Actionability and scope should determine the extent of counselling for presymptomatic genetic testing
DOI: https://doi.org/10.4414/smw.2020.20274
Bettina
Zimmermannab, Georg
Starkea, David
Shawac, Bernice
Elgerad, Insa
Konéa
aInstitute for Biomedical Ethics,
bInstitute for History and Ethics of Medicine, Technical University of Munich School of Medicine,
cCare and Public Health Research Institute,
dCentre for Legal Medicine,
Actionability and scope should determine the extent of counselling for presymptomatic genetic testing
In clinical settings, patients considering undergoing presymptomatic genetic and genomic testing (PGT) commonly receive prior genetic counselling. Counsellors provide comprehensive information regarding the testing procedure, possible outcomes of the test, potential medical and psychological consequences, and implications for family members. After testing, counsellees obtain detailed feedback about the results, their interpretation and advisable further steps. This procedure constitutes standard clinical practice for PGT and is even required by law in certain countries such as Switzerland, Germany and France [1]. According to clinical guidelines [2, 3], prior and post-test counselling are required in PGT because, first, the knowledge that a person is a carrier of a pathogenic variant will affect not only patients themselves, but also family members. Second, it might have important psychosocial effects on patients, potentially affecting life plans and particularly reproductive decision-making. Third, PGT is of considerable complexity in terms of result interpretation and management. Genetic counselling aims to enable people to make informed decisions regarding genetic or genomic testing as an expression of their autonomy. There is a general legal and ethical duty to inform patients and obtain informed consent, whereas an obligation to formal specialised counselling before testing is generally reserved for PGT.
In line with the current discussion on genetic exceptionalism [4], we argue that PGT does not in itself warrant extended genetic counselling. In fact, targeted PGT for actionable phenotypes, such as the BRCA genes, has many similarities with other kinds of clinical tests, such as diagnostic tests for type 2 diabetes mellitus (T2DM). First, both allow inferring similar health-related risk scores for relatives. A positive test result for T2DM implies a 3.5-fold risk for offspring (and a 6-fold increase if both parents are affected) compared with those without parental diabetes [5]. In comparison, having two first-degree relatives with breast cancer indicates a 3.6-fold risk in comparison with the general population [6]. Second, with a pathogenic genetic variant in BRCA1, the risk of developing breast cancer is 44–78% by the age of 70 [7], but because of the high prevalence of T2DM [8], the lifetime risk in the case of family history is (at least) on a similar level even without a specific genetic test. Third, in both cases, patients benefit from established medical actions that reduce the risk of developing a life-threatening condition: preventive surgeries and cancer screening programmes in the case of hereditary breast/ovarian cancer syndrome, and dietary adaptions or insulin replacement for T2DM. Finally, assessing family history is meant to be a standard part of primary care [9], although the analysis of a patient’s pedigree might easily result in severe consequences for patients, life partners and relatives. Despite these similarities, patients undergoing testing for T2DM are rarely counselled by a specialist in these situations; it is part of standard medical practice. Thus, PGT alone does not justify highly specialised and intensive genetic counselling.
In contrast, some PGT applications require additional considerations that, from our perspective, prioritise specialised genetic counselling. Importantly, across different presymptomatic tests, there is a critical difference between medically non-actionable test results, such as Huntington’s disease, and actionable test results such as those for BRCA1 and BRCA2 pathogenic variants. PGT for non-actionable phenotypes does not offer any immediate medical benefit to patients and thus requires expert support. Moreover, exome or genome-wide analyses increase the risk of secondary findings, which can either be actionable or non-actionable. This additional uncertainty and the increased interpretative difficulties also justify exceptional resource allocation for specialised genetic counselling.
Specialised, high-quality and personal counselling is – and should remain – standard practice to ensure informed decision-making. However, given the limited resources within healthcare sytems, considerations of distributive justice demand justification of expenditures for counselling, as well as for other procedures [10]. If there is no adequate justification for the special status, resources should be equalised to other (comparable) contexts. We are not arguing against genetic counselling in PGT for actionable diseases, but we do think that for some types of PGT less formalised methods of counselling could be sufficient. For example, adequately trained healthcare professionals, such as gynaecologists or general practitioners, could provide genetic counselling for well-established and medically actionable PGT applications such as BRCA testing for hereditary breast and ovarian cancer. However, we emphasise that this should not lead to lower communication standards. People undergoing PGT should still have professional counselling, enabling them to address their concerns before and after the testing procedure. Moreover, for results that go beyond standard practice and do not allow for straightforward clinical recommendations, such as variants of unknown significance or secondary findings, counsellees should still be referred to a geneticist for discussing these results. Figure 1 illustrates these different scenarios.
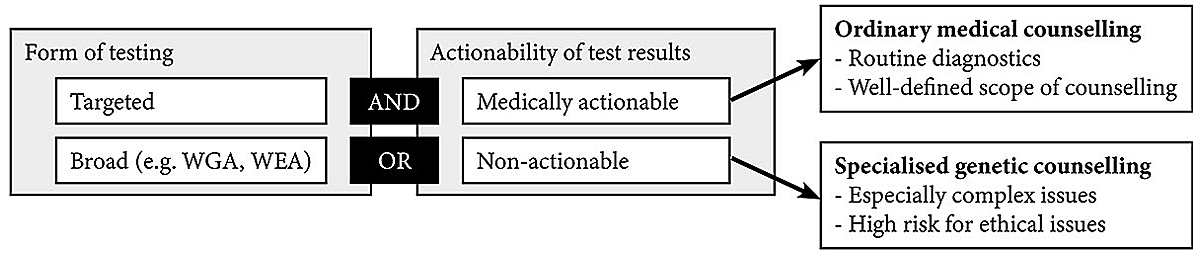
Figure 1 Illustration of factors influencing who should provide genetic counselling for presymptomatic genetic testing. The "AND" operator indicates that both indicators need to be fulfilled for ordinary medical counselling, the "OR" operator means that one of them is sufficient to qualify for specialised genetic counselling. WGA = whole-genome analyses; WEA = whole-exome analyses.
There is a need to discuss further why genetic counselling has such unique and high importance in many countries worldwide. This discussion will help to rationalise resource allocations within healthcare systems and improve patient communication in genetics and other fields. Specialised genetic counselling is justified and required for genomic analyses because of the possibility of secondary findings, and for targeted PGT for non-actionable diseases. However, for well-established, highly penetrant and actionable variants, such as BRCA, specialised counselling could be replaced by ordinary counselling through healthcare professionals not specialised in genetics.
References
1Rössler F, Lemke JR. Legislation on Genetic Testing in Different Countries. In: Kiess W, Bornehag C-G, Gennings C, editors. Pediatric Epidemiology. Pediatric and Adolescent Medicine. Basel: Karger; 2017. p. 30–40.
2
Owens
DK
,
Davidson
KW
,
Krist
AH
,
Barry
MJ
,
Cabana
M
,
Caughey
AB
, et al.; US Preventive Services Task Force. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322(7):652–65. doi:.https://doi.org/10.1001/jama.2019.10987
3
Skirton
H
,
Goldsmith
L
,
Jackson
L
,
Tibben
A
. Quality in genetic counselling for presymptomatic testing--clinical guidelines for practice across the range of genetic conditions. Eur J Hum Genet. 2013;21(3):256–60. doi:.https://doi.org/10.1038/ejhg.2012.174
4
Garrison
NA
,
Brothers
KB
,
Goldenberg
AJ
,
Lynch
JA
. Genomic Contextualism: Shifting the Rhetoric of Genetic Exceptionalism. Am J Bioeth. 2019;19(1):51–63. doi:.https://doi.org/10.1080/15265161.2018.1544304
5
Meigs
JB
,
Cupples
LA
,
Wilson
PW
. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes. 2000;49(12):2201–7. doi:.https://doi.org/10.2337/diabetes.49.12.2201
6
Singletary
SE
. Rating the risk factors for breast cancer. Ann Surg. 2003;237(4):474–82. doi:.https://doi.org/10.1097/01.SLA.0000059969.64262.87
7
Antoniou
A
,
Pharoah
PDP
,
Narod
S
,
Risch
HA
,
Eyfjord
JE
,
Hopper
JL
, et al.
Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–30. doi:.https://doi.org/10.1086/375033
8
Ligthart
S
,
van Herpt
TTW
,
Leening
MJG
,
Kavousi
M
,
Hofman
A
,
Stricker
BHC
, et al.
Lifetime risk of developing impaired glucose metabolism and eventual progression from prediabetes to type 2 diabetes: a prospective cohort study. Lancet Diabetes Endocrinol. 2016;4(1):44–51. doi:.https://doi.org/10.1016/S2213-8587(15)00362-9
9
Guttmacher
AE
,
Collins
FS
,
Carmona
RH
. The family history--more important than ever. N Engl J Med. 2004;351(22):2333–6. doi:.https://doi.org/10.1056/NEJMsb042979
10Brock D. Ethical Issues in the Use of Cost Effectiveness Analysis for the Prioritization of Health Resources. In: Khushf G, editor. Handbook of Bioethics. Philosophy and Medicine. Dordrecht: Kluwer Academic Publishers; 2004. p. 353–80.
