
Figure 1 Initial evaluation pathway for OI patient in the CHUV OI group.; CHUV = Centre Hospitalier Universitaire Vaudois; DXA = dual X-ray absorptiometry; OI = osteogenesis imperfecta
DOI: https://doi.org/10.4414/smw.2020.20285
Osteogenesis imperfecta is a rare genetic connective tissue disorder with a wide phenotypic and molecular heterogeneity. The characteristic features and severity of OI vary greatly from person to person [1]. Skeletal fragility results in an increased risk of bone fracture in early life and progressive bone deformities, and extra-skeletal manifestations can lead to dental alterations (dentinogenesis imperfecta) and/or hearing impairment [2, 3]. Despite expanding research into its treatment, OI remains a burden that limits a person in daily activities and significantly impairs quality of life [4–6].
Over the last decade, numerous efforts have been made, with considerable emphasis, by national institutions, leading to the recognition of rare diseases by the European Union (EU). The “concept of rare disease” was acknowledged by the Swiss Federal Council in 2014, triggering its recognition and implementation in the 2020 Health Strategy Plan. This “concept” highlights intensive efforts to achieve global care for patients with a rare disease. Creating reference centres, enhancing care, adopting an interdisciplinary approach, educating patients and their loved ones, and increasing funding for research and treatment are all desired goals.
By adopting an interdisciplinary approach, we aimed at sharing information and educating patients and family members, as well as recent knowledge and therapeutic advances. We hypothesised that such a global “concept” would improve patients’ management and outcomes, including musculoskeletal health, physical activity and quality of life [7–9]. The interdisciplinary approach for OI patients was created in 2012 (referred as to the “OI CHUV” group), with two primary goals: (1) to provide each and every patient with the same initial evaluation, the same access to genetic testing and the same follow up; (2) to assess the impact of this OI CHUV management on patients’ physical activity and quality of life. Here we report our first results after 7 years of follow up, including a survey addressing patients’ and families’ satisfaction.
Between October 2012 and October 2019, all patients attending an OI-dedicated consultation in a single Swiss centre (Bone and Joint Department, CHUV, Lausanne University Hospital, Switzerland) and seen by one member of the OI team could be included in this prospective study. Informed consent was obtained from each patient or caregiver. This study and its related prospective data collection were approved by the Ethics Committee of Vaud (Switzerland, CER Vaud 2018-01673).
Any patient, whether adult or child, referred to the CHUV (Lausanne University Hospital, Lausanne, Switzerland) for a suspected or confirmed OI diagnosis, was prospectively included in the OI CHUV group cohort. To be included in the present report, patients had to have attended at least one “OI day” (described below) since 2012.
Before the recognition of OI as a rare disease by the Swiss Council, the OI days and OI CHUV team were created in 2012, in our tertiary hospital: the CHUV. At first, the interdisciplinary team was composed of two bone disorder specialists (an adult and a child specialist), one orthopaedic surgeon, one geneticist, and three physiotherapists (two for adults and one for children). Then, in 2013, a dentist joined the team, followed by an otorhinolaryngologist in 2016. Each team member had expertise in clinical OI management. However, by integrating a more global OI network, each specialist shared the same goal: more effective decision making and treatment. All health workers involved had a strong culture of team working, which allowed constructive discussion and optimised coordination of the different agendas. Such information was conveyed through local and national professional networks [4, 10] associations (The Swiss Association of Patients with OI) and the internet (https://www.info-maladies-rares.ch/).
All patients underwent a complete physical examination to confirm the clinical diagnosis of OI and to determine its phenotype according to the Sillence classification system [11, 12] (fig. 1).

Figure 1 Initial evaluation pathway for OI patient in the CHUV OI group.; CHUV = Centre Hospitalier Universitaire Vaudois; DXA = dual X-ray absorptiometry; OI = osteogenesis imperfecta
Each patient was assessed for musculoskeletal and respiratory disorders (spirometry) by a physical therapist. A questionnaire was used to evaluate patient’s physical practice (physical activity and sport), and each patient was counselled on physical and athletic activities and provided with recommendations for physical therapy.
Quality of life refers to an interdisciplinary concept that varies across subject and time. It encompasses multiple notions pertaining to the physical, emotional and social domains [13]. Although discrepancies remain as to which dimensions to include or not in a quality of life evaluation, we chose to use the French version of the EQ-5D [14, 15], a widely used and validated tool for estimating health status preferences (utilities). The EQ-5D questionnaire allows assessment of five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) with three response options for each item: 1 = no problems; 2 = some problems; or 3 = severe problems, resulting in 243 possible health states. The instrument is complemented by a visual analogue scale that allows individuals to rate their overall health status on a scale from 0 to 100. The visual analogue scale provides a purely patient-based rating of health-related quality of life when used with clinical populations. For countries without a national value set, the EuroQol group recommends employing a value set based on geographic proximity. In the absence of a Swiss EQ-5D value set, we chose the French set. We used the EQ-5D version for adults and the EQ-5D version for youth.
In addition, patient’s musculoskeletal function was evaluated using the 36-Item Short Form (SF-36, adult version) [16]. Results are typically used to calculate quality-adjusted life-years (QALYs) in the context of a cost-utility analysis. The SF-36 is comprised of questions covering eight domains: (1) physical functioning;( 2) role limitations due to physical problems; (3) pain; (4) general health; (5) vitality; (6) social functioning; (7) role limitations due to emotional problems; and (8) mental health. Scores vary between 0 and 100 with higher scores indicating better musculoskeletal function and health-related quality of life.
A bone health evaluation was performed for all patients aged 5 years and older. All adults and children underwent a measurement of bone mineral density (BMD) by DXA (dual X-ray absorptiometry, Hologic Discovery A Hologic Inc., Bedford, MA), with measures at the spine and left proximal hip. We systematically added a vertebral fracture assessment based on DXA for all adults and children from 5 years of age. In adults, a trabecular bone score assessment completed the evaluation (TBS iNsight® Version 2.1 Med-Imaps, Pessac, France). The patient’s bone metabolism was evaluated, including measurement of serum calcium, phosphorous, vitamin D, and bone turnover markers, and any anomaly was treated. Based on the clinical examination and bone evaluation results, an orthopaedic surgeon, a dentist and/or an otorhinolaryngologist was consulted for examinations and investigations. If necessary, patients were referred to other specialists for additional investigations (e.g., a cardiological assessment).
All patients were offered a genetic consultation, analysis and counselling.
Results of this initial evaluation were discussed for each patient, during regular meetings of the OI team. A medical summary was provided to the primary care physician with the recommendations and proposals of the OI team, including medication.
After the initial evaluation, all patients were invited annually to a dedicated OI day organised in our clinic for an individualised medical check-up that was designed to address the patient’s situation and needs (fig. 2).
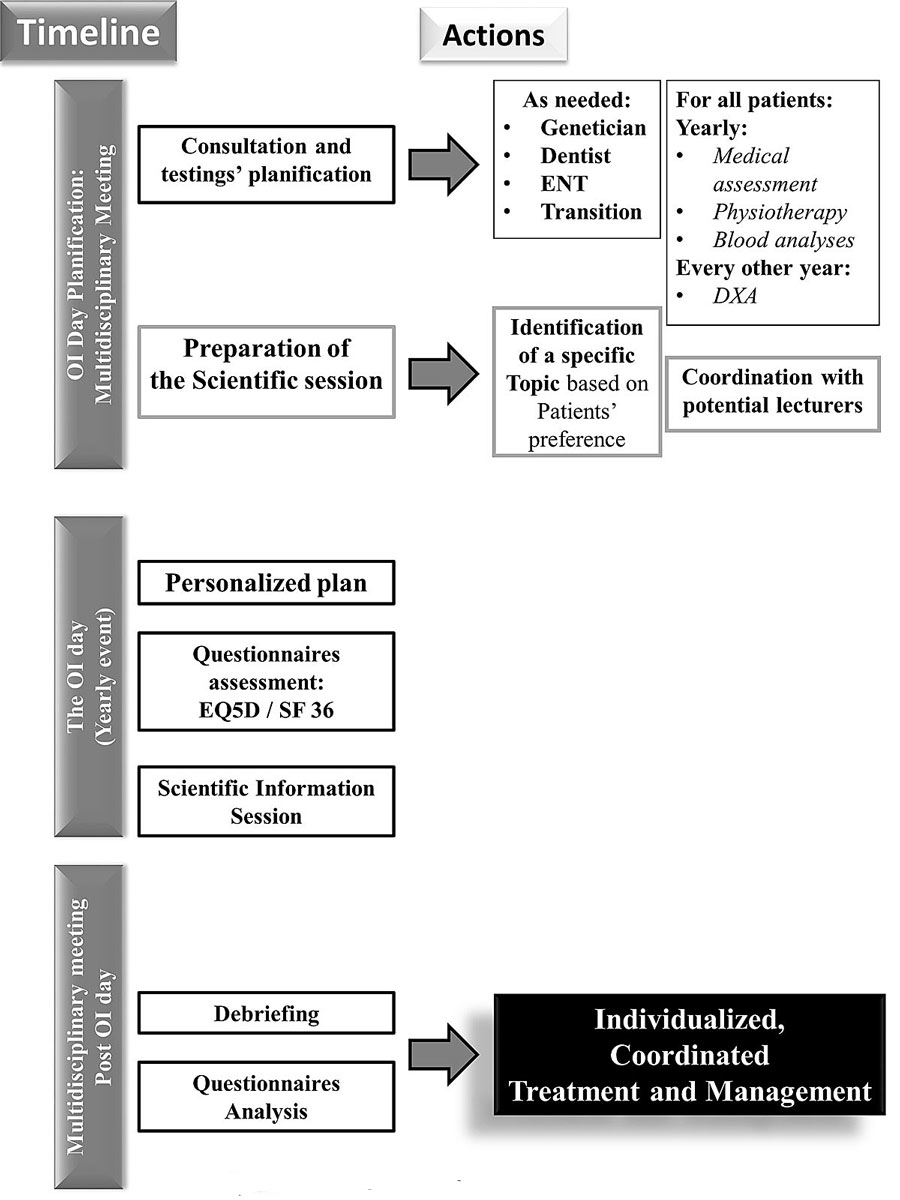
Figure 2 The OI day – planning of individualised follow-up. OI: Osteogenesis Imperfecta. CHUV Centre Hospitalier Universitaire Vaudois. DXA = dual X-ray absorptiometry.
For the OI day, the interdisciplinary team was reinforced by additional clinicians, DXA technicians (with two DXA machines), nurses and physiotherapists. The clinical space was expanded, and the team benefited from a designated meeting room and appropriate consultation rooms, with relevant available technology and equipment.
Some of the examinations prescribed during the initial evaluation were repeated annually (physical examination, physiotherapy evaluation, blood analyses, EQ-5D, and SF-36), and others were done every 2 years (DXA) or as needed (orthopaedic surgeon, dentist and otorhinolaryngologist consultations). Transition consultations with both a paediatric and adult bone specialist were provided for teenagers (17 to 19 years old). A medical summary was provided to the primary care physician with the recommendations and proposals of the OI team, including medication.
On each OI day, a clinical and scientific information session was provided to patients, families and professional caregivers. This was intended to cover the latest advances in understanding and treatment, but also to connect people and to involve representatives of the national patients’ association. To reach a broader audience, patients as well as representative of the national patients’ association are invited to suggest a topic of interest for the next OI day. Satisfaction of patients and their families, restructuring of patients’ psychosocial system and efficiency at providing a continuity of medical and surgical care were assessed by measurement of the adherence to the OI day and the use of a short “OI day” quality survey (appendix 1). A typical timeline of an OI day for one patient is shown in figure S1 in appendix 2.
Statistical analyses were conducted using Stata ICv14® (StataCorp, College Station, Texas, USA) for Windows. Results were expressed as average ± standard deviation (SD). Association between studied parameters was determined using Student’s t-test for continuous variables and chi-square test for categorical variables. Statistical significance was considered for a bivariate test with a p-value <0.05.
Since 2012 we have met 71 new patients with OI, of whom 50 were included in this report. Exclusion criteria were: no consent agreement, loss of follow up (n = 20) or death (n = 1) (fig. 3). For the 50 patients included, the mean follow-up after the initial evaluation was 2.76 years (SD 1.57; minimum OI day = 1, maximum OI days = 5).
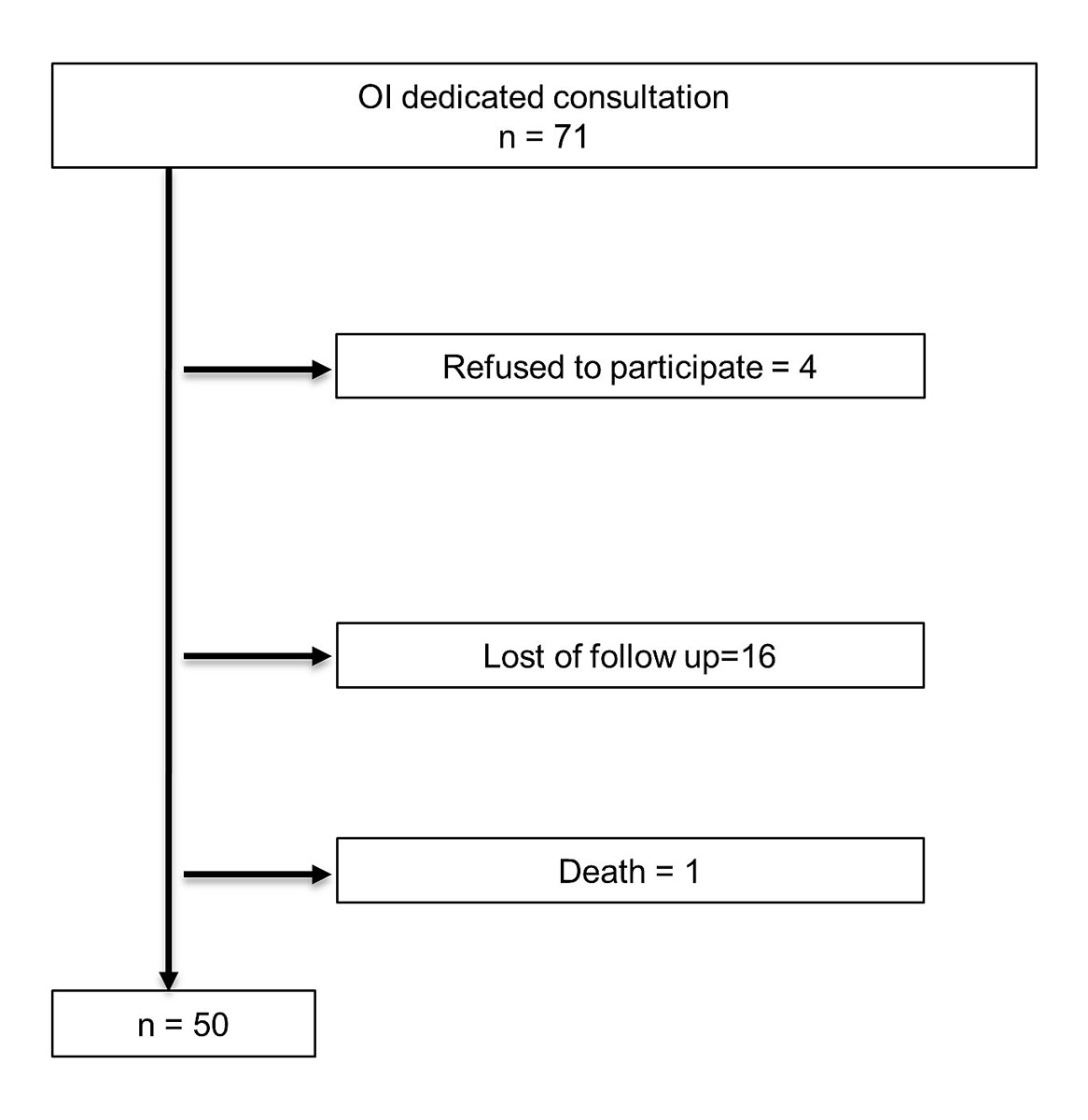
Figure 3 Patients excluded. OI = osteogenesis imperfecta
All the 50 patients included in this analysis received an initial evaluation. At the time of the first evaluation, 12 of these 50 patients were still of paediatric age (age 1 to 17 years, mean 8.5), and 38 were adults (age 18 to 69 years, mean 43.5) (table 1)
Table 1 Characteristics of the sample (n = 50).
| Age (mean, min; max) | 32.8 (1; 69) |
| Sex M/F (%) | 22/68 |
| Multiple previous fracture (%) | 78 |
| First fracture age (mean, min; max) | 3.8 (0; 41) |
| Previous bone active drug (apart calcium / vitamin D) (%) | 76 |
We collected specific data on physical activity and sports for 45 patients (all except very young children). Initially, 24 of the 45 patients were not engaged in any type of specific physical activity (53%), whereas only 14 were participating in a sport (31%). Over time, 62% of the inactive patients started some physical activity and 44% started a sport. As of their last evaluation, the final rates of regular physical activity and sports were 82% and 60%, respectively, within the entire group.
Thirty-six patients (72%; adults, children, teenagers) completed the EQ-5D at their initial evaluation, with an EQ-5D mean score of 0.75 (range 0.16 to 1.00; SD 0.17). However, we observed a tendency towards an improvement in the quality of life, with a mean score increasing from 0.73 to 0.75 (p >0.05) in the group of 26 patients with at least two EQ-5D measurements (mean time between two EQ-5D assessments 3.69 years). Thirty-four patients (68%; adults, teenagers) had an SF-36 evaluation at first evaluation, and 30 had at least two measurements. The initial SF-36 score, obtained during the initial evaluation (n = 34), demonstrated a lower score for the physical domain (mean 59.09; SD 4.15) than for the mental domain (mean 68.06; SD 3.66). Thirty patients presented with at least two SF-36 measurements (mean time between two SF-36 assessments 3.23 years). The physical domain score tended to increase between two assessment (from 59.09 to 65.79; p = 0.08), whereas it remained stable for the mental domain (score varying from 68.06 to 68.02).
About 80% (78%) of the whole cohort experienced multiple fractures in childhood; among these patients, 12 had never received any bone-active drug, apart from calcium / vitamin D substitution. All 38 adults, except one who had no measurable sites, had undergone at least one DXA measurement, with a mean spine T-score of −2.68 (range −5.6 to +0.6; SD 1.34), hip T-score −1.38 (range−3.3 to +1.6; SD 1.17), and neck T-score −1.59 (range −3.5 to +1.3; SD 1.25). Thirty-four adult patients underwent a bone texture measurement using a trabecular bone score method (TBS) with a mean spine TBS of 1.259 (range 1.003 to 1.501; SD 0.13, normal TBS >1.310). Only 5/12 children had a DXA measurement. Mean spine Z-score was −1.3 (range −1.9 to −1), hip Z-score was −2.0 (range −3.1 to −0.9), and neck Z-score −1.4 (only one measurement).
Genetic assessment was obtained in 88% (38 adult patients) of our cohort patients. Eighteen patients from 11 families had a mutation in COL1A1 predicting haploinsufficiency, associated with a mild phenotype. Four patients from three families had missense mutations in other amino acid residues of COL1A1 or COL1A2, associated with a mild to moderate phenotype. A glycine substitution whether in COL1A2 or in COL1A1 (table 2, light grey), associated with a more severe phenotype, was observed in patients from six families. Both families with OI type V had the recurrent hotspot mutation in the 5′UTR region of IFITM5. One family with a dominant mild phenotype (type I) had a mutation in CREB3L1. The family with LRP5 mutations included two affected patients: a heterozygous father with osteoporosis and a homozygous child with osteoporosis-pseudoglioma syndrome and a type I borderline to IV skeletal phenotype (table 2).
Table 2 Phenotype and genotype of 38 patients from 24 families followed up from the osteogenesis imperfecta group.
| Family (number of affected patients) | Skeletal phenotype | Gene | Mutation (cDNA) | Mutation (protein) |
|---|---|---|---|---|
| 1 (1) | I | COL1A1 | c.3536_3540delCCCCC | p.Pro1179ArgfsTer39 |
| 2 (2) | I | COL1A1 | c.2426dupG | p.Ala811CysfsTer10 |
| 3 (4) | I | COL1A1 | c.3540delC | p.Gly1181Alafs*58 |
| 4 (2) | I | COL1A1 | c.2710delG | p.Glu904LysfsTer204 |
| 5 (1) | I | COL1A1 | c.2867delG | p.Gly956AspfsTer152 |
| 6 (1) | I | COL1A1 | c.1491delC | p.Ala498Glnfs*43 |
| 7 (1) | I | COL1A1 | c.2614-1G>A | splicing |
| 8 (1) | I | COL1A1 | c.1477dupC | p.Arg493ProfsTer18 |
| 9 (2) | I | COL1A1 | c.1405C>T | p.Arg469Ter |
| 10 (1) | I | COL1A1 | c.757C>T | p.Arg253Ter |
| 11 (2) | I | COL1A1 | c.658C>T | p.Arg220Ter |
| 12 (1) | I | COL1A2 | c.2797G>A | p.Asp933Asn |
| 13 (1) | IV | COL1A1 | c.3893C>A | p.Thr1298Asn |
| 14 (2) | IV | COL1A2 | c.3814T>C | p.Cys1272Arg |
| 15 (2) | I-IV | COL1A2 | c.964G>A | p.Gly322Ser |
| 16 (1) | III | COL1A2 | c.1378G>A | p.Gly460Ser |
| 17 (1) | III | COL1A2 | c.1678G>A | p.Gly560Ser |
| 18 (1) | I-IV | COL1A1 | c.581G>A | p.Gly194Asp |
| 19 (1) | III | COL1A2 | c.830G>A | p.Gly277Asp |
| 20 (1) | III | COL1A2 | c.1613G>T | p.Gly538Val |
| 21 (3) | V | IFITM5 | c.1-14C>T | Unknown |
| 22 (1) | V | IFITM5 | c.1-14C>T | Unknown |
| 23 (3) | I | CREB3L1 | c.p22C>T | p.Arg308Cys |
| 24 (2) | I-IV | LRP5 | c.1682C>T | p.Tyr581Ile |
Patients’ attendance varied from year to year, from 27 patients in 2012 (creation of the OI day) to 50 in 2018 (seven new patients) and a slight decrease with 38 patients in 2019 (five new patients), this was later explained by a diminution in children’s participation (fig. 4). From 2012 to 2018, the mean attendance to the OI days was 2.76 years (min 1 OI day, max 5 OI days).

Figure 4 Patient attendance at OI days. OI = osteogenesis imperfecta
Thirty-five patients completed the survey, in an anonymous manner. The quality of the OI day was monitored through six main axes. Overall, most of the patients found the OI day useful for them (mean score=3.7 out of 4), improving their continuity of care (3.75 out of 4), their physical health (3.56 out of 4) (fig. 5). The impact of the OI day extended to the patient’s family.
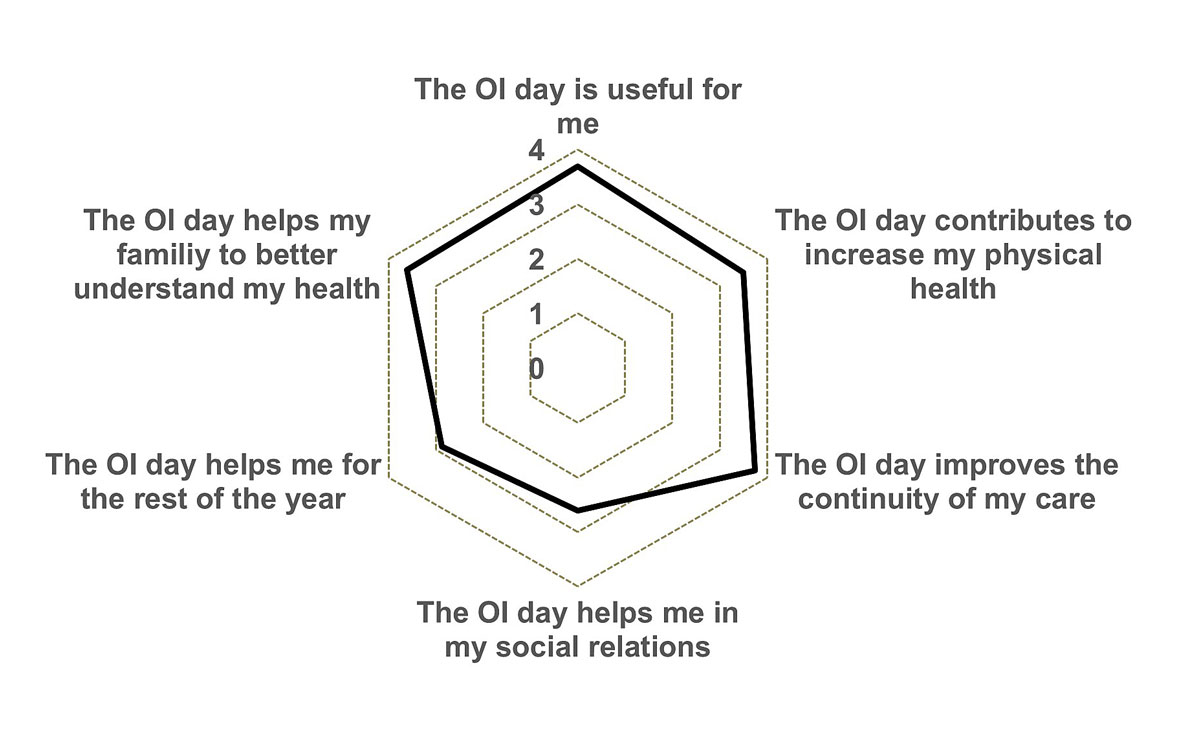
Figure 5 OI day quality related survey. Patients answered with a grading scale: 1 = extremely dissatisfied, 2 = somewhat dissatisfied, 3 = somewhat satisfied, 4 = extremely satisfied.
Coordinated care in OI is sparse and limited. Development of a new interdisciplinary care strategy in a tertiary centre remains challenging and requires motivation driven towards a compelling purpose: patients’ and their families’ satisfaction. The enhanced teamwork provided by the interdisciplinary structure improved communication between the different stakeholders in OI patients’ care. Thereby, it provided not only the ability to decide upon the best treatment plan for the patient, but it also brought satisfaction to the healthcare professionals, the patients and their families. Such impact of an interdisciplinary care strategy has been shown to foster faster [17] and better clinical decision making [18].
Our interdisciplinary team approach allowed us to offer a patient-centred clinical decision making, based on clinical observation, evidence-based guidelines and analyses of data collections. Regular data gathering provides support for optimised and comprehensive care strategies, but also allows the creation of data banks, which are extremely limited in OI. Our belief is that exhaustive data collection on a cohort that seems to be one of the largest in Europe can increase knowledge and improve patient care [9].
All of the participants in this study had the same initial evaluation and participated in the OI day at least once. We evaluated the severity of the disease in order to propose the best treatment and management, using a classification based on the phenotype, the genotype, bone measurements (clinical and radiological) and a physiotherapeutic evaluation. Analyses of the phenotypes revealed a high number of patients with OI types I (58%), followed by type III (18%), and IV (14%). We met only four patients, from two different families, with OI type V. All patients were offered a genetic analysis, and 88% of them accepted. As expected, many of the mutations found were in COL1A1 or COL1A2.
All adult patients but one (because of lack of any measurable site) underwent a complete bone evaluation. As expected, the mean bone mineral density values were in the range of bone fragility, and the mean TBS was low [19, 20]. The molecular findings, coupled with the DXA results, influenced the choice of bone therapy [4], leading to some modifications in all the patients’ bone treatment in order to decrease their risk of fracture.
All of our patients had a physiotherapy evaluation and counselling in physical activities, 62% of inactive patients started some form of routine physical activity and 44% of patients not participating in any sports-related activity started to do so. Although most patients with mild to moderate OI can walk, they frequently report fatigue, diminished exercise capacity, kinesiophobia and exercise intolerance as limiting their regular physical and athletic activities, especially among children [21, 22]. In adult patients, physical activity was below population norms [23–25], even if patients were aware of their need to remain physically active to prevent loss of function and muscle strength. In one observational study, Wekre et al. identified a significant difference between the different types of OI and activity: patients with OI type III had less ability to perform activities than those with types I or IV [26]. In addition, Van Brussel et al. found that significant improvements in aerobic capacity and muscle force were observable after 3 months of training in children with OI. Unfortunately, these effects decreased over time as the intervention was discontinued [27]. As of their last evaluation, 82% of the CHUV OI group were actively participating in some form of routine physical activity and 60% were practicing sport. Among the nine patients with OI type III, only three were participating in at least one sport at their initial evaluation, but this number increased to six at the end of this study.
We discovered that our patients’ quality of life was low in the physical health domain but was much better for mental health. We observed a clear but non-statistically significant trend toward an improved quality of life as reflected by an increase in EQ-5D scores. Although we lacked the statistical power to confirm a significant benefit over time, our results are nonetheless comparable with the literature. As already shown, the quality of life of OI patients is not severely impacted [28], except regarding physical function. Notably, their psychological and mental health is reported to be comparable to the healthy population [24, 25]. Having a coordinated organisation that includes assisting older adolescents transitioning from childhood to adulthood provides one of the best levels of care for children and adults [29]. Offering patients’ group management, which helps to reduce their level of isolation, and personalised physical training has been shown to augment quality of life of OI children and their families [6, 27].
Patient satisfaction and restructuring of their psychosocial systems were measured using attendance at the OI day and its quality-related survey. Although participation varied from year to year, a large number of patients attended each year. For many of them, having all their medical evaluations conducted over the course of a single day was a real benefit in terms of organising, coordinating and simplifying their care. Regarding family and social participation, we originally proposed this OI day in order to address the problems of patient isolation and concerns about the financial burden of their care. In addition, we sought to facilitate the transition from adolescent to adult medical care for both the medical and physiotherapeutic components. This transition, acclaimed by families and caregivers, aimed to ensure continuity in medical management and to restructure psychosocial and work-related systems [29]. Even if we did not perform any economic analysis, the unique opportunity to have all of their evaluations and consultations on a single day once per year is unquestionably convenient and could explain the steady increase in new programme participants. Moreover, the plenary session seemed to be much appreciated by both patients and their families. They could meet other patients, share their experiences and keep in touch through social networking. The presence of a representative of the national patients association increased the association’s visibility and established links with patients.
The main strengths of our interdisciplinary group were originally the OI day, which could answer to the majority of concerns, and the close collaboration with physical therapists. Physical activity and the quality of life improved.
The main limitation of our current programme pertains to the sustainability of the single OI day, given the increasing number of patients and the limited time and resources for organising the event. Even if managing rare diseases with an interdisciplinary approach is encouraged by our institution, reimbursement of medical care is comparable to other diseases despite the increased complexity of many cases. Moreover, the time spent in interdisciplinary meetings and organising the OI day is not recoverable. Reliable funding sources and better visibility would make this OI day sustainable on the long term. The second limitation related to confirming that interdisciplinary management improve the bone health and decrease the bone fragility: we have not enough data to date, but the OI day continues, and new dates are scheduled.
The interdisciplinary approach proposed by our group addressed most of the problems encountered by patients with OI. It satisfied the standard of care for improving the patients’ condition, including their overall health and quality of life, in addition to familial and social participation [30–32]. It made it possible for patients to receive the clinical expertise and research-based wisdom of experts in their disease, resulting in optimised care [7].
This kind of management could help meeting rare disease patients’ special needs. It provides a way to facilitate continuing education for team members and is consistent with the 2020 Rare Diseases Strategy of the Swiss Federal Council.
Appendix 1 is available as a separate file at https://smw.ch/article/doi/smw.2020.20285.
Figure S1 Timeline of an OI day, example for one patient. DXA = dual X-ray absorptiometry
We are very grateful to the patients and their families for their involvement in the organisation of the OI day.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Forlino A , Cabral WA , Barnes AM , Marini JC . New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol. 2011;7(9):540–57. doi:.https://doi.org/10.1038/nrendo.2011.81
2 Hald JD , Folkestad L , Swan CZ , Wanscher J , Schmidt M , Gjørup H , et al. Osteogenesis imperfecta and the teeth, eyes, and ears-a study of non-skeletal phenotypes in adults. Osteoporos Int. 2018;29(12):2781–9. doi:.https://doi.org/10.1007/s00198-018-4663-x
3 Pillion JP , Vernick D , Shapiro J . Hearing loss in osteogenesis imperfecta: characteristics and treatment considerations. Genet Res Int. 2011;2011:983942. doi:.https://doi.org/10.4061/2011/983942
4 Bregou Bourgeois A , Aubry-Rozier B , Bonafé L , Laurent-Applegate L , Pioletti DP , Zambelli PY . Osteogenesis imperfecta: from diagnosis and multidisciplinary treatment to future perspectives. Swiss Med Wkly. 2016;146:w14322.
5 Harsevoort AGJ , Gooijer K , van Dijk FS , van der Grijn DAFM , Franken AAM , Dommisse AMV , et al. Fatigue in adults with Osteogenesis Imperfecta. BMC Musculoskelet Disord. 2020;21(1):6. doi:.https://doi.org/10.1186/s12891-019-3000-7
6 Hill CL , Baird WO , Walters SJ . Quality of life in children and adolescents with Osteogenesis Imperfecta: a qualitative interview based study. Health Qual Life Outcomes. 2014;12(1):54. doi:.https://doi.org/10.1186/1477-7525-12-54
7 Marr C , Seasman A , Bishop N . Managing the patient with osteogenesis imperfecta: a multidisciplinary approach. J Multidiscip Healthc. 2017;10:145–55. doi:.https://doi.org/10.2147/JMDH.S113483
8 Lafage-Proust MH , Courtois I . The management of osteogenesis imperfecta in adults: state of the art. Joint Bone Spine. 2019;86(5):589–93. doi:.https://doi.org/10.1016/j.jbspin.2019.02.001
9 Swezey T , Reeve BB , Hart TS , Floor MK , Dollar CM , Gillies AP , et al. Incorporating the patient perspective in the study of rare bone disease: insights from the osteogenesis imperfecta community. Osteoporos Int. 2019;30(2):507–11. doi:.https://doi.org/10.1007/s00198-018-4690-7
10 Aubry-Rozier B , Unger S , Bregou A , Freymond Morisod M , Vaswani A , Scheider P , et al. [News in osteogenesis imperfecta: from research to clinical management]. Rev Med Suisse. 2015;11(466):657–8, 660–2. Article in French.
11 Van Dijk FS , Sillence DO . Osteogenesis imperfecta: clinical diagnosis, nomenclature and severity assessment. Am J Med Genet A. 2014;164(6):1470–81. doi:.https://doi.org/10.1002/ajmg.a.36545
12 Sillence DO , Senn A , Danks DM . Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;16(2):101–16. doi:.https://doi.org/10.1136/jmg.16.2.101
13Fayer PMD. Quality of Life. The assessment, analysis and interpretation of patient-reported outcomes. 2nd edition. Chichester: John Wiley and Sons Ltd; 2007.
14 Rabin R , de Charro F . EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–43. doi:.https://doi.org/10.3109/07853890109002087
15 Chevalier J , de Pouvourville G . Valuing EQ-5D using time trade-off in France. Eur J Health Econ. 2013;14(1):57–66. doi:.https://doi.org/10.1007/s10198-011-0351-x
16 Ware JE, Jr , Sherbourne CD . The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. doi:.https://doi.org/10.1097/00005650-199206000-00002
17 Stalfors J , Lundberg C , Westin T . Quality assessment of a multidisciplinary tumour meeting for patients with head and neck cancer. Acta Otolaryngol. 2007;127(1):82–7. doi:.https://doi.org/10.1080/00016480600740589
18 van Hagen P , Spaander MC , van der Gaast A , van Rij CM , Tilanus HW , van Lanschot JJ , et al.; Rotterdam Oesophageal Tumour Study Group. Impact of a multidisciplinary tumour board meeting for upper-GI malignancies on clinical decision making: a prospective cohort study. Int J Clin Oncol. 2013;18(2):214–9. doi:.https://doi.org/10.1007/s10147-011-0362-8
19 Folkestad L , Hald JD , Hansen S , Gram J , Langdahl B , Abrahamsen B , et al. Bone geometry, density, and microarchitecture in the distal radius and tibia in adults with osteogenesis imperfecta type I assessed by high-resolution pQCT. J Bone Miner Res. 2012;27(6):1405–12. doi:.https://doi.org/10.1002/jbmr.1592
20 Kocijan R , Muschitz C , Haschka J , Hans D , Nia A , Geroldinger A , et al. Bone structure assessed by HR-pQCT, TBS and DXL in adult patients with different types of osteogenesis imperfecta. Osteoporos Int. 2015;26(10):2431–40. doi:.https://doi.org/10.1007/s00198-015-3156-4
21 Takken T , Terlingen HC , Helders PJ , Pruijs H , Van der Ent CK , Engelbert RH . Cardiopulmonary fitness and muscle strength in patients with osteogenesis imperfecta type I. J Pediatr. 2004;145(6):813–8. doi:.https://doi.org/10.1016/j.jpeds.2004.08.003
22 Engelbert RH , Uiterwaal CS , Gerver WJ , van der Net JJ , Pruijs HE , Helders PJ . Osteogenesis imperfecta in childhood: impairment and disability. A prospective study with 4-year follow-up. Arch Phys Med Rehabil. 2004;85(5):772–8. doi:.https://doi.org/10.1016/j.apmr.2003.08.085
23 Feehan AG , Zacharin MR , Lim AS , Simm PJ . A comparative study of quality of life, functional and bone outcomes in osteogenesis imperfecta with bisphosphonate therapy initiated in childhood or adulthood. Bone. 2018;113:137–43. doi:.https://doi.org/10.1016/j.bone.2018.05.021
24 Balkefors V , Mattsson E , Pernow Y , Sääf M . Functioning and quality of life in adults with mild-to-moderate osteogenesis imperfecta. Physiother Res Int. 2013;18(4):203–11. doi:.https://doi.org/10.1002/pri.1546
25 Tosi LL , Oetgen ME , Floor MK , Huber MB , Kennelly AM , McCarter RJ , et al. Initial report of the osteogenesis imperfecta adult natural history initiative. Orphanet J Rare Dis. 2015;10(1):146. doi:.https://doi.org/10.1186/s13023-015-0362-2
26 Wekre LL , Frøslie KF , Haugen L , Falch JA . A population-based study of demographical variables and ability to perform activities of daily living in adults with osteogenesis imperfecta. Disabil Rehabil. 2010;32(7):579–87. doi:.https://doi.org/10.3109/09638280903204690
27 Van Brussel M , Takken T , Uiterwaal CS , Pruijs HJ , Van der Net J , Helders PJ , et al. Physical training in children with osteogenesis imperfecta. J Pediatr. 2008;152(1):111–6, 116.e1. doi:.https://doi.org/10.1016/j.jpeds.2007.06.029
28 Dahan-Oliel N , Oliel S , Tsimicalis A , Montpetit K , Rauch F , Dogba MJ . Quality of life in osteogenesis imperfecta: A mixed-methods systematic review. Am J Med Genet A. 2016;170(1):62–76. doi:.https://doi.org/10.1002/ajmg.a.37377
29 Shapiro JR , Germain-Lee EL . Osteogenesis imperfecta: effecting the transition from adolescent to adult medical care. J Musculoskelet Neuronal Interact. 2012;12(1):24–7.
30 Zeitlin L , Fassier F , Glorieux FH . Modern approach to children with osteogenesis imperfecta. J Pediatr Orthop B. 2003;12(2):77–87.
31 Montpetit K , Palomo T , Glorieux FH , Fassier F , Rauch F . Multidisciplinary Treatment of Severe Osteogenesis Imperfecta: Functional Outcomes at Skeletal Maturity. Arch Phys Med Rehabil. 2015;96(10):1834–9. doi:.https://doi.org/10.1016/j.apmr.2015.06.006
32 Tsimicalis A , Denis-Larocque G , Michalovic A , Lepage C , Williams K , Yao TR , et al. The psychosocial experience of individuals living with osteogenesis imperfecta: a mixed-methods systematic review. Qual Life Res. 2016;25(8):1877–96. doi:.https://doi.org/10.1007/s11136-016-1247-0
Data acquisition: All. Data interpretation: BAR, LB, BCX and AB. Drafting manuscript: BAR, CR and AB. Revising manuscript: all authors. Approving final version of manuscript: all authors. BAR affirms that the manuscript is an honest, accurate and transparent account of the study being reported.
No financial support and no other potential conflict of interest relevant to this article was reported.