Unexpected liaisons between old cytokines and new deadly virus
DOI: https://doi.org/10.4414/smw.2020.20315
Solongevity Research, Scientific Director, R&D,
Unexpected liaisons between old cytokines and new deadly virus
Summer has come and the virus has apparently decided to give Italy a break, giving rise to controversies as to whether the virus has lost virulence or not. Meanwhile Italian researchers have made some important progress: the immunology group led by Andrea Cossarizza in Modena has not only identified new unexpected liaisons between old cytokines and the new coronavirus that open the way to the use in COVID-19 of drugs already approved for other diseases, but they have also provided valuable indications on how to better prepare ourselves to face a possible second wave of infection [1].
In research the only things that matter are data. Even if they do not always give us unambiguous or definitive answers, the important thing is that they be rigorously and, if possible, impartially verified. In research we have only one way to know if we have discovered something seriously illuminating or (to use an Italian saying) if we are simply chasing a firefly: the peer review process. We hurriedly throw our data in a draft article that we submit to a scientific journal that in turn asks two or at most three colleague-peers (whose identities we do not know) to analyse in detail our draft and conclude whether we have valid findings. I assure you that for us it is a very difficult process, sometimes even painful, however effective; our unknown examiners may pursue details that seemed insignificant to us and force us to re-reflect on our conclusions and sometimes even abandon them. The reviewer may also be wrong, but the process is designed to reduce bias, such that when the data are published we can all draw conclusions with some confidence.
The COVID-19 emergency has changed this basic rule. Partly because of the urgency to quickly share knowledge about potentially effective therapies, but also because of a certain increasing light-heartedness of some colleagues, we have become accustomed to hear about almost miraculous cures in this or that television talk-show or in an interview with this or that newspaper. All this has generated an enormous confusion among the medical community which, not being able to access verified and published data, has often found itself dispersing into a sort of butterfly hunt, which is as useless as it is harmful to patients who rightly want from us some certainties, or almost-certainties.
Let’s come now to the work of Andrea Cossarizza’s group [1]. A first consideration: the work has been published in a highly prestigious journal, Nature Communications. The prestige of a journal is based on its reliability, which in turn is based on the rigour with which it analyses data. One thing that struck me about this work is that, although it is has been recognised by Nature Communications to be significant, I have not yet heard about it in the newspapers or on television. Upon reading the article, I realised that it is indeed dealing with something very serious.
What did they do in Modena? They studied how the immune system of 39 hospitalised patients with not yet severe forms of COVID-19 (sore throat, fever, cough, difficulty breathing, chest pain and radiological signs of pneumonia, but not yet requiring intubation) reacts to the virus. The technique used for the study is very sophisticated, with only few laboratories knowing how to use it; it is essentially a matter of identifying hundreds of different types of cells, understanding how they are behaving, how they coordinate to respond to the virus, and, very importantly, which molecules they produce. The technique used is to pass them, one by one, through a kind of metal detector that, with the help of special probes, allows each cell to be given a name and an identity card. This is a very complicated process that works only thanks to the help of complex algorithms of data analysis, which then require an expert to interpret.
The cells of the immune system, the lymphocytes, are organised in groups that have different functions; all of them, under normal conditions, are coordinated with each other by soluble molecules, the cytokines, thus optimising the defensive response.
The first result that came out of the Modena team showed that in COVID-19, the coordination between the various groups of cells is completely lost. In a normal immune response, the various immune cells act in such a way as to optimise the defensive response without doing any damage. In COVID-19, they all go off to war at the same time and end up fighting each other. A war that damages the lung and many other tissues of the body, with an exaggerated inflammatory reaction, whose name says it all: “cytokine storm”. A sort of fight erupts between cytokines, to decide what signal to give to the various cells. And it ends up that the immune cells, as expected, get confused and trigger a true friendly fire that damages the lung or other tissues (fig. 1). The reasons for the confused coordination of the immune system are very complex, but are consistent with what we have already learned about the destructive capabilities of the virus when entering cells.
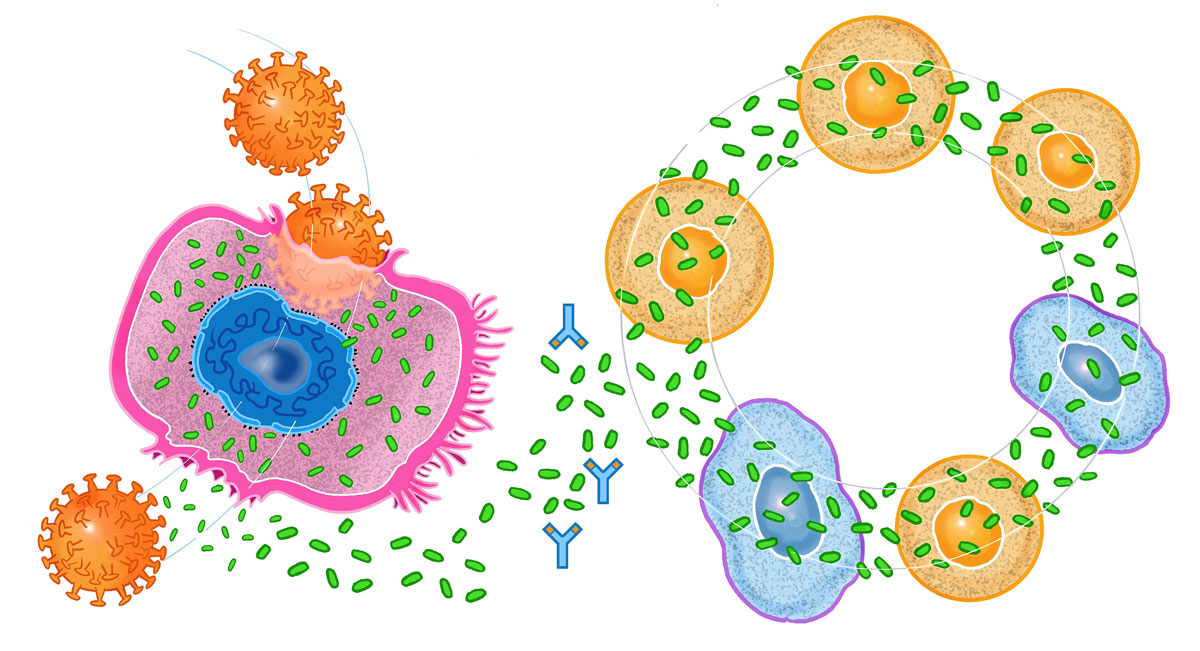
Figure 1 When the COVID-19 virus infects the cell of the lung it induces the release of inflammatory cytokines (depicted as small green discs) that trigger various cells of the immune system into a confused, vicious circle that ultimately damage the host’s tissues. This inflammatory reaction will be worse in persons who already have chronically activated inflammatory cells. This inflammatory reaction can be neutralised (stopped) with the administration of specific monoclonal antibodies (depicted here as the blue Y shaped figures). Illustration by Fulvio Bernardini Fuller©
And here comes the second big news from the study: while we knew that there was a cytokine storm in COVID-19, we still didn't know that one of the most important cytokines was interleukin (IL)-17. The results of clinical trials with a drug called tocilizumab convinced us that another cytokine alone can trigger the inflammatory response, IL-6. We now know that IL-17 also plays a very important role. This is no small news, because against IL-17 we already have at least two drugs available that have already been successfully tested in inflammatory joint diseases. They are called secukinumab and brodalumab, names that are very difficult to remember but are easy to interpret: the term “-mab” indicates that they are monoclonal antibodies. Antibodies are capable of neutralising not only a whole virus, but also single molecules such as cytokines. The advantage of having two drugs already tested, even if on a different disease, is enormous, because it greatly shortens the time needed for clinical trials in COVID-19 patients.
Modena’s third notable finding from their analysis is the detection of another cytokine that damages the lung, called IL-8. IL-8 is an old acquaintance of immunologists, having already been recognised to be guilty of the cytokine storm unleashed by the cousin of SARS-CoV-2, the SARS virus. The good news is that for IL-8 also there is already an investigational drug in the pipeline, in development for another disease: Hu-Max-IL8. I can imagine that after this article, someone’s already thinking about testing it on COVID-19. At this point we can anticipate a future in which a cocktail of monoclonal antibodies capable of blocking not only one, but two or three cytokines, even at low doses, may be the best weapon against this disease.
Important implications: the findings above about the increased cytokines and subsequent inflammation were observed in patients with symptoms but not yet a serious form of the disease (not intubated). We still don’t know when these phenomena start after infection and prior to developing symptoms, but it would not be surprising to find out that already in the early stages of the disease the cells of the immune system start this war with each other. You may have wondered why the elderly are more susceptible to the disease. The findings here would strongly suggest that the elderly are particularly predisposed as a result of an already activated immune system. As we age, lymphocytes are chronically activated in a process called “inflam-aging”, literally, inflammation-aging. It is the result of the accumulation over time of stimuli to the immune system that keep it in a state of chronic activation. When the virus arrives, it only takes a short time to unleash the storm.
This chronic activation of the immune system and thus increased vulnerability to the virus is not limited to the elderly; COVID-19 also affects young people. The condition most frequently associated with COVID-19 in young people is obesity, and it turns out that obesity generates chronic inflammation, mediated by inflammatory cytokines, among them IL-17. The circle is closed. While of course monoclonal antibody treatment is not a practical way to prevent COVID-19 in the young and obese, inflammation can be reduced by setting healthy rules of life-style (weight loss and physical activity) and facilitated by the use of newly available molecules of natural origin that have been found to have a clear impact on inflammation.
In conclusion, an in-depth analysis, carried out using state-of-the-art techniques, has made it possible to identify two new therapeutic targets that can be used in COVID-19. These two targets already have three potential drugs; all that remains now is to see how they work. It will not take long because all the studies necessary for the validation of the three drugs have already been done for their development in other diseases, thus reducing the time to potential clinical application in COVID-19 by 3 to 5 years.
Finally, with regards to the clinical picture of COVID-19 patients in countries, such as Italy, where the virus appears to be now more benign, the problem is that we still do not have a convincing explanation for this phenomenon. Has the virus changed or lost strength? We have no evidence of that. Has the summer temperature weakened it? It may have, but how do we explain the course of the epidemic in Texas, Florida, Iran, Brazil? Air humidity or pollution have been proposed, and are possible explanations, but we do not have clear or definitive data. There is an ongoing discussion between virologists on the interpretation of this phenomenon. Perhaps the simplest hypothesis, now increasingly supported by epidemiological data, is that we are observing the positive effect of social distancing measures (including the use of face masks) that greatly reduce the viral load that transmits the contagion. Further, there appears to be less virulent disease circulating because there is a greater ratio of people who have been tested who have less viral load (compared with the month of March, when more dramatically ill people were tested who had, perhaps not surprisingly, a greater viral load). For the time being, masks are still used by many people, but with summer and mixed messages about the virulence of COVID-19, they are at high risk of being abandoned. While I hope I am wrong, we cannot rule out the risk of new summer outbreaks. What is happening in Germany and Portugal tells us something: caution is a must.
I suggest that we enjoy the summer while we can, taking opportunities to get ourselves (and our inflammatory levels) in good shape.
Reference
1
De Biasi
S
,
Meschiari
M
,
Gibellini
L
,
Bellinazzi
C
,
Borella
R
,
Fidanza
L
, et al.
Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun. 2020;11(1):3434. doi:.https://doi.org/10.1038/s41467-020-17292-4
