Insulin under the influence of light
DOI: https://doi.org/10.4414/smw.2020.20273
Gloria
Ursinoab, Roberto
Coppariab
aDepartment of Cell Physiology and Metabolism,
bDiabetes Centre of the Faculty of Medicine,
Insulin under the influence of light
Summary
The discovery and administration of exogenous insulin has revolutionised diabetes treatment and continues, almost 100 years on, to be the basis for the management of insulin deficiency. However, insulin therapy still has potentially life-threatening side effects such as hypoglycaemia and increased risk of cardiovascular disease. So far, improvements in insulin therapy have focused mainly on modulating its pharmacokinetic and pharmacodynamic properties and improving delivery methods, while variations in the insulin sensitivity of peripheral tissues has received relatively little attention. Notably, tissue insulin sensitivity has been shown to vary considerably around the clock, which could contribute greatly to the effect (and risk of side effects) of a given dose of insulin. Recent evidence suggests that photic inputs regulate diurnal variations in the insulin sensitivity of metabolically relevant tissues via a previously unrecognised mechanism involving the ventromedial hypothalamic nucleus. Therefore, understanding the mechanisms underlying photic control of insulin action is of paramount medical importance. In addition, considering “when” (i.e., the time of day) could assist in deciding “how much” insulin should be administered and hence could aid the fine-tuning of insulin dosage, lowering the risk of side effects, and improving the quality of life of patients with insulin deficiencs.
Physiological actions of insulin
Insulin is a hormone produced by the β-cells of the pancreatic islets [1]. Its secretion is induced when blood glucose levels increase, and is reduced when circulating glucose content decreases. This finely tuned insulin secretion is achieved through a mechanism that starts with glucose entry into the pancreatic β-cells via the low-affinity glucose transporter GLUT2 [2]. Glucose then undergoes glycolysis to produce ATP, which drives a change in membrane potential, triggering calcium influx and insulin release. This mechanism, which couples changes in extracellular glucose content with cellular activity, is also observed in neurons [3]. Furthermore, β-cells are arranged within the islet so that they are in contact with other β-cells [4–6]. Membrane areas of β-cell to β-cell contact also contain gap junctions that allow communication between β-cells, thus enabling the coordination of electrical activity across the islet and the tight regulation of insulin output [4–6]. While the majority of studies have looked at insulin secretion in response to a systemic glucose load, it is important to note that insulin secretion also occurs during fasting [7].
Once secreted into the bloodstream, insulin binds to and activates the insulin receptor (a tyrosine kinase receptor), which is expressed in myocytes, hepatocytes, adipocytes, neurons, and virtually all other cell types [8–13]. Insulin-induced activation of the insulin receptor triggers its auto-phosphorylation and the activation of several phosphorylation signalling cascades, including the phosphoinositide 3-kinase (PI3K) and the mitogen-activated protein kinase (MAPK) pathways that underlie most of the metabolic and anabolic actions of the hormone [14]. The insulin-induced activation of its receptor causes a plethora of downstream effects leading to coordinated glucose and lipid metabolism. This is achieved through insulin-induced changes in the expression and phosphorylation status of key regulatory enzymes, and also through changes in the activity of these enzymes caused by indirectly regulated changes in the amounts of their allosteric modulators (e.g., citrate, ATP, fructose 2,6-bisphosphate) [15, 16]. In hypothalamic neurons, insulin-induced activation of its receptor also leads to changes in membrane potential and firing rates [17, 18].
Insulin plays an important role in carbohydrate metabolism. In the healthy state, insulin-induced activation of its receptor on hepatocytes leads to (i) suppression of the expression and/or activity of gluconeogenic enzymes (e.g., phosphoenolpyruvate carboxykinase and glucose-6-phosphatase), (ii) stimulation of glycolytic enzymes (e.g., glucokinase, phosphofructokinase 1 and pyruvate kinase), and (iii) enhancement of glycogen synthesis (e.g., inhibition of glycogen synthase kinase 3). Hence, in hepatocytes insulin reduces gluconeogenesis and stimulates glucose stocking in the form of glycogen (fig. 1) [12, 14, 20–25]. In myocytes and adipocytes, insulin-induced activation of its receptor causes the specific glucose transporter GLUT4 (a high-affinity glucose transporter) to translocate at the plasma membrane, hence enabling these cells to take up more glucose from the bloodstream. In myocytes, insulin action stimulates the uptake of glucose for glycogen synthesis, while in adipocytes insulin-induced glucose uptake and enhanced glycolysis increase the production of dihydroxyacetone phosphate, a key substrate for the synthesis of triglycerides (TG) (fig. 1) [10, 13, 19, 26, 27]. Overall, the concerted action of insulin on its cognate receptor in adipocytes, myocytes and hepatocytes reduces endogenous glucose production, enhances glucose uptake/storage and lowers glucose concentration in the bloodstream [28]. It is important to note that insulin-independent mechanisms contributing to glucose uptake and homeostasis also exist [29]. For example, glucose entry into myocytes and adipocytes is not limited to the insulin-dependent GLUT4, but can also be mediated by insulin-independent glucose transporters such as GLUT1 [29, 30].
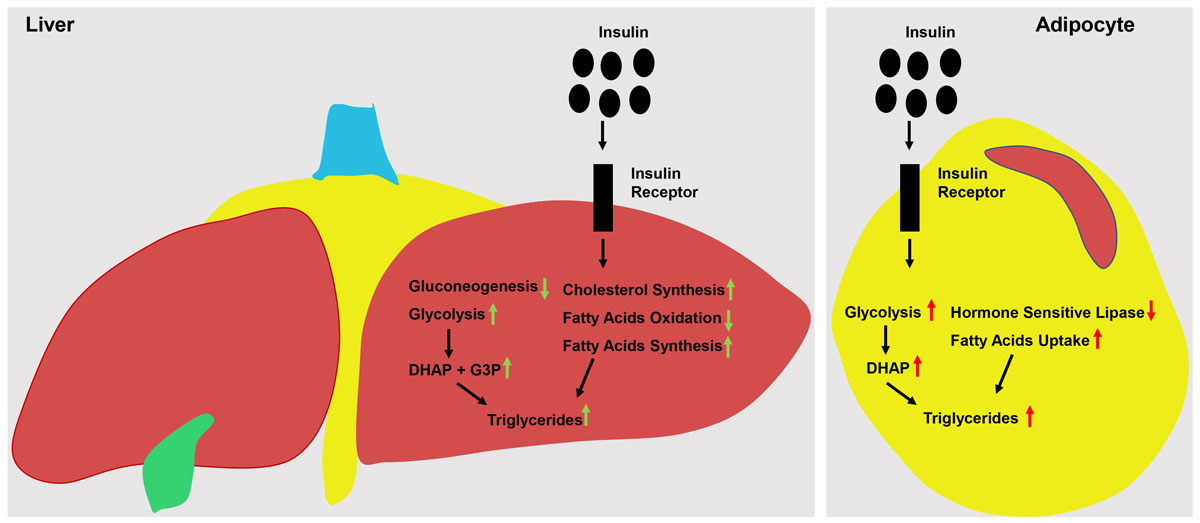
Figure 1
Insulin action in liver and fat tissue. The insulin receptor is a tyrosine kinase that undergoes autophosphorylation and catalyses the phosphorylation of cellular proteins such as Shc and Cbl, members of the IRS family. Upon tyrosine phosphorylation, these proteins interact with signalling molecules through their SH2 domains, resulting in a diverse series of signalling pathways, including activation of PI(3)K and downstream PtdIns(3,4,5)P3-dependent protein kinases, Ras and the MAP kinase cascade, and Cbl/CAP and the activation of TC10. These pathways act in a concerted fashion to coordinate the regulation of vesicle trafficking, protein synthesis, enzyme activation and inactivation, and gene expression, resulting in the regulation of glucose, lipid and protein metabolism [19]. The illustration on the left side of the figure shows the insulin-induced activation of the insulin receptor in hepatocytes. Here, it stimulates glycolysis and therefore the formation of glycolytic intermediates such as dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3 phosphate (G3P), which can be used for triglyceride synthesis. It also suppresses gluconeogenesis and at the same time stimulates cholesterol synthesis and the formation of fatty acids, which can also be used for the synthesis of triglycerides. The illustration on the right side of the figure shows the insulin-induced activation of the insulin receptor in adipocytes. Here, it stimulates glycolysis and the formation of glycolytic intermediates such as DHAP and G3P. However, adipocytes lack glycerol kinase and therefore use DHAP (as they cannot use G3P) as a precursor for triglyceride synthesis. Furthermore, it stimulates fatty acid uptake, hence stimulating the synthesis of triglycerides. Drawings by Vincenzo Antonio Vianna Coppari.
Insulin also effects lipid metabolism. In the healthy state, insulin-induced activation of its receptor on hepatocytes boosts lipogenesis via increased expression of the transcription factor sterol regulatory element-binding transcription factor-1c (SREBP-1c) [31]. Indeed, SREBP-1c promotes the expression of genes involved in fatty acid synthesis and TG production [32]. Insulin action in hepatocytes also reduces the oxidation of fatty acids, hence increasing their availability for TG synthesis (fig. 1) [14]. In adipocytes, insulin reduces the action of hormone-sensitive lipase, an intracellular enzyme that is also activated by, for example, norepinephrine, and that induces the breakdown of TG into fatty acids and glycerol [33]. Also, in fat depots insulin is known to activate lipoprotein lipase, which facilitates lipid and fatty acids uptake (from circulating chylomicrons and lipoproteins) into adipocytes [34]. Thus, via (i) inhibition of hormone-sensitive lipase, (ii) activation of lipoprotein lipase, and (iii) enhanced glycolysis and hence production of dihydroxyacetone phosphate, insulin strongly stimulates the accumulation of TG in adipocytes (fig. 1) [10, 13, 19, 26, 27].
Altered actions of insulin in diabetes mellitus
Diabetes mellitus is a group of metabolic disorders that occur when insulin is either significantly reduced (or lacking) or unable to exert its biological functions, resulting in chronic hyperglycaemia and other serious metabolic defects [35–37]. It is currently one of the most prevalent diseases worldwide and is responsible for an estimated 1.5–2 million deaths per year [38]. There are multiple diabetes subtypes, but these can be broadly classified into two aetiopathogenic categories: type 1 and type 2 diabetes mellitus (T1DM and T2DM, respectively) [39]. T1DM is thought to be caused by the immune-mediated destruction of insulin-producing β-cells, resulting in a rapid and almost complete loss of insulin and the acute onset of symptoms [40]. Conversely, T2DM occurs more gradually, as chronic metabolic pressure associated with obesity [41, 42] and ageing [43, 44] begins to prevent peripheral tissues from responding effectively to insulin. For example, in T2DM the effect of insulin on the hepatic carbohydrate metabolism is reduced, while its effect on lipid metabolism seems to remain intact, a defect termed “selective insulin resistance” [45, 46]. This defect causes uncontrolled and increased hepatic glucose output [47, 48]. It is therefore important to carefully establish what we refer to when we mention insulin resistance. General wisdom is that T2DM is underpinned by insulin resistance. However, as introduced above, the evidence indicates that in T2DM there is not generalised insulin resistance. Indeed, if hepatocytes were to become resistant to insulin, then in the context of T2DM hepatic lipid synthesis should be reduced. However, in T2DM insulin can still promote lipogenesis, and lipid synthesis is increased in line with increased insulin contents [46, 48–51]. Hence, while the ability of insulin to regulate carbohydrate metabolism is impaired in T2DM, its ability to control lipid metabolism is not weakened. This “selective insulin resistance” in hepatocytes contributes to the increased hepatic glucose and lipid output and hence to the hyperglycaemia and hypertriglyceridaemia that (with hyperinsulinaemia) characterise T2DM [46]. Moreover, if insulin’s action on lipid metabolism in adipocytes were to be reduced, then T2DM should be associated with reduced adipose mass [10]. Contrariwise, T2DM is very often associated with increased body fat mass. This apparent conundrum can also be explained by a selective insulin resistance in adipose tissue. In this context, while the ability of insulin to induce GLUT4 translocation and glucose uptake is impaired, its ability to inhibit hormone-sensitive lipase [52] and to stimulate TG accumulation in adipocytes is not (fig. 1).
In addition, as T2DM progresses, this peculiar defect in insulin action is followed by a reduction in insulin secretion and the loss and/or dedifferentiation of β-cells [53]. Eventually, all T1DM and late-stage T2DM patients (estimated to be 100 million people worldwide) develop an absolute or relative insulin deficiency [54]. This defect leads to an inability to regulate blood glucose and lipid levels, with life-threatening short- and long-term metabolic consequences [55]. Without the coordinated actions of insulin in metabolically relevant tissues, glucose and lipid control is severely compromised, leading to hyperglycaemia and dyslipidaemia. For example, following a reduction in the level of circulating insulin and thus of its action in adipocytes and hepatocytes, the body begins to break down TG in adipose tissue, overwhelming the blood with secretions of free fatty acids and glycerol. These free fatty acids and glycerol are then used as substrates for ketogenesis and gluconeogenesis respectively in hepatocytes [56]. Indeed, in addition to the well-known hyperglycaemia, another outcome of untreated/uncontrolled insulin deficiency is hyperketonaemia, which can cause a drop in blood pH, known as ketoacidosis, that often requires hospitalisation and can be fatal [57]. Eventually, chronic hyperglycaemia can also lead to the damage, dysfunction and eventual failure of the retina, kidney, nervous system, heart and blood vessels. This causes several acute and long-term complications such as diabetic retinopathy, cardiovascular disease, foot ulcers, peripheral nerve damage and a host of cutaneous complications [58].
Discovery and development of insulin
Before the discovery of insulin, attempts to normalise blood glucose in patients with insulin deficiency were limited to fasting and calorie restriction [59]. This approach did little to improve glycosuria and ketoacidosis, and patient prognosis remained very poor, with most affected individuals not living beyond a year [60]. Although it was long hypothesised that the pancreas secretes a substance that controls carbohydrate metabolism, attempts to use pancreatic extracts to lower blood glucose were unsuccessful due to impurities and toxicities [61, 62]. This was until Banting et al. successfully isolated islets from canine pancreas to produce a more purified insulin extract that was able to reduce blood glucose, glycosuria and ketonuria, enabling patients with insulin deficiency to live a pseudonormal life for the first time [63, 64]. This remarkable discovery prompted the isolation and purification of insulin from animal pancreas after the development of an isoelectric precipitation method that led to a purer and more potent animal insulin and decreased the variation between lots [63]. By 1923 and 1924, pharmaceutical laboratories in Germany, Denmark, Austria, and later Hungary, had already begun purifying insulin on a commercial scale [65]. This was followed by attempts by investigators to prolong the duration of insulin action, thus reducing the need for multiple daily injections. This began with the addition of protamine and zinc to insulin preparations to sequester insulin in its hexameric conformation and delay its solubility in the bloodstream and subsequent receptor binding [66]. These efforts led to the commercialisation of both Neutral Protamine Hagedorn (NPH) insulin and the “lente” insulin series, consisting of semilente, lente and ultralente insulin, which had increasing lengths of duration depending on the proportion of zinc added [67].
The ability to administer exogenous insulin has greatly extended the lifespan of patients with insulin deficiency. However, once patients with diabetes started to live longer, the complications of prolonged insulin therapy became prevalent [60]. Specifically, episodes of life-threatening hypoglycaemia brought on by excessive amounts of circulating exogenous insulin and/or diurnal changes in insulin sensitivity emerged as a major limiting factor for successful glucose control in patients with insulin deficiency [68]. Indeed, studies such as the 1993 Diabetes Control and Complications Trial showed a direct relationship between the degree of glycaemic control and the incidence of long-term complications of insulin therapy [69]. These complications shifted the focus to developing insulin analogues that matched the endogenous pattern of insulin secretion as closely as possible, leading to the creation of many classes of modified insulins, each with different pharmacokinetic/pharmacodynamic profiles and durations of action. For instance, long-acting “basal” insulins with very long durations of action (on average 24 hours), such as insulin glargine, insulin detemir and insulin degludec, started to reach the market [7]. Insulin glargine (approved in the USA in 2000) has glycine instead of asparagine at position A21, an extra two arginine molecules at position B30 and a pH of 4.0 [70, 71]. These changes cause it to form micro precipitates at the site of injection, resulting in a prolonged absorption with little peak activity [72]. Similarly, insulin detemir has a 14-carbon fatty acid chain attached to the lysine at position B29 [73]. The fatty acid side chain increases the self-association of the insulin into hexamers and di-hexamers and allows reversible binding to albumin, all of which contribute to its prolonged action [7, 74]. Later, insulin degludec, an even longer-acting insulin (24-48 hours) was developed by Novo Nordisk and approved by the US Food and Drug Administration in 2015 [75]. It is used as a “once daily basal insulin” and has a deletion of the threonine at B30 and a glutamic acid spacer that links a 16-carbon fatty di-acid chain to the B29 amino acid residue [76, 77]. These changes allow it to form a slowly soluble multihexameric chain after subcutaneous injection [77].
In addition to basal insulins, fast-acting insulin analogues were also developed to improve post-prandial glycaemic control in patients with insulin deficiency. These are characterised by their ability to dissolve rapidly in the blood stream and reach target tissues promptly [78]. Fast-acting insulins such as lispro, aspart and glulisine contain amino acid modifications that instead cause charge repulsion at the sites where insulin monomers would normally associate to form dimers [79]. This weakens the propensity for the insulin to self-associate, favouring rapid absorption from the subcutaneous tissue [80]. Insulin lispro is formed by switching the lysine and proline amino acids at positions B28 and B29, and insulin aspart by substituting proline with a negatively charged aspartic acid in position B28 [74, 80]. Insulin glulisine differs from human insulin in that it has aspargine substituted for lysine at position B3 and lysine substituted for glutamic acid at position B29, but unlike the other two rapid-acting analogues, it does not contain zinc [81].
The development of new insulin delivery methods such as insulin pens and pumps has also enabled patients to more conveniently and accurately fine-tune their glucose levels [82]. Insulin pens are safer, easier to use and more accurate compared to traditional syringe injections [83]. Newer smart pens can also help guide patient dosage, remember the amounts and timings of insulin doses, and transmit this information to mobile logbooks using Bluetooth technology [84, 85].
Continuous subcutaneous insulin infusion (CSII) (also known as the insulin pump) enables a continuous and more physiologic delivery of insulin. The first pumps were developed in the 1980s and replaced the need for multiple daily injections [86]. Since then, sensor augmented insulin pumps have been developed which combine the technology of an insulin pump with a continuous glucose monitoring (CGM) sensor that transmits glucose readings to the person wearing the device. This allows the user to have real-time glucose readings that enhance their ability to monitor blood glucose levels and reduce the risk of hypoglycaemic episodes [87]. Recent advances in CSII devices, CGM and insulin delivery algorithms have resulted in the development of the artificial pancreas or closed-loop system of insulin administration. This combines a real-time glucose sensing component, an insulin delivery device (pump) and a computer that calculates the amount of insulin needed in response to the blood glucose concentration [88]. Closed-loop systems were found to be safe, as well as effective at improving both glycaemic control and the proportion of time spent within the target glucose concentration range [89]. However, despite these advancements in CSII technology, they remain costly and invasive, and no significant improvements in mean glucose concentration or in the frequency of hypoglycaemic events were found compared with multi-dose insulin regimens [90].
More recently, combinatorial approaches, whereby insulin is administered alongside other adjuvants that improve insulin sensitivity, lower blood sugar or fine-tune insulin secretion, such as metformin, sodium-glucose transport protein (SGLT) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists, have been considered (table 1) [91]. Metformin and GLP-1 receptor agonists are currently approved in the USA and Europe. SGLT2 inhibitors are approved in the USA for treatment of T2DM but not T1DM, but a dual SGLT1 and SGLT2 inhibitor has recently been approved in Europe for treatment of T1DM (with restrictions) [92]. Although not devoid of side effects, the use of these pharmacotherapies alongside insulin can contribute to improving glycaemic control and reducing insulin requirements in patients with insulin deficiency [93, 94]. In most studies, the use of these adjuvants has improved glycaemic control more than using insulin alone. Although not effective in correcting the harmful circulating lipid and lipoprotein profiles commonly seen in patients with insulin deficiency (such as increased triglycerides, HDL and LDL cholesterol and ketones), recent large-scale clinical trials have revealed that these medications have the potential to prevent very serious diabetic complications such as adverse cardiovascular outcomes (e.g. heart failure) and nephropathy [95, 96].
Table 1 Pharmacological adjuvants for glycaemic control.
|
Class of antidiabetic
|
Representative agents
|
Mechanism of action
|
Risk of hypoglycaemia
|
Metabolic alterations
|
Cardiovascular benefit and risk
|
Other adverse effects
|
| Biguanide |
Metformin |
Insulin sensitizer
Numerous effects on inhibition of hepatic glucose production |
None |
Lactic acidosis (very rare)
May cause nausea/vomiting or diarrhoea after introduction, which may result in electrolyte or pH alterations |
Reduce MI by 39% and coronary deaths by 50% |
Vitamin B12 deficiency, which may cause anaemia and neuropathy (risk in elderly) |
| Sodium-glucose cotransporter (SGLT1/2) inhibitor |
Canagliflozin
Dapagliflozin
Empagliflozin |
Glucosuria due to blocking (90%) of glucose reabsorption in renal proximal tubules
Insulin-independent mechanism of action |
Low |
N/A |
Positive CV effect due to reduction of sodium and uric acid absorption and reduction of BP |
Ketoacidosis (rare)
Genital mycosis
May increase LDLc
Bone fractures |
| GLP-1 agonists |
Liraglutide
Exenatide
Dulaglutide |
Activate GLP1 receptor
Increased insulin secretion
Decreased glucagon
Delayed gastric emptying
Increased satiety |
No (risk if used in combination with sulphonylureas) |
N/A |
Reduce CV risk |
Nausea,
vomiting,
Pancreatitis
C cell tumour of thyroid (contraindicated in MEN type 2) |
Today, the global human insulin market is estimated at 100 million people, comprising all T1DM and 10–25% of T2DM sufferers [97]. Currently, Eli Lilly, Novo Nordisk and Sanofi control over 90% of the world’s insulin production [98]. In 2018, the insulin market was valued at almost 26 billion Swiss francs. Furthermore, it is growing rapidly and is expected to reach 48 billion Swiss francs by 2020 [99]. This is due to a number of factors, including an increase in the prevalence of diabetes, an increase in the size of the geriatric population, rising awareness of diabetes and rising prevalence of obesity (a huge risk factor for developing T2DM) [97].
Challenges of insulin therapy
It is clear that significant advances in insulin therapy have been made over the last century. Nevertheless, continual challenges associated with its long-term use remain. Patient- and clinician-related challenges still present numerous obstacles to optimal insulin deficiency management. There is a greater need for clinicians to remain informed about new therapies and emerging technologies [100, 101]. Patients’ understanding of the importance and rationale of insulin therapy should also be improved, so that they can monitor their blood glucose better and self-manage their diabetes more effectively [102]. Another problem is patient non-compliance due to psychological factors such as insulin distress (apprehension and dejection due to a perceived inability to cope with the requirements of insulin therapy [103]), which causes significant lack of motivation [104]. There remains a need for clinicians to use tools such as counselling and motivational interviewing to collaborate with and empower their patients and to improve non-compliance [103]. Indeed, a study of 116 T2DM patients who were initially non-adherent to therapy found that after one month of counselling, 90% of them began adhering to treatment [105].
Additionally, and of greater concern, insulin therapy is still unable to restore metabolic homeostasis in patients with insulin deficiency, and many still experience wide fluctuations in their blood glucose levels. In the USA alone, almost 40% of patients diagnosed with diabetes did not reach accepted glycaemic targets in 2008 [106]. Current studies in the Czech Republic and Slovakia also indicate that as few as 29.9% and 33.4% of T1DM and T2DM patients respectively achieve their recommended blood sugar targets with insulin therapy [107]. Although the relative contributions of patient non-adherence to insulin therapy to these results cannot be determined, statistics such as these suggest that there is an urgent need to improve insulin deficiency treatments, as this inability to control blood glucose is putting these patients at risk of hyperglycaemia/hypoglycaemia and several other comorbidities [108].
Hypoglycaemia in particular is one of the most common serious adverse effects of insulin therapy and remains a major limiting factor in diabetes treatment. Hypoglycaemia can trigger symptoms such as sweating, confusion and palpitations, and may require hospitalisation. Between the late 90s and 2013, hospital admission rates for hypoglycaemia rose by 11.7% and 8.6% in the USA and England, respectively [109, 110]. In the last two years, hypoglycaemia has accounted for as many as 4,804 emergency hospital visits in the USA alone [111]. Severe hypoglycaemia (glucose ≤50mg/dl) can also result in seizures and ventricular tachycardia, and is often fatal [112]. Specifically, hypoglycaemia has been shown to be responsible for approximately 5% of deaths in childhood onset T1DM patients [113]. Patients with insulin deficiency also experience frequent episodes of hypoglycaemia outside of hospital settings. Studies by Karter et al. revealed that although hypoglycaemia related hospitalisations amount to on average only 0.8% of total hospital visits, 95% of severe hypoglycaemic events reported do not result in hospitalisation, indicating that current surveillance grossly underestimates the overall burden of severe hypoglycaemia [107]. In fact, it is estimated that T1DM patients may spend as much as 10% of their time in a state of hypoglycaemia, although this is usually asymptomatic [114]. This pathological outcome is underpinned by hypoglycaemia-associated autonomic failure, which contributes to hypoglycaemia unawareness (e.g. impaired hypoglycaemia-mediated stimulation of food-seeking behaviour) and reduced hormonal-mediated glucose counterregulation (e.g. dampened hypoglycaemia-induced epinephrine and glucagon secretion), hence leading to impaired homeostatic defences against hypoglycaemia [115, 116].
Besides hypoglycaemia, patients with insulin deficiency are at greater risk of developing other serious complications such as kidney failure, weight gain, blindness, nerve damage and cardiovascular disease (CVD). It is not too farfetched to suggest that some of these conditions, such as weight gain and CVD, may be promoted by insulin therapy itself, as insulin is known to exert anabolic actions on adipocytes and have lipogenic effects on hepatocytes [10, 46, 48–51]. Due to its stimulatory actions on lipid and cholesterol synthesis, it is not surprising that insulin therapy promotes weight gain in almost all patients with insulin deficiency [117, 118]. Chronic insulin therapy could also increase lipid deposition in tissues other than adipose tissue, and this is a major contributing factor to CVD. The majority of large observational studies show strong dose-dependent associations between injected insulin and increased risk of CVD complications such as coronary artery disease (CAD) in T2DM patients [119]. CAD is also extremely elevated in T1DM patients [120, 121].
Over time, the lipogenic actions of insulin can also lead to the development of lipid-induced insulin resistance, whereby the ability of peripheral tissues to respond effectively to insulin decreases. This effect promotes a vicious cycle in which continuously increasing insulin requirements are necessary in long-term diabetes care [122]. The development of insulin resistance is also a major risk factor for the development of CVD due to its effects on endothelial cells [123]. Ten-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study demonstrated that insulin resistance-related factors (rather than glycaemia) were predictive of CAD in patients with insulin deficiency [124]. Insulin resistance decreases the production of nitric oxide from endothelial cells and increases the release of pro-coagulant factors, leading to platelet aggregation [125]. In addition, studies have shown that in an insulin-resistant state, the PI3K pathway (responsible for modulating glucose uptake) is defective, while the MAPK pathway remains intact [126]. MAPK signalling in endothelial cells promotes their growth and activation, which accelerates the atherosclerotic process [127]. Therefore, the imbalance in these two pathways can lead to increased CVD risk for patients with insulin deficiency.
Insulin sensitivity in insulin therapy
The fact that the responsiveness of peripheral tissues to insulin (and the consequent risk of hypoglycaemia) depends also on insulin sensitivity and that cardiovascular complications of insulin therapy are associated with insulin resistance underscores the need to understand more about the factors regulating the responsiveness of insulin target tissues to insulin (i.e. insulin sensitivity). Insulin sensitivity is known to be affected by diet, smoking, body weight (particularly abdominal adiposity) and exercise [128]. However, numerous other factors can also have profound effects on the responsiveness of tissues to insulin. The circadian rhythm, for example, is emerging as an important and previously overlooked factor affecting insulin resistance. Circadian rhythms are 24-hour rhythms entrained to the local environment by external cues, called zeitgebers (ZT) or “time-givers”, such as light, temperature or feeding inputs [129]. In mammals, they are regulated by the cyclical oscillations of transcription factors such as CLOCK and BMAL1, which regulate the transcription of other circadian genes that control the sleep/wake cycle and various physiological and biochemical processes [130–132].
Human studies from as early as the 1970s suggest the importance of the time of day in regulating how the body responds to a glucose overload or an insulin injection. Indeed, both Gibson et al. (1972) and Carroll et al. (1973) observed that the rates of change in blood glucose levels after an intravenous glucose or insulin administration vary between injections done in the morning and in the afternoon [133, 134]. Remarkably, the authors stated that “judged by normally accepted criteria for diagnosis of diabetes mellitus based on glucose disposal rates in the early part of the day, the normal subjects reacted in the evening as mild diabetics” [133]. This clearly indicates that whole-body responsiveness to insulin varies during the circadian period. More recent studies have also suggested circadian involvement in the insulin sensitivity of mouse skeletal muscle, as well as of human and murine adipose tissue [135–138]. However, because these studies did not directly test whether the tissues’ insulin sensitivities oscillated in a diurnal fashion in vivo, the mechanism underlying the phenomenon they observed remained elusive.
The first study to provide direct experimental evidence supporting the notion that insulin sensitivity varies around the clock was conducted by Aras et al. They provided data indicating that the insulin sensitivity of several metabolically relevant tissues (gastrocnemius and soleus skeletal muscle, liver and perigonadal white adipose tissue; pWAT) fluctuates substantially over a 24-hour period in vivo in mice (fig. 2) [139]. Moreover, by performing challenging gain- and loss-of-light-action experiments in mice with the NAD+-dependent deacetylase SIRT1 [140] either intact or lacking only in steroidogenic factor 1 (SF1) neurons of the ventromedial hypothalamic nucleus, they unveiled key elements of the neuronal mechanism underlying this important phenomenon. These key elements are (i) photic inputs, (ii) SIRT1 in SF1 neurons, and (iii) insulin action in gastrocnemius skeletal muscle [139]. Aras et al. revealed that SIRT1 in SF1 neurons conveys photic inputs to entrain the action of insulin at this peripheral site. This light-driven effect on insulin-induced glucose uptake in skeletal muscle via SIRT1 in ventromedial hypothalamic nucleus SF1 neurons represents a previously unrecognised neuronal mechanism which enables changes in light inputs to govern circadian insulin sensitivity in skeletal muscle [139] (fig. 3). Notably, this mechanism is of pathophysiological relevance as it is required for normal insulin and glucose homeostasis in vivo [139, 141]. This finding of Aras et al. agrees well with, and provides a potential neuronal and molecular explanation for, recent studies showing that altered core clock gene expression (in either a whole-body or in a tissue-specific manner) and exposure to photic inputs at the wrong time cause insulin resistance and negatively affect glucose metabolism [142–147].
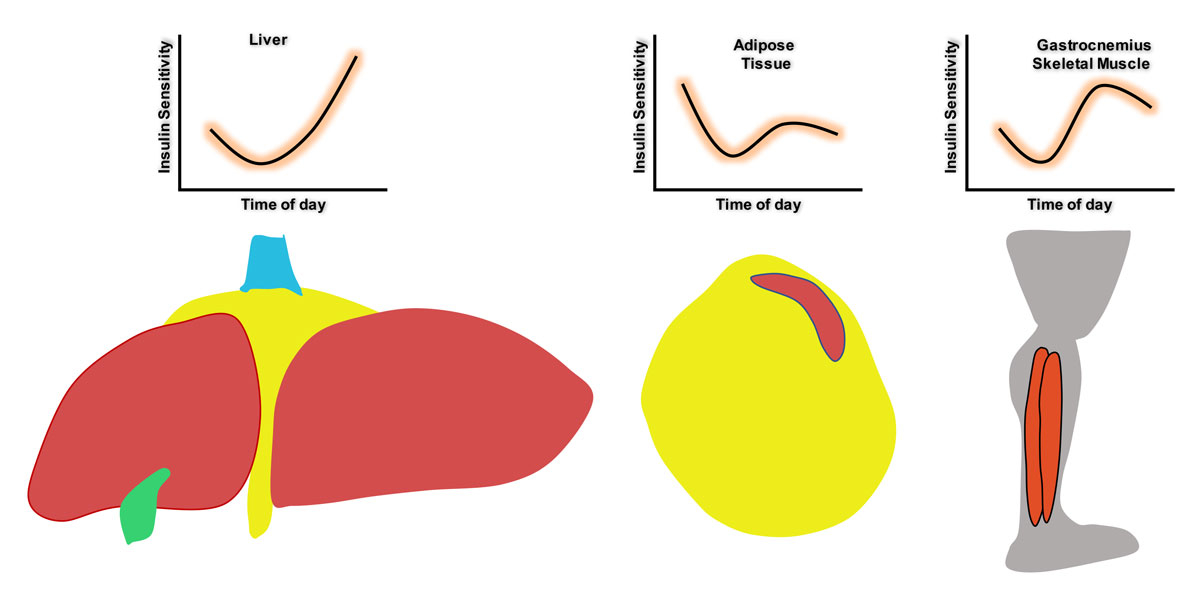
Figure 2
Tissue insulin sensitivity varies around the clock in vivo
. Illustrations representing diurnal fluctuations in the insulin sensitivity of liver, adipose tissue and gastrocnemius skeletal muscle, as indicated by Aras et al. [139]. Drawings by Vincenzo Antonio Vianna Coppari.
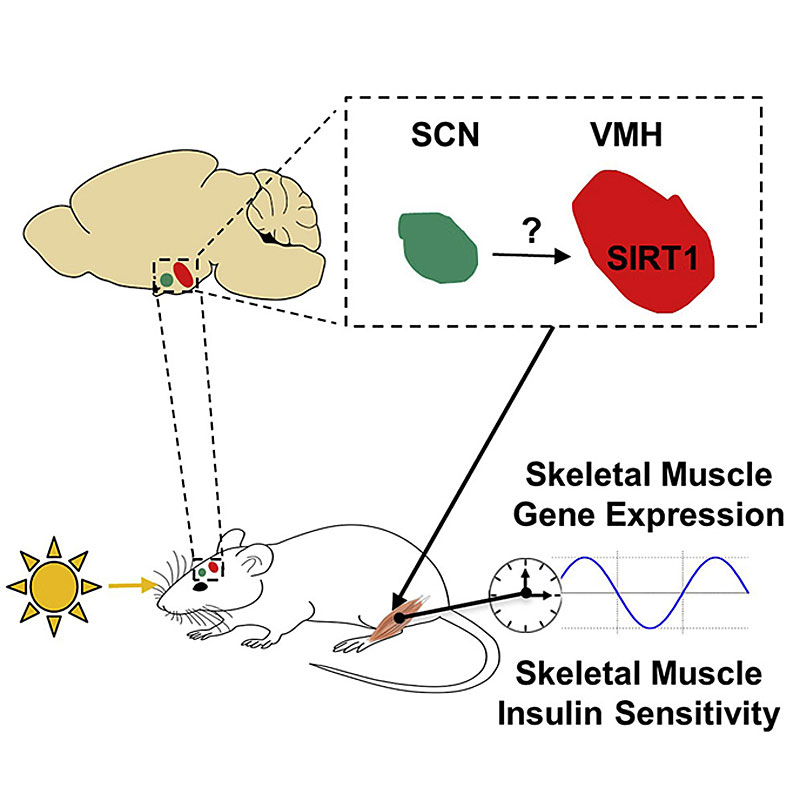
Figure 3
Schematic representation of the photic-induced and hypothalamic-mediated control of skeletal muscle insulin sensitivity. Illustration taken from Aras et al. [139].
The discovery that tissue insulin sensitivity varies around the clock brings to light a major, previously neglected factor to be considered when administering insulin. In practice, the amount of insulin administered is a strict function of blood glucose and carbohydrate intake, but the importance of the time of day at which insulin is given may be under-appreciated [148]. However, if insulin sensitivity varies around the clock, considering the time of day would be a simple, non-pharmacological method of fine-tuning insulin dosage and reducing the deleterious side effects of insulin therapy. Furthermore, the finding that photic inputs can regulate the insulin sensitivity of myocytes in mice raises the possibility that light could also entrain insulin sensitivity in other cells such as hepatocytes and adipocytes. Indeed, murine liver and pWAT display daily fluctuations in insulin sensitivity in vivo [139, 141], with liver in particular showing great variation, up to 15-fold changes in sensitivity at different times of day (fig. 2) [139]. There is also strong evidence that liver insulin sensitivity is directly influenced by the circadian rhythm, as lesions of the superchiasmatic nucleus (SCN) reduce insulin’s ability to inhibit endogenous glucose production and therefore cause severe hepatic insulin resistance in mice [149]. Other studies by Zhou et al. have shown that SIRT1 in hepatocytes regulates their responsiveness to insulin and that SIRT1 transcription in hepatocytes is regulated by CLOCK/BMAL1 [150]. This indicates that SIRT1, and possibly other proteins, can modulate hepatic insulin sensitivity in a diurnal fashion. Notably, while SIRT1 in SF1 neurons does not seem to be directly involved in the link between changes in photic inputs and changes in hepatic responsiveness to insulin [139], it is possible that SIRT1 in other hypothalamic neurons [9, 151] controls insulin action in hepatocytes via direct and/or indirect mechanisms.
Another important mediator of hepatic insulin sensitivity is protein tyrosine phosphatase receptor gamma (PTPRγ) [152]. PTPRs regulate a variety of cellular processes, including cell growth, cell differentiation, the mitotic cycle and oncogenic transformation [153]. Hepatic PTPRγ expression, however, is induced by inflammatory signalling and is increased in the context of obesity. Experiments by Brenachot et al. also identified PTPRγ as a powerful negative regulator of hepatic insulin sensitivity [152]. Loss of PTPRγ increases insulin sensitivity in liver (as well as in muscle) tissue and protects mice from developing insulin resistance when placed on a high calorie diet [152]. Hepatic-specific overexpression of PTPRγ at levels similar to those associated with obesity is sufficient to cause systemic insulin resistance [152]. Hepatic PTPRγ expression also fluctuates around the clock, and importantly, there is an inverse relationship between its expression and hepatic insulin sensitivity (G.U. and R.C. unpublished data). This raises the possibility that PTPRγ could be a potential molecular link between changes in photic inputs and changes in hepatic insulin sensitivity. Experiments involving gain- and loss-of-light-action to assess the effects of changes in photic inputs on hepatic PTPRγ expression, and to monitor circadian changes in hepatic insulin sensitivity in genetically engineered mice overexpressing or lacking hepatic PTPRγ, will be important in determining the relevance of this receptor. Similarly, discovering the factors regulating diurnal changes in insulin sensitivity in adipose tissues would be useful. For instance, ChIP sequencing has shown cross-talk between hypoxia-inducible factor 1α (HIF1α) and circadian core clock proteins such as BMAL1, and up-regulation of HIF1α increases inflammation, induces fibrosis and reduces insulin sensitivity in white adipose tissue [154, 155]. Future experiments aimed at assessing the relevance of HIF1α to the photic-mediated control of insulin action in adipose tissue [139] are warranted.
Outlook and conclusions
Many advancements have been made in insulin therapy since its discovery, yet we still have to acknowledge that it is unsatisfactory, and research aimed at improving insulin deficiency treatment is urgently needed. The prevalence of side effects and complications associated with the long-term use of insulin therapy continues to place a burden on the health system and greatly compromises the quality of life of patients with insulin deficiency. The discovery that insulin sensitivity fluctuates around the clock suggests that considering the time of day of administration could be a simple method to fine-tune insulin dosage and reduce the incidence of undesirable side effects. Also, given the major effect that photic inputs have on insulin action in metabolically relevant tissues [139], understanding the mechanisms underlying this phenomenon may be of medical importance and could help provide patients with insulin deficiency with better-tailored insulin therapy. We believe that the time of day and exposure to photic inputs should be considered when determining the amount of insulin administered.
Acknowledgments
We thank the funding agencies that supported and are supporting research activities performed in the Coppari laboratory. These include the European Commission, Geneva Cancer League, La Fondation pour la Recherche sur le Diabète, Swiss Cancer League, Swiss National Science Foundation, Foundation Gertrude von Meissner, Fondation Pour Recherches Medicales of the University of Geneva, the American Heart Association, National Institutes of Health (National Institute of Diabetes and Digestive and Kidney diseases) of the United States of America, the Juvenile Diabetes Research Foundation, and European Research Council. We thank Dr Giorgio Ramadori at the University of Geneva for his critical reading of this manuscript and Vincenzo Antonio Vianna Coppari for his artwork.
References
1
Unger
RH
,
Orci
L
. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci USA. 2010;107(37):16009–12. doi:.https://doi.org/10.1073/pnas.1006639107
2
Mueckler
M
,
Thorens
B
. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34(2-3):121–38. doi:.https://doi.org/10.1016/j.mam.2012.07.001
3
Parton
LE
,
Ye
CP
,
Coppari
R
,
Enriori
PJ
,
Choi
B
,
Zhang
CY
, et al.
Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449(7159):228–32. doi:.https://doi.org/10.1038/nature06098
4
Hoang Do
O
,
Thorn
P
. Insulin secretion from beta cells within intact islets: location matters. Clin Exp Pharmacol Physiol. 2015;42(4):406–14. doi:.https://doi.org/10.1111/1440-1681.12368
5
Orci
L
,
Amherdt
M
,
Henquin
JC
,
Lambert
AE
,
Unger
RH
,
Renold
AE
. Pronase effect on pancreatic beta cell secretion and morphology. Science. 1973;180(4086):647–9. doi:.https://doi.org/10.1126/science.180.4086.647
6
Meda
P
,
Halban
P
,
Perrelet
A
,
Renold
AE
,
Orci
L
. Gap junction development is correlated with insulin content in the pancreatic B cell. Science. 1980;209(4460):1026–8. doi:.https://doi.org/10.1126/science.6773144
7
Pettus
J
,
Santos Cavaiola
T
,
Tamborlane
WV
,
Edelman
S
. The past, present, and future of basal insulins. Diabetes Metab Res Rev. 2016;6(32):478–96. doi:. https://doi.org/10.1002/dmrr.2763
8
Balland
E
,
Chen
W
,
Dodd
GT
,
Conductier
G
,
Coppari
R
,
Tiganis
T
, et al.
Leptin Signaling in the Arcuate Nucleus Reduces Insulin’s Capacity to Suppress Hepatic Glucose Production in Obese Mice. Cell Rep. 2019;26(2):346–355.e3. doi:.https://doi.org/10.1016/j.celrep.2018.12.061
9
Coppari
R
. Hypothalamic neurones governing glucose homeostasis. J Neuroendocrinol. 2015;27(6):399–405. doi:.https://doi.org/10.1111/jne.12276
10
Blüher
M
,
Michael
MD
,
Peroni
OD
,
Ueki
K
,
Carter
N
,
Kahn
BB
, et al.
Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3(1):25–38. doi:.https://doi.org/10.1016/S1534-5807(02)00199-5
11
Guerra
C
,
Navarro
P
,
Valverde
AM
,
Arribas
M
,
Brüning
J
,
Kozak
LP
, et al.
Brown adipose tissue-specific insulin receptor knockout shows diabetic phenotype without insulin resistance. J Clin Invest. 2001;108(8):1205–13. doi:.https://doi.org/10.1172/JCI13103
12
Michael
MD
,
Kulkarni
RN
,
Postic
C
,
Previs
SF
,
Shulman
GI
,
Magnuson
MA
, et al.
Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6(1):87–97. doi:.https://doi.org/10.1016/S1097-2765(05)00015-8
13
Brüning
JC
,
Michael
MD
,
Winnay
JN
,
Hayashi
T
,
Hörsch
D
,
Accili
D
, et al.
A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2(5):559–69. doi:.https://doi.org/10.1016/S1097-2765(00)80155-0
14
Taniguchi
CM
,
Emanuelli
B
,
Kahn
CR
. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7(2):85–96. doi:.https://doi.org/10.1038/nrm1837
15
Brady
MJ
,
Bourbonais
FJ
,
Saltiel
AR
. The activation of glycogen synthase by insulin switches from kinase inhibition to phosphatase activation during adipogenesis in 3T3-L1 cells. J Biol Chem. 1998;273(23):14063–6. doi:.https://doi.org/10.1074/jbc.273.23.14063
16
Depré
C
,
Veitch
K
,
Hue
L
. Role of fructose 2,6-bisphosphate in the control of glycolysis. Stimulation of glycogen synthesis by lactate in the isolated working rat heart. Acta Cardiol. 1993;48(1):147–64.
17
Vogt
MC
,
Brüning
JC
. CNS insulin signaling in the control of energy homeostasis and glucose metabolism - from embryo to old age. Trends Endocrinol Metab. 2013;24(2):76–84. doi:.https://doi.org/10.1016/j.tem.2012.11.004
18
Spanswick
D
,
Smith
MA
,
Mirshamsi
S
,
Routh
VH
,
Ashford
ML
. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci. 2000;3(8):757–8. doi:.https://doi.org/10.1038/77660
19
Saltiel
AR
,
Kahn
CR
. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. doi:.https://doi.org/10.1038/414799a
20
Anderson
JG
,
Ramadori
G
,
Ioris
RM
,
Galiè
M
,
Berglund
ED
,
Coate
KC
, et al.
Enhanced insulin sensitivity in skeletal muscle and liver by physiological overexpression of SIRT6. Mol Metab. 2015;4(11):846–56. doi:.https://doi.org/10.1016/j.molmet.2015.09.003
21
Abel
ED
,
Peroni
O
,
Kim
JK
,
Kim
YB
,
Boss
O
,
Hadro
E
, et al.
Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409(6821):729–33. doi:.https://doi.org/10.1038/35055575
22
Rossetti
L
,
Rothman
DL
,
DeFronzo
RA
,
Shulman
GI
. Effect of dietary protein on in vivo insulin action and liver glycogen repletion. Am J Physiol. 1989;257(2 Pt 1):E212–9.
23
Foufelle
F
,
Ferré
P
. New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Biochem J. 2002;366(2):377–91. doi:.https://doi.org/10.1042/bj20020430
24
Foretz
M
,
Guichard
C
,
Ferré
P
,
Foufelle
F
. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci USA. 1999;96(22):12737–42. doi:.https://doi.org/10.1073/pnas.96.22.12737
25
Weber
G
,
Singhal
RL
. Insulin: inducer of phosphofructokinase. The integrative action of insulin at the enzyme biosynthetic level. Life Sci. 1965;4(20):1993–2002. doi:.https://doi.org/10.1016/0024-3205(65)90057-3
26
Ahmadian
M
,
Duncan
RE
,
Jaworski
K
,
Sarkadi-Nagy
E
,
Sul
HS
. Triacylglycerol metabolism in adipose tissue. Future Lipidol. 2007;2(2):229–37. doi:.https://doi.org/10.2217/17460875.2.2.229
27
Yechoor
VK
,
Patti
ME
,
Ueki
K
,
Laustsen
PG
,
Saccone
R
,
Rauniyar
R
, et al.
Distinct pathways of insulin-regulated versus diabetes-regulated gene expression: an in vivo analysis in MIRKO mice. Proc Natl Acad Sci USA. 2004;101(47):16525–30. doi:.https://doi.org/10.1073/pnas.0407574101
28
Henquin
JC
. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49(11):1751–60. doi:.https://doi.org/10.2337/diabetes.49.11.1751
29
Fujikawa
T
,
Berglund
ED
,
Patel
VR
,
Ramadori
G
,
Vianna
CR
,
Vong
L
, et al.
Leptin engages a hypothalamic neurocircuitry to permit survival in the absence of insulin. Cell Metab. 2013;18(3):431–44. doi:.https://doi.org/10.1016/j.cmet.2013.08.004
30
Ezaki
O
,
Fukuda
N
,
Itakura
H
. Role of two types of glucose transporters in enlarged adipocytes from aged obese rats. Diabetes. 1990;39(12):1543–9. doi:.https://doi.org/10.2337/diab.39.12.1543
31
Matsumoto
M
,
Ogawa
W
,
Teshigawara
K
,
Inoue
H
,
Miyake
K
,
Sakaue
H
, et al.
Role of the insulin receptor substrate 1 and phosphatidylinositol 3-kinase signaling pathway in insulin-induced expression of sterol regulatory element binding protein 1c and glucokinase genes in rat hepatocytes. Diabetes. 2002;51(6):1672–80. doi:.https://doi.org/10.2337/diabetes.51.6.1672
32
Shimomura
I
,
Bashmakov
Y
,
Ikemoto
S
,
Horton
JD
,
Brown
MS
,
Goldstein
JL
. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci USA. 1999;96(24):13656–61. doi:.https://doi.org/10.1073/pnas.96.24.13656
33
Meijssen
S
,
Cabezas
MC
,
Ballieux
CG
,
Derksen
RJ
,
Bilecen
S
,
Erkelens
DW
. Insulin mediated inhibition of hormone sensitive lipase activity in vivo in relation to endogenous catecholamines in healthy subjects. J Clin Endocrinol Metab. 2001;86(9):4193–7. doi:.https://doi.org/10.1210/jcem.86.9.7794
34
Spooner
PM
,
Chernick
SS
,
Garrison
MM
,
Scow
RO
. Insulin regulation of lipoprotein lipase activity and release in 3T3-L1 adipocytes. Separation and dependence of hormonal effects on hexose metabolism and synthesis of RNA and protein. J Biol Chem. 1979;254(20):10021–9.
35
Kharroubi
AT
,
Darwish
HM
. Diabetes mellitus: The epidemic of the century. World J Diabetes. 2015;6(6):850–67. doi:.https://doi.org/10.4239/wjd.v6.i6.850
36
Fujikawa
T
,
Coppari
R
. Living without insulin: the role of leptin signaling in the hypothalamus. Front Neurosci. 2015;9:108. doi:.https://doi.org/10.3389/fnins.2015.00108
37
Coppari
R
. Diabetes present and future. Int J Biochem Cell Biol. 2017;88:197. doi:.https://doi.org/10.1016/j.biocel.2017.05.012
38WHO. Diabetes. [cited 2019 August 30]; Available from: https://www.who.int/health-topics/diabetes.
39
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. doi:.https://doi.org/10.2337/dc14-S081
40
Daneman
D
. Type 1 diabetes. Lancet. 2006;367(9513):847–58. doi:.https://doi.org/10.1016/S0140-6736(06)68341-4
41
Vianna
CR
,
Coppari
R
. A treasure trove of hypothalamic neurocircuitries governing body weight homeostasis. Endocrinology. 2011;152(1):11–8. doi:.https://doi.org/10.1210/en.2010-0778
42
Coppari
R
,
Ramadori
G
,
Elmquist
JK
. The role of transcriptional regulators in central control of appetite and body weight. Nat Clin Pract Endocrinol Metab. 2009;5(3):160–6.
43
Biessels
GJ
,
van der Heide
LP
,
Kamal
A
,
Bleys
RL
,
Gispen
WH
. Ageing and diabetes: implications for brain function. Eur J Pharmacol. 2002;441(1-2):1–14. doi:.https://doi.org/10.1016/S0014-2999(02)01486-3
44
Ramadori
G
,
Coppari
R
. Does hypothalamic SIRT1 regulate aging?
Aging (Albany NY). 2011;3(3):325–8. doi:.https://doi.org/10.18632/aging.100311
45
Taylor
R
. Insulin resistance and type 2 diabetes. Diabetes. 2012;61(4):778–9. doi:.https://doi.org/10.2337/db12-0073
46
Brown
MS
,
Goldstein
JL
. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7(2):95–6. doi:.https://doi.org/10.1016/j.cmet.2007.12.009
47
Cherrington
AD
. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999;48(5):1198–214. doi:.https://doi.org/10.2337/diabetes.48.5.1198
48
Matsumoto
M
,
Han
S
,
Kitamura
T
,
Accili
D
. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116(9):2464–72. doi:.https://doi.org/10.1172/JCI27047
49
Schwarz
JM
,
Linfoot
P
,
Dare
D
,
Aghajanian
K
. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr. 2003;77(1):43–50. doi:.https://doi.org/10.1093/ajcn/77.1.43
50
Iozzo
P
,
Turpeinen
AK
,
Takala
T
,
Oikonen
V
,
Bergman
J
,
Grönroos
T
, et al.
Defective liver disposal of free fatty acids in patients with impaired glucose tolerance. J Clin Endocrinol Metab. 2004;89(7):3496–502. doi:.https://doi.org/10.1210/jc.2003-031142
51
Ginsberg
HN
,
Zhang
YL
,
Hernandez-Ono
A
. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch Med Res. 2005;36(3):232–40. doi:.https://doi.org/10.1016/j.arcmed.2005.01.005
52
Kraemer
FB
,
Shen
WJ
. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res. 2002;43(10):1585–94. doi:.https://doi.org/10.1194/jlr.R200009-JLR200
53
Cho
J-H
,
Kim
JW
,
Shin
JA
,
Shin
J
,
Yoon
KH
. β-cell mass in people with type 2 diabetes. J Diabetes Investig. 2011;2(1):6–17. doi:.https://doi.org/10.1111/j.2040-1124.2010.00072.x
54
Ramadori
G
,
Ljubicic
S
,
Ricci
S
,
Mikropoulou
D
,
Brenachot
X
,
Veyrat-Durebex
C
, et al.
S100A9 extends lifespan in insulin deficiency. Nat Commun. 2019;10(1):3545. doi:.https://doi.org/10.1038/s41467-019-11498-x
55
Coppari
R
,
Bjørbæk
C
. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat Rev Drug Discov. 2012;11(9):692–708. doi:.https://doi.org/10.1038/nrd3757
56
Agius
L
,
Chowdhury
MH
,
Davis
SN
,
Alberti
KG
. Regulation of ketogenesis, gluconeogenesis, and glycogen synthesis by insulin and proinsulin in rat hepatocyte monolayer cultures. Diabetes. 1986;35(11):1286–93. doi:.https://doi.org/10.2337/diab.35.11.1286
57
Turton
JL
,
Raab
R
,
Rooney
KB
. Low-carbohydrate diets for type 1 diabetes mellitus: A systematic review. PLoS One. 2018;13(3):e0194987. doi:.https://doi.org/10.1371/journal.pone.0194987
58
Chawla
A
,
Chawla
R
,
Jaggi
S
. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum?
Indian J Endocrinol Metab. 2016;20(4):546–51. doi:.https://doi.org/10.4103/2230-8210.183480
59
Mazur
A
. Why were “starvation diets” promoted for diabetes in the pre-insulin period?
Nutr J. 2011;10(1):23. doi:.https://doi.org/10.1186/1475-2891-10-23
60
Quianzon
CC
,
Cheikh
I
. History of insulin. J Community Hosp Intern Med Perspect. 2012;2(2):18701. doi:.https://doi.org/10.3402/jchimp.v2i2.18701
61
Bliss
M
. The history of insulin. Diabetes Care. 1993;16(Suppl 3):4–7. doi:.https://doi.org/10.2337/diacare.16.3.4
62
Rosenfeld
L
. Insulin: discovery and controversy. Clin Chem. 2002;48(12):2270–88. doi:.https://doi.org/10.1093/clinchem/48.12.2270
63
Banting
FG
,
Best
CH
,
Collip
JB
,
Campbell
WR
,
Fletcher
AA
. Pancreatic Extracts in the Treatment of Diabetes Mellitus. Can Med Assoc J. 1922;12(3):141–6.
64
Banting
FG
,
Campbell
WR
,
Fletcher
AA
. Further Clinical Experience with Insulin (Pancreatic Extracts) in the Treatment of Diabetes Mellitus. BMJ. 1923;1(3236):8–12. doi:.https://doi.org/10.1136/bmj.1.3236.8
65
Vecchio
I
,
Tornali
C
,
Bragazzi
NL
,
Martini
M
. The Discovery of Insulin: An Important Milestone in the History of Medicine. Front Endocrinol (Lausanne). 2018;9:613. doi:.https://doi.org/10.3389/fendo.2018.00613
66
Zinman
B
. Newer insulin analogs: advances in basal insulin replacement. Diabetes Obes Metab. 2013;15(s1, Suppl 1):6–10. doi:.https://doi.org/10.1111/dom.12068
67
Deckert
T
. Intermediate-acting insulin preparations: NPH and lente. Diabetes Care. 1980;3(5):623–6. doi:.https://doi.org/10.2337/diacare.3.5.623
68
Cryer
PE
,
Davis
SN
,
Shamoon
H
. Hypoglycemia in diabetes. Diabetes Care. 2003;26(6):1902–12. doi:.https://doi.org/10.2337/diacare.26.6.1902
69
Nathan
DM
,
Genuth
S
,
Lachin
J
,
Cleary
P
,
Crofford
O
,
Davis
M
, et al., Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86. doi:.https://doi.org/10.1056/NEJM199309303291401
70
Hirsch
IB
. Insulin analogues. N Engl J Med. 2005;352(2):174–83. doi:.https://doi.org/10.1056/NEJMra040832
71Levemir (Internet) Silver Spring. M.U.F.a.D.A. [cited 2019 September 3]; Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails.
72Prescribing information: Insulin glargine injection for subcutaneous injection 2000 [cited 2019 September 3]; Available from: http://products.sanofi.us/lantus/lantus.html.
73
Kurtzhals
P
. Pharmacology of insulin detemir. Endocrinol Metab Clin North Am. 2007;36(Suppl 1):14–20. doi:.https://doi.org/10.1016/S0889-8529(07)80004-1
74
Evans
M
,
Schumm-Draeger
PM
,
Vora
J
,
King
AB
. A review of modern insulin analogue pharmacokinetic and pharmacodynamic profiles in type 2 diabetes: improvements and limitations. Diabetes Obes Metab. 2011;13(8):677–84. doi:.https://doi.org/10.1111/j.1463-1326.2011.01395.x
75
Kalra
S
,
Gupta
Y
. Clinical use of Insulin Degludec: Practical Experience and Pragmatic Suggestions. N Am J Med Sci. 2015;7(3):81–5. doi:.https://doi.org/10.4103/1947-2714.153918
76
Kalra
S
,
Baruah
MP
,
Niazi
AK
. Degludec: a novel basal insulin. Recent Pat Endocr Metab Immune Drug Discov. 2012;6(1):18–23. doi:.https://doi.org/10.2174/187221412799015326
77
Kalra
S
. Insulin degludec: a significant advancement in ultralong-acting Basal insulin. Diabetes Ther. 2013;4(2):167–73. doi:.https://doi.org/10.1007/s13300-013-0047-6
78
Kruger
DF
,
Novak
LM
. Role of ultrafast-acting insulin analogues in the management of diabetes. J Am Assoc Nurse Pract. 2019;31(9):537–48. doi:.https://doi.org/10.1097/JXX.0000000000000261
79
Senior
P
,
Hramiak
I
. Fast-Acting Insulin Aspart and the Need for New Mealtime Insulin Analogues in Adults With Type 1 and Type 2 Diabetes: A Canadian Perspective. Can J Diabetes. 2019;43(7):515–23. doi:.https://doi.org/10.1016/j.jcjd.2019.01.004
80
Brange
J
,
Ribel
U
,
Hansen
JF
,
Dodson
G
,
Hansen
MT
,
Havelund
S
, et al.
Monomeric insulins obtained by protein engineering and their medical implications. Nature. 1988;333(6174):679–82. doi:.https://doi.org/10.1038/333679a0
81
van Bon
AC
,
Bode
BW
,
Sert-Langeron
C
,
DeVries
JH
,
Charpentier
G
. Insulin glulisine compared to insulin aspart and to insulin lispro administered by continuous subcutaneous insulin infusion in patients with type 1 diabetes: a randomized controlled trial. Diabetes Technol Ther. 2011;13(6):607–14. doi:.https://doi.org/10.1089/dia.2010.0224
82
Shah
RB
,
Patel
M
,
Maahs
DM
,
Shah
VN
. Insulin delivery methods: Past, present and future. Int J Pharm Investig. 2016;6(1):1–9. doi:.https://doi.org/10.4103/2230-973X.176456
83
Selam
JL
. Evolution of diabetes insulin delivery devices. J Diabetes Sci Technol. 2010;4(3):505–13. doi:.https://doi.org/10.1177/193229681000400302
84
Hirsch
IB
. Does size matter? Thoughts about insulin pen needles. Diabetes Technol Ther. 2012;14(12):1081. doi:.https://doi.org/10.1089/dia.2012.0274
85
Aronson
R
,
Gibney
MA
,
Oza
K
,
Bérubé
J
,
Kassler-Taub
K
,
Hirsch
L
. Insulin pen needles: effects of extra-thin wall needle technology on preference, confidence, and other patient ratings. Clin Ther. 2013;35(7):923–933.e4. doi:.https://doi.org/10.1016/j.clinthera.2013.05.020
86
Moser
EG
,
Morris
AA
,
Garg
SK
. Emerging diabetes therapies and technologies. Diabetes Res Clin Pract. 2012;97(1):16–26. doi:.https://doi.org/10.1016/j.diabres.2012.01.027
87
Steineck
I
,
Ranjan
A
,
Nørgaard
K
,
Schmidt
S
. Sensor-Augmented Insulin Pumps and Hypoglycemia Prevention in Type 1 Diabetes. J Diabetes Sci Technol. 2017;11(1):50–8. doi:.https://doi.org/10.1177/1932296816672689
88
Peyser
T
,
Dassau
E
,
Breton
M
,
Skyler
JS
. The artificial pancreas: current status and future prospects in the management of diabetes. Ann N Y Acad Sci. 2014;1311(1):102–23. doi:.https://doi.org/10.1111/nyas.12431
89
Mibu
K
,
Yatabe
T
,
Hanazaki
K
. Blood glucose control using an artificial pancreas reduces the workload of ICU nurses. J Artif Organs. 2012;15(1):71–6. doi:.https://doi.org/10.1007/s10047-011-0611-7
90
Umpierrez
GE
,
Klonoff
DC
. Diabetes Technology Update: Use of Insulin Pumps and Continuous Glucose Monitoring in the Hospital. Diabetes Care. 2018;41(8):1579–89. doi:.https://doi.org/10.2337/dci18-0002
91
Tosur
M
,
Redondo
MJ
,
Lyons
SK
. Adjuvant Pharmacotherapies to Insulin for the Treatment of Type 1 Diabetes. Curr Diab Rep. 2018;18(10):79. doi:.https://doi.org/10.1007/s11892-018-1041-1
92Sotagliflozin Approved in EU for Adults With Type 1 Diabetes. 2019 [cited 2019 September 5]; Available from: https://www.ajmc.com/newsroom/sotagliflozin-approved-in-eu-for-adults-with-type-1-diabetes.
93
Ang
KH
,
Sherr
JL
. Moving beyond subcutaneous insulin: the application of adjunctive therapies to the treatment of type 1 diabetes. Expert Opin Drug Deliv. 2017;14(9):1113–31. doi:.https://doi.org/10.1080/17425247.2017.1360862
94
Chaudhury
A
,
Duvoor
C
,
Reddy Dendi
VS
,
Kraleti
S
,
Chada
A
,
Ravilla
R
, et al.
Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front Endocrinol (Lausanne). 2017;8:6. doi:.https://doi.org/10.3389/fendo.2017.00006
95
Zinman
B
,
Wanner
C
,
Lachin
JM
,
Fitchett
D
,
Bluhmki
E
,
Hantel
S
, et al.; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117–28. doi:.https://doi.org/10.1056/NEJMoa1504720
96
Hernandez
AF
,
Green
JB
,
Janmohamed
S
,
D’Agostino
RB, Sr
,
Granger
CB
,
Jones
NP
, et al.; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–29. doi:.https://doi.org/10.1016/S0140-6736(18)32261-X
97
Beran
D
,
Laing
RO
,
Kaplan
W
,
Knox
R
,
Sharma
A
,
Wirtz
VJ
, et al.
A perspective on global access to insulin: a descriptive study of the market, trade flows and prices. Diabet Med. 2019;36(6):726–33. doi:.https://doi.org/10.1111/dme.13947
98Globally, Top 10 Insulin Manufacturers Are Dominated By Europe and North America. 2017 [cited 2019 September 2]; Available from: https://www.envisioninteligence.com/blog/globally-top-10-insulin-manufacturers/.
99Global Human Insulin Market to Worth $48 billion by 2020 – Leading Players are Pfizer, Inc., Novo Nordisk, Eli Lily and Company, Sanofi, GlaxoSmithKline and Merck & Co. 2018 [cited 2019 September 2]; Available from: https://www.medgadget.com/2018/05/global-human-insulin-market-to-worth-48-billion-by-2020-leading-players-are-pfizer-inc-novo-nordisk-eli-lily-and-company-sanofi-glaxosmithkline-and-merck-co.html.
100
Cuddihy
RM
,
Philis-Tsimikas
A
,
Nazeri
A
. Type 2 diabetes care and insulin intensification: is a more multidisciplinary approach needed? Results from the MODIFY survey. Diabetes Educ. 2011;37(1):111–23. doi:.https://doi.org/10.1177/0145721710388426
101
Standl
E
,
Owen
DR
. New Long-Acting Basal Insulins: Does Benefit Outweigh Cost?
Diabetes Care. 2016;39(Suppl 2):S172–9. doi:.https://doi.org/10.2337/dcS15-3011
102
Sorli
C
,
Heile
MK
. Identifying and meeting the challenges of insulin therapy in type 2 diabetes. J Multidiscip Healthc. 2014;7:267–82. doi:.https://doi.org/10.2147/JMDH.S64084
103
Kalra
S
,
Bajaj
S
,
Sharma
SK
,
Priya
G
,
Baruah
MP
,
Sanyal
D
, et al.
A Practitioner’s Toolkit for Insulin Motivation in Adults with Type 1 and Type 2 Diabetes Mellitus: Evidence-Based Recommendations from an International Expert Panel. Diabetes Ther. 2020;11(3):585–606. doi:.https://doi.org/10.1007/s13300-020-00764-7
104
Polonsky
WH
,
Fisher
L
,
Guzman
S
,
Villa-Caballero
L
,
Edelman
SV
. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care. 2005;28(10):2543–5. doi:.https://doi.org/10.2337/diacare.28.10.2543
105
Mathew
EM
,
Rajiah
K
. Assessment of medication adherence in type-2 diabetes patients on poly pharmacy and the effect of patient counseling given to them in a multispecialty hospital. J Basic Clin Pharm. 2013;5(1):15–8. doi:.https://doi.org/10.4103/0976-0105.128251
106
Hoerger
TJ
,
Segel
JE
,
Gregg
EW
,
Saaddine
JB
. Is glycemic control improving in U.S. adults?
Diabetes Care. 2008;31(1):81–6. doi:.https://doi.org/10.2337/dc07-1572
107
Brož
J
,
Janíčková Žďárská
D
,
Urbanová
J
,
Brabec
M
,
Doničová
V
,
Štěpánová
R
, et al.
Current Level of Glycemic Control and Clinical Inertia in Subjects Using Insulin for the Treatment of Type 1 and Type 2 Diabetes in the Czech Republic and the Slovak Republic: Results of a Multinational, Multicenter, Observational Survey (DIAINFORM). Diabetes Ther. 2018;9(5):1897–906. doi:.https://doi.org/10.1007/s13300-018-0485-2
108
Stratton
IM
,
Adler
AI
,
Neil
HA
,
Matthews
DR
,
Manley
SE
,
Cull
CA
, et al.
Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12. doi:.https://doi.org/10.1136/bmj.321.7258.405
109
Zhong
VW
,
Juhaeri
J
,
Cole
SR
,
Kontopantelis
E
,
Shay
CM
,
Gordon-Larsen
P
, et al.
Incidence and Trends in Hypoglycemia Hospitalization in Adults With Type 1 and Type 2 Diabetes in England, 1998-2013: A Retrospective Cohort Study. Diabetes Care. 2017;40(12):1651–60. doi:.https://doi.org/10.2337/dc16-2680
110
Gregg
EW
,
Li
Y
,
Wang
J
,
Rios Burrows
N
,
Ali
MK
,
Rolka
D
, et al.
Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014;370(16):1514–23. doi:.https://doi.org/10.1056/NEJMoa1310799
111
Mahoney
GK
,
Henk
HJ
,
McCoy
RG
. Severe Hypoglycemia Attributable to Intensive Glucose-Lowering Therapy Among US Adults With Diabetes: Population-Based Modeling Study, 2011-2014. Mayo Clin Proc. 2019;94(9):1731–42. doi:.https://doi.org/10.1016/j.mayocp.2019.02.028
112
Kana Kadayakkara
D
,
Balasubramanian
P
,
Araque
KA
,
Davis
K
,
Javed
F
,
Niaki
P
, et al.
Multidisciplinary strategies to treat severe hypoglycemia in hospitalized patients with diabetes mellitus reduce inpatient mortality rate: Experience from an academic community hospital. PLoS One. 2019;14(8):e0220956. doi:.https://doi.org/10.1371/journal.pone.0220956
113
Gagnum
V
,
Stene
LC
,
Jenssen
TG
,
Berteussen
LM
,
Sandvik
L
,
Joner
G
, et al.
Causes of death in childhood-onset Type 1 diabetes: long-term follow-up. Diabet Med. 2017;34(1):56–63. doi:.https://doi.org/10.1111/dme.13114
114
Perlmuter
LC
,
Flanagan
BP
,
Shah
PH
,
Singh
SP
. Glycemic control and hypoglycemia: is the loser the winner?
Diabetes Care. 2008;31(10):2072–6. doi:.https://doi.org/10.2337/dc08-1441
115
Cryer
PE
. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57(12):3169–76. doi:.https://doi.org/10.2337/db08-1084
116
Cryer
PE
. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes. 2005;54(12):3592–601. doi:.https://doi.org/10.2337/diabetes.54.12.3592
117
Horton
JD
,
Goldstein
JL
,
Brown
MS
. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–31. doi:.https://doi.org/10.1172/JCI0215593
118
Hodish
I
. Insulin therapy, weight gain and prognosis. Diabetes Obes Metab. 2018;20(9):2085–92. doi:.https://doi.org/10.1111/dom.13367
119
Herman
ME
,
O’Keefe
JH
,
Bell
DSH
,
Schwartz
SS
. Insulin Therapy Increases Cardiovascular Risk in Type 2 Diabetes. Prog Cardiovasc Dis. 2017;60(3):422–34. doi:.https://doi.org/10.1016/j.pcad.2017.09.001
120
Orchard
TJ
,
Costacou
T
,
Kretowski
A
,
Nesto
RW
. Type 1 diabetes and coronary artery disease. Diabetes Care. 2006;29(11):2528–38. doi:.https://doi.org/10.2337/dc06-1161
121
Larsen
J
,
Brekke
M
,
Sandvik
L
,
Arnesen
H
,
Hanssen
KF
,
Dahl-Jorgensen
K
. Silent coronary atheromatosis in type 1 diabetic patients and its relation to long-term glycemic control. Diabetes. 2002;51(8):2637–41. doi:.https://doi.org/10.2337/diabetes.51.8.2637
122
Coppari
R
,
Bjørbæk
C
. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat Rev Drug Discov. 2012;11(9):692–708. doi:.https://doi.org/10.1038/nrd3757
123
Dongerkery
SP
,
Schroeder
PR
,
Shomali
ME
. Insulin and Its Cardiovascular Effects: What Is the Current Evidence?
Curr Diab Rep. 2017;17(12):120. doi:.https://doi.org/10.1007/s11892-017-0955-3
124
Orchard
TJ
,
Olson
JC
,
Erbey
JR
,
Williams
K
,
Forrest
KY
,
Smithline Kinder
L
, et al.
Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2003;26(5):1374–9. doi:.https://doi.org/10.2337/diacare.26.5.1374
125
Wu
G
,
Meininger
CJ
. Nitric oxide and vascular insulin resistance. Biofactors. 2009;35(1):21–7. doi:.https://doi.org/10.1002/biof.3
126
Boucher
J
,
Kleinridders
A
,
Kahn
CR
. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6(1):a009191. doi:.https://doi.org/10.1101/cshperspect.a009191
127
Stoekenbroek
RM
,
Rensing
KL
,
Bernelot Moens
SJ
,
Nieuwdorp
M
,
DeVries
JH
,
Zwinderman
AH
, et al.
High daily insulin exposure in patients with type 2 diabetes is associated with increased risk of cardiovascular events. Atherosclerosis. 2015;240(2):318–23. doi:.https://doi.org/10.1016/j.atherosclerosis.2015.03.040
128
Wilcox
G
. Insulin and insulin resistance. Clin Biochem Rev. 2005;26(2):19–39.
129
Vitaterna
MH
,
Takahashi
JS
,
Turek
FW
. Overview of circadian rhythms. Alcohol Res Health. 2001;25(2):85–93.
130
Orozco-Solis
R
,
Aguilar-Arnal
L
,
Murakami
M
,
Peruquetti
R
,
Ramadori
G
,
Coppari
R
, et al.
The Circadian Clock in the Ventromedial Hypothalamus Controls Cyclic Energy Expenditure. Cell Metab. 2016;23(3):467–78. doi:.https://doi.org/10.1016/j.cmet.2016.02.003
131
Orozco-Solis
R
,
Ramadori
G
,
Coppari
R
,
Sassone-Corsi
P
. SIRT1 Relays Nutritional Inputs to the Circadian Clock Through the Sf1 Neurons of the Ventromedial Hypothalamus. Endocrinology. 2015;156(6):2174–84. doi:.https://doi.org/10.1210/en.2014-1805
132
King
DP
,
Takahashi
JS
. Molecular genetics of circadian rhythms in mammals. Annu Rev Neurosci. 2000;23(1):713–42. doi:.https://doi.org/10.1146/annurev.neuro.23.1.713
133
Carroll
KF
,
Nestel
PJ
. Diurnal variation in glucose tolerance and in insulin secretion in man. Diabetes. 1973;22(5):333–48. doi:.https://doi.org/10.2337/diab.22.5.333
134
Gibson
T
,
Jarrett
RJ
. Diurnal variation in insulin sensitivity. Lancet. 1972;300(7784):947–8. doi:.https://doi.org/10.1016/S0140-6736(72)92472-5
135
Dyar
KA
,
Ciciliot
S
,
Wright
LE
,
Biensø
RS
,
Tagliazucchi
GM
,
Patel
VR
, et al.
Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab. 2014;3(1):29–41. doi:.https://doi.org/10.1016/j.molmet.2013.10.005
136
Liu
J
,
Zhou
B
,
Yan
M
,
Huang
R
,
Wang
Y
,
He
Z
, et al.
CLOCK and BMAL1 Regulate Muscle Insulin Sensitivity via SIRT1 in Male Mice. Endocrinology. 2016;157(6):2259–69. doi:.https://doi.org/10.1210/en.2015-2027
137
Carrasco-Benso
MP
,
Rivero-Gutierrez
B
,
Lopez-Minguez
J
,
Anzola
A
,
Diez-Noguera
A
,
Madrid
JA
, et al.
Human adipose tissue expresses intrinsic circadian rhythm in insulin sensitivity. FASEB J. 2016;30(9):3117–23. doi:.https://doi.org/10.1096/fj.201600269RR
138
Shostak
A
,
Meyer-Kovac
J
,
Oster
H
. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes. 2013;62(7):2195–203. doi:.https://doi.org/10.2337/db12-1449
139
Aras
E
,
Ramadori
G
,
Kinouchi
K
,
Liu
Y
,
Ioris
RM
,
Brenachot
X
, et al.
Light Entrains Diurnal Changes in Insulin Sensitivity of Skeletal Muscle via Ventromedial Hypothalamic Neurons. Cell Rep. 2019;27(8):2385–2398.e3. doi:.https://doi.org/10.1016/j.celrep.2019.04.093
140
Coppari
R
. Metabolic actions of hypothalamic SIRT1. Trends Endocrinol Metab. 2012;23(4):179–85. doi:.https://doi.org/10.1016/j.tem.2012.01.002
141
Ramadori
G
,
Fujikawa
T
,
Anderson
J
,
Berglund
ED
,
Frazao
R
,
Michán
S
, et al.
SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metab. 2011;14(3):301–12. doi:.https://doi.org/10.1016/j.cmet.2011.06.014
142
Cheung
IN
,
Zee
PC
,
Shalman
D
,
Malkani
RG
,
Kang
J
,
Reid
KJ
. Morning and Evening Blue-Enriched Light Exposure Alters Metabolic Function in Normal Weight Adults. PLoS One. 2016;11(5):e0155601. doi:.https://doi.org/10.1371/journal.pone.0155601
143
Opperhuizen
A-L
,
Stenvers
DJ
,
Jansen
RD
,
Foppen
E
,
Fliers
E
,
Kalsbeek
A
. Light at night acutely impairs glucose tolerance in a time-, intensity- and wavelength-dependent manner in rats. Diabetologia. 2017;60(7):1333–43. doi:.https://doi.org/10.1007/s00125-017-4262-y
144
Versteeg
RI
,
Stenvers
DJ
,
Visintainer
D
,
Linnenbank
A
,
Tanck
MW
,
Zwanenburg
G
, et al.
Acute Effects of Morning Light on Plasma Glucose and Triglycerides in Healthy Men and Men with Type 2 Diabetes. J Biol Rhythms. 2017;32(2):130–42. doi:.https://doi.org/10.1177/0748730417693480
145
Carrasco-Benso
MP
,
Rivero-Gutierrez
B
,
Lopez-Minguez
J
,
Anzola
A
,
Diez-Noguera
A
,
Madrid
JA
, et al.
Human adipose tissue expresses intrinsic circadian rhythm in insulin sensitivity. FASEB J. 2016;30(9):3117–23. doi:.https://doi.org/10.1096/fj.201600269RR
146
Marcheva
B
,
Ramsey
KM
,
Buhr
ED
,
Kobayashi
Y
,
Su
H
,
Ko
CH
, et al.
Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466(7306):627–31. doi:.https://doi.org/10.1038/nature09253
147
Masri
S
,
Sassone-Corsi
P
. The circadian clock: a framework linking metabolism, epigenetics and neuronal function. Nat Rev Neurosci. 2013;14(1):69–75. doi:.https://doi.org/10.1038/nrn3393
148
Petznick
A
. Insulin management of type 2 diabetes mellitus. Am Fam Physician. 2011;84(2):183–90.
149
Coomans
CP
,
van den Berg
SA
,
Lucassen
EA
,
Houben
T
,
Pronk
AC
,
van der Spek
RD
, et al.
The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes. 2013;62(4):1102–8. doi:.https://doi.org/10.2337/db12-0507
150
Zhou
B
,
Zhang
Y
,
Zhang
F
,
Xia
Y
,
Liu
J
,
Huang
R
, et al.
CLOCK/BMAL1 regulates circadian change of mouse hepatic insulin sensitivity by SIRT1. Hepatology. 2014;59(6):2196–206. doi:.https://doi.org/10.1002/hep.26992
151
Fujikawa
T
,
Coppari
R
. Hypothalamic-mediated control of glucose balance in the presence and absence of insulin. Aging (Albany NY). 2014;6(2):92–7. doi:.https://doi.org/10.18632/aging.100641
152
Brenachot
X
,
Ramadori
G
,
Ioris
RM
,
Veyrat-Durebex
C
,
Altirriba
J
,
Aras
E
, et al.
Hepatic protein tyrosine phosphatase receptor gamma links obesity-induced inflammation to insulin resistance. Nat Commun. 2017;8(1):1820. doi:.https://doi.org/10.1038/s41467-017-02074-2
153
LaForgia
S
,
Morse
B
,
Levy
J
,
Barnea
G
,
Cannizzaro
LA
,
Li
F
, et al.
Receptor protein-tyrosine phosphatase gamma is a candidate tumor suppressor gene at human chromosome region 3p21. Proc Natl Acad Sci USA. 1991;88(11):5036–40. doi:.https://doi.org/10.1073/pnas.88.11.5036
154
Wu
Y
,
Tang
D
,
Liu
N
,
Xiong
W
,
Huang
H
,
Li
Y
, et al.
Reciprocal Regulation between the Circadian Clock and Hypoxia Signaling at the Genome Level in Mammals. Cell Metab. 2017;25(1):73–85. doi:.https://doi.org/10.1016/j.cmet.2016.09.009
155
Halberg
N
,
Khan
T
,
Trujillo
ME
,
Wernstedt-Asterholm
I
,
Attie
AD
,
Sherwani
S
, et al.
Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29(16):4467–83. doi:.https://doi.org/10.1128/MCB.00192-09


