COVID-19 and the role of imaging: early experiences in Central Switzerland
DOI: https://doi.org/10.4414/smw.2020.20304
Carsten
Fechnera, Klaus
Strobela, Thomas
Treumanna, Beat
Sondereggerb, Andrea
Azzolac, Jürgen
Fornaroa, Simone
Schradinga, Justus E.
Roosa
aRadiology and Nuclear Medicine,
bInstitute of Infectious Diseases,
cInstitute of Pulmonology,
COVID-19 and the role of imaging: early experiences in Central Switzerland
Background
The SARS-CoV-2 virus (COVID-19) was initially observed in a group of Chinese patients with unclear pneumonia in Wuhan, Hubei [1] in late December 2019. The first positive case in Switzerland was confirmed on 25 February 2020 in a patient from canton Tessin, who most likely caught the virus during a visit to Milan, Italy [2]. The country has since been preparing for an imminent public health emergency caused by the pandemic. As of 14 May 2020, the Swiss healthcare system is facing a total of 30,463 corona virus-positive people [3]. With numbers of new infections decreasing after the first pandemic wave, the continuing endemic situation will continue to be a major challenge for the Swiss healthcare system. It remains crucial to separate the clinically low-symptomatic from the severely affected patients in order to offer a specific therapeutic strategy to every SARS-CoV-2 patient. Reports from Chinese cohorts describe an increasing role of imaging strategies in the detection and surveillance of COVID-19 patients because of insufficient testing sensitivity of real-time reverse transcription polymerase chain reaction (RT-PCR) tests [4]. Chest computed tomography (CT), with a reported sensitivity of up to 97% [5, 6], gained importance particularly in patients with false negative RT-PCR results.
In this short communication, we describe our first clinical experiences with 55 COVID-19 patients in Central Switzerland, who were either imaged with a standard chest x-ray, chest CT, or both. We provide an illustrative and schematic description of typical COVID-19 imaging features and suggest that imaging plays an important role in the clinical work-up of suspected or confirmed COVID-19 patients. This study was approved by the national ethics review committee (EKNZ, Switzerland) and patients’ informed consent was waived.
Clinical findings
Patients and symptoms
Fifty-five COVID-19 patients admitted to our hospital were retrospectively identified in our digital hospital information system (EPIC), which provides real-time COVID-19 dashboard statistics. The patients’ demographic characteristics (age and risk groups), their clinical symptoms and laboratory test results are summarised in table 1. The mean age was 63 ± 15.7 years (range 31–94). Fort-five percent were in the critical age group older than 65 years. Most of them could be assigned to a risk group defined by the Swiss Federal Office of Public Health FOPH [7].
Table 1 Demographic data, symptoms and laboratory test data of our patient cohort in Central Switzerland (n = 55).
|
COVID-19 patients Central Switzerland
|
Chinese reference
|
|
% (number of patients)
|
|
Patient age
|
|
|
| >65 years old |
45% (25) |
|
| <65 years old |
55% (30) |
|
| Gender female |
25% (14) |
|
| Gender male |
75% (41) |
|
|
Comorbidities
|
|
|
| Arterial hypertension |
14% (3) |
|
| Diabetes |
30% (8) |
|
| Cardiovascular disease |
32% (9) |
|
| Chronic airway disease |
27% (7) |
|
| Cancer |
24% (6) |
|
| Immunomodulation |
10% (2) |
|
|
Symptoms
|
|
WHO-China report*
|
| Fever |
69% (38) |
87.9% |
| Cough |
69% (38) |
67.7% |
| Fatigue |
35% (19) |
38.1% |
| Sputum production |
11% (6) |
33.4% |
| Shortness of breath |
29% (16) |
18.6% |
| Myalgia / Arthralgia |
15% (8) |
14.8% |
| Headache |
11% (6) |
13.6% |
| Sore throat |
2% (1) |
13.9% |
| Chills |
9% (5) |
11.4% |
| Pleuritic pain |
6% (3) |
– |
| Diarrhoea |
36% (19) |
3.7% |
|
Laboratory results
|
|
|
| Lymphopenia (<1.0 Giga/l) |
60% (32) |
70.3% |
| Increased prothrombin time (<70%) |
17% (9) |
58% |
| Increased LDH (>480 U/l) |
49% (26) |
39.9% |
| CRP (>5 mg/l) |
77% (41) |
– |
| D-dimer (measured later) (>500 ng/l) |
43% (23) |
– |
| Increased ferritin (>400 µg/l) |
21% (3) |
– |
|
RT-PCR testing and CT
|
|
Chest CT and RT-PCR†
|
| PCR positive at baseline |
92% (47) |
– |
| PCR positive, CT negative |
0% (0) |
– |
| PCR positive at follow-up, preceding CT positive |
2% (1) |
– |
| PCR pending, CT positive |
9% (4) |
– |
| PCR positive, CT positive |
100% |
97% |
|
Time interval in days
|
|
|
| Symptoms to baseline chest x-ray or CT |
7.41 |
|
The mean time from development of initial symptoms to hospital admission and first medical imaging was 7.2 ± 4.2 days (range 2–21). The most frequent symptoms were dry cough and fever (69%), followed by fatigue (35%) and shortness of breath (29%). These findings closely parallel the clinical data observed in China. Interestingly the number of patients complaining of diarrhoea was larger in our patient cohort (36%) than initially reported in China (3.7%). This observation supports newer studies suggesting a possible faecal transmission [8]. Noteworthy is that in both the Chinese patients and in our patient group, lymphopenia (60%), elevated levels of D-dimer (43%) and C-reactive protein (77%) were highly suggestive of a SARS-CoV-2 infection. Also, there was a correlation between lymphopenia and disease severity [9], which also increased the risk for a secondary complication. Literature suggests that about every fourth SARS-CoV-2 patient will develop a complication on follow-up: 61.1% acute respiratory distress syndrome, 44.4% heart problems such as arrhythmia, and 30.6% cardiovascular shock [10]. A study by Klok et al. [11] showed that COVID-19 patients tend to be in a procoagulatory state, even with standard doses of thromboprophylaxis, and therefore have a much higher incidence for thromboembolic events such as pulmonary embolism – up to 30% of their patients. It is advised that every in-hospital patient receives at least thromboprophylaxis with low molecular weight heparin [12]. In our cohort, we did not witness any embolic events even though high levels of D-dimer were observed.
Clinical work-up
The initial diagnostic work-up in our cohort included RT-PCR tests to detect viral nucleotides from oropharyngeal and/or nasopharyngeal swabs. In rare cases, the specimens were obtained from tracheal secretions or even bronchoalveolar lavage fluid. Three days after hospital admission, 92% of the patients were tested positive for SARS-CoV-2 infection. For 9% of our patients, the RT-PCR result was pending while CT was already positive for COVID-19 according to the imaging guidelines published by the British Institute of Radiology [13]. One patient (2%) remained RT-PCR-negative after three nasopharyngeal swabs for up to 1 week after a positive thoracic CT result. He turned RT-PCR positive after bronchoalveolar lavage was used to obtain enough viral nucleotides (see fig. 6 below).
With sensitivity levels from 59–71% reported [5], RT-PCR is prone to several possible pre-diagnostic errors (wrong swabbing technique, wrong transport media, wrong extraction, etc.) and leaves a substantial gap for false negative test results. This limitation currently jeopardises quarantine efforts and requires repetitive testing, the latter further accentuating the lack of sufficient test kits. In our patient cohort (table 1), the RT-PCR sensitivity was higher, at 92%, and consequently yielded only a small number of false negative test results.
Role of medical imaging
Chest x-ray
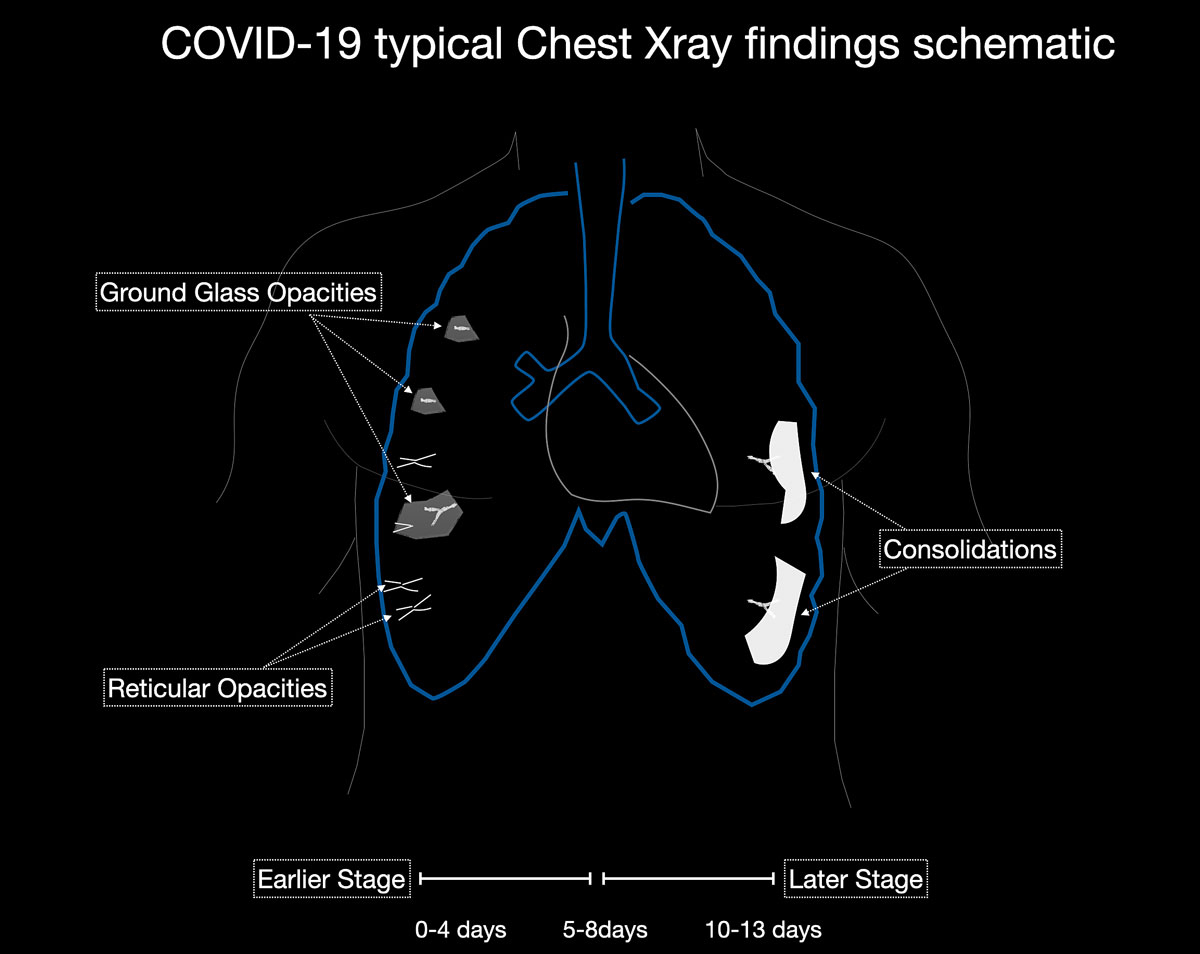
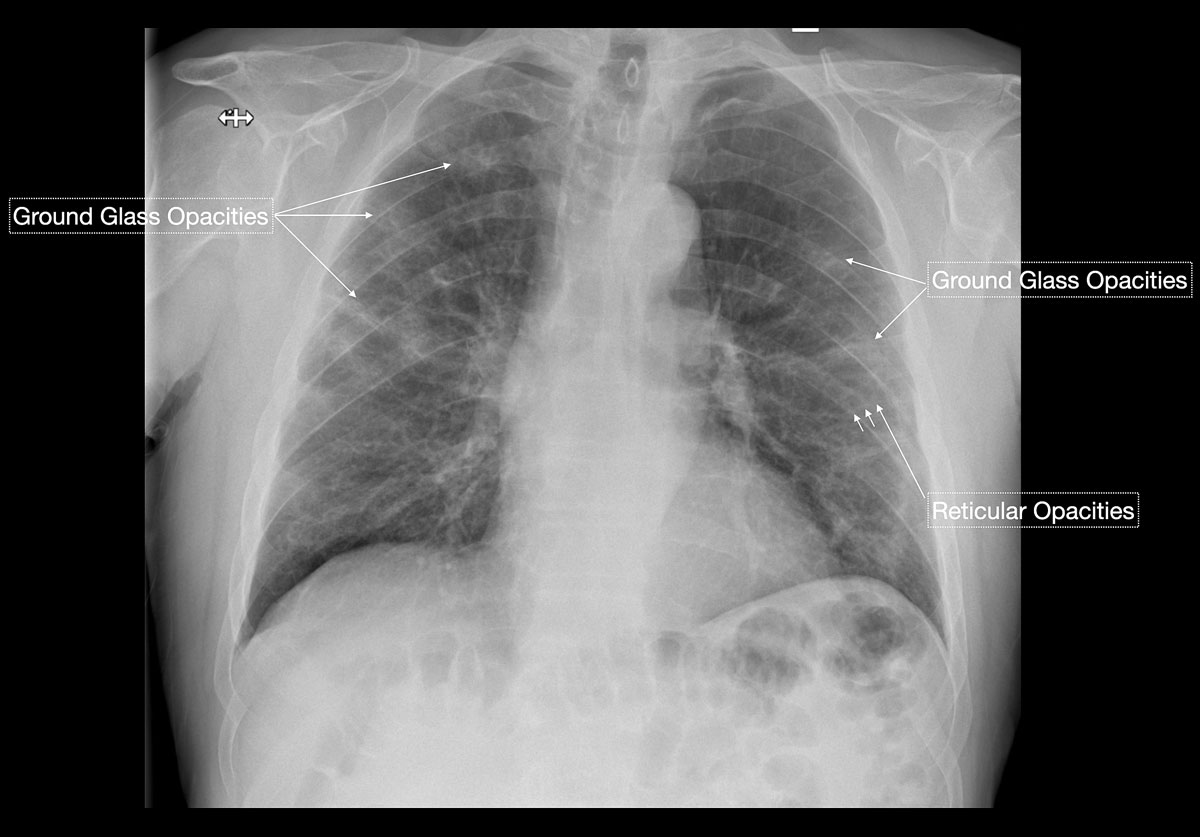
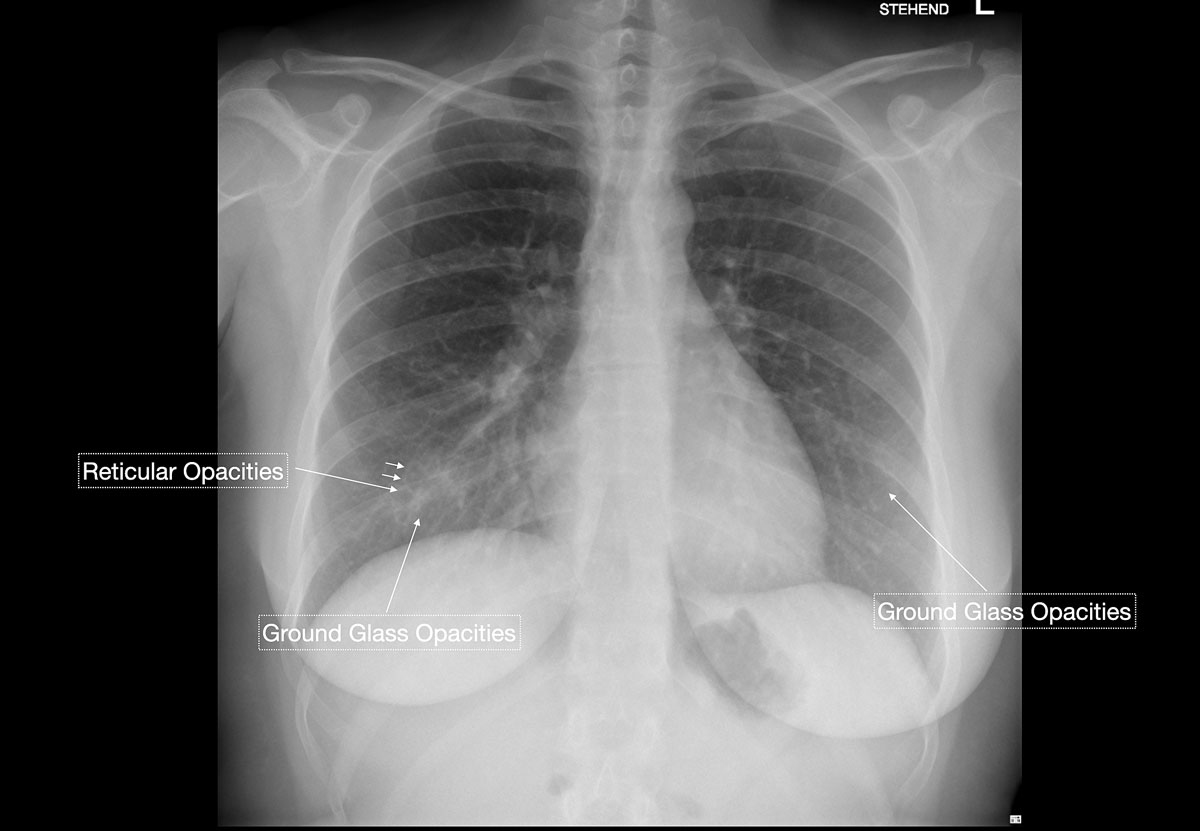
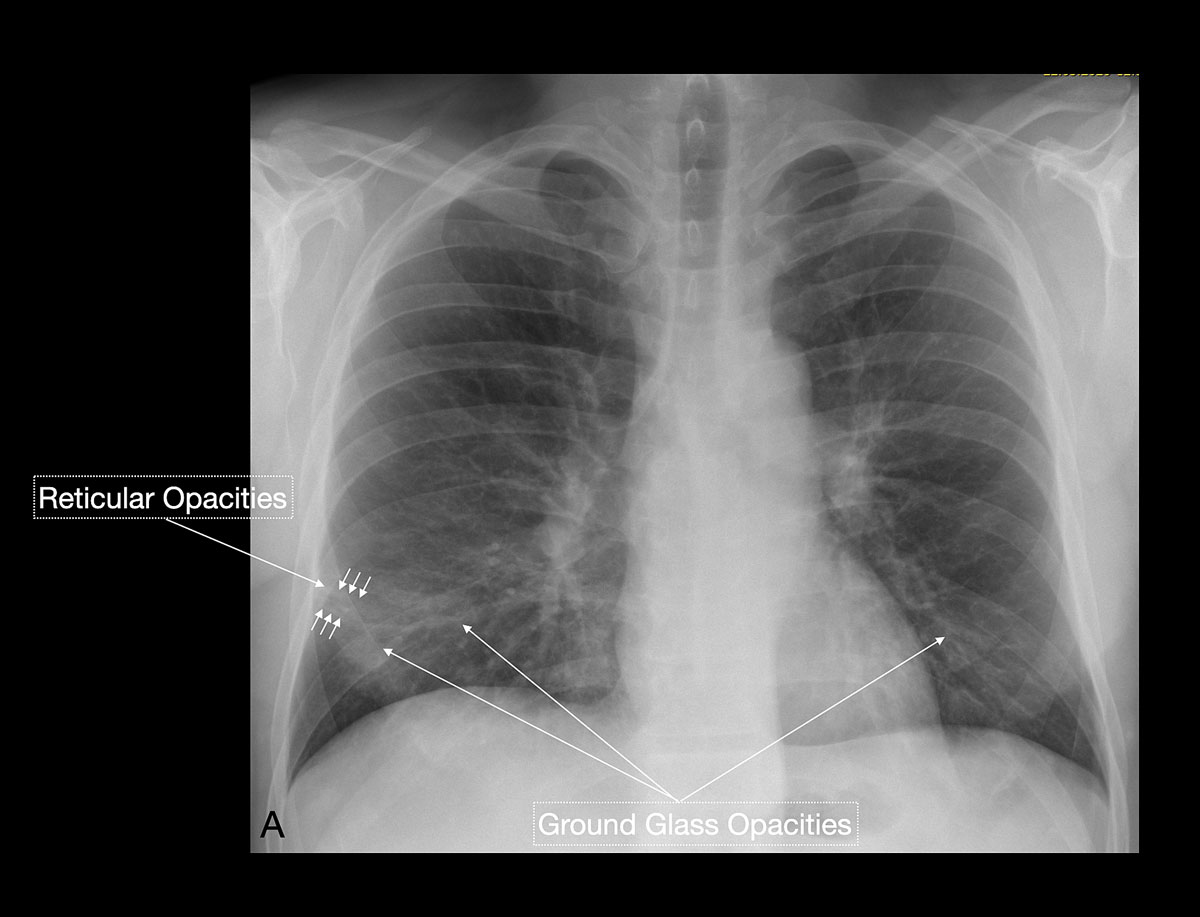
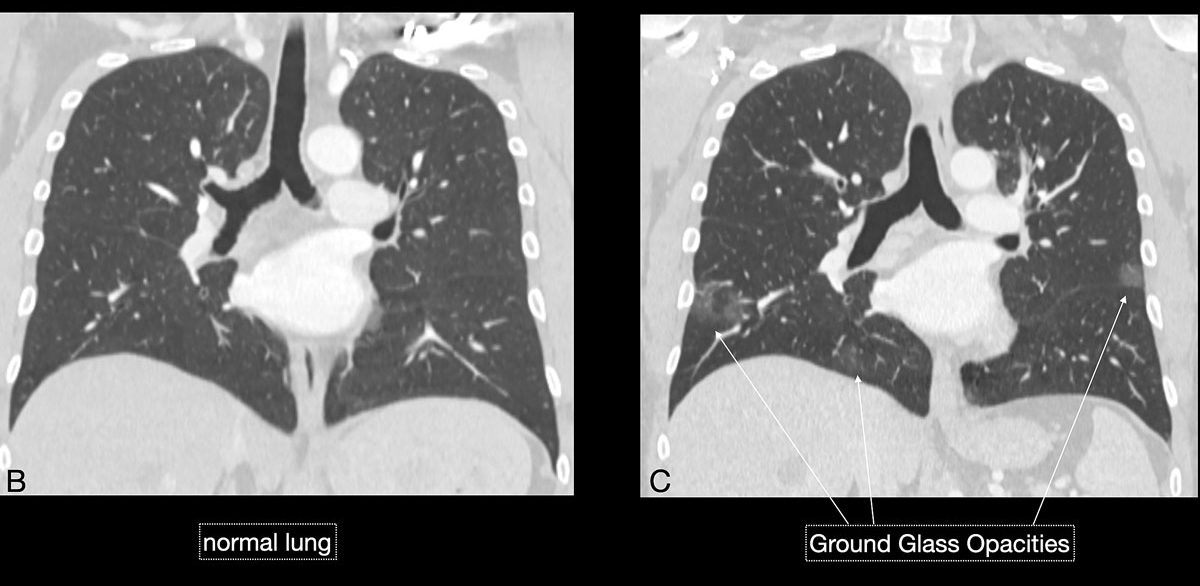
Studies [4, 13, 14] have shown that in early stages, COVID-19 and atypical, interstitial pneumonia share the same primary findings. Typically our patients presented with bilateral reticular (64%) and patchy either ground glass (64%) or consolidative air space opacities (45%), with a peripheral and basal distribution (detailed definitions of these features are illustrated in figs 1 and 2
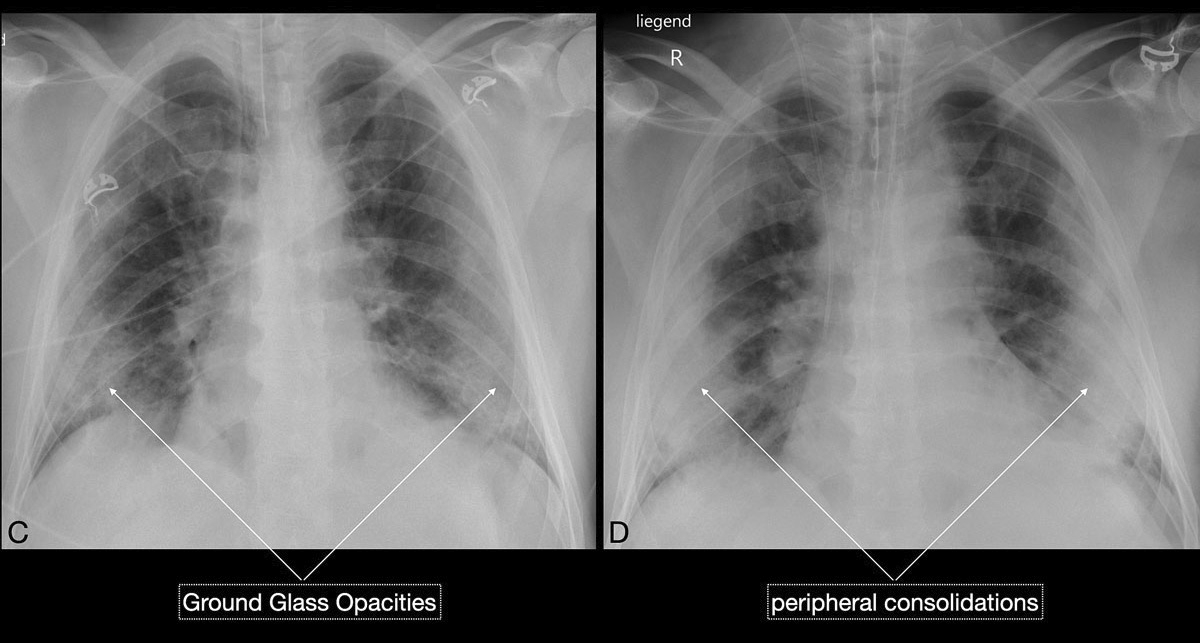
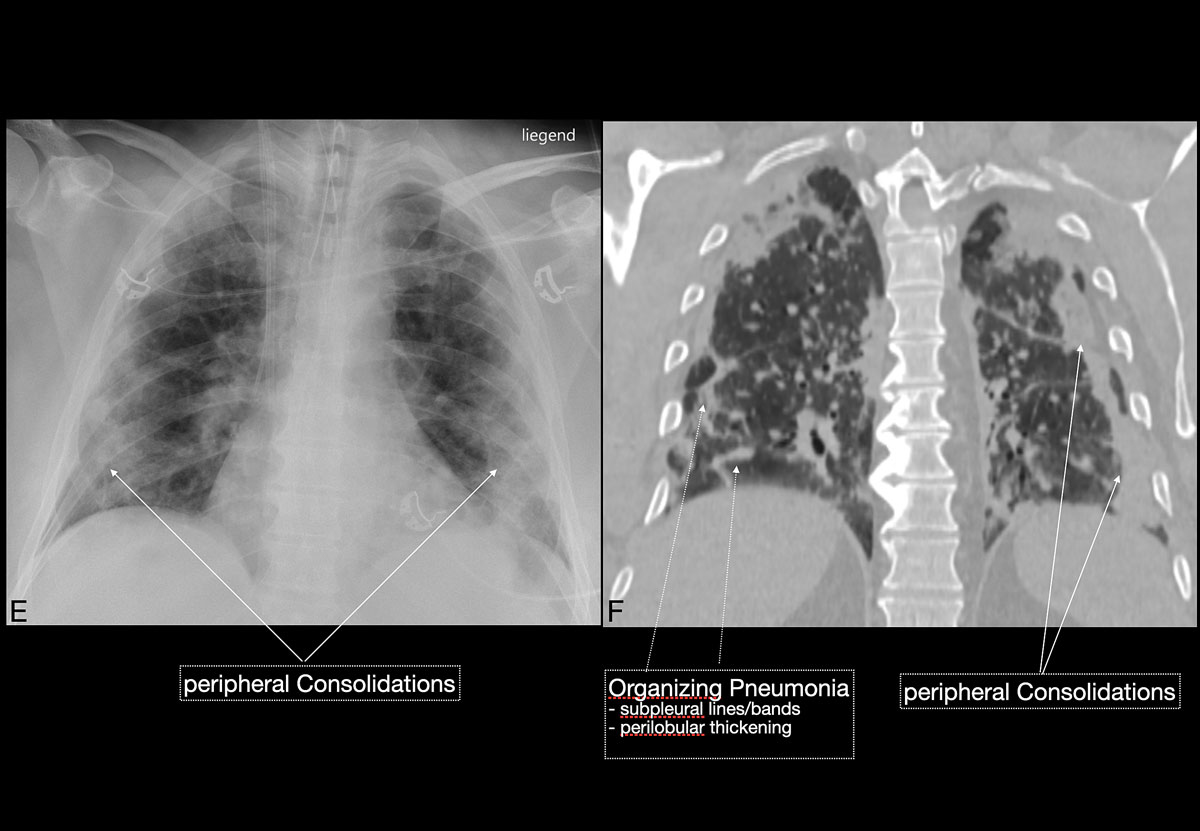
). Since ground glass opacities may be very subtle in appearance, their detection on a chest x-ray is sometimes uncertain. Pleural effusions are rare and were seen in only 13% of our COVID-19 patients. In later stages, features of organising pneumonia with accentuated bilateral patchy consolidations were observed (fig. 3).
In the recent study of Wong et al. [15], chest x-ray had a baseline sensitivity of 69%, compared with RT-PCR testing at 91%, in COVID-19 patients. In a substantial minority (9%) of patients, chest x-ray abnormalities preceded a positive RT-PCR test (fig. 4). In light of the overwhelming numbers of patients, Italian and British hospitals consider chest x-ray as a first-line triage; this is also due to long RT-PCR turnaround times. Since the current test capacity in Switzerland is sufficient, we do not recommend chest x-ray as a screening method. Although the appropriateness of chest radiography in COVID-19 patients is currently being discussed, there is an indication that radiographic recovery parallels clinical recovery [13]. Clinicians of all specialties should familiarise themselves with the spectrum on COVID-19 features seen on chest x-rays.
Computed tomography
According to the treatment protocol of the National Health Commission in China [16], a chest CT scan may also be used to monitor disease progression and to evaluate potential therapeutic measures (fig. 3). Whereas experts from the epidemic-stricken areas in China recommend the use of chest CT as a potential COVID-19 screening tool [17], experts from other countries, such as the American College of Radiology [18], do not advise the same. The latter opine that the need for CT suite decontamination after every patient is scanned severely limits radiological service availability.
However, compared with moderate COVID-19 sensitivities for chest x-rays, the study of Ai et al. on 1014 COVID-19 patients [5] underlined the importance of chest CT in the diagnostic workup, with a high sensitivity of 97% compared with conventionally used RT-PCR tests. RT-PCR achieved a moderate sensitivity of between 59% and 71% compared with the number of patients considered positive for COVID-19 according to clinical and imaging findings. However, the radiologists in this study achieved a low specificity of 25% and an overall accuracy of 68%. This implies that the CT patterns are not exclusively pathognomonic for COVID-19 infection and that they can also be seen with alternative pulmonary infectious and non-infectious diseases. At the moment sensitivities could be also falsely increased owing to a high-prevalence environment. A low specificity might cause additional strain on medical infrastructure and health worker capacity because of an increased number of false positive cases. Nevertheless, this has changed with the increasing knowledge of COVID-19 in relation to chest CT. The subsequent study of Bai et al. [19] demonstrated significantly better specificities in discriminating COVID-19 from non-COVID-19 pulmonary infections. They rated 219 COVID-19 patients against 204 non-COVID patients with viral pneumonias (such as influenza, human rhinovirus, etc.) and achieved higher maximum specificities of up to 100% for some radiologists, at comparable sensitivities from 72% to 93%. In our patient population, all RT-PCR positive patients showed CT findings suggestive of COVID-19 infection; no false negative chest CT test was observed.
Common CT imaging patterns in COVID-19 patients
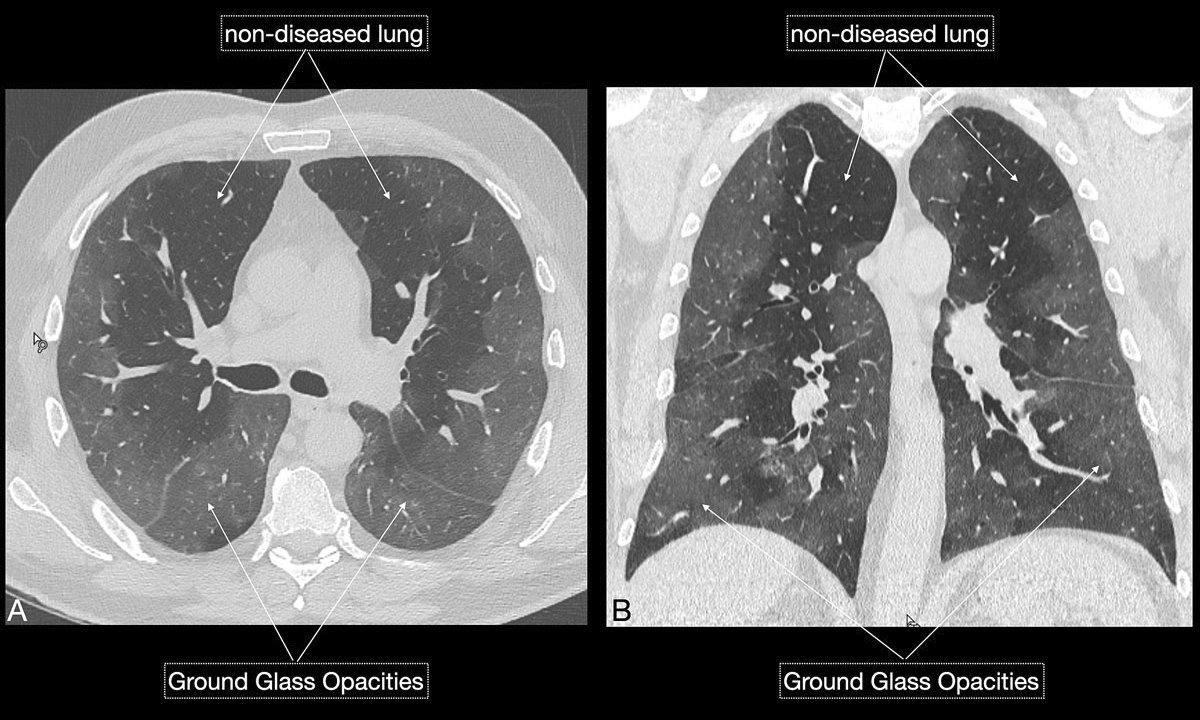
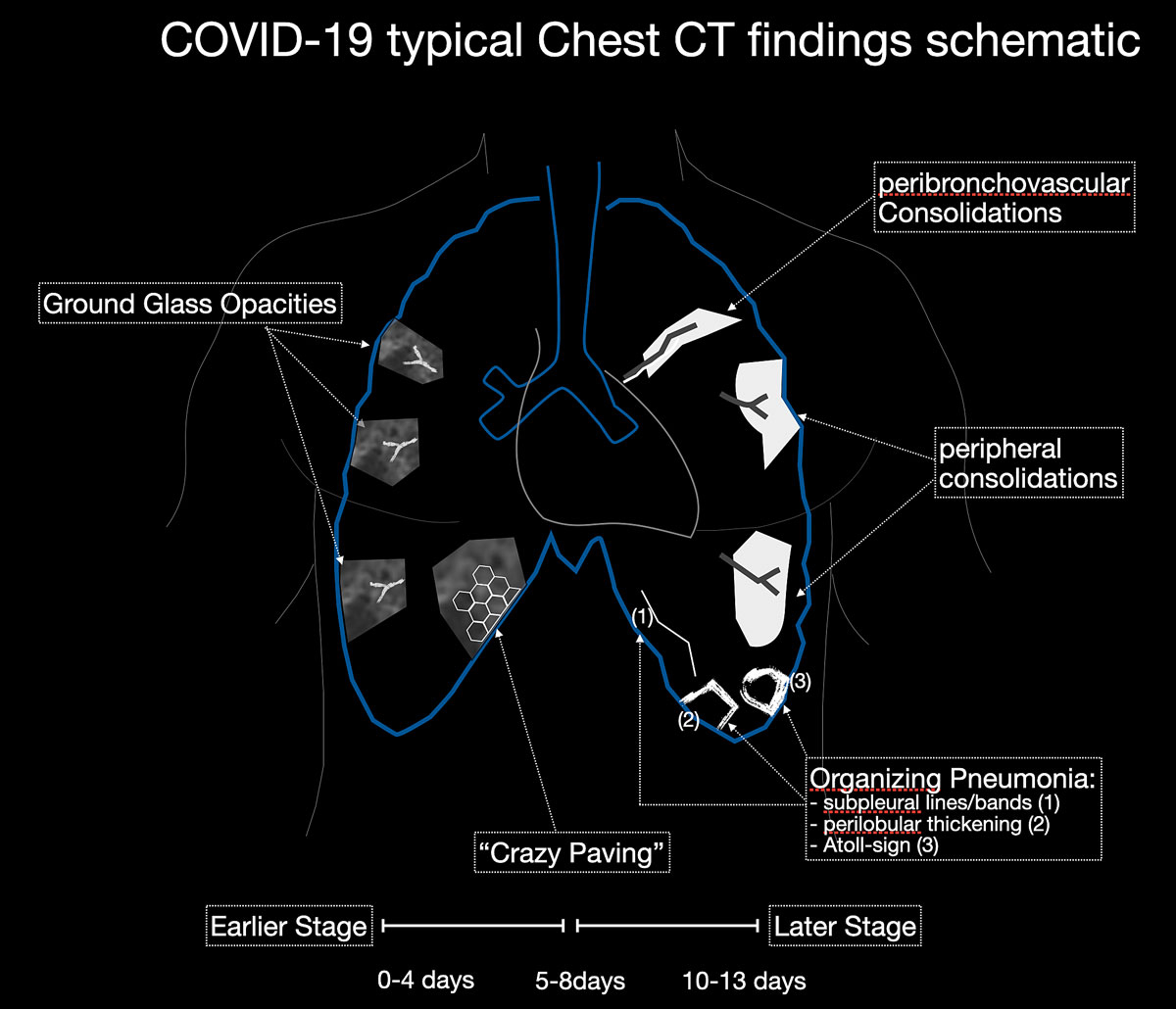
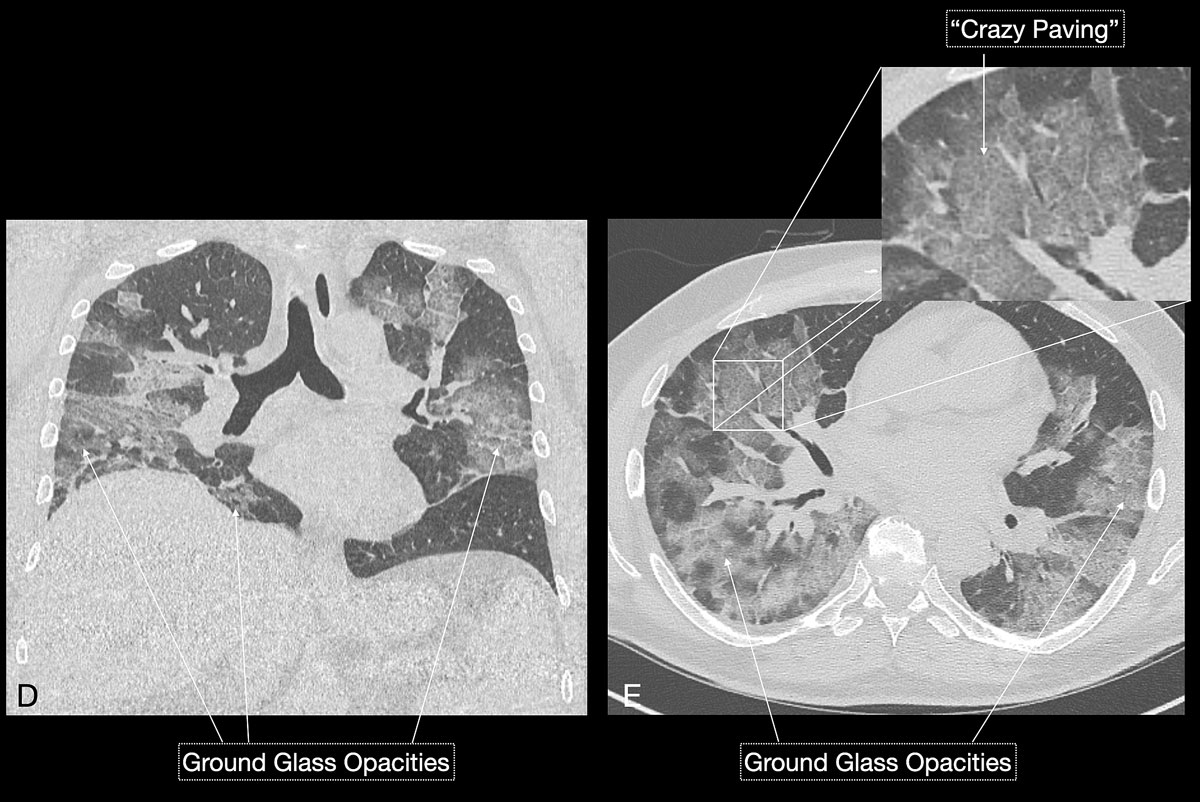
Imaging features in our patient cohort are summarised in table 2 and compared with manifestations observed on CT in Chinese patients [20]. Classic imaging findings are bilateral ground glass opacities with or without areas of consolidation, predominantly with a peripheral or basal lower lobe distribution (figs 3 and 5
). Unilateral or bilateral ground glass opacities – the hallmark of an early infection (0–4 days) – were observed in almost all COVID-19 patients on chest CT examinations (97% positivity). Given the very subtle appearance of ground glass opacities, their detection on chest x-ray is difficult and often not made prospectively. The second most apparent imaging patterns are consolidations, which are typically multifocal, subpleural/peripheral, or in a peribronchovascular distribution (figs 3 and 5
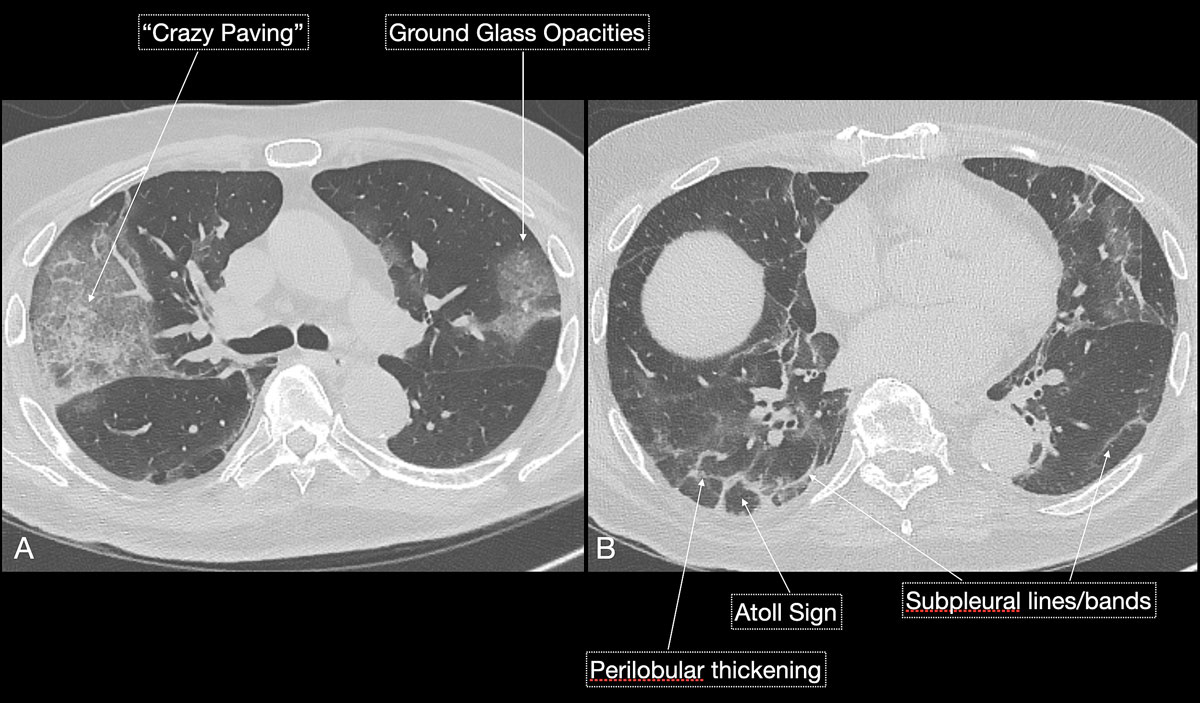
). As the disease progresses (5–8 days), the extent of ground glass opacities increases and the amount of consolidation rises (fig. 6). The ground glass opacities progressively transform into consolidative opacities featuring patterns of organising pneumonia of varying extents in the peak stage (10–13 days), before gradually resolving with the patient’s recovery. Typical patterns of organising pneumonia include subpleural lines/bands, perilobular thickening, arcade and atoll signs. Generally, the development of consolidations is a sign of disease progression and is more common in patients above 50 years old and those who have experienced a longer duration of symptoms.
Table 2 Chest computed tomography (CT) imaging findings in our COVID-19 patient cohort compared with Chinese experience (n = 30).
| |
COVID-19 patients Central Switzerland
|
Chinese reference
|
|
% (number of patients)
|
Chest CT manifestations*
|
|
Modality
|
|
|
| CT only |
27% (15) |
|
| X-ray only |
46% (25) |
|
| CT + x-ray |
27% (15) |
|
|
CT findings
|
|
|
| Ground glass opacities) |
97% (29) |
55% |
| Crazy paving pattern |
33% (10) |
17% |
| Consolidation |
60% (18) |
42% |
| Peribronchovascular distribution |
33% (10) |
– |
| Peripheral distribution |
80% (24) |
– |
| Traction bronchiectasis |
10% (3) |
11% |
| Perilobular thickening |
30% (9) |
– |
| Arcade sign or atoll sign |
27% (8) |
2% |
| Interlobular septal thickening |
3% (1) |
15% |
| Lymphadenopathy |
13% (4) |
6% |
| Pleural / pericardial effusion |
13% (4) |
8% |
|
Mean Total Severity Score (TSS 0–20)†
|
|
|
| TSS |
7.03 (30) |
|
| Right upper lobe |
1.30 (29) |
|
| Right middle lobe |
1.20 (26) |
|
| Right lower lobe |
1.53 (29) |
|
| Left upper lobe |
1.30 (28) |
|
| Left lower lobe |
1.70 (30) |
|
| TSS score <7.5 |
63% (19) |
|
| TSS score >7.5 |
37% (11) |
|
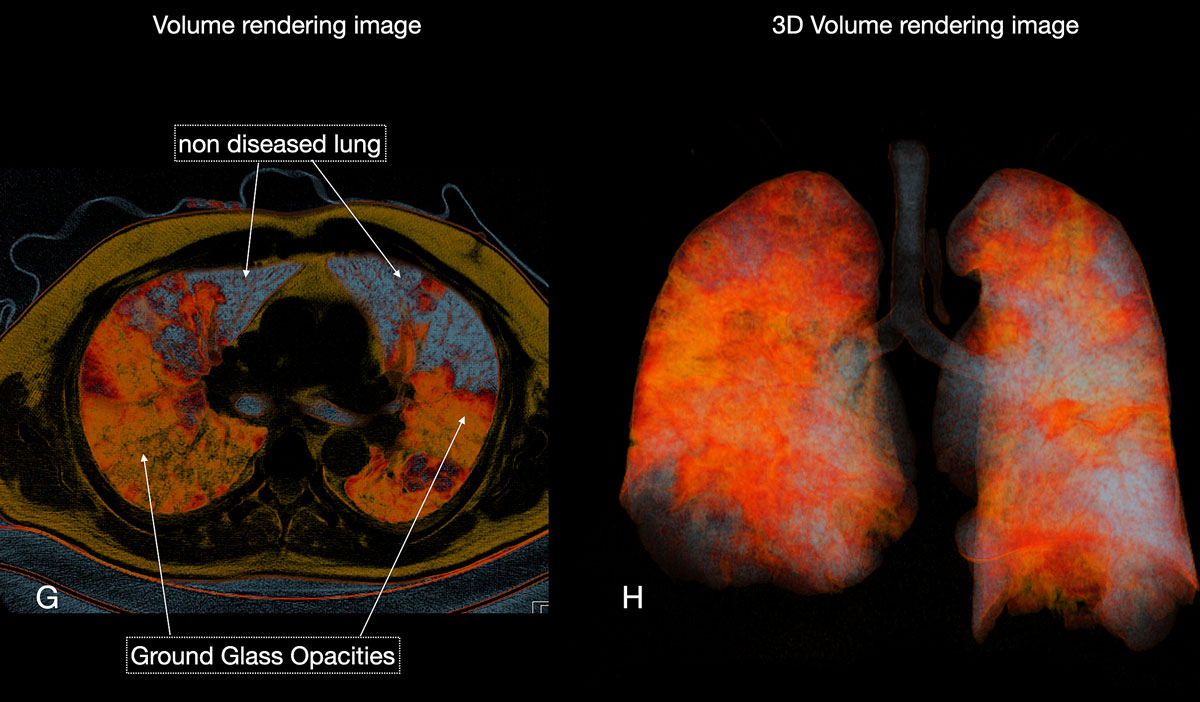
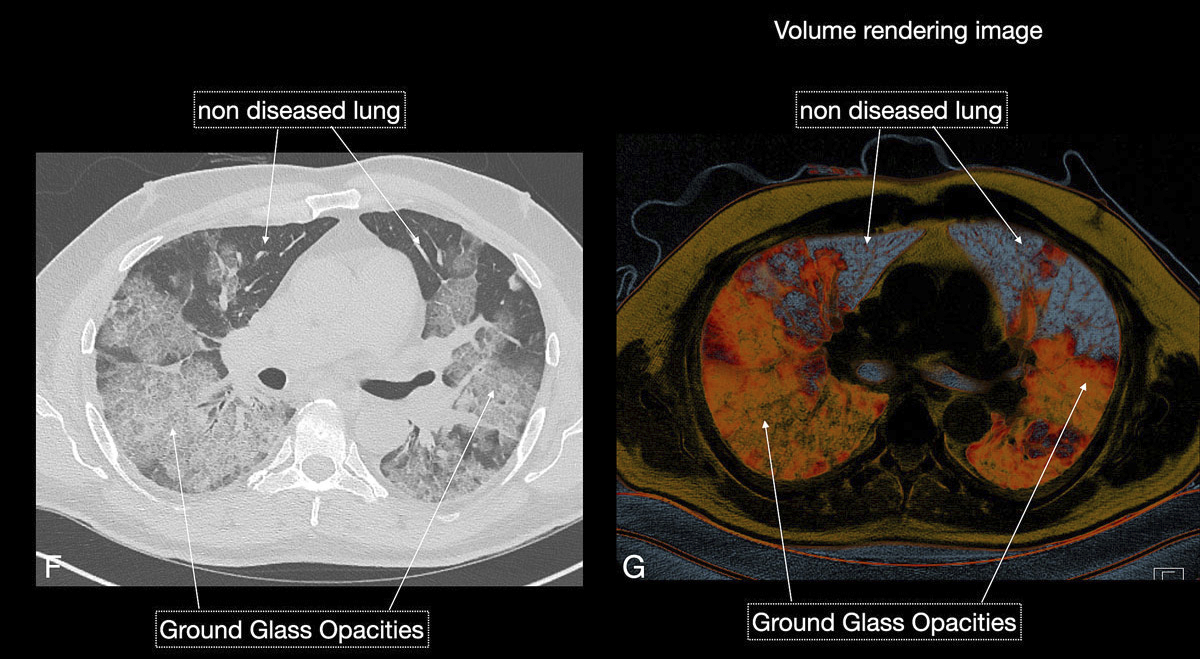
Figure 6 GH Three-dimensional volume rendering images (H) gave an overview of diseased total lung. The patient required no respiratory assistance.
Reticular opacities are also often seen with COVID-19 patients and likely represent lymphocytic interstitial infiltration, similar to other viral infections (fig. 6). A unique CT pattern with a thickened intra- and interlobular interstitium superimposed on background ground glass opacity was observed in 5–36% of COVID-19 patients. The CT pattern had a specific appearance of “crazy paving”, since it resembles a mosaic of irregular paving stones (figs 6 and 7
). This sign likely represents the combination of alveolar oedema and interstitial inflammatory changes in the setting of diffuse alveolar damage and is most commonly seen in the progressive stage of the disease [22, 23].
Reported atypical CT manifestations [20] of COVID-19 include airway changes with bronchial wall thickening, bronchiectasis, pleural changes and pleural effusions. The latter are observed in only 5% of COVID-19 patients [24]. However, pleural effusions suggest an inferior prognosis in COVID-19 patients. Findings such as lymphadenopathy, tree-in-bud or centrilobular nodularity or lobar consolidations are considered not typical of a COVID-19 infection.
Based on imaging experience with COVID-19 type and the temporal change of specific imaging findings, the British Institute of Radiology provided a confidence level categorisation scheme [25] for diagnosing COVID-19 patients according to typical and atypical CT findings; this might prove very helpful in clinical practice (table 3).
Table 3 Chest computed tomography (CT) imaging patterns typical and atypical for COVID-19 pulmonary infections.
|
CT pattern type
|
Confidence for COVID-19
|
Description
|
|
Classical COVID-19
|
100% |
Ground glass opacities: lower lobe, peripheral predominant, bilateral |
| ± Crazy paving |
| ± Peripheral consolidation (organising pneumonia) |
| ± Reverse halo / perilobular pattern (organising pneumonia) |
|
Probable COVID-19
|
71–99% |
Lower lobe predominant mix of bronchocentric and peripheral consolidations |
| Reverse halo / periloblar pattern (organising pneumonia) |
| Minimal ground glass opacities |
|
Intermediate COVID-19
|
<70% |
Not classical, probable or non-COVID type |
| Clinical context is wrong or suggests alternative diagnosis (interstitial lung disease) |
|
Non-COVID-19
|
70% confidence of alternative |
Lobar pneumonia |
| Cavitating infections |
| Tree-in-bud / centrilobular nodularity |
| Lymphadenopathy |
| Pleural effusions |
| Established pulmonary fibrosis |
Experience is also emerging with regard to a visual quantitative analysis of the extent of the disease as seen on CT, relative to clinical classification. Li et al. [21, 26] suggest a scoring system (TTS = Total Severity Score) per pulmonary lobe (extent of opacities per lobe are graded on a 4-point scale (0: no opacity; 1: <25% of lobe diseased; 2: 25–50% of lobe diseased; 3: 50–75% diseased; 4: >75% diseased). Based on their results, a TTS >7.5 was fairly sensitive (83%) and highly specific (100%) for predicting a severe and critical course of COVID-19 infection. In our patient cohort, a mean TTS of 7.0, predominantly with higher scores in the lower lobes, was observed. Ongoing research is needed to further validate quantitative image analysis in COVID-19 patients and to combine this with other stratification measures in order to better classify clinical severity, improve the course of clinical treatment and, ultimately, better predict patients’ clinical outcome
Conclusions
The worldwide mortality rates of COVID-19 are unprecedented. Every country is taking measures to confine viral spread and avoid overloading their healthcare system, particularly intensive care units with limited personnel capacities and artificial respiration resources. Since the number of confirmed cases is also increasing in Switzerland, we must absorb valuable knowledge from other countries to help us better understand the disease, its detection and its treatment. Previous studies have shown the importance of chest imaging, especially given that current RT-PCR testing sometimes renders false negative results. This problem seems to be reducible because of increasing knowledge about the virus and improved standardisation of pre-diagnostic workup.
The use of chest imaging at the general practitioners’ office or in the hospital plays an increasing role in the assessment of symptomatic COVID-19 patients [24]. This report shares our experience with 55 COVID-19 patients who have been evaluated using either a chest x-ray or CT. We compared the concordance of our clinical and imaging findings with existing COVID-19 literature. In general, the clinical and imaging features were concordant with the current literature reports of COVID-19 viral pneumonia. Most patients had ground glass opacities and consolidations in a subpleural or peribronchovascular distribution, changing over time. Specific patterns of organising pneumonia were comparable to observations in the early cohorts (tables 1 and 2
). Similarly, we also observed COVID-19 patients with initial negative RT-PCR tests who had classical positive findings on their chest CT results; 4 days later, the infections of such patients were confirmed using RT-PCR on bronchoalveolar lavage fluid (fig. 1).
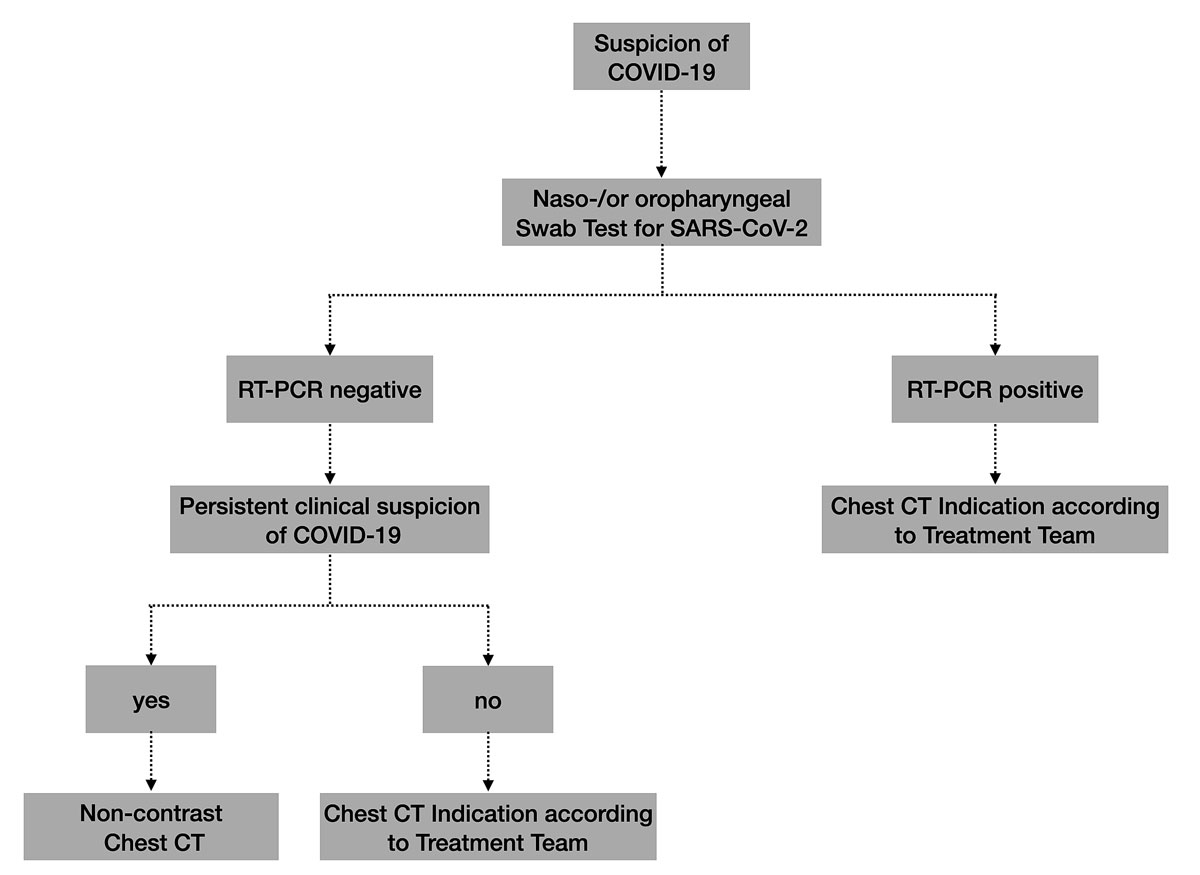
Based on the current available literature and our early clinical and radiological experience with the first COVID-19 patients in Central Switzerland, an interdisciplinary local taskforce of experts in infectious diseases, emergency medicine, pneumonology and radiology defined the indications and analysed a radiological workup of COVID-19 patients (fig. 8). We determined that a chest X-ray remains the primary imaging modality in patients with all types of respiratory infections. Currently, we use neither chest CT nor x-rays as a screening method in asymptomatic patients. The decision to perform a chest CT or x-ray examination must be made by the medical team treating the patient, for example in the event of clinical deterioration or suspicion of possible superinfection. Additionally, chest CT may be used in patients with a negative RT-PCR test, but with persisting clinical suspicion of COVID-19 pneumonia (fig. 6).
In this short communication, we report our early experiences with use of medical imaging in the current COVID-19 pandemic. Our observations in Central Switzerland parallel those from China regarding the increased importance of medical imaging to assess clinically suspicious patients with severe symptoms or a negative RT-PCR result. By describing typical imaging features on chest X-ray and chest CT studies, we hope to aid the readership in familiarising themselves with these findings in order to make the right diagnosis when faced with similar cases, whether at the general practitioners’ office or in a hospital setting. A major indication for chest CT is ongoing clinical suspicion of COVID-19 infection, but a negative RT-PCR test; this is likewise important in cases of clinical deterioration or possible superinfection. Our hospital does not recommend the use of chest CT as a screening tool or alternative to swab tests.
References
1
Zhu
N
,
Zhang
D
,
Wang
W
,
Li
X
,
Yang
B
,
Song
J
, et al.; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. doi:.https://doi.org/10.1056/NEJMoa2001017
2Tagblatt. Tessin meldet ersten bestätigten Fall von Corona-Virus in der Schweiz. https://www.tagblatt.ch/news-service/inland-schweiz/tessin-meldet-ersten-bestaetigten-fall-von-corona-virus-in-der-schweiz-ld.1198115
3Bundesamt für Gesundheit BAG. Situationsbericht zur epidemiologischen Lage in der Schweiz und im Fürstentum Liechtenstein Coronavirus Krankheit 2019 (COVID-19). www.bag.admin.ch
4
Kanne
JP
,
Little
BP
,
Chung
JH
,
Elicker
BM
,
Ketai
LH
. Essentials for Radiologists on COVID-19: An Update-Radiology Scientific Expert Panel. Radiology. 2020;200527:200527. doi:.https://doi.org/10.1148/radiol.2020200527
5
Ai
T
,
Yang
Z
,
Hou
H
,
Zhan
C
,
Chen
C
,
Lv
W
, et al.
Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;200642:200642. doi:.https://doi.org/10.1148/radiol.2020200642
6
Fang
Y
,
Zhang
H
,
Xie
J
,
Lin
M
,
Ying
L
,
Pang
P
, et al.
Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020;200432:200432. doi:.https://doi.org/10.1148/radiol.2020200432
7Bundesamt für Gesundheit BAG. Neues Coronavirus: Besonders gefährdete Personen. www.bag.admin.ch
8
Wang
D
,
Hu
B
,
Hu
C
,
Zhu
F
,
Liu
X
,
Zhang
J
, et al.
Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi:.https://doi.org/10.1001/jama.2020.1585
9
Ruan
Q
,
Yang
K
,
Wang
W
,
Jiang
L
,
Song
J
. Correction to: Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(6):1294–7. doi:.https://doi.org/10.1007/s00134-020-06028-z
10
Gu
J
,
Han
B
,
Wang
J
. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158(6):1518–9. doi:.https://doi.org/10.1053/j.gastro.2020.02.054
11
Klok
FA
,
Kruip
MJHA
,
van der Meer
NJM
,
Arbous
MS
,
Gommers
DAMPJ
,
Kant
KM
, et al.
Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–7. doi:.https://doi.org/10.1016/j.thromres.2020.04.013
12
Casini
A
,
Alberio
L
,
Angelillo-Scherrer
A
,
Fontana
P
,
Gerber
B
,
Graf
L
, et al.
Thromboprophylaxis and laboratory monitoring for in-hospital patients with COVID-19 - a Swiss consensus statement by the Working Party Hemostasis. Swiss Med Wkly. 2020;150(1516):w20247. doi:.https://doi.org/10.4414/smw.2020.20247
13
Pan
F
,
Ye
T
,
Sun
P
,
Gui
S
,
Liang
B
,
Li
L
, et al.
Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology. 2020;295(3):715–21. doi:.https://doi.org/10.1148/radiol.2020200370
14
Huang
C
,
Wang
Y
,
Li
X
,
Ren
L
,
Zhao
J
,
Hu
Y
, et al.
Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:.https://doi.org/10.1016/S0140-6736(20)30183-5
15
Wong
HYF
,
Lam
HYS
,
Fong
AH-T
,
Leung
ST
,
Chin
TW-Y
,
Lo
CSY
, et al.
Frequency and Distribution of Chest Radiographic Findings in COVID-19 Positive Patients. Radiology. 2019;201160:201160. doi:.https://doi.org/10.1148/radiol.2020201160
16National Health Commission of the People’s Republic of China. (2020) The diagnostic and treatment protocol of COVID-19. China. Available at http://www.gov.cn/
17
Huang
Y
,
Cheng
W
,
Zhao
N
,
Qu
H
,
Tian
J
. CT screening for early diagnosis of SARS-CoV-2 infection. Lancet Infect Dis. 2020:S1473-3099(20)30241-3. doi:.https://doi.org/10.1016/S1473-3099(20)30241-3
18American College of Radiology. Recommendations for Chest Radiography and CT for Suspected COVID19 Infection. 11.03.2020. www.acr.org
19
Bai
HX
,
Hsieh
B
,
Xiong
Z
,
Halsey
K
,
Choi
JW
,
Tran
TML
, et al.
Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020;200823:200823. doi:.https://doi.org/10.1148/radiol.2020200823
20
Ye
Z
,
Zhang
Y
,
Wang
Y
,
Huang
Z
,
Song
B
. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020:1–9. doi:.https://doi.org/10.1007/s00330-020-06801-0
21
Li
K
,
Fang
Y
,
Li
W
,
Pan
C
,
Qin
P
,
Zhong
Y
, et al.
CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19). Eur Radiol. 2020:1–10. doi:.https://doi.org/10.1007/s00330-020-06817-6
22
Bernheim
A
,
Mei
X
,
Huang
M
,
Yang
Y
,
Fayad
ZA
,
Zhang
N
, et al.
Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020;295(3):200463. doi:.https://doi.org/10.1148/radiol.2020200463
23
Pan
Y
,
Guan
H
,
Zhou
S
,
Wang
Y
,
Li
Q
,
Zhu
T
, et al.
Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020;30(6):3306–9. doi:.https://doi.org/10.1007/s00330-020-06731-x
24
Shi
H
,
Han
X
,
Jiang
N
,
Cao
Y
,
Alwalid
O
,
Gu
J
, et al.
Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–34. doi:.https://doi.org/10.1016/S1473-3099(20)30086-4
25British Institute of Radiology. Thoracic Imaging in COVID-19 Infection Guidance for the Reporting. Version 2 16th March 2020. http://www.bsti.org.uk/
26
Zhou
Z
,
Guo
D
,
Li
C
,
Fang
Z
,
Chen
L
,
Yang
R
, et al.
Coronavirus disease 2019: initial chest CT findings. Eur Radiol. 2020:1–9. doi:.https://doi.org/10.1007/s00330-020-06816-7