Figure 1 Dimensions for an evaluation of the disease in question.
DOI: https://doi.org/10.4414/smw.2020.20298
Approved by the Executive Board of the SAMS on 21 April 2020.
The German text is the authentic version.
The Swiss Society of Reproductive Medicine (SGRM), the Swiss Society of Obstetrics and Gynecology (SGGG) and the Swiss Society of Medical Genetics (SGMG) welcome these recommendations and advise their members to note and follow them.
The following recommendations are addressed to professionals who use preimplantation genetic testing (PGT), advise couples in this connection, or perform PGT analyses. The use of PGT is regulated by Art. 5a of the Reproductive Medicine Act (RMA).1 However, for implementation in practice, the text of this legislation leaves certain questions open. Mention should be made in particular of the different restrictions applying to PGT-M/PGT-SR and to PGT-A, and of discrepancies between these provisions and the legal permissibility of terminations of pregnancy.
Against this background, the recommendations of the Swiss Academy of Medical Sciences (SAMS) are designed to draw attention, from an ethical perspective, to important aspects of the PGT counselling and decision-making situation, and to promote ethical awareness in the performance of PGT. In addition, they discuss how, for example, surplus information should be managed.
Being able to fulfil the desire to have children is an important goal in many people’s lives. Here, reproductive medicine can offer assistance if pregnancy cannot be achieved naturally. In addition, PGT makes it possible to analyse the genome of an embryo before it is transferred to the uterus. This option becomes relevant in cases where there is a risk of transmitting a serious disease, or the couple suffers from infertility. The fear of transmitting a serious disease to a child, or an unfulfilled desire for children, places a considerable burden on a couple. Accordingly, many couples are prepared to undergo elaborate treatments.
PGT may not be performed at will. It is important to resist societal “bottom-up eugenics”, where human life is classified by medicine and society as either “worth living” or “not worth living”. This view and decisions made on this basis have impacts both on people with disabilities and on how they are perceived, valued and integrated into society. To ensure that such developments are counteracted as far as possible, the selection of embryos on the basis of genetic characteristics is possible only as an ethically demanding individual decision, made within the legal framework by the couple concerned and the attending physician in a shared decision-making process. The couple’s personal situation, experience and values are taken into account in this process. The rejection of embryos – undertaken within the legally permissible framework – on the basis of their genetic characteristics is not to be interpreted as an assessment by society as a whole of a disease or disability considered not worthy of life.
Given the complexity of the medical situation, the psychological burden on the couple and the (social) ethical implications of decisions relating to PGT, counselling is of great importance. The aim is to enable the couple concerned to make an appropriate, informed decision. Counselling will focus on matters of genetics and reproductive medicine, also covering psychosocial aspects. The couple should be guided by their possibilities, limits and ideas, which are influenced by cultural, moral, religious, biographical and financial factors. At the same time, the scope for action is subject to legal, ethical and medical/professional constraints, which must be explained and respected by the attending physician. The physician’s responsibility also includes reflecting on and becoming aware of his or her own values and personal attitude to PGT, so that these do not (consciously or unconsciously) influence the couple’s decision-making. Counselling must not be directive. In addition, conflicts of interest need to be recognised and taken into account, or made transparent vis-à-vis the couple.
During counselling, the couple’s attention is to be drawn to the option of forgoing PGT and their right not to know, and the benefits and drawbacks of PGT are to be discussed. The latter include the fact that manipulation and cell removal may possibly impair the embryo and its capacity for development.
It should be mentioned that, with regard to the potential for establishing a successful pregnancy, the genetic findings from PGT may in some cases be difficult to interpret (e.g. mosaic embryos). It should therefore be carefully examined whether, in a particular case, the goals pursued by means of PGT offset the disadvantages. The couple are to be informed about alternatives to PGT, such as prenatal diagnosis in a pregnancy established naturally or via sperm donation.2 In addition, it should be explained to the couple that neither PGT nor the alternatives offer a guarantee of a healthy child.
The consequences arising from the decision should be anticipated and discussed with the couple before assisted reproductive treatment is started. After the couple have been fully informed, they should be granted an appropriate period for reflection. If a couple are ambivalent or in disagreement, this period may be extended and additional counselling offered. Here, the involvement of third parties close to those affected, or of other experts (e.g. representatives of patient organisations, disability associations and parents’ groups), is to be recommended. The individual counselling steps and results are to be documented and made available to the couple.
If a couple with a family history of genetic disease request PGT, and if it is technically feasible for the condition concerned, the attending physician must examine whether the legally specified eligibility criteria are met: the condition in question must be a hereditary, serious disease which is likely to be manifested before the age of 50, and for which no effective and appropriate treatment is available. In addition, the couple must state in writing that they consider unacceptable the risk of a child being affected by the disease.
The questions of hereditariness and age at onset can generally be answered by consulting the literature. Treatment options, however, are subject to change. If new therapeutic approaches are developed for genetic disorders, this may affect the evaluation of whether an effective treatment is available. Given the sometimes complex and burdensome nature of such therapies, this evaluation requires careful consideration.
The question whether a particular disease is to be considered serious cannot be answered objectively with reference to medical criteria. It is true that characteristics such as severe pain, dependence, or severe impairments of motor functions, mobility, cognition and emotion provide evidence of the seriousness of a disease. However, the actual degree to which a disease is manifested cannot be predicted, nor the suffering which could be experienced by those affected. This is particularly apparent in the case of genetic conditions with variable expressivity. The establishment of a list of pathologies which are to be deemed serious genetic diseases is to be rejected from an ethical viewpoint, as this could promote societal acceptance of the idea of embryos being rejected as a result of specific genetic characteristics.
Analogously to late termination of pregnancy, the physician and the couple concerned should be assigned the responsibility for jointly assessing whether, in a particular case, the degree of seriousness is sufficient for PGT-M/PGT-SR to be indicated. What is decisive for this assessment is not the genetic disease in itself, but whether the birth of a child with this disease would place the couple in an unacceptable situation. The decision-making process thus largely remains within the personal physician-patient relationship.
To evaluate the seriousness of the disease and to estimate individual acceptability, it may be helpful for a couple to consult experts in the condition concerned. As well as specialists in the relevant field (e.g. neurology), this could include people affected, or disability/patient organisations. Such advice may help the couple to reflect on information concerning the possible severity of the disease in an affected child (penetrance and expressivity) in relation to their own particular circumstances.
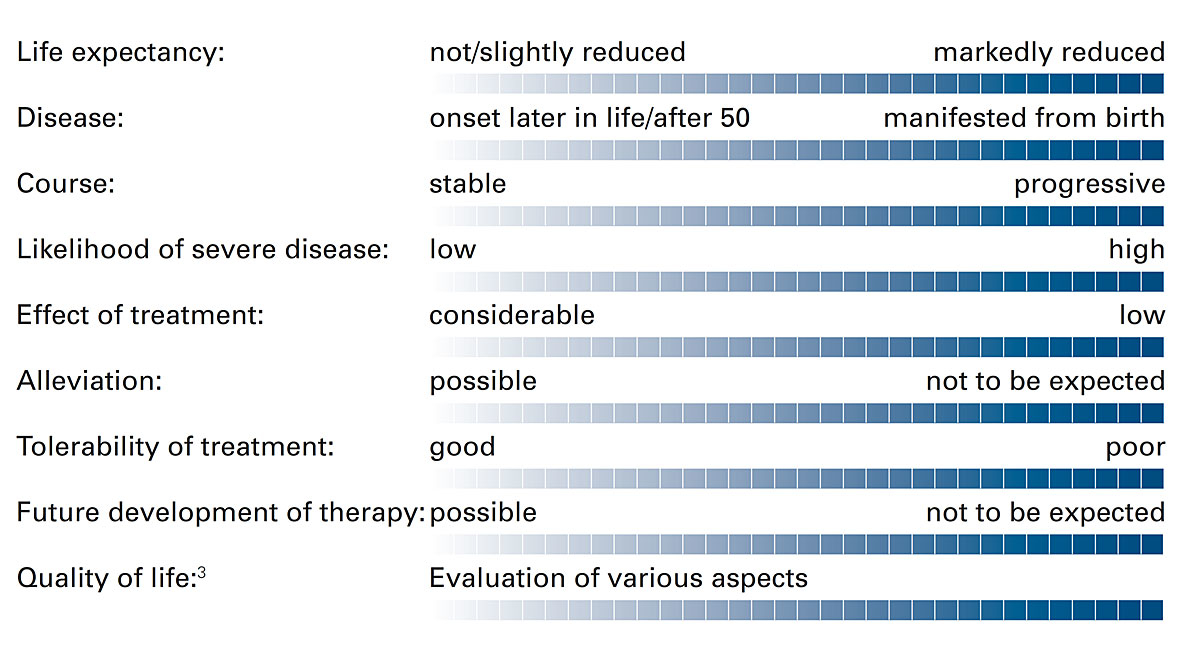
Consideration of the following dimensions may be useful for an evaluation of the disease in question (figure 1). The various points are not designed as a checklist for decision-making, but to provide support for discussions with the couple or for the deliberations of a PGT board. They may also be used for documentation of the decision. The possibility that nothing can be said about certain points always exists.

Figure 1 Dimensions for an evaluation of the disease in question.
If physicians lack adequate expertise with regard to the genetic disease, they must consult geneticists and specialists in the relevant areas. Depending on the particular circumstances, it may be helpful to obtain advice from a PGT board, bringing together representatives of various medical disciplines, as well as ethics and psychology (cf. Section 8).
It should be borne in mind that, within the board, the couple’s perspective is only represented indirectly by the attending physicians, but in many cases the couple will have “expert knowledge”, as they may already have an affected child or have grown up with an affected family member, or one partner may be affected by the genetic disease him/herself. The board plays an important role, particularly in complex cases, in assessing the indication in the individual situation; however, responsibility for deciding whether the couple can be offered PGT remains with the attending physician and cannot be delegated to a board.
As well as the eligibility criteria for PGT (hereditariness, serious disease, lack of treatment options, unacceptable situation for the couple), further points are to be considered. Topics to be discussed are the reliability of the genetic tests, the possibility of false findings, the procedure in the event of any surplus information (cf. Section 6), mosaic findings, or difficult-to-interpret results. The couple must be fully informed about the in vitro fertilisation (IVF) treatment procedure and the associated physical, psychological and financial burdens and risks. It should be discussed with the couple how they view the opportunities and burdens of IVF-PGT compared with a natural pregnancy in which – in the event of prenatal diagnostic findings – they would be confronted with the question of whether to proceed with or terminate the pregnancy.
Under the legislation, PGT-A may be used to screen embryos for impaired developmental capacity prior to implantation. Depending on the circumstances, and with an adequate number of embryos, PGT-A can permit more rapid achievement of pregnancy and a reduced miscarriage rate. By shortening the treatment time, PGT-A can alleviate physical burdens for the woman and psychological and financial burdens for the couple. Overall, however, IVF with PGT-A is not associated with a higher birth rate than IVF without PGT-A.
PGT-A is not indicated for every couple using IVF; rather, the indication depends, inter alia, on the medical history, the woman’s age and the number of oocytes or embryos. During counselling, it should be pointed out that, after the performance of PGT-A, it is possible that no viable embryos free of abnormalities will be available.
Given the rapid changes in technology and the state of scientific knowledge, physicians are advised to inform themselves regularly about the latest developments. In the definition of Good Clinical Practice, a key role is played by the professional societies and their working groups. In establishing the indication for PGT-A, the attending physician may also find it appropriate to seek the advice of a PGT board so as to obtain the views of additional specialists – not least also in cases where the ideas of the couple differ from the assessment of the professional responsible for treatment.
PGT-A can also be performed in addition to PGT-M/PGT-SR. Couples pursuing PGT-M/PGT-SR on account of a genetic disease should be made aware of the diagnostic option of detecting a chromosomal abnormality which impairs the developmental capacity of the embryo. It should be determined in advance whether or not the couple wish to avail themselves of this option. The couple are to be informed of their right not to know.
In PGT-M/PGT-SR, the selection criterion is the known familial genetic abnormality. PGT-A can exclude embryos with a reduced capacity for development. If, after genetic testing has been performed, more than one embryo not meeting any exclusion criteria is available, it must be decided which embryo is to be transferred to the uterus. This requires embryo ranking. As well as morphological criteria, the question arises whether surplus information (see below) may or should be taken into account for this decision.
Ranking poses an ethical challenge and should be discussed with the couple prior to PGT, so that the procedure can be individually determined. After ranking, it needs to be decided which embryos should continue to be stored and which, in view of the test results, should be directly destroyed. Decisions on destruction and storage are made by the couple.
Particularly careful assessment is required in cases where none of the embryos produced are free of abnormalities and the couple wish to have an embryo with an impairment transferred.
In order to avoid divergent evaluations and conflict situations as far as possible, the various possible eventualities should be discussed before the start of treatment, and a procedure should be defined which is acceptable to the physician and the couple.
When PGT is performed, surplus information may be generated, i.e. results of a genetic test which are not required for the purpose thereof. The generation of surplus information is to be avoided as far as possible.4 In general, surplus information can be divided into the following categories:
For the management of surplus information, the SAMS recommends the following procedure: before PGT is performed, the couple are to be informed that surplus information may be generated. Such information can influence decisions as to which embryos are excluded, transferred or stored. It should be discussed that there is also a right not to know, and that additional information may increase the complexity of decision-making and does not automatically lead to greater decision-making freedom. This applies, for example, to information whose relevance for health cannot be unequivocally interpreted, or to statements of probability. Surplus information can give rise to uncertainties or ethical conflicts and affect the child’s right to an “open future”.
The decision to pass on surplus information from laboratory tests to the attending physician or the couple, thus allowing it to be taken into account in selection decisions, should be guided by the health relevance of the information. This leads to the following recommendations:
It is recommended that written agreements be concluded with the couple on the one hand, and laboratories on the other, to the effect that surplus information in categories 1 (sex), 2 (carrier status) and 3b (genetic characteristics neither indicating a serious hereditary disease nor impairing the developmental capacity of the embryo) will not be disclosed to the attending physician or the couple.
PGT is necessarily linked to an IVF treatment. This should be carried out in such a way as to minimise the likelihood of multiple pregnancies, as these increase the risk of maternal and fetal/neonatal complications, which are associated with possible long-term sequelae for the children. The fact that, under the revised RMA, up to twelve embryos may be produced and cryopreserved means that this objective can now be realised with elective single embryo transfer (eSET). The eSET procedure leads to a marked decrease in multiple pregnancies, without reducing the chances of a birth. Given this evidence base, and from the perspective of medical responsibility, the exclusive use of eSET is strongly recommended.
While laboratory diagnostic procedures must always be subject to quality control, quality assurance is of crucial importance in an area as sensitive as embryo testing. The SAMS suggests that it should be explored whether discarded embryos could be used, with the couple’s consent, for purposes of quality control in PGT.
As PGT requires highly specialised knowledge, the SAMS recommends the expansion of basic and specialist training programmes for medical professionals and the promotion of sharing of experience at the national level among the various disciplines concerned. In addition, the findings expected from the evaluation process conducted by the FOPH (in accordance with Art. 14a RMA) should be taken into account in the future design of PGT practice.
In the area of PGT, efforts should be made to harmonise Good Clinical Practice at all centres in Switzerland. With growing experience and increasing PGT case numbers, it will become apparent what constellations pose particular challenges for attending physicians seeking to establish an indication. These could be collected by a national expert committee. In addition, a body of this kind could observe to what extent differing approaches or forms of unequal treatment emerge, indicating problematic trends at the societal level. Such a committee could also provide advisory services for the cantons, which act as supervisory authorities vis-à-vis centres performing PGT. Also essential – to ensure that PGT continues to be used in an ethically acceptable manner in the future – are public ethical debates on the development of PGT practice and its effects on couples wishing to have children, on children and on people with disabilities.
eSET = elective single embryo transfer
HGTA = Federal Act on Human Genetic Testing
IVF = in vitro fertilisation
PGT = preimplantation genetic testing, i.e. testing for genetic and/or genomic abnormalities
PGT-A = screening for aneuploidies (abnormalities of chromosome number)
PGT-M = testing for monogenic (single gene) disorders
PGT-SR = testing for structural rearrangements (familial chromosomal abnormalities)
RMA = Reproductive Medicine Act
With the entry into force of the revised Reproductive Medicine Act in September 2017, preimplantation genetic testing became permissible in Switzerland under certain conditions. The revision of the legislation had been advocated and supported by the Central Ethics Committee (CEC) of the SAMS. As it was apparent that the implementation of the Act would raise practical and ethical questions, the CEC decided in June 2017 to prepare guidance and, for this purpose, it appointed a subcommittee which operated between January 2018 and August 2019.
A first draft of the recommendations was presented to experts for discussion at a national workshop held on 29 October 2019. This event was attended by almost 70 physicians from fertility centres and the field of medical genetics, as well as experts from human genetic laboratories. The workshop was co-organised by the Swiss Society of Reproductive Medicine (SGRM), the Swiss Society of Obstetrics and Gynecology (SGGG) and the Swiss Society of Medical Genetics (SGMG).
The final version of these recommendations was approved by the Central Ethics Commission (CEC) of the SAMS on 28 February 2020 and by the Executive Board of the SAMS on 21 April 2020.
1 For a detailed discussion of these new provisions cf. Andrea Büchler/Bernhard Rütsche, Kommentar zu Art. 5a FMedG, in: Büchler/Rütsche (eds), Fortpflanzungsmedizingesetz, Stämpfli, Bern 2020.
2 The alternatives also include gamete analysis (i.e. polar body biopsy). Under Art. 5a para. 1 RMA, this is permissible in order to detect chromosomal abnormalities affecting the developmental capacity of the embryo to be produced, or to avoid the risk of transmitting a serious disease. Whether these criteria are met is decided by the attending physician in the counselling discussions with the couple.
3 This refers to elements of human functioning according to the WHO framework; cf. www.who.int/classifications/icf/en/
4 The revised RMA which came into effect on 1 September 2017 makes no mention of surplus information. This topic is, however, included in the revised Human Genetic Testing Act (HGTA) – cf. Art. 3 let. n of the HGTA of 15 June 2018 and the newly formulated Art. 6b of the RMA, which is expected to come into force with the revised HGTA in 2021.
5 This recommendation is in line with the restricted right to knowledge in connection with prenatal diagnosis (sex determination via a blood test before the twelfth week of pregnancy is not permissible).