rehabilitation [9]. Importantly, the recovery of functions is only party predictable, so a highly flexible rehabilitation process is required [10, 11]. The complexity of the rehabilitation process in patients with a spinal cord injury means that clear recommendations on when to perform examinations and assessments are needed. Within the framework of a quality improvement project, international initiatives agreed, as a first step, to reach a consensus based on common data sets describing the relevant areas of impairment [12]. In addition, national and international cohort studies were initiated to increase the understanding of health conditions and to develop better outcome prediction models (e.g., Swiss Spinal Cord Injury Cohort Study [SwiSCI], European Multicenter Study about Spinal Cord Injury [EMSCI]) [13–15]. Recently, assessments such as lung function testing to detect subclinical impairment have been added to reduce complications and to increase patients’ quality of life [16–18]. As a first step in this quality improvement project, a situational analysis of routinely applied assessments during first rehabilitation of patients with a newly acquired spinal cord injury was performed [19]. This study by Lampart et al. indicated that some recommended examinations and assessments were performed in accordance with the guidelines (e.g., the Spinal Cord Independence Measure III [SCIM III]), whereas others (e.g., ISNCSCI) were not performed as recommended [19]. In preparing to implement the institutional standards, it became evident that as well as the choice of examinations and assessments, the time frames of their performance should also be included. This led to the research question of which examinations and assessments are performed within the recommended time frame and the need to identify subgroups where examinations and assessments are not performed as recommended.
The main aim of the current study was therefore to identify the time to clinical assessments in an acute and rehabilitation clinic and to compare the results with the time frames recommended for patients after a newly acquired spinal cord injury in guidelines. Secondary objectives were to analyse subgroups (e.g., paraplegic versus quadriplegic patients) and to evaluate reasons why the assessments were not performed within the recommended time frames. Ultimately, this line of research will provide criteria for concrete guideline recommendations and data to initiate a tailored quality improvement project.
Materials and methods
Study design
This is a retrospective chart analysis performed within the framework of a quality improvement project for patients with a newly acquired spinal cord injury [19]. The main objective of this study was to investigate the time to selected examinations and assessments in these patients.
Participants
Patients over 18 years of age with a newly acquired spinal cord injury who were admitted for their first rehabilitation to a single specialised acute care and rehabilitation spinal cord injury clinic in Switzerland between December 2013 and December 2014 were eligible and were followed until their discharge in 2015. Patients with a neuroinflammatory disease (e.g., Guillain-Barre syndrome, critical illness polyneuropathy) were excluded, unlike in the earlier publication [19], as these syndromes are considered spinal cord injury-similar diseases and therefore the analysed recommendations and guidelines do not apply to this patient population.
The Ethics Committee of Northwest and Central Switzerland approved this study (EKNZ UBE-15/109). We conducted the study according to the ICH-GCP guidelines and all national legal regulatory requirements, and excluded patients who denied their consent to the retrospective analysis of their data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines [20].
Data collection
Data were reversibly pseudonymised and stored locally in a database of the hospital’s clinical trial unit.
Patient data
The following patient characteristics were obtained from medical records [21]: age on admission, sex, aetiology of spinal cord injury (i.e., traumatic versus non-traumatic), level of spinal cord injury (i.e., paraplegic versus quadriplegic), completeness of spinal cord injury (i.e., motor complete versus sensor-motor incomplete according to ISNCSCI), time to hospital admission after spinal cord injury, length of hospital stay, and date of examination and assessment. Data were retrieved from four electronic databases used in clinical routine: Medfolio (Nexus, Switzerland), a database for medical information; WicareDoc (Wigasoft, Switzerland), a documentation database used by nurses and therapists; PACS, (Phönix-PACS GmbH, Germany), a radiological data storing system; and Patient Management Cockpit (SPARE GmbH, Switzerland), a system linking the above databases.
Assessments
The following examinations and assessments were identified as being performed insufficiently often [19] within the time frame recommended by the EMSCI [22] or by the clinic’s internal recommendation list [14]: magnetic resonance imaging (MRI) and computed tomography (CT) of the spine during initial treatment (trauma protocol), recommended within eight days after newly acquired spinal cord injury [23]; manual muscle test of the lower extremity, recommended within 28 days after newly acquired spinal cord injury [24]; International Standards For Neurological Classification of Spinal Cord Injury (ISNCSCI), recommended either within eight days after admission to a specialised acute and rehabilitation clinic or within 40 days after newly acquired spinal cord injury [25–27]; electrophysiological evaluation of the lower extremity, recommended within 40 days after newly acquired spinal cord injury [28]; urodynamic testing, recommended within 84 days after newly acquired spinal cord injury [18]; and lung function testing, recommended within 40 days after newly acquired spinal cord injury [29].
Statistical analysis
Discrete variables are expressed as frequencies (percentage and number of participants, n) and continuous variables as means with standard deviation or medians with interquartile range (IQR 25th–75th percentiles), according to the data distribution. A time-to-event analysis was carried out to assess the median time, with 95% confidence interval (95% CI), to examination/assessment after injury and/or hospital admission.
Graphical analysis was done by creating Kaplan-Meier plots. The recommended time of each examination/assessment was shown with grey shading. Visual and statistical subgroup analyses were performed for aetiology of spinal cord injury (i.e., traumatic versus non-traumatic), level of spinal cord injury (i.e., paraplegic versus quadriplegic) and completeness of spinal cord injury (i.e., motor complete versus sensor-motor incomplete according to ISNCSCI).
Two-group comparison of continuous data was performed using an unpaired two-sample t-test or a Wilcoxon rank sum test according to the data distribution. Two-group comparison of categorical data was performed using the chi-square test. A two-sided p-value <0.05 was considered to indicate statistical significance. Analyses were performed using R statistical software [30].
Results
Baseline characteristics
A total of 105 patients with a median age of 58 years (IQR 33; 70), 30 (29%) of whom were female, were included in this study. 61% of patients had a traumatic and 39% a non-traumatic spinal cord injury; 39% of patients were paraplegic and 61% were quadriplegic; and 59% of patients had a motor complete (ISNCSCI A and B) and 41% a sensor-motor incomplete (ISNCSCI C and D) lesion (table 1). Compared to patients with a non-traumatic spinal cord injury, patients with a traumatic spinal cord injury were significantly younger, more often quadriplegic, more often had a motor complete lesion and had a longer hospital stay (table 2). Quadriplegic patients more often had a traumatic spinal cord injury and were admitted to a specialised rehabilitation clinic earlier (supplementary table S1 in appendix 1).
Table 1 Baseline characteristics.
|
Characteristic
|
Total (n = 105)
|
| Age (years), median (IQR) |
58 (33; 70) |
| Sex (female), n (%) |
30 (29) |
| Paraplegia, n (%) |
41 (39) |
| Quadriplegia, n (%) |
64 (61) |
| Traumatic SCI, n (%) |
64 (61) |
| Non-traumatic SCI, n (%) |
41 (39) |
| Completeness of SCI, n (%) |
|
| ISNCSCI A |
36 (34) |
| ISNCSCI B |
26 (25) |
| ISNCSCI C |
21 (20) |
| ISNCSCI D |
22 (21) |
| Time to hospital admission after SCI (days), median (IQR) |
13 (5; 24) |
| Length of hospital stay (days), median (IQR) |
179 (111; 244) |
Table 2 Baseline characteristics – traumatic versus non-traumatic spinal cord injury.
|
Characteristic
|
Traumatic SCI
(n = 64)
|
Non-traumatic SCI (n = 41)
|
p-value*
|
| Age (years), median (IQR) |
50 (30; 66) |
65 (51; 75) |
0.001
|
| Sex (female), n (%) |
14 (22) |
16 (40) |
0.09 |
| Paraplegia, n (%) |
18 (28) |
23 (56) |
0.008
|
| Quadriplegia, n (%) |
46 (72) |
18 (44) |
0.008
|
| Completeness of SCI, n (%) |
|
|
0.002
|
| ISNCSCI A |
31 (49) |
5 (12) |
| ISNCSCI B |
13 (20) |
13 (32) |
| ISNCSCI C |
11 (17) |
10 (24) |
| ISNCSCI D |
9 (14) |
13 (32) |
| Time to hospital admission after SCI (days), median (IQR) |
10 (4; 23) |
16 (9; 31) |
0.06 |
| Length of hospital stay (days), median (IQR) |
191 (148; 263) |
147 (96; 185) |
0.009
|
Clinical examination or assessment schedule
An overview of the clinical examinations and assessments schedule for all patients is displayed in table 3.
Table 3 Time to examination and assessment after hospital admission and spinal cord injury.
Examination or
assessment*
|
Number of assessments performed†
|
Time to assessment after SCI (days)
|
Recommended time to assessment after SCI (days)
|
Assessments performed in recommended time after SCI†
|
Time to assessment after hospital admission (days)
|
Recommended time to assessment after hospital admission (days)
|
Assessments performed in recommended time after hospital admission‡
|
|
n (%)
|
Median (95% CI)
|
Range
|
n (%)
|
Median (95% CI)
|
Range
|
n (%)
|
| MRI of the spine [22] |
94 (90) |
1 (1; 5) |
0-8 |
67 (71) |
NR |
NR |
NR |
| CT of the spine [22] |
89 (85) |
1 (0; 1) |
0-8 |
80 (90) |
NR |
NR |
NR |
| Manual muscle test of the lower extremity [23] |
91 (87) |
NR |
NR |
NR |
5 (4; 8) |
0-14 |
72 (79) |
| ISNCSCI [24–26] |
100 (95) |
37 (31; 43) |
0-40 |
59 (59) |
20 (16; 24) |
0-8 |
28 (28) |
| Electro-physiological evaluation of the lower extremity [27] |
88 (84) |
43 (38; 48) |
0-40 |
44 (50) |
NR |
NR |
NR |
| Urodynamic testing [17] |
81 (73) |
67 (59; 73) |
0-84 |
73 (90) |
NR |
NR |
NR |
| Lung function testing [28] |
51 (49) |
117 (57; inf) |
0-40 |
27 (53) |
NR |
NR |
NR |
Magnetic resonance imaging and computed tomography of the spine
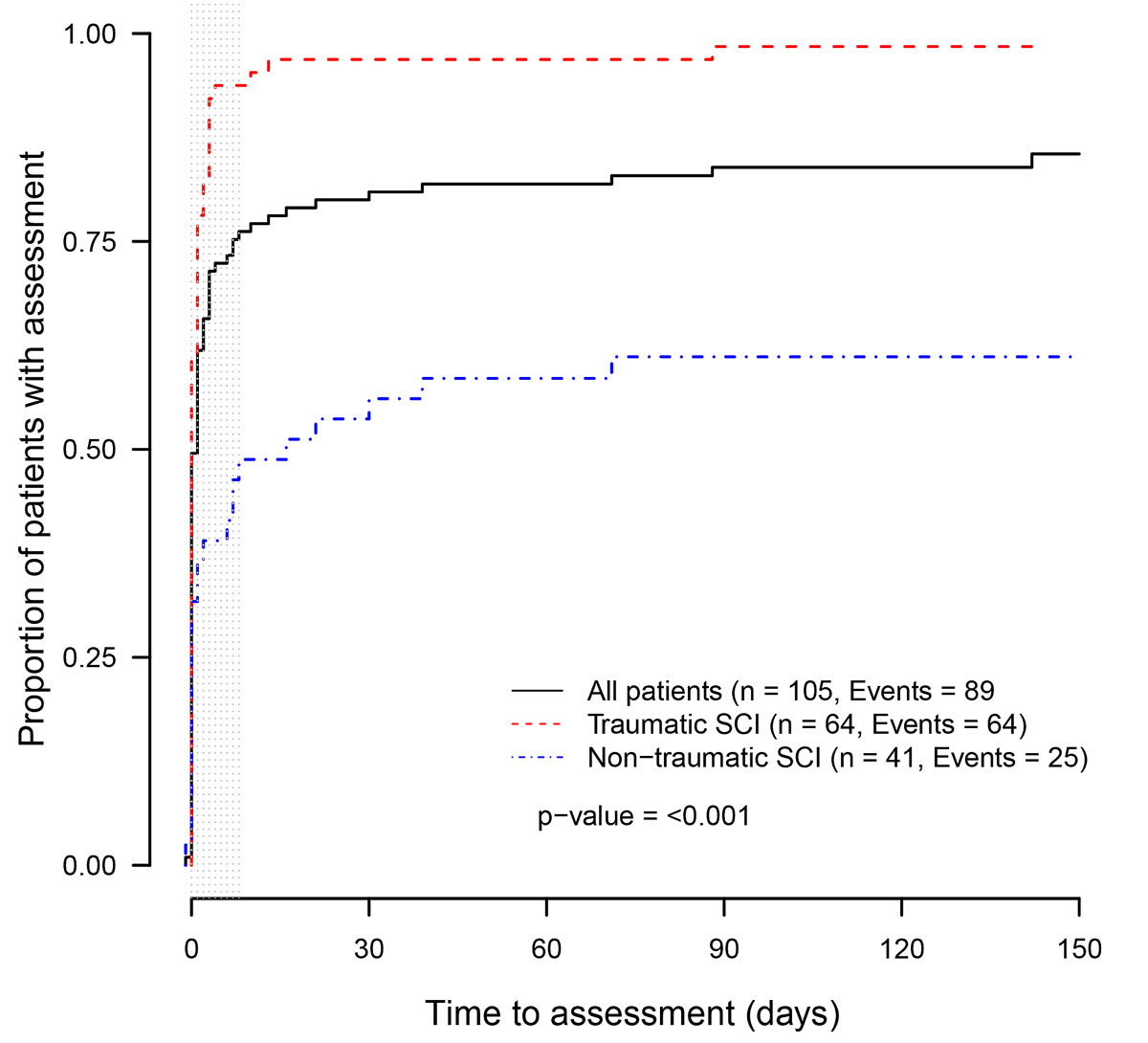
Ninety percent of all included patients had an MRI scan during their acute care and first rehabilitation, and 71% of these patients had the examination within the recommended time of eight days after spinal cord injury (fig. 1). Patients with a non-traumatic spinal cord injury received an MRI of the spine within a shorter time frame and more frequently compared to patients with a traumatic spinal cord injury (p = 0.0015; fig. 1). Quadriplegic patients tended to receive an MRI within a shorter time frame and more frequently compared to paraplegic patients (p = 0.09). There was no significant difference between the subgroups of completeness of spinal cord injury (p = 0.9).
Eighty-five percent of all included patients had a CT scan of the spine during their acute care and first rehabilitation, and 90% of these patients had the examination within the recommended time of eight days after spinal cord injury (fig. 2). Patients with a traumatic spinal cord injury received a CT scan of the spine within a shorter time frame and more frequently compared to patients with a non-traumatic spinal cord injury (p <0.001: fig. 2). Quadriplegic patients had CT within a shorter time frame and more frequently compared to paraplegic patients (p = 0.03). There was no significant difference between the subgroups of completeness of spinal cord injury (p = 0.16).
Manual muscle test of the lower extremity
Eighty-seven percent of all included patients had a manual muscle test during their first rehabilitation, and 79% of these patients had the assessment within the recommended time of 14 days after admission to the specialised acute care and rehabilitation clinic (fig. 3A and B). There were no statistically significant differences between the subgroups of aetiology of spinal cord injury (p = 0.07; fig. 3A), level of spinal cord injury (p = 0.9) or completeness of spinal cord injury (p = 0.11).
International Standards for Neurological Classification of Spinal Cord Injury
Ninety-five percent of all included patients had an ISNCSCI during their first rehabilitation; 28% of these patients had the assessment within the recommended time of eight days after admission to the specialised acute care and rehabilitation clinic and 59% of them had the assessment within the recommended time of 40 days after spinal cord injury. There were no statistically significant differences between the subgroups of aetiology of spinal cord injury (p = 0.44; fig. 4A and B) or completeness of spinal cord injury (p = 0.11; fig. 3b). Paraplegic patients received the ISNCSCI earlier and more often than quadriplegic patients (p = 0.003).
Electrophysiological evaluation of the lower extremity
Eighty-four percent of all included patients had an electrophysiological evaluation of the lower extremity during their first rehabilitation, and 50% of these patients had the examination within the recommended time of 40 days after spinal cord injury. There were no statistically significant differences between the subgroups of aetiology (p = 0.4; supplementary fig. S1 in appendix 1), level of spinal cord injury (p = 0.11) or completeness of spinal cord injury (p = 0.83).
Urodynamic testing
Seventy-three percent of all patients had urodynamic testing during their first rehabilitation and 90% of these patients had the examination within the recommended time of 84 days after spinal cord injury. There were no statistically significant differences between the subgroups of aetiology of spinal cord injury (p = 0.88; fig. S2) or completeness of spinal cord injury (p = 0.5). Patients with a paraplegic spinal cord injury received urodynamic testing earlier and more often than patients with a quadriplegic spinal cord injury (p = 0.004).
Lung function testing
Forty-nine percent of all patients had lung function testing during their first rehabilitation, and 53% of these patients had the examination within the recommended time of 40 days after spinal cord injury. There were no statistically significant differences between the subgroups for aetiology of spinal cord injury (p = 0.8), level of spinal cord injury (p = 0.1; fig. S3) or completeness of spinal cord injury (p = 0.6).
Discussion
The three main findings of this study are: first, some spinal cord injury-specific examinations, such as CT for traumatic spinal cord injury and MRI for non-traumatic spinal cord injury, and some assessments, such as manual muscle status or urodynamic testing, are conducted within the proposed time frame in nearly all patients. Second, other assessments specific to therapeutic planning, such as the ISNCSCI or the electrophysiological evaluation of the lower extremity, are conducted in a large number of patients, but often later than recommended. Third, some spinal cord injury-specific assessments, such as lung function testing, are performed less often, and if they are conducted it is often later than recommended.
Our data show that MRI and CT of the spine, as well as the manual muscle test, were performed within a reasonable time frame in nearly all patients. A possible reason for that is a high awareness of the importance of these examinations and assessments for the planning of treatment. While the results of the MRI and CT are used to decide between surgical or conservative treatment, the manual muscle test is used to guide individual physiotherapy [17].
The ISNCSCI and electrophysiological evaluation of the lower extremity were performed in nearly all patients, but often later than recommended. The ISNCSCI is only one of many assessments that form part of the rehabilitation process overseen by the ward physicians, and the neurologist is responsible for the electrophysiological evaluation of the lower extremity. Both assessments are used initially to predict recovery and for goal setting, but they do not have immediate therapeutic consequences [28]. Young physicians especially could be overwhelmed by the large number of assessments to be considered when rehabilitating patients with an acute spinal cord injury. Guidelines explaining the benefits of these assessments to both the treating physician and the patient could help to raise awareness of these assessments. Additionally, the ISNCSCI is not part of the medical students’ curriculum, and is a complex tool that requires adequate training programs [31, 32]. Including thorough information about the importance of this assessment may remind young physicians of the importance of this assessment.
Urodynamic testing and lung function testing are complex, spinal cord injury-specific examinations that require specialised physicians. Recent data have highlighted the importance of both assessments, especially for predicting recovery, improving subclinical dysfunctions, preventing complications and improving quality of life [33–35]. Complications such as urinary tract infection, hydronephrosis, renal failure, pneumonia, sleep-disordered breathing and dyspnoea are associated with a reduced quality of life and increased morbidity and mortality [33, 34, 36, 37]. While urodynamic testing is performed largely in accordance with the guidelines, lung function testing, especially in paraplegic patients, is neglected. One explanation for this difference might be that only recent data have highlighted the consequences of subclinical lung function impairment (36, 37). Furthermore, patients with a paraplegic spinal cord injury tend to receive even less lung function testing. A relation with the underlying diagnosis and a lack of awareness of lung complications could explain this difference. The small sample size is likely the reason why the divergent lines in the graph are not statistically significant.
The results of this analysis indicate that the majority of examinations and assessments used for treatment planning are performed in accordance with existing guidelines. Complex examinations and assessments which are not directly linked to immediate treatment planning seem to require better control systems and clear implementation strategies. One way to improve guideline adherence could be to define clear guidelines on when to perform examinations and assessments based on clinical data. Additionally, raising awareness of the importance of the examinations/assessments with regards to treatment planning and preventing complications could improve the guideline adherence of physicians. As a next step, we propose building a national and international consensus on the appropriate timing of examinations and assessments, as well as studies comparing assessment schedules between different centres. Furthermore, control systems should be established to optimise patient care.
Some limitations of our study must be mentioned. First, this is a secondary analysis of a retrospective chart analysis of adult patients with newly acquired spinal cord injury admitted to a single specialised spinal cord injury clinic for their first rehabilitation. As this is a rather specific cohort, and as the data were collected several years ago, the generalisability of the results is limited. However, this situational analysis demonstrates important principles that can be used as a foundation for prospective, multicentre studies to compare health-related outcomes and improve the quality of healthcare. Additionally, since until recently no quality improvement project was implemented in this setting, additional data collection after 2015 would most likely not change our results. Second, we have no information about the specific reasons for not conducting assessments and can only speculate that comorbidities or lack of consent from the patient might have been the reason. This is an important part of the process of guideline development and should be addressed in a further study. Third, we only looked at selected assessments. However, these were the most relevant ones with which to examine times to assessments in patients with newly acquired spinal cord injury. To build a more complete picture of the complex process of evaluating outcomes further assessments, namely mental health assessments, could be included in future studies. Lastly, there are currently no outcomes specified which assess whether the regularly performed assessments improve the quality of the rehabilitation process. This is of high importance for the future development of clinical guidelines.
The main strength of this study is its investigation of the time to clinical assessments in patients with a newly acquired spinal cord injury in a consecutive sample. This study might be important for re-defining guidelines and ultimately improving quality management.
In conclusion, to improve the quality of healthcare in patients with a newly acquired spinal cord injury, guidelines should include data from clinical practice. Our data suggest a relevant gap between recommendations and clinical routine regarding the time to some examinations/assessments after spinal cord injury. Raising awareness of the clinical relevance of these assessments might be a beneficial starting point. Within the framework of a quality improvement project, the next steps should be to build a consensus on a national and international standard for examinations and assessments after newly acquired spinal cord injury and to develop an implementation strategy based on institutional situation analysis.
Appendix 1 Supplementary data
Table S1: Baseline characteristics – paraplegic versus quadriplegic spinal cord injury.
Figure S1: Electrophysiology of the lower extremity after spinal cord injury (SCI) for patients with a traumatic versus non-traumatic SCI.
Figure S2: Urodynamic assessment after spinal cord injury (SCI) for patients with a traumatic versus non-traumatic SCI.
Figure S3: Lung function after spinal cord injury (SCI) for patients with a paraplegic versus quadriplegic SCI.
The appendix is available as a separate file at https://smw.ch/article/doi/smw.2020.20291.
Acknowledgments
We thank the patients for their valuable support in the conception of the study and in the discussion of the results. We also thank the interdisciplinary team and the varied health professionals for their assistance with the data collection. This work is part of a master’s thesis for the Master’s Programme in Clinical Research, Centre for Clinical Research and Management Education, Division of Health Care Sciences, Dresden International University, Dresden, Germany.
*
Equally contributing first authors
References
1Maritz R, Scheel-Sailer A, Schmitt K, Prodinger B. Overview of quality management models for inpatient healthcare settings. A scoping review. Int J Qual Heal Care. 2018.
2
Steiner
WA
,
Ryser
L
,
Huber
E
,
Uebelhart
D
,
Aeschlimann
A
,
Stucki
G
. Use of the ICF model as a clinical problem-solving tool in physical therapy and rehabilitation medicine. Phys Ther. 2002;82(11):1098–107. doi:.https://doi.org/10.1093/ptj/82.11.1098
3
Meyer
T
,
Gutenbrunner
C
,
Bickenbach
J
,
Cieza
A
,
Melvin
J
,
Stucki
G
. Towards a conceptual description of rehabilitation as a health strategy. J Rehabil Med. 2011;43(9):765–9. doi:.https://doi.org/10.2340/16501977-0865
4
Stucki
G
,
Sangha
O
. Clinical quality management: putting the pieces together. Arthritis Care Res. 1996;9(5):405–12. doi:.https://doi.org/10.1002/1529-0131(199610)9:5<405::AID-ANR1790090510>3.0.CO;2-2
5
Uitz
E
,
Fransen
J
,
Langenegger
T
,
Stucki
G
; Swiss Clinical Quality Management in Rheumatoid Arthritis. Clinical quality management in rheumatoid arthritis: putting theory into practice. Rheumatology (Oxford). 2000;39(5):542–9. doi:.https://doi.org/10.1093/rheumatology/39.5.542
6
Nelson
EC
,
Godfrey
MM
,
Batalden
PB
,
Berry
SA
,
Bothe
AE, Jr
,
McKinley
KE
, et al.
Clinical microsystems, part 1. The building blocks of health systems. Jt Comm J Qual Patient Saf. 2008;34(7):367–78. doi:.https://doi.org/10.1016/S1553-7250(08)34047-1
7
Wasson
JH
,
Anders
SG
,
Moore
LG
,
Ho
L
,
Nelson
EC
,
Godfrey
MM
, et al.
Clinical microsystems, part 2. Learning from micro practices about providing patients the care they want and need. Jt Comm J Qual Patient Saf. 2008;34(8):445–52. doi:.https://doi.org/10.1016/S1553-7250(08)34055-0
8
Kirchberger
I
,
Cieza
A
,
Biering-Sørensen
F
,
Baumberger
M
,
Charlifue
S
,
Post
MW
, et al.
ICF Core Sets for individuals with spinal cord injury in the early post-acute context. Spinal Cord. 2010;48(4):297–304. doi:.https://doi.org/10.1038/sc.2009.128
9
Fehlings
MG
,
Tetreault
LA
,
Wilson
JR
,
Kwon
BK
,
Burns
AS
,
Martin
AR
, et al.
A Clinical Practice Guideline for the Management of Acute Spinal Cord Injury: Introduction, Rationale, and Scope. Global Spine J. 2017;7(3, Suppl):84S–94S. doi:.https://doi.org/10.1177/2192568217703387
10
Curt
A
,
Van Hedel
HJA
,
Klaus
D
,
Dietz
V
; EM-SCI Study Group. Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J Neurotrauma. 2008;25(6):677–85. doi:.https://doi.org/10.1089/neu.2007.0468
11Chhabra HS. ISCoS textbook on comprehensive management of spinal cord injuries. First edition. New Delhi: Wolters Kluwer; 2015.
12
Biering-Sørensen
F
,
Alai
S
,
Anderson
K
,
Charlifue
S
,
Chen
Y
,
DeVivo
M
, et al.
Common data elements for spinal cord injury clinical research: a National Institute for Neurological Disorders and Stroke project. Spinal Cord. 2015;53(4):265–77. doi:.https://doi.org/10.1038/sc.2014.246
13
Noonan
VK
,
Kwon
BK
,
Soril
L
,
Fehlings
MG
,
Hurlbert
RJ
,
Townson
A
, et al.; RHSCIR Network. The Rick Hansen Spinal Cord Injury Registry (RHSCIR): a national patient-registry. Spinal Cord. 2012;50(1):22–7. doi:.https://doi.org/10.1038/sc.2011.109
14
Post
MWM
,
Brinkhof
MWG
,
von Elm
E
,
Boldt
C
,
Brach
M
,
Fekete
C
, et al.; SwiSCI study group. Design of the Swiss Spinal Cord Injury Cohort Study. Am J Phys Med Rehabil. 2011;90(11, Suppl 2):S5–16. doi:.https://doi.org/10.1097/PHM.0b013e318230fd41
15
Cieza
A
,
Boldt
C
,
Ballert
CS
,
Eriks-Hoogland
I
,
Bickenbach
JE
,
Stucki
G
. Setting up a cohort study on functioning: deciding what to measure. Am J Phys Med Rehabil. 2011;90(11, Suppl 2):S17–28. doi:.https://doi.org/10.1097/PHM.0b013e318230fddb
16
Mueller
G
,
de Groot
S
,
van der Woude
LH
,
Perret
C
,
Michel
F
,
Hopman
MT
. Prediction models and development of an easy to use open-access tool for measuring lung function of individuals with motor complete spinal cord injury. J Rehabil Med. 2012;44(8):642–7. doi:.https://doi.org/10.2340/16501977-1011
17Scheel-Sailer A. Rehabilitation der unteren Extremität, der Steh- und Gehfunktion von Menschen mit Querschnittlähmung. AMWF. 2018:179-009.
18Böthig R. Querschnittgelähmte Patienten, neuro-urologische Versorgung. AWMF. 2016:179-001.
19
Lampart
P
,
Gemperli
A
,
Baumberger
M
,
Bersch
I
,
Prodinger
B
,
Schmitt
K
, et al.
Administration of assessment instruments during the first rehabilitation of patients with spinal cord injury: a retrospective chart analysis. Spinal Cord. 2018;56(4):322–31. doi:.https://doi.org/10.1038/s41393-017-0039-x
20
von Elm
E
,
Altman
DG
,
Egger
M
,
Pocock
SJ
,
Gøtzsche
PC
,
Vandenbroucke
JP
; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–9. doi:.https://doi.org/10.1016/j.ijsu.2014.07.013
21
Chamberlain
JD
,
Ronca
E
,
Brinkhof
MW
. Estimating the incidence of traumatic spinal cord injuries in Switzerland: Using administrative data to identify potential coverage error in a cohort study. Swiss Med Wkly. 2017;147:w14430.
22EMSCI. International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI).
23
Walters
BC
,
Hadley
MN
,
Hurlbert
RJ
,
Aarabi
B
,
Dhall
SS
,
Gelb
DE
, et al.; American Association of Neurological Surgeons; Congress of Neurological Surgeons. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery. 2013;60(CN_suppl_1):82–91. doi:.https://doi.org/10.1227/01.neu.0000430319.32247.7f
24
Kahn
JH
,
Tappan
R
,
Newman
CP
,
Palma
P
,
Romney
W
,
Tseng Stultz
E
, et al.
Outcome Measure Recommendations From the Spinal Cord Injury EDGE Task Force. Phys Ther. 2016;96(11):1832–42. doi:.https://doi.org/10.2522/ptj.20150453
25
Betz
R
,
Biering-Sørensen
F
,
Burns
SP
,
Donovan
W
,
Graves
DE
,
Guest
J
, et al., ASIA and ISCoS International Standards Committee. The 2019 revision of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)-What’s new?
Spinal Cord. 2019;57(10):815–7. doi:.https://doi.org/10.1038/s41393-019-0350-9
26
Eng
JJ
,
Teasell
R
,
Miller
WC
,
Wolfe
DL
,
Townson
AF
,
Aubut
JA
, et al.; the SCIRE Research Team. Spinal cord injury rehabilitation evidence: Method of the SCIRE systematic review. Top Spinal Cord Inj Rehabil. 2007;13(1):1–10. doi:.https://doi.org/10.1310/sci1301-1
27EMSCI. European Multicenter Study about Spinal Cord Injury [Internet]. Available from: https://www.emsci.org/index.php
28
Curt
A
,
Dietz
V
. Electrophysiological recordings in patients with spinal cord injury: significance for predicting outcome. Spinal Cord. 1999;37(3):157–65. doi:.https://doi.org/10.1038/sj.sc.3100809
29
Raab
AM
,
Krebs
J
,
Perret
C
,
Pfister
M
,
Hopman
M
,
Mueller
G
. Evaluation of a clinical implementation of a respiratory muscle training group during spinal cord injury rehabilitation. Spinal Cord Ser Cases. 2018;4(1):40. doi:.https://doi.org/10.1038/s41394-018-0069-4
30R Core T. R. A Language and Environment for Statistical Computing. 2018 [cited 2019 Jan 14]; Available from: https://www.r-project.org/
31
Rupp
R
; ASIA International Standards Committee; ASIA Education Committee. Assessor accuracy of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)-recommendations for reporting items. Spinal Cord. 2018;56(8):819–20. doi:.https://doi.org/10.1038/s41393-018-0133-8
32
Schuld
C
,
Franz
S
,
van Hedel
HJA
,
Moosburger
J
,
Maier
D
,
Abel
R
, et al.; EMSCI study group. International standards for neurological classification of spinal cord injury: classification skills of clinicians versus computational algorithms. Spinal Cord. 2015;53(4):324–31. doi:.https://doi.org/10.1038/sc.2014.221
33
Pavese
C
,
Schneider
MP
,
Schubert
M
,
Curt
A
,
Scivoletto
G
,
Finazzi-Agrò
E
, et al.
Prediction of Bladder Outcomes after Traumatic Spinal Cord Injury: A Longitudinal Cohort Study. PLoS Med. 2016;13(6):e1002041. doi:.https://doi.org/10.1371/journal.pmed.1002041
34
Raab
AM
,
Krebs
J
,
Perret
C
,
Michel
F
,
Hopman
MT
,
Mueller
G
. Maximum Inspiratory Pressure is a Discriminator of Pneumonia in Individuals With Spinal-Cord Injury. Respir Care. 2016;61(12):1636–43. doi:.https://doi.org/10.4187/respcare.04818
35
Pannek
J
,
Kullik
B
. Does optimizing bladder management equal optimizing quality of life? Correlation between health-related quality of life and urodynamic parameters in patients with spinal cord lesions. Urology. 2009;74(2):263–6. doi:.https://doi.org/10.1016/j.urology.2009.02.047
36
Brommer
B
,
Engel
O
,
Kopp
MA
,
Watzlawick
R
,
Müller
S
,
Prüss
H
, et al.
Spinal cord injury-induced immune deficiency syndrome enhances infection susceptibility dependent on lesion level. Brain. 2016;139(3):692–707. doi:.https://doi.org/10.1093/brain/awv375
37
Mueller
G
,
de Groot
S
,
van der Woude
L
,
Hopman
MTE
. Time-courses of lung function and respiratory muscle pressure generating capacity after spinal cord injury: a prospective cohort study. J Rehabil Med. 2008;40(4):269–76. doi:.https://doi.org/10.2340/16501977-0162