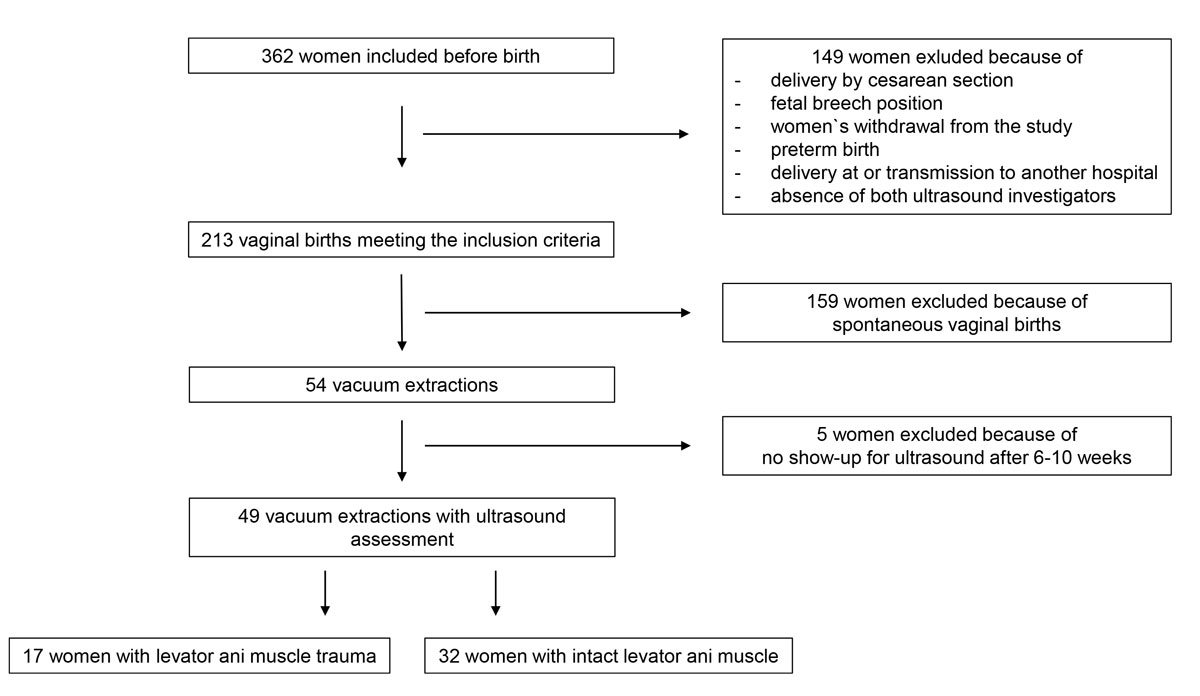
Figure 1 Study flow chart.
DOI: https://doi.org/10.4414/smw.2020.20280
Trauma of the pelvic floor is common after vaginal birth and mainly occurs during a woman's first birth [1–3]. Subsequent births rarely cause substantial additional trauma to the pelvic floor [1, 4]. Pelvic floor trauma after birth appears in the form of a widening of the genital hiatus or, in 6–63%, of partial or complete avulsions of the levator ani muscle [2, 3, 5–12]. These defects can cause immense short- and long-term morbidity in women, including uterine prolapse, incontinence, pain, sexual disorders, psychological distress and the need for repetitive surgery with tremendous costs [2, 13–19]. Diagnosis of levator ani muscle avulsions can most reliably be made using three-dimensional (3D) translabial ultrasound, as described by Dietz et al. [20–23]. Ultrasound shows moderate to very good interobserver agreement and agreement with magnetic resonance imaging [18]. Named risk factors for such levator ani muscle avulsions are advanced maternal age, lower body mass index, a higher fetal weight and head circumference, a prolonged second stage of labour and vaginal-operative delivery, especially forceps extraction [24–29]. In a recent meta-analysis regarding delivery mode and the risk of complete levator ani muscle avulsion, the odds ratio for forceps versus spontaneous vaginal birth was 6.94, for forceps versus vacuum extraction it was 4.57 and for vacuum extraction versus spontaneous vaginal birth it was 1.31 [30]. Regarding operative assisted births, little information is available for the associations between the techniques and procedures of operative assisted vaginal births and levator ani muscle trauma. Some studies exist, but only evaluating single factors relating to the vacuum procedure, such as the number of tractions, the height of the fetal head at cup application or different cup types [10, 31, 32].
Therefore, the aim of our study was to evaluate associations of multiple combined parameters of the technique and process of vacuum extraction with the occurrence of any form of levator ani muscle avulsion in order to better advise obstetricians regarding the optimal technique.
Between March 2017 and April 2019, we asked nulliparous women ≥18 of age with singletons in vertex presentation at ≥36+0 gestational weeks (gw) at the University Hospital of Zurich who planned to have a vaginal birth in our institution to participate in a prospective cohort study for the evaluation of levator ani muscle trauma after vaginal birth. The study was approved by the local ethics board and was performed according to the Declaration of Helsinki. Within this prospective observational cohort study, all women who gave birth with the help of vacuum extraction were included in the final analysis for the presented study. Clinical data were extracted from the institutional obstetric database (Perinat version 6.1.9.45) with the help of different observation and monitoring tools, as described by our group previously [33]. In our institution, we document in detail every step of vacuum extraction and its accompanying procedures in a special computerised report, including photo documentation of cup placement on the fetal head [33, 34]. Therefore, multiple parameters of the vacuum extraction procedure, as well as fetal and maternal characteristics, could be evaluated in our study. Levator ani muscle injury was assessed by 3D translabial ultrasound 6–10 weeks after birth, conducted by two well-trained pelvic floor sonographers. Prior to the examination, women were asked to empty their bladder and were placed in the lithotomy position. A covered 4–7 MHz 3D abdominal probe (Voluson S10, GE Healthcare, Zipf, Austria) was then placed between the labia. Acquisition and interpretation of 3D tomographic volumes was performed as described previously by Dietz et al. [20, 22]. We documented each discontinuity (a break in the normal texture of the pubococcygeal-puborectalis muscle, evident as an ultrasound hypo-/anechogenic lesion interrupting the hyperechogenic course of muscle fibres) involving the pubococcygeus-puborectalis muscle recognisable in the coronal C-plane slice (unilateral if the defect involves one side, bilateral if both sides are damaged). Levator ani muscle status was then classified into levator ani muscle trauma in the form of partial or complete avulsion or intact levator ani muscle, as described by Dietz et al. [20, 21, 35]. Levator ani muscle avulsion was diagnosed as a partial avulsion if an abnormal insertion of the levator ani muscle to the pubic bone was evident in at least one slice, and as a complete avulsion if an abnormal insertion was found in all three central slices at the level of the plane of minimal hiatal dimension and at 2.5 and 5 mm above this plane. A univariate analysis was performed to evaluate the association of levator ani muscle injury with different possible risk factors of interest, using a chi-square test for categorical variables and Student's t-test for continuous variables. In the case of several single parameters being statistically significant, a multivariate analysis was planned in as second step. Statistical significance was set at a level of <0.05. No power calculations were performed, as this was a secondary analysis of the above-mentioned main prospective study.
Approved by the district’s local ethical board (“Kantonale Ethikkommission Zürich”) under the registration number BASEC-Nr.2016-00908. All study participants gave their written informed consent for the study.
In total, 362 nulliparous women agreed to take part in the main study regarding levator ani muscle trauma and signed the informed consent form before birth. Of these, 149 had to be excluded from the study because of delivery by caesarean section, absence of both ultrasound investigators, fetal breech position, women’s withdrawal from the study, preterm birth, delivery at or transmission to another hospital, or because they did not show up for the ultrasound evaluation after 6–10 weeks. Of the remaining 213 women with vaginal birth, 54 gave birth with the help of vacuum extraction. Five of these had to be excluded because they did not show up for ultrasound evaluation 6–10 weeks postpartum. Forty-nine women remained for the final analysis in this study, as can be seen in the study flow chart (fig.1). The characteristics of the remaining 49 women are shown in table 1.

Figure 1 Study flow chart.
Table 1 Characteristics of the 49 women after vacuum-assisted birth with or without levator ani muscle (LAM) trauma.
|
Intact LAM
(n = 32) |
LAM trauma
(n = 17) |
p-value | |
|---|---|---|---|
| Age in years | |||
| <35 | 21 (65.6) | 12 (70.6) | 0.724 |
| ≥35 | 11 (34.4) | 5 (29.4) | |
| Body mass index in kg/m2 | 22.8 ± 4.3 | 21.3 ± 2.4 | 0.197 |
| Ethnicity | |||
| Caucasian | 27 (84.4) | 12 (70.6) | 0.251 |
| Asian | 0 (0) | 0 (0) | |
| Mediterranean | 2 (6.3) | 3 (17.6) | |
| Afro-Caribbean | 2 (6.3) | 0 (0) | |
| Oriental | 1 (3.1) | 2 (11.8) | |
| Gestational age in days | 279 ± 8.1 | 280 ± 5.5 | 0.460 |
| Duration of second stage in min | 150.8 ± 69.4 | 169.2 ± 60.1 | 0.341 |
| Duration of pushing phase in min | 75.3 ± 42.5 | 86.3 ± 49.2 | 0.423 |
| Epidural during birth | 26 (81.3) | 13 (76.5) | 0.693 |
| Application of intravaginal gel | 21 (65.6) | 8 (47.1) | 0.133 |
| Perineal length | 4.3 ± 1.2 | 4.7 ± 1.3 | 0.381 |
| Moulding of fetal cranial bones | 16 (50) | 8 (47.1) | 0.679 |
| Presence of caput succedaneum | |||
| No | 5 (15.6) | 4 (23.5) | 0.064 |
| Small | 7 (21.9) | 5 (29.4) | |
| Moderate | 17 (53.1) | 4 (23.5) | |
| Large | 1 (3.1) | 4 (23.5) | |
| Fetal position according to Hodge's planes | |||
| +1 | 12 (37.5) | 6 (35.3) | 0.899 |
| +2 | 12 (37.5) | 7 (41.2) | |
| +3 | 6 (18.8) | 3 (17.6) | |
| +4 | 1 (3.1) | 0 (0) | |
| Type of vacuum cup | |||
| Bird’s metal cup | 13 (40.6) | 6 (35.3) | 0.860 |
| KiwiOmnicup | 18 (56.3) | 10 (58.8) | |
| Not documented | 1 (3.1) | 1 (5.9) | |
| Number of vacuum tractions | 2.7 ± 1.1 | 2.8 ± 1.3 | 0.779 |
| Duration of vacuum in min | 6.8 ± 3.7 | 6.8 ± 4.1 | 0.982 |
| Sufficient uterine contractions | 28 (87.5) | 9 (52.9) | 0.014* |
| Traction forces | |||
| Light | 8 (25.0) | 5 (29.4) | 0.159 |
| Moderate | 20 (62.5) | 7 (41.2) | |
| Strong | 3 (9.4) | 5 (29.4) | |
| Maternal pushing effort | |||
| Moderate | 12 (37.5) | 4 (23.5) | 0.286 |
| Good | 19 (59.4) | 13 (76.5) | |
| Births with Kristeller manoeuvre | 4 (12.5) | 5 (29.4) | 0.145 |
| Fetal head position during extraction | |||
| Occipitoanterior, sagittal suture vertical | 7 (21.9) | 5 (29.4) | 0.652 |
| Occipitoanterior, sagittal suture oblique left | 14 (43.8) | 8 (47.1) | |
| Occipitoanterior, sagittal suture oblique right | 3 (9.4) | 0 (0) | |
| Occipitoposterior, sagittal suture vertical | 1 (3.1) | 1 (5.9) | |
| Occipitoposterior, sagittal suture oblique left | 2 (6.3) | 0 (0) | |
| Occipitoposterior, sagittal suture oblique right | 4 (12.5) | 3 (17.6) | |
| Abnormal fetal heart rate tracing in the second stage | 20 (62.5) | 11 (64.7) | 0.797 |
| Fetal weight in g | |||
| ≤4000 | 30 (93.8) | 15 (88.2) | 0.502 |
| >4000 | 2 (6.2) | 2 (11.8) | |
| Fetal head circumference in cm | |||
| ≤36 | 25 (78.1) | 12 (70.6) | 0.559 |
| >36 | 7 (21.2) | 5 (29.4) |
Values are mean ± standard deviation or n (%); p-values <0.05 are of statistical significance.
Of the 49 women with vacuum-assisted births in our cohort, 32 (65.3%) had an intact levator ani muscle, as shown in the illustration in fig. 2a and in the 3D ultrasound scan in fig. 2b. In contrast, 17 (34.7%) sustained levator ani muscle trauma, with 9 women (18.4%) having a partial levator ani muscle avulsion and 8 women (16.3%) having a complete levator ani muscle avulsion, as shown in the illustration in fig. 3a and in the 3D ultrasound scan in fig. 3b. No significant differences between the two groups were found, except for the state of their uterine contractions. Women without any levator ani muscle injury after vacuum extraction had more efficient uterine contractions compared to women with levator ani muscle trauma.
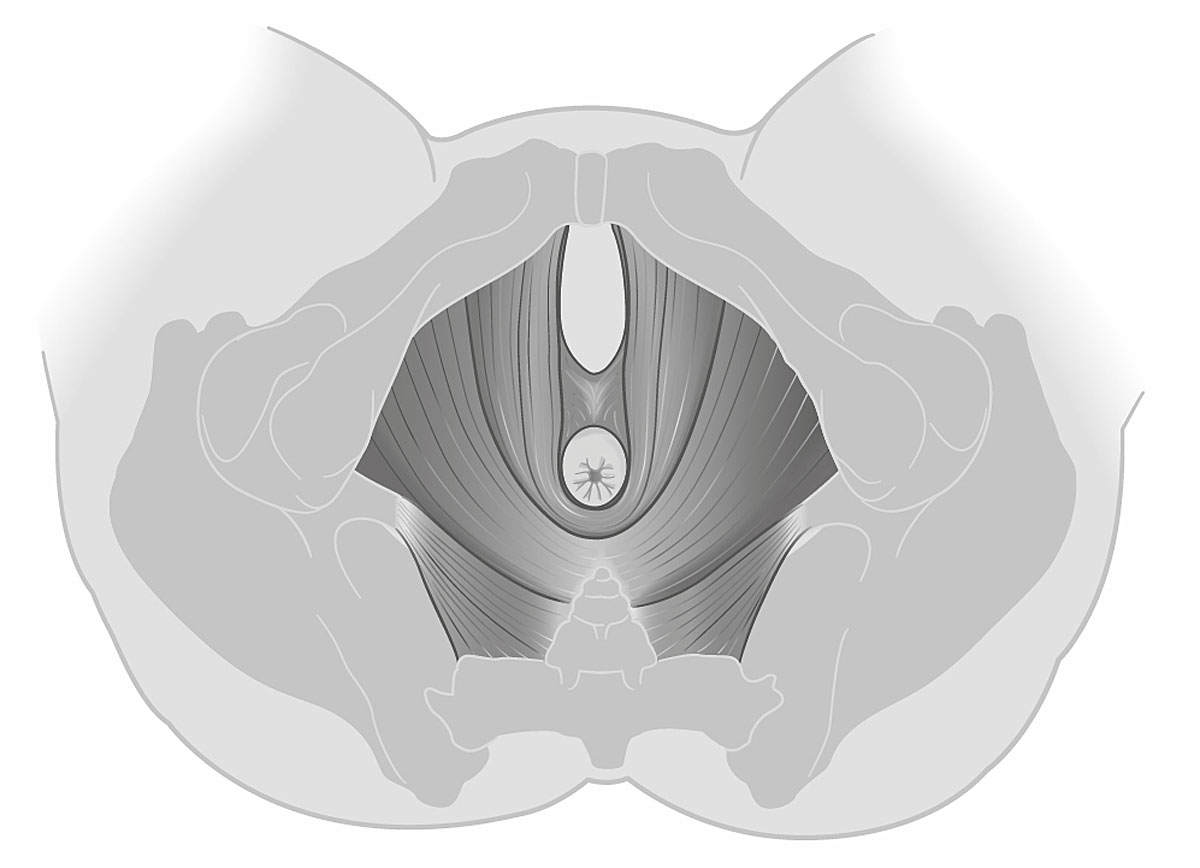
Figure 2a Illustration of an intact bilateral levator ani muscle.
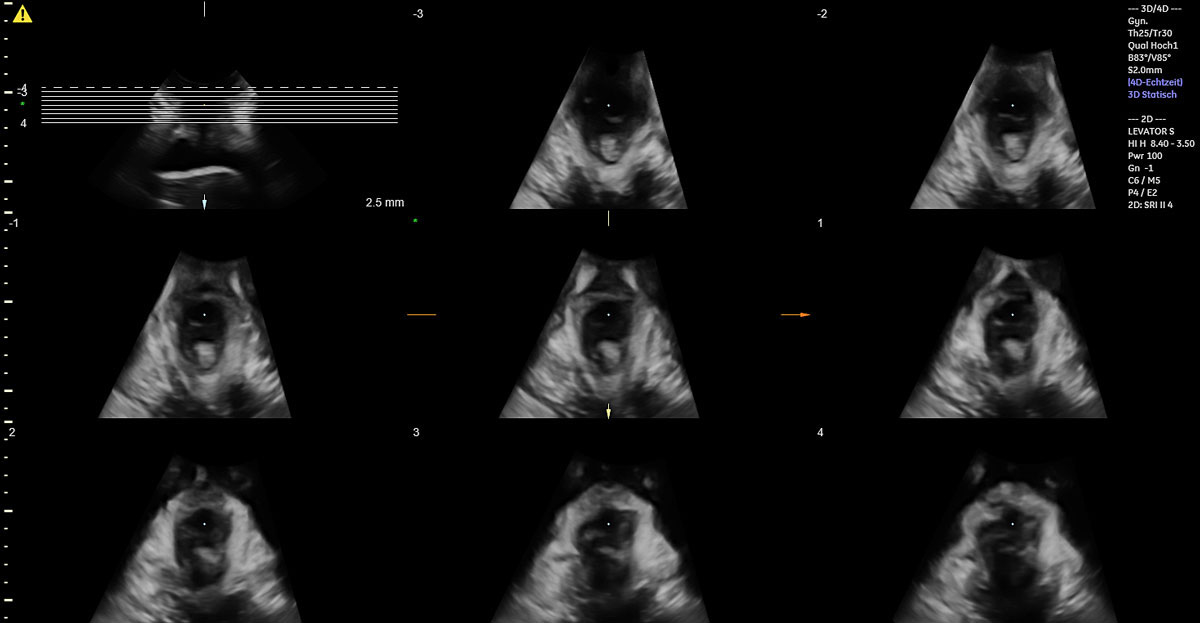
Figure 2b 3D translabial ultrasound image of an intact bilateral levator ani muscle.
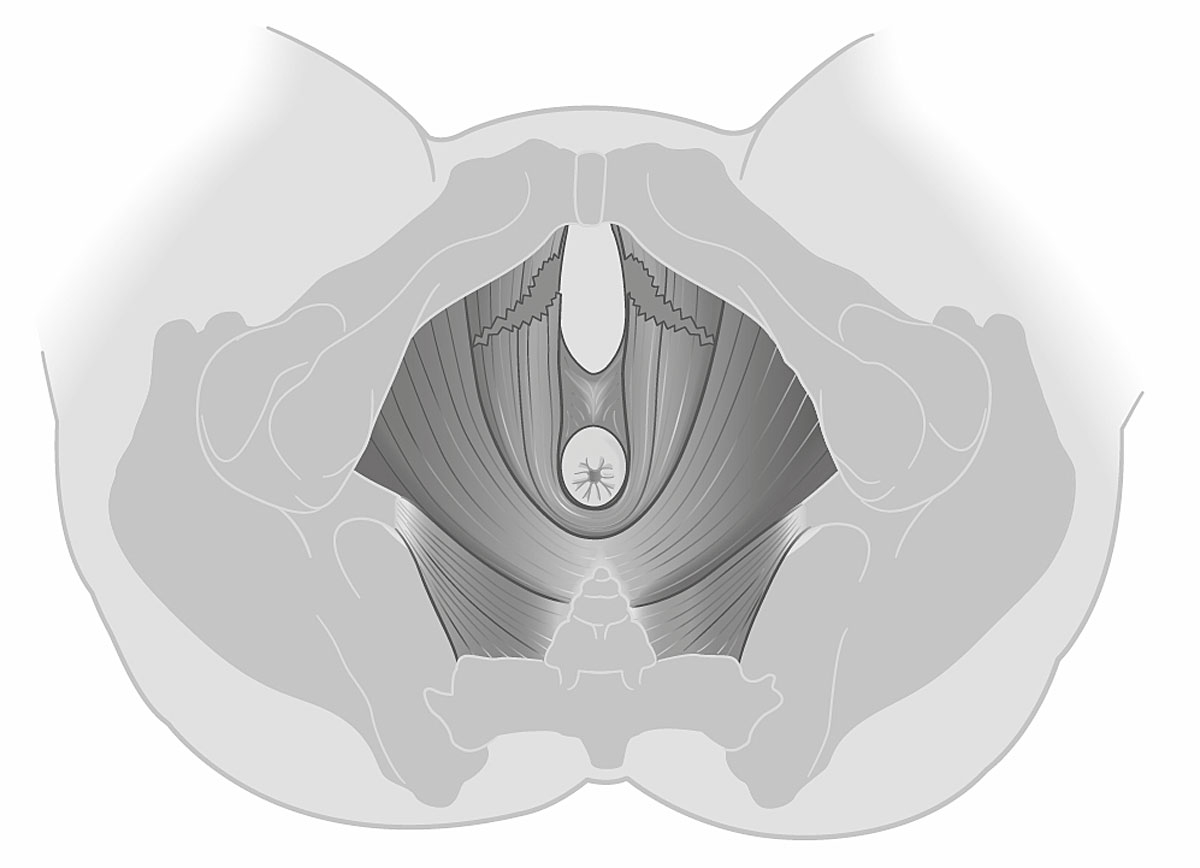
Figure 3a Illustration of a complete bilateral levator ani muscle avulsion.
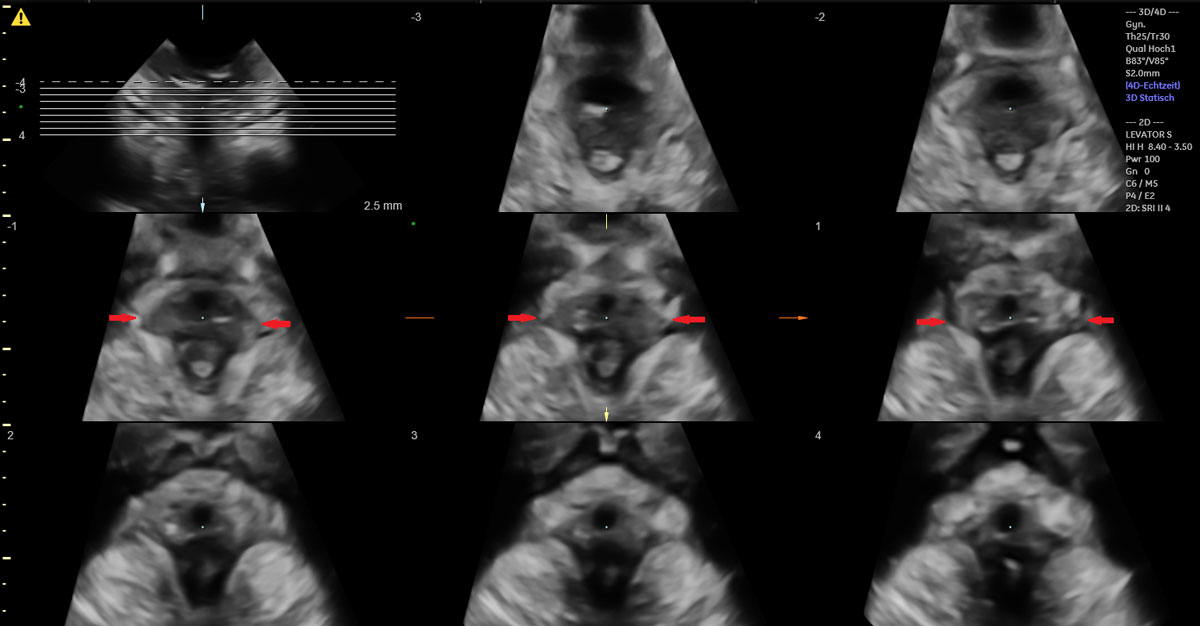
Figure 3b 3D translabial ultrasound image of a complete bilateral levator ani muscle avulsion.
The total rate of partial and complete avulsions after vacuum extraction in our study was 34.7%. This rate is consistent with those reported in other studies [2, 5–10]. However, it must be mentioned that these studies focused only on complete levator ani muscle avulsions and did not evaluate any partial avulsions.
In the study by Gonzalez-Diaz et al., a complete avulsion was found in 33% of women after vacuum extraction with Malmstrom’s cup and in 29% after vacuum extraction with the KiwiOmnicup; partial avulsions were not included [9]. Garcia et al. reported the rate of complete levator ani muscle avulsions according to the difficulty of instrumentation and the number of necessary tractions (less than 3 or ≥3) in vacuum extractions and found no differences between the groups (36.7% vs 30%) [10]. Garcia et al. evaluated the rate of complete levator ani muscle avulsions according to whether vacuum extraction or forceps were used in vaginal-assisted births [32]. They found no differences in levator ani muscle avulsion rates between the two groups or regarding the height of the fetal head at the moment of cup or forceps placement (Hodge's planes +2 and lower vs above +2) [32]. Our results support their findings concerning their individually evaluated factors. In contrast to the studies mentioned above, we evaluated multiple parameters of vacuum extraction technique and procedure simultaneously. We found no factors significantly associated with levator ani muscle avulsions, except insufficient uterine contractions during the second stage of labour.
During birth, the fetus performs different rotational movements while passing through the maternal pelvis and birth canal. These movements are to position the fetus optimally in relation to the shape of the bony and soft tissue structures of the birth canal in order to minimise space requirements, and therefore fetal and maternal trauma. These adaptations might take a certain amount of time, but the duration required in every single section of the birth canal is unknown. We hypothesise that if the birth canal has not enough time to adapt to the passage of the fetus or if the fetus has not enough time to adapt by rotations to the shape of the birth canal, this might cause harm to the above-mentioned structures.
Vacuum extractions are indicated in situations where the mother's pushing efforts are limited, uterine contractions are not efficient enough, the second stage of labour is prolonged or fetal compromise is highly expected. In the first three situations, there is no need to accelerate the vacuum manoeuvre too much, so there is time to slowly pull the fetus down through the birth canal and let it rotate into the ideal position. In contrast, in cases with expected fetal compromise, there is a need to extract the fetus swiftly, but obstetricians sometimes tend to proceed too quickly. Although the clinical situation requires rapid action, it is not necessary to pull the fetus through the whole pelvis in 1–2 tractions, especially when the fetal head is placed at a higher Hodge's plane. From biomechanical finite element studies, we know that the greatest extension of the levator ani muscle occurs when the fetal head is positioned at Hodge's plane +4 [36]. Furthermore, translabial ultrasound examinations of the pelvic floor during birth have shown that the maximum dimension of the hiatus of the levator ani muscle is also at Hodge's plane +4 [37]. Therefore, it might be worthwhile being particularly careful and pulling slowly when the fetal head descends to Hodge’s plane +4 and further on. It is possible that the rotation of the shoulders into a vertical axis should be awaited before passing through the sites of insertion of the levator ani muscle to the pubic bones in order not to cause any avulsions when pulling the shoulders through the hiatus in a transverse or oblique position. Unfortunately, there is currently no way of observing inside the birth canal to answer this question. Additionally, birth attendants should refrain from asking women to push too strongly when the fetal head is passing through the hiatus of the levator ani muscle and through the introitus of the vulva in order to preserve maternal structures. This knowledge suggests that performing future studies which provide insights into the timing of the in vivo adaptations of the fetus within the birth canal in the final phase of the second stage of labor is of great importance.
Strengths of our study are the longitudinal, prospective design with the antenatal inclusion of the participants into the study, a very low dropout rate, and the validated and very detailed assessment methods. Furthermore, our study adds important information to the current knowledge by evaluating the effects of multiple detailed parameters of vacuum extraction technique and procedure on the occurrence of levator ani muscle trauma. A limitation is the relatively small cohort number and the missing power calculation regarding the sample size for this sub-analysis. Additionally, the vacuum manoeuvre was performed by different obstetricians and the categorisation of some of the factors, for example “traction force”, was to some extent subjective.
So far, no fetal, maternal or obstetric characteristics and no parameters of vacuum technique have been found to be associated with the occurrence of levator ani muscle trauma after vacuum extraction, except for insufficient uterine contractions. Nevertheless, there may be influencing factors that have not yet been evaluated, or are not easily accessible for evaluation, like the timing of adaptations of the fetus inside the birth canal and within the hiatus of the levator ani muscle, and the adaptations of the birth canal to the fetus passing through. This should be the subject of further research with a sample size adequately powered to answer this question properly.
GE Healthcare, Zipf, Austria supported this study by providing the ultrasound device (Voluson S10) and the probes for the ultrasound examinations during the whole study period. The Heartbay Foundation (Vaduz, Liechtenstein) supported the project financially.
The authors of this manuscript have no conflict of interest to disclose.
1 Kamisan Atan I , Gerges B , Shek KL , Dietz HP . The association between vaginal parity and hiatal dimensions: a retrospective observational study in a tertiary urogynaecological centre. BJOG. 2015;122(6):867–72. doi:.https://doi.org/10.1111/1471-0528.12920
2 Dietz HP . Pelvic floor trauma in childbirth. Aust N Z J Obstet Gynaecol. 2013;53(3):220–30. doi:.https://doi.org/10.1111/ajo.12059
3 de Araujo CC , Coelho SA , Stahlschmidt P , Juliato CRT . Does vaginal delivery cause more damage to the pelvic floor than cesarean section as determined by 3D ultrasound evaluation? A systematic review. Int Urogynecol J Pelvic Floor Dysfunct. 2018;29(5):639–45. doi:.https://doi.org/10.1007/s00192-018-3609-3
4 Horak TA , Guzman-Rojas RA , Shek KL , Dietz HP . Pelvic floor trauma: does the second baby matter? Ultrasound Obstet Gynecol. 2014;44(1):90–4. doi:.https://doi.org/10.1002/uog.13252
5 van Delft K , Thakar R , Sultan AH , Schwertner-Tiepelmann N , Kluivers K . Levator ani muscle avulsion during childbirth: a risk prediction model. BJOG. 2014;121(9):1155–63, discussion 1163. doi:.https://doi.org/10.1111/1471-0528.12676
6 van Delft KW , Thakar R , Sultan AH , IntHout J , Kluivers KB . The natural history of levator avulsion one year following childbirth: a prospective study. BJOG. 2015;122(9):1266–73. doi:.https://doi.org/10.1111/1471-0528.13223
7 Shek KL , Dietz HP . Intrapartum risk factors for levator trauma. BJOG. 2010;117(12):1485–92. doi:.https://doi.org/10.1111/j.1471-0528.2010.02704.x
8 Kearney R , Fitzpatrick M , Brennan S , Behan M , Miller J , Keane D , et al. Levator ani injury in primiparous women with forceps delivery for fetal distress, forceps for second stage arrest, and spontaneous delivery. Int J Gynaecol Obstet. 2010;111(1):19–22. doi:.https://doi.org/10.1016/j.ijgo.2010.05.019
9 González-Diaz E , García-Mejido JA , Martín-Martínez A , Fernández-Fernández C , Ortega I , Medina M , et al. Are there differences in the damage to the pelvic floor between malmstrom’s and kiwi omnicup vacuums? A multicenter study. Neurourol Urodyn. 2020;39(1):190–6. doi:.https://doi.org/10.1002/nau.24167
10 García Mejido JA , De la Fuente Vaquero P , Fernández Palacín A , Aquise Pino A , Bonomi Barby MJ , Sainz Bueno JA . Influence of difficulty of instrumentation with vacuum on the rate of levator ani muscle avulsion identified by 3-4 d transperineal ultrasound. J Matern Fetal Neonatal Med. 2018;31(5):591–6. doi:.https://doi.org/10.1080/14767058.2017.1293022
11 Garcia-Mejido JA , Gutierrez L , Fernandez-Palacín A , Aquise A , Sainz JA . Levator ani muscle injuries associated with vaginal vacuum assisted delivery determined by 3/4D transperineal ultrasound. J Matern Fetal Neonatal Med. 2017;30(16):1891–6. doi:.https://doi.org/10.1080/14767058.2016.1228104
12 Michalec I , Simetka O , Navratilova M , Tomanova M , Gartner M , Salounova D , et al. Vacuum-assisted vaginal delivery and levator ani avulsion in primiparous women. J Matern Fetal Neonatal Med. 2016;29(16):2715–8.
13 Rådestad I , Olsson A , Nissen E , Rubertsson C . Tears in the vagina, perineum, sphincter ani, and rectum and first sexual intercourse after childbirth: a nationwide follow-up. Birth. 2008;35(2):98–106. doi:.https://doi.org/10.1111/j.1523-536X.2008.00222.x
14 Skinner EM , Barnett B , Dietz HP . Psychological consequences of pelvic floor trauma following vaginal birth: a qualitative study from two Australian tertiary maternity units. Arch Women Ment Health. 2018;21(3):341–51. doi:.https://doi.org/10.1007/s00737-017-0802-1
15 Skinner EM , Dietz HP . Psychological and somatic sequelae of traumatic vaginal delivery: A literature review. Aust N Z J Obstet Gynaecol. 2015;55(4):309–14. doi:.https://doi.org/10.1111/ajo.12286
16 Dietz HP , Schierlitz L . Pelvic floor trauma in childbirth - myth or reality? Aust N Z J Obstet Gynaecol. 2005;45(1):3–11. doi:.https://doi.org/10.1111/j.1479-828X.2005.00363.x
17 Dannecker C , Lienemann A , Fischer T , Anthuber C . Influence of spontaneous and instrumental vaginal delivery on objective measures of pelvic organ support: assessment with the pelvic organ prolapse quantification (POPQ) technique and functional cine magnetic resonance imaging. Eur J Obstet Gynecol Reprod Biol. 2004;115(1):32–8. doi:.https://doi.org/10.1016/j.ejogrb.2003.09.047
18 Notten KJB , Vergeldt TFM , van Kuijk SMJ , Weemhoff M , Roovers JWR . Diagnostic Accuracy and Clinical Implications of Translabial Ultrasound for the Assessment of Levator Ani Defects and Levator Ani Biometry in Women With Pelvic Organ Prolapse: A Systematic Review. Female Pelvic Med Reconstr Surg. 2017;23(6):420–8. doi:.https://doi.org/10.1097/SPV.0000000000000402
19 Rortveit G , Hannestad YS , Daltveit AK , Hunskaar S . Age- and type-dependent effects of parity on urinary incontinence: the Norwegian EPINCONT study. Obstet Gynecol. 2001;98(6):1004–10. doi:.https://doi.org/10.1097/00006250-200112000-00004
20 Dietz HP . Ultrasound imaging of the pelvic floor. Part II: three-dimensional or volume imaging. Ultrasound Obstet Gynecol. 2004;23(6):615–25. doi:.https://doi.org/10.1002/uog.1072
21 Dietz HP , Abbu A , Shek KL . The levator-urethra gap measurement: a more objective means of determining levator avulsion? Ultrasound Obstet Gynecol. 2008;32(7):941–5. doi:.https://doi.org/10.1002/uog.6268
22 Dietz HP , Bernardo MJ , Kirby A , Shek KL . Minimal criteria for the diagnosis of avulsion of the puborectalis muscle by tomographic ultrasound. Int Urogynecol J Pelvic Floor Dysfunct. 2011;22(6):699–704. doi:.https://doi.org/10.1007/s00192-010-1329-4
23 Dietz HP , Garnham AP , Rojas RG . Is the levator-urethra gap helpful for diagnosing avulsion? Int Urogynecol J Pelvic Floor Dysfunct. 2016;27(6):909–13. doi:.https://doi.org/10.1007/s00192-015-2909-0
24 Valsky DV , Lipschuetz M , Bord A , Eldar I , Messing B , Hochner-Celnikier D , et al. Fetal head circumference and length of second stage of labor are risk factors for levator ani muscle injury, diagnosed by 3-dimensional transperineal ultrasound in primiparous women. Am J Obstet Gynecol. 2009;201(1):91.e1–7. doi:.https://doi.org/10.1016/j.ajog.2009.03.028
25 Aydın S , Tuncel MA , Aydın CA , Ark C . Do we protect the pelvic floor with non-elective cesarean? A study of 3-D/4-D pelvic floor ultrasound immediately after delivery. J Obstet Gynaecol Res. 2014;40(4):1037–45. doi:.https://doi.org/10.1111/jog.12303
26 Kearney R , Miller JM , Ashton-Miller JA , DeLancey JO . Obstetric factors associated with levator ani muscle injury after vaginal birth. Obstet Gynecol. 2006;107(1):144–9. doi:.https://doi.org/10.1097/01.AOG.0000194063.63206.1c
27 Falkert A , Endress E , Weigl M , Seelbach-Göbel B . Three-dimensional ultrasound of the pelvic floor 2 days after first delivery: influence of constitutional and obstetric factors. Ultrasound Obstet Gynecol. 2010;35(5):583–8. doi:.https://doi.org/10.1002/uog.7563
28 Caudwell-Hall J , Kamisan Atan I , Brown C , Guzman Rojas R , Langer S , Shek KL , et al. Can pelvic floor trauma be predicted antenatally? Acta Obstet Gynecol Scand. 2018;97(6):751–7. doi:.https://doi.org/10.1111/aogs.13315
29 Caudwell-Hall J , Kamisan Atan I , Martin A , Guzman Rojas R , Langer S , Shek K , et al. Intrapartum predictors of maternal levator ani injury. Acta Obstet Gynecol Scand. 2017;96(4):426–31. doi:.https://doi.org/10.1111/aogs.13103
30 Friedman T , Eslick GD , Dietz HP . Delivery mode and the risk of levator muscle avulsion: a meta-analysis. Int Urogynecol J Pelvic Floor Dysfunct. 2019;30(6):901–7. doi:.https://doi.org/10.1007/s00192-018-3827-8
31 Garcia-Mejido JA , Martin-Martinez A , Gonzalez-Diaz E , Fernandez-Fernandez C , Ortega I , Medina M , et al. Malmstrom’s vacuum or Kielland’s forceps: what causes more damage to the pelvic floor? Ultrasound Obstet Gynecol. 2019. doi:https://doi.org/10.1002/uog.20404
32 García-Mejido JA , Fernández-Palacín A , Bonomi Barby MJ , Castro L , Aquise A , Sainz JA . A comparable rate of levator ani muscle injury in operative vaginal delivery (forceps and vacuum) according to the characteristics of the instrumentation. Acta Obstet Gynecol Scand. 2019;98(6):729–36. doi:.https://doi.org/10.1111/aogs.13544
33 Kimmich N , Burkhardt T , Kreft M , Zimmermann R . Reducing birth trauma by the implementation of novel monitoring and documentation tools. Acta Obstet Gynecol Scand. 2019;98(10):1223–6. doi:.https://doi.org/10.1111/aogs.13660
34 Burkhardt T , Zimmermann R . Eine strukturierte und fotografische Dokumentation der Vakuumentbindung [A Structured and Photographic Documentation of the Vacuum-Assisted Vaginal Delivery]. Z Geburtshilfe Neonatol. 2018;222(1):25–7. Article in German. doi:.https://doi.org/10.1055/s-0043-124123
35 Dietz HP , Moegni F , Shek KL . Diagnosis of levator avulsion injury: a comparison of three methods. Ultrasound Obstet Gynecol. 2012;40(6):693–8. doi:.https://doi.org/10.1002/uog.11190
36 Parente MP , Natal Jorge RM , Mascarenhas T , Silva-Filho AL . The influence of pelvic muscle activation during vaginal delivery. Obstet Gynecol. 2010;115(4):804–8. doi:.https://doi.org/10.1097/AOG.0b013e3181d534cd
37 García Mejido JA , Suárez Serrano CM , Fernéndez Palacín A , Aquise Pino A , Bonomi Barby MJ , Sainz Bueno JA . Evaluation of levator ani muscle throughout the different stages of labor by transperineal 3D ultrasound. Neurourol Urodyn. 2017;36(7):1776–81. doi:.https://doi.org/10.1002/nau.23175
NK: Study conception, ultrasound performance, data acquisition and management, statistical analysis, manuscript writing. JB: Data acquisition and management. RZ: Study conception, manuscript editing. MK: Study conception, ultrasound performance, data acquisition and management, manuscript editing
GE Healthcare, Zipf, Austria supported this study by providing the ultrasound device (Voluson S10) and the probes for the ultrasound examinations during the whole study period. The Heartbay Foundation (Vaduz, Liechtenstein) supported the project financially.
The authors of this manuscript have no conflict of interest to disclose.