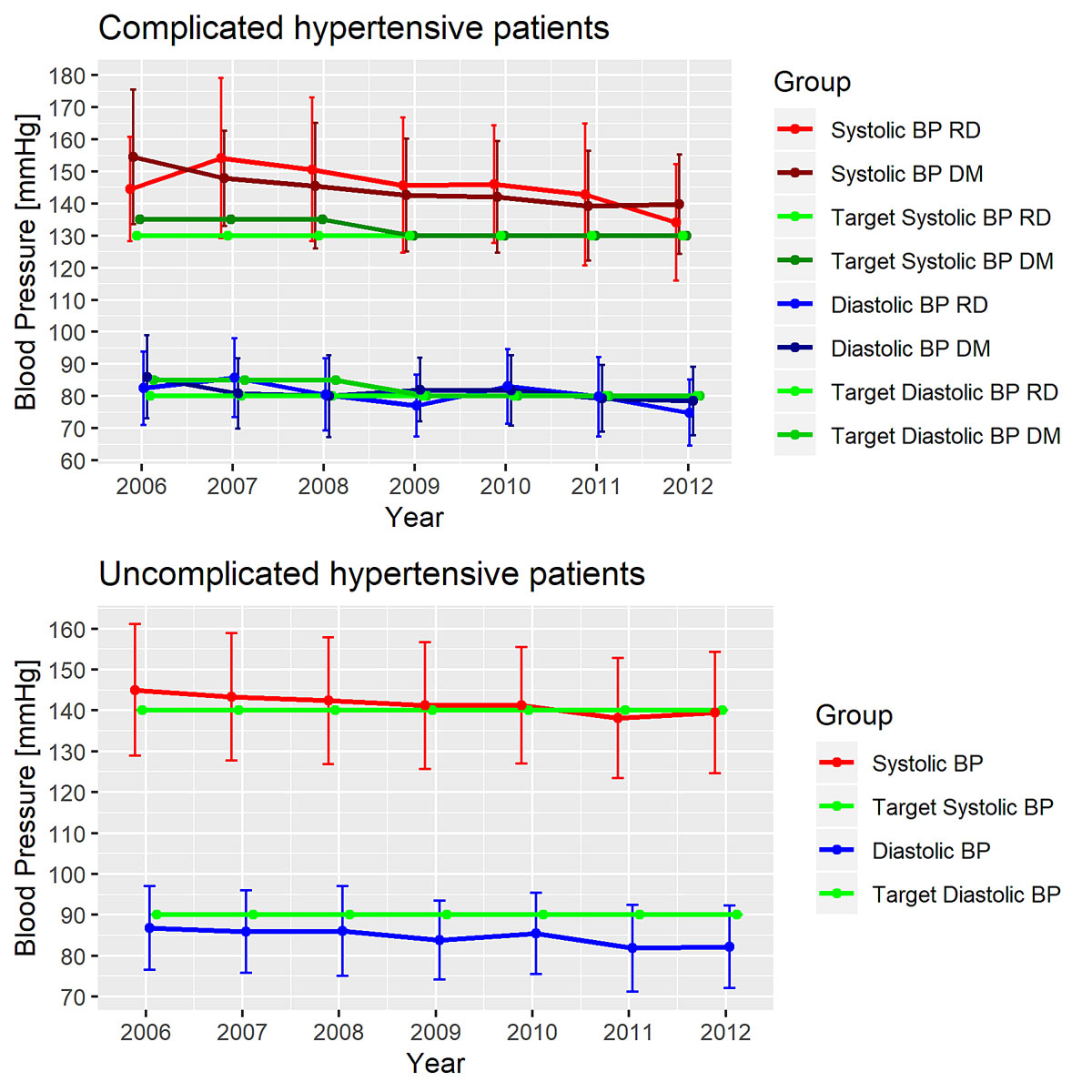
Figure 1 Change in mean blood pressure over time in patients with complicated hypertension and patients with uncomplicated hypertension.
DOI: https://doi.org/10.4414/smw.2020.20279
In Switzerland and other countries in the western world hypertension affects around a quarter to a third of the adult population and is one of the most common conditions seen in primary care [1–5]. Hypertension is one of the major risk factors for cardiovascular and renal morbidity and mortality [6–8]. In patients with diabetes, hypertension affects more than 60% [9]. In particular, hypertensive patients with diabetes and renal dysfunction have a higher risk for cardiovascular morbidity and mortality than those without [10, 11]. Convincing evidence suggests that decreasing blood pressure below recommended target levels reduces cardiovascular morbidity and mortality [12]. Nevertheless, worldwide data suggest that blood pressure target levels are rarely reached [13]. In Switzerland, a cross-sectional study of hypertensive primary care patients indicated that half of all hypertensive patients and only one fifth of patients with diabetes, chronic kidney disease or proteinuria achieved recommended blood pressure target levels [14, 15].
Blood pressure goal recommendations have substantially changed during recent years [16]. In 2003, guidelines of the Swiss Society of Hypertension (SSH) for the management of arterial hypertension aimed at blood pressure values below <135/85 mm Hg for hypertensive patients with complicated hypertension (e.g., diabetes mellitus) [17]. In 2009, SSH guidelines recommended lowering blood pressure to <130/80 mm Hg in hypertension complicated by other diseases, such as diabetes mellitus or renal disease. In 2015, SSH guidelines were revised [18] again based on the 2013 guidelines of the European Society of Hypertension (ESH) / European Society of Cardiology (ESC) [19]. The revised 2015 guidelines recommended a blood pressure target of <140/85 mm Hg in hypertensive patients with diabetes and/or renal dysfunction. The revised blood pressure treatment goals were due to a lack of solid evidence from randomised controlled trials for an aggressive blood pressure lowering approach [20]. In particular, with more aggressive goals (<130 mm Hg, as recommended in 2009) target organ heterogeneity was observed, with a decreased risk of stroke, but no advantage regarding the risk of other macrovascular or microvascular events such as microalbuminuria, overt nephropathy, end-stage renal disease / dialysis, doubling of serum creatinine, neuropathy and retinopathy, and the risk of serious adverse events even increased [21, 22]. In 2018, the Swiss guidelines recommended a further loosening of blood pressure goals with a blood pressure reduction <140/90 within 3 months for all patients (uncomplicated hypertension and hypertensive patients with diabetes and/or renal dysfunction).
The aim of this article was to evaluate whether SSH guideline changes in 2009 had an effect on blood pressure measurements and on the practice of antihypertensive therapy among high-risk patients with hypertension complicated by diabetes mellitus and/or renal dysfunction. Further, we evaluated the association of demographic, lifestyle, clinical and therapeutic characteristics with blood pressure goal attainment among high-risk patients and uncomplicated hypertensive patients.
This was a prospective cohort study assessing hypertensive patients visiting their general practitioner (GP) in the north-western part of Switzerland. The Swiss Hypertensive Cohort Study started in 2006 and ended in early 2013. GPs were part of the Primary Care Network Northwest Switzerland. Eligible patients were approached during routine visits to their GPs. One thousand and three patients were recruited by 87 GPs. The data were recorded via an internet-based database. Data were collected at baseline and thereafter at a minimum annually during routine consultations. All participants gave written informed consent, and the institutional ethics committee approved the study protocol.
The following inclusion criteria were applied: a minimum age of 18 years, with an established diagnosis of arterial hypertension or newly diagnosed arterial hypertension. Newly detected hypertension was defined as mean through-sitting office blood pressure measurement ≥140/90 mm Hg. Diagnosis relied on the judgment of the treating physician, and treatment could be pharmacological and/or non-pharmacological (lifestyle modifications). Exclusion criteria encompassed: (1) acute coronary syndrome (myocardial infarction or unstable angina), stroke or transient ischaemic attack in the previous 3 months; (2) percutaneous transluminal coronary angioplasty, coronary artery bypass grafting in the previous 3 months; (3) acute hepatic or renal disease in the previous 3 months; (4) inability to provide informed consent; (5) other severe concomitant disease such as terminal illness, if life expectancy was <1 year.
Subgroups of interest were defined as follows:
The average of a minimum of two blood pressure readings were obtained in sitting position after at least 5 minutes of rest using calibrated aneroid or mercury sphygmomanometers or validated automatic devices, depending on availability in the GP practice.
During the baseline visit, GPs assessed demographic characteristics such as age, sex, weight, height and waist circumference. Information on socioeconomic and marital status and several lifestyle factors were recorded. The latter included tobacco smoking, alcohol use and physical activity. Alcohol consumption was classified into four categories: none, occasional, moderate (≤2 units of alcoholic beverages per day on average) and high (>3 units per day on average). Similarly, physical activity was classified according to three categories at the discretion of the GPs: little, medium and high. Clinical history, physical examination, routine laboratory profile and urine specimens were obtained. If possible, heart rate, 12-lead electrocardiography and transthoracic echocardiography were obtained. Also, information on socioeconomic characteristics, family history of cardiovascular events, presence of target organ damage and cardiovascular risk factors was collected. Lifestyle modifications, antihypertensive drug therapy and concomitant treatments were documented.
The variable “established cardiovascular disease” included a history (>3 months) of cerebrovascular disease, coronary heart disease, cardiac failure, symptomatic lower extremity peripheral artery disease and advanced retinopathy. Judgment on the presence of established diseases was at the discretion of the physician involved. Diabetes mellitus was defined at the discretion of the GPs (as use of insulin or oral antidiabetics or by laboratory values [blood glucose levels over 7 mmol/l, postprandial blood glucose levels over 11 mmol/l or glycated haemoglobin over 6.5%]) [23]. Dyslipidaemia was defined as total cholesterol over 4.9 mmol/l and/or low-density lipoprotein (LDL) cholesterol over 3.0 mmol/l, and/or high-density lipoprotein (HDL) cholesterol in men <1.0 mmol/l (40 mg/dl) and in women lower than 1.2 mmol/l and/or triglycerides over 1.7 mmol/l (150 mg/dl). Microalbuminuria was defined on the basis of semi-quantitative dipstick testing or the albumin/creatinine ratio in spot urine samples (as albumin-creatinine ratio ≥2.26 mmol/l or a urinary dipstick test ≥2+) [24, 25]. Left ventricular hypertrophy (LVH) was based on criteria on the 12-lead electrocardiogram or echocardiography and was defined as a positive Sokolow-Lyon index (SV1 + RV5/V6 ≥3.5 mV) or a positive Cornell Index (RaVL + SV3) >2440 mV3*ms. Echocardiographic LVH was defined as a left ventricular mass (LVM) index >115g/m2 in men, and >95 g/m2 in women. The eGFR was based on serum creatinine laboratory values using the estimated creatinine clearance rate (eCCr) and Cockcroft-Gault formula: eCCr = ((140−age) × mass (in kg) × [0.85 if female]) / (72 × serum creatinine (in mg/dl)). Impaired eGFR was defined as an eGFR below 60 ml/min/1.73 m2 [26] and was used as a proxy for renal dysfunction. Body mass index (BMI) was defined as the body mass divided by the square of the body height (kg/m2).
The target blood pressure for the general population was defined as blood pressure <140/90 mm Hg according to all Swiss guidelines (2003, 2009, 2015 and 2018). According to 2003 SSH guidelines, blood pressure goals for patients with complicated hypertensiion patients was <135/85 mm Hg for those with diabetes mellitus and <130/80 mm Hg for patients with renal dysfunction [17]. According to 2009 SSH guidelines, blood pressure goal for patients with diabetes mellitus and/or renal dysfunction was <130/80 mm Hg. All guidelines considered the target blood pressure for isolated systolic blood pressure in the elderly to be <150/90 mm Hg.
The database used in HccH was developed by the Centre for Primary Health Care in cooperation with GPs and hosted by IBM SPSS Switzerland. Data entry was by GPs participating in HccH via a password protected website. Anonymised data collection was ensured by using individual patient codes known only by the treating GP. Complete patient datasets were transferred via a secured channel to the study centre.
Statistical analyses were conducted using R version 3.6.2. Continuous and categorical variables were expressed as mean ± standard deviation (SD), as median and interquartile range (IQR) for ordinal variables and as number and percentage (%) of participants, respectively.
To investigate the multivariable association of blood pressure with guideline change (years before/after 2009), linear and logistic mixed-effects regression models were fitted. We used the linear regression for systolic and diastolic blood pressure and the generalised linear model with a log link from the binomial family for blood pressure target achievement. We used mixed‐effects models with subject random intercepts in order to account for the correlation of repeated measures within subjects. For this purpose, the R functions lmer and glmer from the package “lme4” were used. We ran separate models for patients with uncomplicated hypertension and patients with complicated hypertension (diabetes mellitus / renal dysfunction). In secondary analyses, we investigated the relationship between guideline change and antihypertensive pharmacological therapy type via a cumulative link model with one random term to account for the correlation of repeated measures within subjects using the R function clmm from the R package “ordinal”.
The principal dichotomous explanatory variable was guideline change (before/after 2009). Multivariable models were adjusted for the number of follow-up examination (baseline, and first and second visit), age, sex, blood pressure (systolic if the outcome was diastolic blood pressure, diastolic if the outcome was systolic blood pressure, none if the outcome was blood pressure target achievement, or both if the outcome was antihypertensive pharmacological therapy), total cholesterol, fasting plasma glucose, BMI, LVH, diabetes mellitus, years with hypertension diagnosis, established cardiovascular disease, antihypertensive therapy type, lipid lowering therapy, physical activity, alcohol consumption and smoking. Linear models additionally included the following non-pharmacological therapies: weight reduction, increased physical activity, smoking cessation, alcohol reduction.
Two regression models were fitted for each of the four outcome measure (systolic and diastolic blood pressure, blood pressure target achievement (yes/no) and type of antihypertensive combination therapy (mono, duo, triple, quadruple or more)): firstly, “univariable” models including the predictor guideline change adjusted for the variable follow-up visit, second, the multivariable models with the adjusted measures. Eight models were thus fit in total. Statistical significance was a 2-sided p-value of less than 0.05.
In secondary analyses, we investigated the relationship between demographic characteristics, cardiovascular risk factors, diabetes mellitus or subclinical organ damage, pharmacological therapy and blood pressure target achievement using multivariable mixed-effects logistic regression separately within the group of uncomplicated hypertensive patients and the group of high-risk patients.
The study population consisted of 1003 participants with a total of 2237 observations. Of these, 1886 (84%) had missing values in one or more variables, with missing data ranging from 0% (systolic blood pressure) to 60.3% (eGFR) at baseline (supplementary table S1 in appendix 1). We decided to exclude variables with more than 40% missing data at baseline from further multivariable analyses (eGFR and positive family history) because as the percent of missing data increases, the root-mean-square deviation of multiple imputation also linearly increases [27]. To account for missing data of the other variables our primary analyses were conducted on an imputed dataset where missing values were generated using multiple imputation by chained equations (MICE) [28]. In the MICE procedure, a series of regression models are run whereby each variable with missing data is modelled conditional upon the other variables in the data [29]. Imputed datasets were generated under the missing at random assumption, that the probability of data being missing is dependent on the observed data [30]. The imputation model included all variables of interest including the outcome variables. Linear regression was used for imputation of continuous variables, logistic regression for dichotomous variables and multiple logistic regression for categorical variables with more than two categories. MICE estimated a high fraction of missing information for LVH (51%), years with hypertension diagnosis (46%), physical activity (37%), cholesterol (37%), fasting plasma glucose (22%). The other variables of interest were below 6%. Following recommendations by van Buuren [31], 100 datasets were imputed and combined according to Rubin’s rules [32]. We also performed a complete case sensitivity analysis without imputed data.
Table 1 describes the demographic, lifestyle, clinical and therapeutic characteristics of the study population at baseline and follow-up visits. Overall, 87 GPs provided data on hypertensive patients attending their practices. A total of 1003 hypertensive patients were included in the cohort.
Table 1 Characteristics of study participants at baseline and follow-up examinations.
| Characteristics | Missing % | Baseline | First follow-up | Second follow-up | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| n | 1003 | 100 | 753 | 75.07 | 481 | 47.95 | |
| Years after baseline (mean, SD) | 0 | 0 | 1.64 | 0.87 | 2.84 | 0.87 | |
| Demographic data | |||||||
| Sex male | 0 | 558 | 55.63 | 409 | 54.32 | 264 | 54.89 |
| Age (years) (mean, SD) | 0.1 | 64.47 | 13.17 | 67.07 | 12.82 | 68.73 | 12.92 |
| Blood pressure variables | |||||||
| SBP (mm Hg) (mean, SD) | 0 | 145.45 | 17.38 | 139.26 | 15.61 | 138.93 | 15.83 |
| DBP (mm Hg) (mean, SD) | 0 | 85.12 | 11.19 | 81.85 | 10.63 | 80.55 | 10.97 |
| Blood pressure target achievement | 0 | 344 | 34.30 | 346 | 45.95 | 221 | 45.95 |
| Laboratory values | |||||||
| Total cholesterol (mmol/l) (mean, SD) | 12.76 | 5.25 | 1.12 | 5.06 | 1.01 | 5.28 | 1.14 |
| Total cholesterol >4.9 mmol/l | 12.76 | 538 | 61.49 | 218 | 52.91 | 146 | 61.09 |
| Non-HDL cholesterol (mmol/l) | 25.92 | 3.88 | 1.09 | 3.68 | 0.97 | 3.8 | 1.14 |
| LDL (mmol/l) (mean, SD) | 37.49 | 3.12 | 1.01 | 2.98 | 0.96 | 3.12 | 1.08 |
| HDL (mmol/l) (mean, SD) | 25.62 | 1.38 | 0.43 | 1.37 | 0.41 | 1.48 | 0.49 |
| Triglycerides (mmol/l) (mean, SD) | 21.14 | 1.79 | 1.06 | 1.74 | 1.19 | 1.68 | 0.89 |
| Fasting plasma glucose (mmol/l) (mean, SD) | 22.83 | 5.94 | 1.54 | 6.29 | 2.56 | 6.05 | 1.42 |
| eGFR (ml/min/1.73 m2) (mean, SD) | 60.32 | 77.88 | 32.58 | 77.22 | 37.77 | 76.16 | 38.43 |
| Clinical features | |||||||
| BMI (kg/m2) (mean, SD) | 0 | 28.08 | 5.03 | 28.2 | 5.21 | 27.62 | 4.97 |
| Dyslipidaemia | 12.26 | 670 | 76.14 | 302 | 73.12 | 170 | 70.54 |
| LVH | 21.24 | 100 | 12.66 | 36 | 15.45 | 16 | 13.11 |
| DM | 0 | 267 | 26.62 | 212 | 28.15 | 140 | 29.11 |
| Renal dysfunction | 57.03 | 157 | 36.43 | 110 | 45.83 | 59 | 37.82 |
| DM and/or renal dysfunction | 0 | 367 | 36.59 | 279 | 37.05 | 179 | 37.21 |
| Positive family history | 46.66 | 222 | 41.5 | 188 | 46.19 | 107 | 44.96 |
| Years with hypertension diagnosis (mean, SD) | 34.7 | 10.25 | 8.21 | 11.23 | 8.22 | 12.71 | 8.46 |
| Established CV disease | 0.1 | 209 | 20.86 | 158 | 20.98 | 103 | 21.41 |
| Therapy | |||||||
| No antihypertensive therapy | 1.99 | 52 | 5.29 | 29 | 3.88 | 27 | 5.68 |
| Antihypertensive therapy type (no therapy = 1, mono = 2, duo = 3, triple = 4, quadruple = 5) (median, IQR) | 1.99 | 3 | 2 | 3 | 2 | 3 | 2 |
| Lipid lowering therapy | 0 | 299 | 29.81 | 245 | 32.54 | 156 | 32.43 |
| Insulin therapy | 0 | 42 | 4.19 | 36 | 4.78 | 20 | 4.16 |
| Oral antidiabetic therapy | 0 | 167 | 16.65 | 136 | 18.06 | 93 | 19.33 |
| Compliance good | 6.92 | NA | NA | 632 | 84.49 | 316 | 75.96 |
| Non-pharmacological therapy | |||||||
| Weight reduction | 0 | 280 | 27.92 | 124 | 16.47 | 49 | 10.19 |
| Increased physical activity | 0 | 227 | 22.63 | 98 | 13.01 | 53 | 11.02 |
| Smoking cessation | 0 | 59 | 5.88 | 26 | 3.45 | 10 | 2.08 |
| Alcohol reduction | 0 | 43 | 4.29 | 21 | 2.79 | 13 | 2.70 |
| Lifestyle factors | |||||||
| Smoking | 0.1 | 163 | 16.27 | 113 | 15.03 | 71 | 14.79 |
| Alcohol (none = 1, occasionally = 2, frequent = 3, excessive = 4) (median, IQR) | 0.1 | 2 | 0 | 2 | 0 | 2 | 0 |
| Physical activity (little = 1, medium = 2, high = 3) (median, IQR) | 39.68 | 2 | 1 | 2 | 2 | 2 | 1 |
BMI = body mass index; CV = cardiovascular; DBP = diastolic blood pressure; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; IQR = interquartile range; LDL = low-density lipoprotein; LVH = left ventricular hypertrophy; Missing = percent of missing values at baseline; NA = not available; SBP = systolic blood pressure; SD = standard deviation
We included first and second follow-up examinations with a loss to follow-up of 25% and 52%, respectively. Differences between completers and non-completers are reported in appendix 1 (tables S1 and S2 ). Among the whole sample, the first follow-up visit was in average 1.64 (SD 0.87) years after baseline and second follow-up visit 2.84 (SD 0.87) years after baseline. At baseline, mean age was 64.47 (SD 13.17) years and 56% of patients were male (see table 1).
Across the whole sample, systolic blood pressure decreased from 145 mm Hg at baseline by an average of −3.17 (95% CI −3.87 to −2.47) mm Hg per follow-up examination, and diastolic blood pressure decreased from 85 mm Hg by an average of −1.99 (95% CI −2.42 to −1.57) mm Hg per follow-up examination. The percentage of participants achieving target blood pressure increased from 34% at baseline to 46% at second follow-up (see table 1). Blood pressure target achievement increased across follow-up visits in both subgroups. In uncomplicated hypertension 46.85% achieved blood pressure goals at baseline, 61.39% at first follow-up, 61.92% at second follow-up. In complicated hypertension 12.53% achieved blood pressure goals at baseline, 19.71% at first follow-up and 18.99% at second follow-up. Table 2 summarises demographic, lifestyle, clinical and therapeutic characteristics at baseline according to type of hypertension /complicated/uncomplicated). There were 367 (37%) of patients at baseline with complicated hypertension. Of these, 267 had diabetes, 157 had renal disease and 57 had both.
Table 2 Differences between the groups with complicated and uncomplicated hypertension at baseline.
|
Uncomplicated hypertension
(n = 636) |
Complicated hypertension
(n = 367) |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Demographic data | ||||
| Sex male | 286 | 61.9 | 165 | 44.96 |
| Age (years) (mean, SD) | 59.33 | 12.83 | 69.69 | 12.34 |
| Blood pressure variables | ||||
| SBP (mm Hg) (mean, SD) | 142.56 | 17.49 | 146.95 | 19.52 |
| DBP (mm Hg) (mean, SD) | 88.58 | 11.24 | 82.86 | 11.52 |
| Blood pressure target achievement | 298 | 46.85 | 46 | 12.53 |
| Laboratory values | ||||
| Total cholesterol (mmol/l) (mean, SD) | 5.39 | 1.08 | 5.06 | 1.17 |
| Total cholesterol >4.9 mmol/l | 264 | 67.18 | 176 | 53.66 |
| Non-HDL cholesterol (mmol/l) | 4.06 | 1.06 | 3.66 | 1.09 |
| LDL (mmol/l) (mean, SD) | 3.29 | 0.98 | 2.91 | 1.03 |
| HDL (mmol/l) (mean, SD) | 1.38 | 0.41 | 1.37 | 0.47 |
| Triglycerides (mmol/l) (mean, SD) | 1.79 | 1.14 | 1.79 | 0.96 |
| Fasting plasma glucose (mmol/l) (mean, SD) | 5.3 | 0.66 | 6.88 | 1.99 |
| eGFR (ml/min/1.73 m2) (mean, SD) | 88.65 | 26.3 | 64.6 | 31.22 |
| Clinical features | ||||
| BMI (kg/m2) (mean, SD) | 27.83 | 4.65 | 28.33 | 5.59 |
| Dyslipidaemia | 312 | 78.79 | 239 | 72.64 |
| LVH | 30 | 8.43 | 51 | 17.06 |
| DM | 0 | 0 | 267 | 72.75 |
| Renal dysfunction | 0 | 0 | 157 | 68.86 |
| DM and/or renal dysfunction | 109 | 41.6 | 71 | 39.89 |
| Positive family history | 8.76 | 7.1 | 11.72 | 8.69 |
| Years with hypertension diagnosis (mean, SD) | 61 | 13.23 | 113 | 30.79 |
| Established CV disease | ||||
| Therapy | 37 | 8.25 | 8 | 2.19 |
| No antihypertensive therapy | 3 | 1 | 3 | 2 |
| Antihypertensive therapy type (no therapy = 1, mono = 2, duo = 3, triple = 4, quadruple = 5) (median, IQR) | 89 | 19.26 | 154 | 41.96 |
| Lipid lowering therapy | 0 | 0 | 42 | 11.44 |
| Insulin therapy | 0 | 0 | 167 | 45.5 |
| Oral antidiabetic therapy | ||||
| Compliance good | 168 | 26.42 | 112 | 30.52 |
| Non-pharmacological therapy | 143 | 22.48 | 84 | 22.89 |
| Weight reduction | 42 | 6.60 | 17 | 4.63 |
| Increased physical activity | 28 | 4.40 | 15 | 4.09 |
| Smoking cessation | ||||
| Alcohol reduction | 81 | 17.57 | 57 | 15.53 |
| Lifestyle factors | 2 | 0 | 2 | 0 |
| Smoking | 2 | 1 | 2 | 1 |
BMI = body mass index; CV = cardiovascular; DBP = diastolic blood pressure; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; IQR = interquartile range; LDL = low-density lipoprotein; LVH = left ventricular hypertrophy; Missing = percent of missing values at baseline; NA = not available; SBP = systolic blood pressure; SD = standard deviation
Table 3 summarises univariable and multivariable regression results for guideline change in 2009 predicting blood pressure measurements and pharmacological treatment type. The guideline change in 2009 was significantly associated with systolic office blood pressure in high-risk patients, namely by reducing systolic office blood pressure from 147 mm Hg at baseline by 6.85 mm Hg (95% CI −9.92 to −3.77) or adjusted for confounding variables by 5.4 mm Hg (95% CI −8.08 to −2.73) (table 3 and fig. 1). Diastolic office blood pressure in high-risk patients was 83 mm Hg at baseline and decreased by −2.49 mm Hg (95% CI −4.35 to −0.63) after 2009. However, after adjustment for confounding variables, the association disappeared in diastolic office blood pressure (β = 0.26, 95% CI −1.25 to 1.77). Similarly, the cumulative probability of combination pharmacological treatment increased significantly after 2009 in the univariable model (OR 2.22, 95% CI 1.15 to 4.29), but did not remain significant after adjusting for covariates (OR1.85, 95% CI 0.94 to 3.63; p = 0.073). Achievement of blood pressure targets were not significantly associated with guideline change in high-risk patients. Results from multivariable analyses regarding systolic/diastolic blood pressure and pharmacological treatment in complicated and uncomplicated hypertension are reported in the supplementary tables S3–S7 in appendix 1.
Table 3 Pooled multivariable mixed-effects regression results of guideline change (2009 and after vs. before 2009) predicting (1) systolic blood pressure, (2) diastolic blood pressure, (3) the likelihood of blood pressure target achievement and (4) the cumulative probability of pharmacological therapy type using imputed data in complicated hypertension (n = 367).
| Predictor: year of examination (2009 and after vs before 2009) | Univariable model 1* | Multivariable model 2†,‡ | ||||||
|---|---|---|---|---|---|---|---|---|
| Outcome: | β estimates | 95% CI | p-value | β estimates | 95% CI | p-value | ||
| 1. Systolic blood pressure† | −6.85 | −9.92 | −3.77 | <0.001 | −5.40 | −8.08 | −2.73 | <0.001 |
| 2. Diastolic blood pressure† | −2.49 | −4.35 | −0.63 | 0.009 | 0.26 | −1.25 | 1.77 | 0.735 |
| OR | 95% CI | p-value | OR | 95% CI | p-value | |||
| 3. Bloodpressure target achievement‡ | 1.26 | 0.43 | 3.69 | 0.667 | 1.28 | 0.65 | 2.53 | 0.481 |
| 4. Antihypertensive pharmacological therapy§ | 2.22 | 1.15 | 4.29 | 0.017 | 1.85 | 0.94 | 3.63 | 0.073 |
BMI = body mass index; CI = confidence interval; OR = adjusted odds ratio * Adjusted for follow-up visit † Model adjusted for either systolic or diastolic blood pressure, sex, age, follow-up visit, cholesterol, fasting plasma glucose, BMI, left ventricular hypertrophy, diabetes mellitus, years with hypertension diagnosis, established cardiovascular disease, number of antihypertensive medications, lipid lowering therapy, smoking, alcohol, physical activity, non-pharmacological treatment such as reducing weight, increased activity, smoking cessation, alcohol reduction. ‡ For blood pressure target achievement the model was adjusted for sex, age, follow-up visit, cholesterol, fasting plasma glucose, BMI, left ventricular hypertrophy, diabetes mellitus, years with hypertension diagnosis, established cardiovascular disease, number of antihypertensive medications, lipid lowering therapy, smoking, alcohol, physical activity. § For antihypertensive pharmacological therapy the model was adjusted for sex, age, follow-up visit, systolic and diastolic BP cholesterol, fasting plasma glucose, BMI, left ventricular hypertrophy, diabetes mellitus, years with hypertension diagnosis, established cardiovascular disease, number of antihypertensive medication, lipid lowering therapy, smoking, alcohol, physical activity.

Figure 1 Change in mean blood pressure over time in patients with complicated hypertension and patients with uncomplicated hypertension.
Results and inferences from the multivariable complete case sensitivity analyses in patients with complicated hypertension with n = 116 were comparable to those found using the imputed data (supplementary tables S8–S11 ), except for antihypertensive combination therapy with opposite odds ratios.
In patients with uncomplicated hypertension we found independent significant covariates of blood pressure achievement in the multivariate model; patients with LVH were 42% less likely to achieve blood pressure goals (OR 0.58, 95% CI 0.36 to 0.93), older patients were more likely to achieve blood pressure goals (1.19 per 10 years, 95% CI 1.02 to 1.40), patients with an increased use of pharmacological combination therapy were more likely to achieve blood pressure goals (OR 1.19 per combination increase: e.g., dual therapy vs monotherapy, 95% CI 1.02 to 1.40) and patients at later follow-up examinations were more likely to achieve blood pressure goals (OR 1.44 per follow-up visit [e.g. first follow-up visit vs baseline], 95% CI 1.21 TO 1.72) (table 4). The latter association was also observed in high-risk hypertensive patients but was not significant (OR 1.35, 95% CI = 0.98 to 1.86; p = 0.068). In high-risk patients, we found no variables significantly associated with blood pressure target achievement.
Table 4 Pooled multivariable logistic mixed-effect regression results describing the association between blood pressure goal attainment and demographic/health-related variables in (1) patients with complicated hypertension (n = 367) and (2) patients with uncomplicated hypertension (n = 636).
| OR | 95% CI | p-value | ||
|---|---|---|---|---|
| Predictors for patients with complicated hypertension (n = 367) | ||||
| Follow-up visit | 1.35 | 0.98 | 1.86 | 0.068 |
| Year of examination (2009 and after vs before 2009) | 1.28 | 0.65 | 2.53 | 0.481 |
| Sex (male vs female) | 1.16 | 0.54 | 2.51 | 0.702 |
| Age (per 10 years) | 1.35 | 0.96 | 1.88 | 0.082 |
| Cholesterol | 0.79 | 0.55 | 1.15 | 0.206 |
| Fasting plasma glucose (mmol/l) | 0.96 | 0.82 | 1.12 | 0.577 |
| BMI (per 5 kg/m2) | 0.98 | 0.72 | 1.34 | 0.890 |
| LVH | 0.81 | 0.34 | 1.94 | 0.631 |
| DM | 0.69 | 0.30 | 1.58 | 0.381 |
| Years with hypertension diagnosis (per 10 years) | 1.01 | 0.58 | 1.78 | 0.961 |
| Established CV disease | 2.24 | 1.16 | 4.35 | 0.017 |
| Number of antihypertensive medication | 0.89 | 0.65 | 1.22 | 0.472 |
| Lipid lowering therapy | 0.90 | 0.47 | 1.72 | 0.756 |
| Smoking | 0.76 | 0.32 | 1.85 | 0.550 |
| Alcohol | 1.32 | 0.82 | 2.14 | 0.252 |
| Physical activity | 0.88 | 0.58 | 1.35 | 0.564 |
| Predictors for patients with uncomplicated hypertension (n = 636) | ||||
| Follow-up visit | 1.44 | 1.21 | 1.72 | <0.001 |
| Year of examination (2009 and after vs before 2009) | 1.21 | 0.89 | 1.64 | 0.224 |
| Sex (male vs female) | 0.89 | 0.64 | 1.24 | 0.495 |
| Age (per 10 years) | 1.32 | 1.15 | 1.52 | <0.001 |
| Cholesterol | 0.87 | 0.71 | 1.08 | 0.188 |
| Fasting plasma glucose (mmol/l) | 1.03 | 0.82 | 1.29 | 0.815 |
| BMI (per 5 kg/m2) | 1.03 | 0.87 | 1.21 | 0.733 |
| LVH | 0.58 | 0.36 | 0.93 | 0.025 |
| Years with hypertension diagnosis (per 10 years) | 0.79 | 0.63 | 0.99 | 0.044 |
| Established CV disease | 1.31 | 0.81 | 2.12 | 0.266 |
| Number of antihypertensive medication | 1.19 | 1.02 | 1.40 | 0.032 |
| Lipid lowering therapy | 1.29 | 0.87 | 1.92 | 0.198 |
| Smoking | 0.97 | 0.63 | 1.49 | 0.895 |
| Alcohol | 1.18 | 0.92 | 1.52 | 0.197 |
| Physical activity | 1.09 | 0.88 | 1.34 | 0.431 |
| BMI = body mass index; CI = confidence interval; OR = Adjusted odds ratio; | ||||
The analysis of patients enrolled for Swiss Hypertensive Cohort Study provided detailed information on the effect of the revision of Swiss clinical practice guidelines on blood pressure and its control in a primary care setting. In this “real life setting”, the Swiss Society of Hypertension guideline changes resulted in a reduction of systolic blood pressure and increased use of antihypertensive combination therapy among patients with diabetes mellitus or renal dysfunction. Despite antihypertensive treatment of the great majority for an average of 10 years, blood pressure targets were reached in only 34% of all patients (13% in complicated and 47% in uncomplicated hypertensive patients). Blood pressure target achievement in complicated hypertensive patients was not associated with guideline changes, but with established cardiovascular disease. At follow-up examinations, patients were more likely to have achieved blood pressure targets than at baseline in both groups. Namely, they were twice as likely to have achieved blood pressure goals at the second follow-up visit as at baseline.
In general, clinical guidelines are intended to support and assist clinicians to appropriately manage specific condition such as high blood pressure. The main objective of guidelines is to improve the quality of care received by patients based on the requirements of evidence-based medicine. Although there is evidence that clinical practice guidelines can improve quality of care [33], the implementation of specific guidelines into daily practice remains challenging. Our results show this difficulty; the guideline change in 2009 was associated with systolic blood pressure reductions and increased use of pharmacological combination treatment; however changes in diastolic blood pressure and higher blood pressure achievement could not be detected. As pointed out by Woolf et al., practice guidelines clearly have the potential to improve health outcomes, but there are possible limitations or even harm [34]. Further, the fact that guidelines are largely developed by specialists for one specific condition and are supposed to be implemented by generalists /general practitioners is a matter of debate, particularly in patients with multimorbidity [35]. Fragmentation of health care, challenges in delivering patient-centred care and shared decision making are crucial issues, particularly in the multimorbid population.
A population with high cardiovascular risk due to diabetes and/or renal disease needs particular attention and close clinical follow-up. Indeed, our results showed a trend for better blood pressure goal achievement after each follow-up examination. As stated by Kjeldsen et al., more “personalised medicine” might be the effective way to manage high-risk hypertensive patients until more comprehensive evidence on blood pressure goals in this specific population are available [36]. Reasonable adherence to clinical practice guideline by GPs as a guide for the management of hypertensive patients is undoubtedly indispensable. For the GP in daily clinical practice this uncertainty regarding blood pressure goals in high-risk patients remains a difficult issue. Although the prevalence of blood pressure target achievement in hypertensive patients with diabetes mellitus and/or renal disease must have risen in accordance with new Swiss guidelines with the goal of <140/90 mm Hg for all patients after 3 months [37], the majority of these patients may still not achieve blood pressure targets. Walther’s population-based cohort study on high blood pressure emphasised the problem of medical inertia and the need of continual GP education [4]. It showed in particular that a full quarter of surveyed patients were treated with beta-blocker monotherapy although this was no longer an acceptable indication for first line beta-blockade [38].
Similar to previous studies, in this study only half of uncomplicated hypertensive primary care patients reached goals for blood pressure and even fewer high-risk hypertensive patients reached the more stringent goals prevailing during the study [14, 39–41]. A similar Swiss cross-sectional visit-based survey of ambulatory hypertensive patients also found that blood pressure control (<140/90 mm Hg) was significantly better in patients with uncomplicated hypertension (59%) compared with 19% in complicated hypertension (<130/80 mm Hg) [14]. Another Swiss study reported that in people with doctor-diagnosed hypertension, 82% received drug treatment but only 41% had blood pressure control (<140/90 mm Hg) [4]. A cross-sectional study examined the treatment and goals attainments related to cardiovascular risk factors within chronic kidney disease stages in type 2 diabetic patients followed up by primary care physicians in Switzerland [15]. This found that 18% reached the Swiss or American Diabetes Association 2013 blood pressure goals (<140/80 mm Hg). The results of a German study assessing the prevalence and control of hypertension indicated that 43% of primary care patients did not have blood pressure control despite treatment [40]. Another German population-based cohort study evaluating hypertensive patients over 5 years found that 42% of patients with uncomplicated hypertension reached blood pressure targets (<140/90 mm Hg) and 22% of patients with any risk comorbidity reached the target blood pressure of <130/80 mm Hg [42]. In a survey on GPs’ decision to treat hypertension in oldest-old across 29 countries, GPs’ choice to treat/not treat was explained by differences in country-specific health characteristics [43]. The authors reported that GPs in countries with a high cardiovascular disease burden were three times more likely to treat their patients, but this was not the case in countries with high life expectancy. Switzerland has a comparatively low cardiovascular disease burden combined with a relatively high life expectancy at age 60 compared with other countries, which might also contribute to lower prevalence of blood pressure goal attainment.
Among high-risk patients, better blood pressure control was found in our study in those with established coronary heart disease. This finding is in agreement with other studies [44, 45] and may reflect increased attention of both physician and patient with regard to risk factor management in secondary prevention [46]. Compared with patients with controlled uncomplicated hypertensive, high-risk patients not at goal more frequently had LVH and were diagnosed with hypertension for a longer time. The latter may reflect the effect of vessel stiffening resulting in difficulties achieving blood pressure targets [47]. Previous studies have shown that LVH increases with age [48]. Further, data from a longitudinal cohort study of very elderly hypertensive women indicated a high prevalence of LVH, which appeared to be related to levels of systolic blood pressure [49]. Thus, increases in arterial stiffness might be reflected by increased pulse pressure and favour the development of LVH [50, 51]. A study from France showed that patients with not‐at‐goal hypertension in primary care had a heavy burden of cardiovascular diseases [52], with 15% current smokers, 26% obese, 23% with diabetes mellitus, 35% with dyslipidaemia, 12% with left ventricular hypertrophy and 5% with renal insufficiency. Similarly, a cardiovascular risk stratification across the whole sample of our study population [53] has shown that 79% of patients presented with either more than three additional cardiovascular risk factors, diabetes mellitus or subclinical organ damage, whereas 44% of patients had a high or very high overall cardiovascular risk. On the one hand, such patients at high cardiovascular risk need adequate treatment of arterial hypertension. On the other hand, patients with not‐at‐goal hypertension may benefit from effective countermeasures to those concomitant cardiovascular risk factors to reach blood pressure targets [52].
Some thoughts on strengths and limitations of the present study must be considered. The present study reflects “real-life” data from primary care practices assessing a reasonable number of GPs and patients with arterial hypertension in busy clinical practice. It was a specific goal of the Swiss Hypertension Cohort Study to investigate a “real-life” situation in a typical primary care setting. Some limitations of this study should be considered. First, missing data led to the rejection of some important variables for the assessment of cardiovascular risk factors, such as microalbuminuria. A recent study by Abdel-Kader et al. revealed that one in four primary care physicians are reluctant to quantify urine albumin in non-diabetic hypertensive patients with eGFR <60ml/min due to lacking impact on further management [58]. Second, 95% of the patients included in the current cohort were pharmacologically treated for arterial hypertension. Therefore, cardiovascular risk factors were likely to be underestimated compared with an untreated population. Third, there could be a possible selection bias of patients since the hypertensive patients included were not selected in a consecutive way but at the discretion of the GPs. However, patient characteristics at baseline were comparable with a Swiss study consecutively including hypertensive patients attending the practices of GPs for blood pressure follow-up [14]. Therefore, we feel that our population is likely to be comparable to previously studied cohorts. Fourth, the Swiss Society of Hypertension guidelines did not specify adaptations of blood pressure target levels for elderly patients as did the ESH guidelines for patients ≥80 years of age with a blood pressure target <150/90 mm Hg [18]. To our knowledge and according to the literature there are very sparse recommendations on blood pressure target levels in elderly patients with diabetes [59]. Thus, blood pressure targets for this specific subpopulation should be addressed in future Swiss guidelines more explicitly. Moreover, the Swiss Society of Hypertension guidelines did not specify the treatment goal for patients who present with combined conditions, namely isolated systolic hypertension and diabetes mellitus and/or renal dysfunction. Thus, we applied a target blood pressure of <150/90 mm Hg for patients with isolated systolic hypertension without diabetes mellitus and/or renal dysfunction for the current study. Fifth, several parameters were not standardised (e.g., blood pressure devices were different depending of the GP practice; physical activity was classified at the discretion of the GPs; the presence/absence of established diseases was also at the discretion of the GP). As described above this was a real-world observational study from primary care and therefore it is difficult to standardise parameters as in a randomised clinical trial. Randomised clinical trial have restrictive inclusion and exclusion criteria; thus, they are not fully representative of an unselected real-world population. Limitations of real-world observational studies, however, can include low internal validity, lack of quality control regarding data collection and vulnerability to several sources of bias for comparing outcomes [60].
In conclusion, the revision of Swiss clinical practice guidelines on hypertension in 2009 with the goal of <130/80 mm Hg in hypertensive patients with diabetes mellitus or renal dysfunction was associated with a significant reduction of systolic blood pressure and a trend towards increased use of pharmacological combination therapy. These results indicate the efforts of primary care physicians to implement 2009 guidelines in their practices. However, the rate of blood pressure target achievement remained at the same (low) level after 2009. This was mainly owing to high systolic blood pressure at baseline (146 mm Hg) and a relatively small reduction of 6 mm Hg, which was not sufficient to achieve blood pressure targets of <130 mm Hg. The newest 2018 SSH blood pressure targets of <140/90 within 3 months might be more realistic to achieve in patients with complicated hypertension. It seems important to note that all hypertensive patients were more likely to achieve blood pressure goals at later follow up visits compared to baseline indicating the need of regular follow-up visits to meet blood pressure goals.
The appendix is available as a separate file at https://smw.ch/article/doi/smw.2020.20279
We wish to thank all participating GPs and patients for their contribution to this study.
This work was supported by Schweizerische Gesellschaft für Hypertonie, Universität Basel, Gottfried und Julia Bangerter-Rhyner-Stiftung, Labor Rothen Basel, MSD Chibret AG Schweiz, Novartis Pharma Schweiz, Pfizer Pharma Schweiz, Robapharm Schweiz, Sandoz Pharmaceuticals AG Schweiz, Sankyo Pharma Schweiz, Spirig Pharma AG. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors report no competing interest.
1 Estoppey D , Paccaud F , Vollenweider P , Marques-Vidal P . Trends in self-reported prevalence and management of hypertension, hypercholesterolemia and diabetes in Swiss adults, 1997-2007. BMC Public Health. 2011;11(1):114 .https://doi.org/10.1186/1471-2458-11-114
2 Danon-Hersch N , Marques-Vidal P , Bovet P , Chiolero A , Paccaud F , Pécoud A , et al. Prevalence, awareness, treatment and control of high blood pressure in a Swiss city general population: the CoLaus study. Eur J Cardiovasc Prev Rehabil. 2009;16(1):66–72. doi:.https://doi.org/10.1097/HJR.0b013e32831e9511
3 Guessous I , Bochud M , Theler JM , Gaspoz JM , Pechère-Bertschi A . 1999-2009 Trends in prevalence, unawareness, treatment and control of hypertension in Geneva, Switzerland. PLoS One. 2012;7(6):e39877 .https://doi.org/10.1371/journal.pone.0039877
4 Walther D , Curjuric I , Dratva J , Schaffner E , Quinto C , Rochat T , et al. High blood pressure: prevalence and adherence to guidelines in a population-based cohort. Swiss Med Wkly. 2016;146:w14323 .https://doi.org/10.4414/smw.2016.14323
5 Wolf-Maier K , Cooper RS , Banegas JR , Giampaoli S , Hense H-W , Joffres M , et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289(18):2363–9 .https://doi.org/10.1001/jama.289.18.2363
6 Liu L , Zhang Y , Liu G , Li W , Zhang X , Zanchetti A ; FEVER Study Group. The Felodipine Event Reduction (FEVER) Study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens. 2005;23(12):2157–72 .https://doi.org/10.1097/01.hjh.0000194120.42722.ac
7 Mancia G , Parati G . Importance of smooth and sustained blood pressure control in preventing cardiovascular morbidity and mortality. Blood Press Suppl. 2001;10(3):26–32 .https://doi.org/10.1080/08037050152518339
8 Zanchetti A . Hypertension-related mortality and morbidity. J Hypertens. 2015;33(10):1979–80 .https://doi.org/10.1097/HJH.0000000000000725
9 Horr S , Nissen S . Managing hypertension in type 2 diabetes mellitus. Best Pract Res Clin Endocrinol Metab. 2016;30(3):445–54 .https://doi.org/10.1016/j.beem.2016.06.001
10 Chen G , McAlister FA , Walker RL , Hemmelgarn BR , Campbell NR . Cardiovascular outcomes in framingham participants with diabetes: the importance of blood pressure. Hypertension. 2011;57(5):891–7 .https://doi.org/10.1161/HYPERTENSIONAHA.110.162446
11 Weiner DE , Tighiouart H , Amin MG , Stark PC , MacLeod B , Griffith JL , et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15(5):1307–15 .https://doi.org/10.1097/01.ASN.0000123691.46138.E2
12 Cushman WC , Whelton PK , Fine LJ , Wright JT, Jr , Reboussin DM , Johnson KC , et al.; SPRINT Study Research Group. SPRINT Trial Results: Latest News in Hypertension Management. Hypertension. 2016;67(2):263–5 .https://doi.org/10.1161/HYPERTENSIONAHA.115.06722
13 Moran AE . Still on the Road to Worldwide Hypertension Control. Circulation. 2016;134(6):451–4 doi:.https://doi.org/10.1161/CIRCULATIONAHA.116.023960
14 Brenner R , Waeber B , Allemann Y . Medical treatment of hypertension in Switzerland. The 2009 Swiss Hypertension Survey (SWISSHYPE). Swiss Med Wkly. 2011;141:w13169 .https://doi.org/10.4414/smw.2011.13169
15 Corcillo A , Pivin E , Lalubin F , Pitteloud N , Burnier M , Zanchi A . Glycaemic, blood pressure and lipid goal attainment and chronic kidney disease stage of type 2 diabetic patients treated in primary care practices. Swiss Med Wkly. 2017;147:w14459. doi:.https://doi.org/10.4414/smw.2017.14459
16 Papadopoulou E , Angeloudi E , Karras S , Sarafidis P . The optimal blood pressure target in diabetes mellitus: a quest coming to an end? J Hum Hypertens. 2018;32(10):641–50 .https://doi.org/10.1038/s41371-018-0079-5
17 Pechère-Bertschi A , Stalder H . Nachweis einer arteriellen Hypertonie. Primary Care. 2003;3:547–53. doi:https://doi.org/10.4414/pc-d.2003.05803
18Swiss Society of Hypertension. Guidelines 2015 [cited 2016 August 31]. Available from: http://www.swisshypertension.ch/docs/guidelines_2015_d_leaflet.pdf.
19 Mancia G , Fagard R , Narkiewicz K , Redon J , Zanchetti A , Böhm M , et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159–219 .https://doi.org/10.1093/eurheartj/eht151
20 Liakos CI , Grassos CA , Babalis DK . ESH/ESC Guidelines for the Management of Arterial Hypertension: What Has Changed in Daily Clinical Practice? High Blood Press Cardiovasc Prev. 2015;22(1):43–53. doi:.https://doi.org/10.1007/s40292-014-0071-2
21 Bangalore S , Kumar S , Lobach I , Messerli FH . Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and bayesian random-effects meta-analyses of randomized trials. Circulation. 2011;123(24):2799–810, 9, 810 .https://doi.org/10.1161/CIRCULATIONAHA.110.016337
22 Gerstein HC , Miller ME , Genuth S , Ismail-Beigi F , Buse JB , Goff DC, Jr , et al.; ACCORD Study Group. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818–28 .https://doi.org/10.1056/NEJMoa1006524
23 Borm K , Lüscher S , Müller B . Erste Behandlungsschritte beim neuentdeckten Diabetes mellitus Typ 2 – praktische Tipps. Schweiz Med Forum. 2012;12(48):929–35. doi:.https://doi.org/10.4414/smf.2012.01343
24 Zeller A , Haehner T , Battegay E , Martina B . Diagnostic significance of transferrinuria and albumin-specific dipstick testing in primary care patients with elevated office blood pressure. J Hum Hypertens. 2005;19(3):205–9 .https://doi.org/10.1038/sj.jhh.1001803
25 Zeller A , Sigle JP , Battegay E , Martina B . Value of a standard urinary dipstick test for detecting microalbuminuria in patients with newly diagnosed hypertension. Swiss Med Wkly. 2005;135(3-4):57–61.
26 Levey AS , Coresh J , Balk E , Kausz AT , Levin A , Steffes MW , et al.; National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–47 .https://doi.org/10.7326/0003-4819-139-2-200307150-00013
27 Lee JH , Huber J . Multiple imputation with large proportions of missing data: How much is too much? United Kingdom Stata Users’ Group meetings. 2011:23.
28 Raghunathan TE , Lepkowski JM , Van Hoewyk J , Solenberger P . A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27(1):85–96.
29 Azur MJ , Stuart EA , Frangakis C , Leaf PJ . Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–9 .https://doi.org/10.1002/mpr.329
30 Rubin DB . Inference and missing data. Biometrika. 1976;63(3):581–92. doi:.https://doi.org/10.1093/biomet/63.3.581
31van Buuren S. How many imputations? Flexible Imputation of Missing Data. Boca Raton, FL: Chapman & Hall/CRC Press; 2012.
32Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: J. Wiley & Sons; 1987.
33 Grimshaw JM , Russell IT . Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet. 1993;342(8883):1317–22 .https://doi.org/10.1016/0140-6736(93)92244-N
34 Woolf SH , Grol R , Hutchinson A , Eccles M , Grimshaw J . Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318(7182):527–30 .https://doi.org/10.1136/bmj.318.7182.527
35 Sinnott C , Mc Hugh S , Browne J , Bradley C . GPs’ perspectives on the management of patients with multimorbidity: systematic review and synthesis of qualitative research. BMJ Open. 2013;3(9):e003610 .https://doi.org/10.1136/bmjopen-2013-003610
36 Kjeldsen SE , Aksnes TA , Ruilope LM . Clinical implications of the 2013 ESH/ESC hypertension guidelines: targets, choice of therapy, and blood pressure monitoring. Drugs R D. 2014;14(2):31–43 .https://doi.org/10.1007/s40268-014-0049-5
37Swiss Society of Hypertension. Guidelines: Arterial Hypertension (General) 2019 [cited 2019 December]. Available from: http://www.swisshypertension.ch/DOCS_PUBLIC/Pocketcard_Uebersicht_D_WEB.pdf.
38 Argulian E , Grossman E , Messerli FH . Misconceptions and facts about treating hypertension. Am J Med. 2015;128(5):450–5 .https://doi.org/10.1016/j.amjmed.2014.11.015
39 Balijepalli C , Bramlage P , Lösch C , Zemmrich C , Humphries KH , Moebus S . Prevalence and control of high blood pressure in primary care: results from the German Metabolic and Cardiovascular Risk Study (GEMCAS). Hypertens Res. 2014;37(6):580–4 .https://doi.org/10.1038/hr.2014.40
40 Sharma AM , Wittchen HU , Kirch W , Pittrow D , Ritz E , Göke B , et al.; HYDRA Study Group. High prevalence and poor control of hypertension in primary care: cross-sectional study. J Hypertens. 2004;22(3):479–86 .https://doi.org/10.1097/00004872-200403000-00009
41 Tocci G , Rosei EA , Ambrosioni E , Borghi C , Ferri C , Ferrucci A , et al. Blood pressure control in Italy: analysis of clinical data from 2005-2011 surveys on hypertension. J Hypertens. 2012;30(6):1065–74 .https://doi.org/10.1097/HJH.0b013e3283535993
42 van den Berg N , Meinke-Franze C , Fiss T , Baumeister SE , Hoffmann W . Prevalence and determinants of controlled hypertension in a German population cohort. BMC Public Health. 2013;13(1):594 .https://doi.org/10.1186/1471-2458-13-594
43 Streit S , Gussekloo J , Burman RA , Collins C , Kitanovska BG , Gintere S , et al. Burden of cardiovascular disease across 29 countries and GPs’ decision to treat hypertension in oldest-old. Scand J Prim Health Care. 2018;36(1):89–98 .https://doi.org/10.1080/02813432.2018.1426142
44 Jaussi A , Noll G , Meier B , Darioli R . Current cardiovascular risk management patterns with special focus on lipid lowering in daily practice in Switzerland. Eur J Cardiovasc Prev Rehabil. 2010;17(3):363–72 .https://doi.org/10.1097/HJR.0b013e328333c1d9
45 Fang J , Alderman MH , Keenan NL , Ayala C , Croft JB . Hypertension control at physicians’ offices in the United States. Am J Hypertens. 2008;21(2):136–42 .https://doi.org/10.1038/ajh.2007.35
46 Berlowitz DR , Ash AS , Hickey EC , Friedman RH , Glickman M , Kader B , et al. Inadequate management of blood pressure in a hypertensive population. N Engl J Med. 1998;339(27):1957–63 .https://doi.org/10.1056/NEJM199812313392701
47 Chobanian AV . Clinical practice. Isolated systolic hypertension in the elderly. N Engl J Med. 2007;357(8):789–96 .https://doi.org/10.1056/NEJMcp071137
48 Levy D , Garrison RJ , Savage DD , Kannel WB , Castelli WP . Left ventricular mass and incidence of coronary heart disease in an elderly cohort. The Framingham Heart Study. Ann Intern Med. 1989;110(2):101–7 .https://doi.org/10.7326/0003-4819-110-2-101
49 Leibowitz D , Bursztyn M , Jacobs JM , Ein-Mor E , Stessman J . High prevalence of left ventricular hypertrophy in octogenarian women: The Jerusalem Longitudinal Cohort Study. Blood Press. 2010;19(2):86–91 .https://doi.org/10.3109/08037050903516292
50 de Simone G , Roman MJ , Koren MJ , Mensah GA , Ganau A , Devereux RB . Stroke volume/pulse pressure ratio and cardiovascular risk in arterial hypertension. Hypertension. 1999;33(3):800–5 .https://doi.org/10.1161/01.HYP.33.3.800
51 Gosse P , Pichot V , Guilhot M , Dauphinot V , Da Costa A , Barthelemy JC , et al. Relationship of cardiac involvement with arterial stiffness in a general population of 65-year-olds in the PROOF study. J Hypertens. 2010;28(2):389–94 .https://doi.org/10.1097/HJH.0b013e328333d1a4
52 Zhang Y , Lelong H , Kretz S , Agnoletti D , Mourad JJ , Safar ME , et al. Characteristics and future cardiovascular risk of patients with not-at-goal hypertension in general practice in France: the AVANT’AGE study. J Clin Hypertens (Greenwich). 2013;15(4):291–5 .https://doi.org/10.1111/jch.12082
53 Handschin A , Brighenti-Zogg S , Mundwiler J , Giezendanner S , Gregoriano C , Martina B , et al. Cardiovascular risk stratification in primary care patients with arterial hypertension: Results from the Swiss Hypertension Cohort Study (HccH). Eur J Prev Cardiol. 2019;26(17):1843–51 .https://doi.org/10.1177/2047487319856732
58 Abdel-Kader K , Greer RC , Boulware LE , Unruh ML . Primary care physicians’ familiarity, beliefs, and perceived barriers to practice guidelines in non-diabetic CKD: a survey study. BMC Nephrol. 2014;15(1):64 .https://doi.org/10.1186/1471-2369-15-64
59 Solini A , Grossman E . What Should Be the Target Blood Pressure in Elderly Patients With Diabetes? Diabetes Care. 2016;39(Suppl 2):S234–43 .https://doi.org/10.2337/dcS15-3027
60 Camm AJ , Fox KAA . Strengths and weaknesses of ‘real-world’ studies involving non-vitamin K antagonist oral anticoagulants. Open Heart. 2018;5(1):e000788 .https://doi.org/10.1136/openhrt-2018-000788
This work was supported by Schweizerische Gesellschaft für Hypertonie, Universität Basel, Gottfried und Julia Bangerter-Rhyner-Stiftung, Labor Rothen Basel, MSD Chibret AG Schweiz, Novartis Pharma Schweiz, Pfizer Pharma Schweiz, Robapharm Schweiz, Sandoz Pharmaceuticals AG Schweiz, Sankyo Pharma Schweiz, Spirig Pharma AG. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors report no competing interest.