A contemporary perspective on the diagnosis and treatment of diffuse gliomas in adults
DOI: https://doi.org/10.4414/smw.2020.20256
Patrick
Rotha, Andreas F.
Hottingerb, Thomas
Hundsbergerc, Heinz
Läublid, Philippe
Schuchte, Michael
Reinertf, Christoph
Mamotg, Ulrich
Roelckeh, Gianfranco
Pescej, Silvia
Hoferai, Michael
Wellera
aDepartment of Neurology and Brain Tumour Centre,
bBrain Tumour Centre, Department of Clinical Neurosciences and Oncology,
cDepartments of Neurology and Department of Haematology/Oncology,
dDivision of Oncology and Department of Biomedicine,
eDepartment of Neurosurgery,
fNeurosurgery NSI, EOC Lugano Switzerland, Biomedical Faculty University of Southern Switzerland,
gDivision of Oncology,
hDivision of Neurology,
iDivision of Medical Oncology,
jRadiation Oncology,
A contemporary perspective on the diagnosis and treatment of diffuse gliomas in adults
Summary
Gliomas are intrinsic brain tumours, which are classified by the World Health Organization (WHO) into different grades of malignancy, with glioblastoma being the most frequent and most malignant subtype (WHO grade IV). Mutations in the isocitrate dehydrogenase (IDH) 1 or 2 genes are frequent in lower (WHO II/III) grade tumours but typically absent in classical glioblastoma. IDH mutations are associated with a better prognosis compared with IDH wild-type tumours of the same WHO grade. Following detection of a tumour mass by imaging, maximum safe surgery as feasible is commonly performed to reduce mass effect and to obtain tissue allowing histopathological diagnosis and molecular assessment. Radiotherapy has been the mainstay in the treatment of diffuse gliomas for several decades. It provides improved local control, but is not curative. Furthermore, several randomised trials have shown that the addition of alkylating chemotherapy, either temozolomide or nitrosourea-based regimens, to radiotherapy results in prolonged survival. Tumour-treating fields (TTFields) have emerged as an additional treatment option in combination with maintenance temozolomide treatment for patients with newly diagnosed glioblastoma. Treatment at recurrence is less standardised and depends on the patient’s performance status, symptom burden and prior treatments. Bevacizumab prolongs progression-free survival in newly diagnosed and recurrent glioblastoma, but does not impact overall survival. However, in Switzerland and some other countries, it is still considered a valuable treatment option to reduce clinical symptom burden. Given the generally poor outcome for these patients, various novel treatment approaches are currently being explored within clinical trials including immunotherapeutic strategies such as immune checkpoint inhibition and the brain-penetrant proteasome inhibitor marizomib.
Introduction
The present manuscript results from two ad hoc meetings of Swiss neuro-oncologists that aimed to define current standards of clinical practice as well as challenges in the diagnosis and management of gliomas in adulthood, including specific considerations for Switzerland. The neuro-oncologists involved considered the European Association of Neuro-Oncology (EANO) recommendations [1] and, at single centres, NCCN guidelines valid for Switzerland, but there are country-specific challenges discussed herein, as well as several recent developments not covered in the currently available versions of these guidelines. This consensus paper addresses the clinical and scientific evidence, but does not seek to value interventions by relating efficacy and cost.
Classification of gliomas
Gliomas are intrinsic brain tumours that most likely develop from neuroglial progenitor cells. Traditionally, the diagnosis of gliomas was based on histopathological features alone according to the World Health Organization (WHO) classification of primary brain tumours. The most recent version of the WHO classification includes molecular markers, which allows for a more accurate diagnosis and prognosis. The vast majority of grade II gliomas and approximately 60–70% of all anaplastic gliomas (WHO grade III) harbour a mutation in the isocitrate dehydrogenase (IDH) 1 or 2 genes, whereas only a minority of glioblastomas, the most frequent and most malignant type of glioma, are IDH mutant [2]. A co-deletion of chromosome arms 1p/19q, also referred to as loss of heterozygosity (LOH) 1p/19q, assigns the diagnosis of an oligodendroglial tumour [3]. Recently, mutations in the histone H3 gene (H3K27M) have been identified as a subgroup of midline gliomas. These tumours show an extremely poor prognosis and must be considered as high grade gliomas [4]. It can be assumed that additional molecular markers, such as the presence of telomerase reverse transcriptase (TERT) promoter mutations or homozygous deletion of CDKN2a, will allow for even more refined diagnoses and become part of an updated version of the WHO classification [5, 6]. In glioblastoma, and probably also other IDH wild-type gliomas, methylation of the promoter region of the O6-methylguanine DNA methyltransferase (MGMT) gene is a predictive marker for benefit from alkylating agents. Recently, genome-wide DNA methylation profiling has been described as a valuable tool to classify primary brain tumours and may be integrated into routine diagnostics in the coming years [7].
Diagnosis and work-up
Gliomas may present with various neurological symptoms or signs, including seizures, focal deficits, cognitive alterations or any other focal neurological symptom or sign that triggers an imaging procedure. Magnetic resonance imaging (MRI) is the gold standard for the detection and monitoring of gliomas and evaluation should be according to RANO criteria [8]. Only patients who are unable to undergo MRI should be examined by computed tomography (CT). Amino acid positron emission tomography (PET) has become increasingly available and may be used in selected patients for various purposes including the delineation of tumour extension, the definition of appropriate biopsy spots in non-contrast-enhancing tumours and radiotherapy planning, as well as monitoring of tumour growth and response assessment (fig. 1) [9].
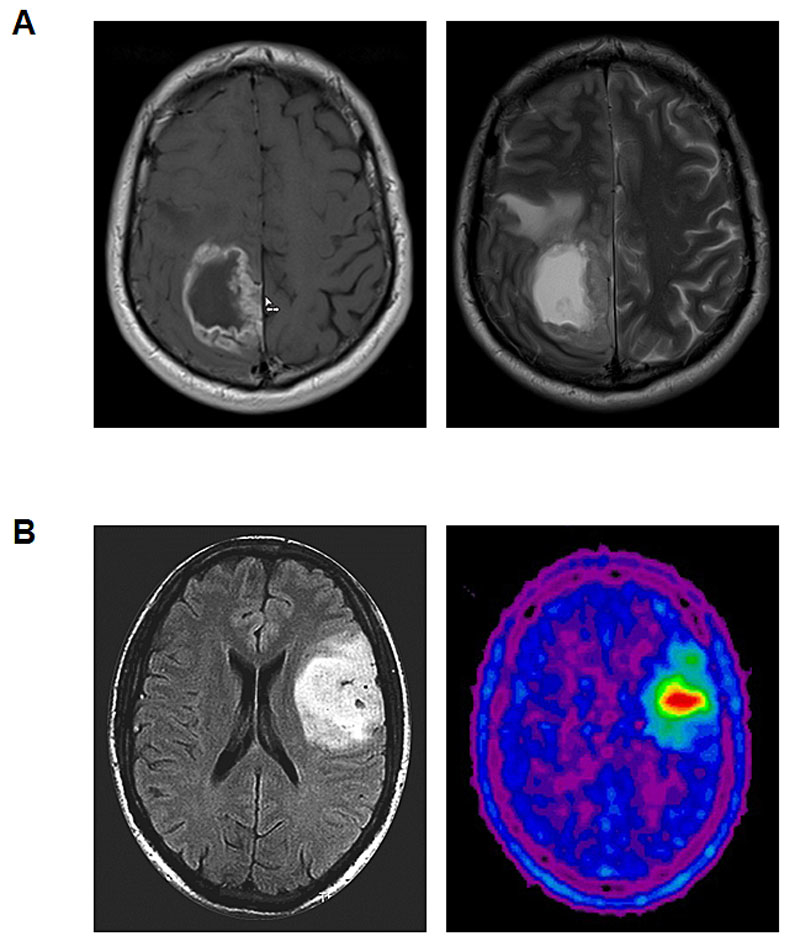
Figure 1
Representative imaging findings in glioma patients. A. Glioblastoma. T1-weighted contrast-enhanced MRI (left) and T2-weighted MRI sequence (right). B. Oligodendroglioma WHO grade 2. T2/FLAIR-weighted MRI (left) and 18F-fluoroethyl-tyrosine (FET)-PET (right).
Once tumour tissue is available, a standard histopathological examination will be performed (fig. 2), which subsequently should be supplemented by molecular assessments. These include the determination of the IDH status, first by immunohistochemistry, which will reveal IDH R132H-mutant tumours, representing approximately 90% of all IDH-mutant gliomas. In patients younger than 60 years with negative immunohistochemistry, additional sequencing of the IDH genes is recommened to exclude less frequent IDH 1 as well as IDH 2 mutations. IDH-mutant tumours should be further assessed for the presence of a 1p/19q co-deletion [10]. Some sites prefer to determine the 1p/19q status only in tumours which are α-thalassaemia/mental-retardation-syndrome-X-linked gene (ATRX) wild-type by immunohistochemistry [11]. The determination of the MGMT promoter methylation status may guide clinical decision making, particularly in elderly glioblastoma patients (see below), but does not aid in diagnosis. TERT promoter mutations may help to increase the diagnostic accuracy because they are common in oligodendrogliomas and glioblastomas, but rare in lower grade astrocytomas. The search for potentially “actionable” molecular alterations, such mutations in the BRAF gene or NTRK gene fusions may be considered, particularly in younger patients [12]. Larger next generation sequencing (NGS) panels are used at different centres to identify such molecular alterations, which are rare overall.
Following the diagnosis of a glioma, regular assessment of the Karnofsky performance status (KPS) and a clinical examination are required to monitor the occurrence or development of symptoms that may be related to the tumour or therapy. The Neurologic Assessment in Neuro-Oncology (NANO) scale provides a tool for a standardised clinical evaluation of glioma patients [13]. Glioblastoma patients are typically followed-up every 2–3 months, but longer intervals may be warranted in patients with lower grade tumours and those with prolonged stable disease.
Treatment
Surgery
Maximum safe surgical resection has been considered a standard of care despite lack of evidence from randomised clinical trials. In glioblastoma, 5-aminolevulinic acid-guided resection resulted in a higher rate of gross total resections and in prolonged progression-free survival (PFS) [14], but there was no effect on overall survival (OS) and this trial was conducted prior to the introduction of temozolomide. Similarly, the use of intraoperative MRI guidance increased the percentage of patients with gross total resection [15], but survival data were not reported. Thus, any impact of extent of resection and residual tumour on survival remains a matter of debate. The extent of resection should be determined by MRI within 24–72 hours after the intervention.
Radiotherapy
Radiotherapy has been used as a treatment for gliomas for several decades. It is typically administered in 1.8–2 Gy fractions up to a dose of 54–60 Gy. Prolongation of OS by radiotherapy was demonstrated in high-grade gliomas 40 years ago [16]. In contrast, radiotherapy prolonged PFS but not OS in patients with WHO grade II gliomas [17]. Particularly in elderly and frail patients affected by glioblastoma, hypofractionated radiotherapy with a dose of 40 Gy given in 15 fractions of 2.67 Gy has been established as a standard of care [18]. In glioblastoma patients, several studies failed to demonstrate an impact on survival by dose escalation or high precision techniques including brachytherapy, stereotactic boosts or hypofractionated schemes [19, 20]. However, techniques such as intensity-modulated radiotherapy (IMRT) or volumetric modulated arc therapy (VMAT) may improve safety via reduction of the dose to healthy tissue. Dose escalation also did not prolong OS in patients with low grade gliomas in two randomised trials [21, 22]. While the number of studies demonstrating superiority of combined radiochemotherapy compared to radiotherapy alone across various WHO grades has continuously increased (see below), it has remained unclear whether irradiation may be safely deferred in subgroups of patients, particularly with WHO grade II and III tumours. This strategy has been partially integrated into standards of care in elderly patients with MGMT promoter-methylated glioblastoma who are not considered candidates for combined radiochemotherapy. These patients benefit from treatment with temozolomide alone and may receive radiotherapy as salvage therapy [23, 24].
Systemic treatment
Data from several randomised trials have defined the role of systemic therapy in the treatment of diffuse gliomas [1]. A randomised trial enrolling patients with a diagnosis of a WHO grade II glioma considered to be at increased risk of further progression (age of 40 years or more, or residual tumour following resection) demonstrated that the addition of procarbazine, lomustine and vincristine (PCV) to radiotherapy prolongs PFS and OS compared with radiotherapy alone [25]. In the European Organisation for Research and Treatment of Cancer (EORTC) trial 22033-26033, temozolomide alone yielded similar results to radiotherapy alone [26]. Altogether, these data suggest that combined modality treatment is uniformly superior to either radiotherapy alone or alkylating agent chemotherapy alone. Several studies have also demonstrated a survival benefit upon addition of alkylating chemotherapy to radiotherapy in patients with anaplastic gliomas (fig. 3). Long-term analyses of two randomised trials showed that the combination of radiotherapy and PCV was superior to radiotherapy alone in patients with 1p/19q co-deleted anaplastic gliomas [27, 28]. In patients with 1p/19q intact anaplastic gliomas, radiotherapy followed by up to 12 cycles of maintenance treatment with temozolomide prolonged PFS and OS compared with radiotherapy alone [29]. Whether the addition of concomitant treatment with temozolomide to radiotherapy provides an additional benefit in subgroups of patients requires further analyses and longer follow-up of the EORTC 26053 trial (CATNON).
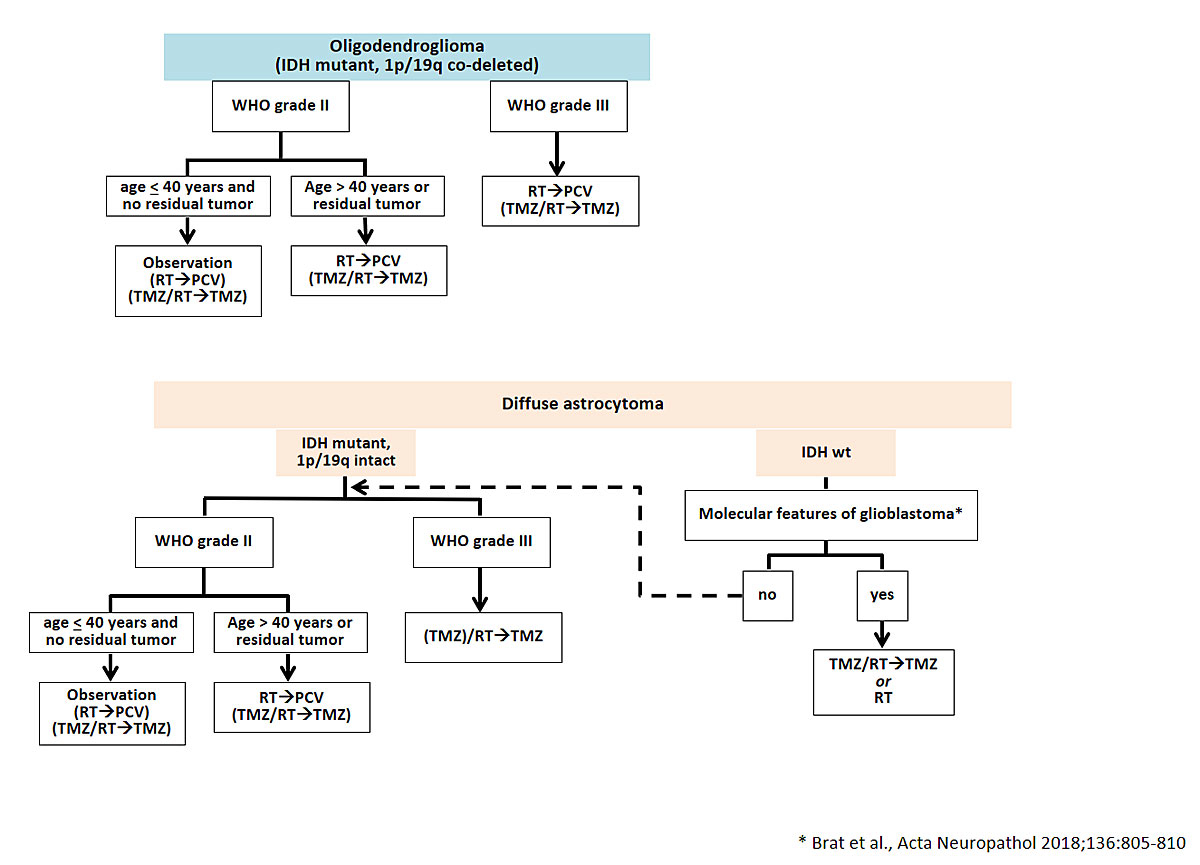
Figure 3
Therapeutic options for patients with WHO grade II and III diffuse gliomas. Alternative treatment options which may considered on an invididual base are indicated in brackets. The dotted arrow indicates that for IDH wild-type tumours without molecular features of glioblastoma, the same therapeutic approach as for IDH mutant tumours should be considered.
IDH = isocitrate dehydrogenase; WHO = World Health Organization; RT = radiotherapy; TMZ = temozolomide; PCV = procarbazine; CCNU = vincristine.
Temozolomide became part of the standard treatment for glioblastoma patients in 2005. Concomitant treatment during radiotherapy followed by maintenance treatment with temozolomide resulted in prolonged PFS and OS compared with radiotherapy alone, a finding that was also recently confirmed in elderly glioblastoma patients [30, 31]. Benefit from temozolomide is largely restricted to patients with glioblastoma with methylation of the MGMT promoter [32]. However, in the absence of convincing alternatives, such as within a clinical trial, and given the overall good tolerability, most neuro-oncological centres treat all glioblastoma patients with combined temozolomide-based radiochemotherapy up to approximately 70 years of age as long as the performance status is favourable (fig. 4). Based on the CeTeG trial, in younger patients with good performance status and MGMT promoter-methylated tumours, the addition of the combination of lomustine and temozolomide chemotherapy to and after radiotherapy may be considered [33].
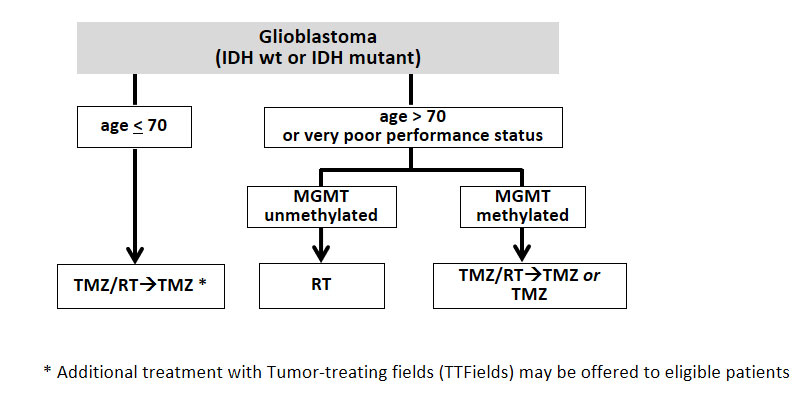
Figure 4
First-line therapy in patients with newly diagnosed glioblastoma. Patients older than 70 years with MGMT-methylated glioblastoma may receive combined temozolomide-based radiochemotherapy or monotherapy with temozolomide, depending on the performance status as considered appropriate by the treating physician.
IDH = isocitrate dehydrogenase; RT = radiotherapy; TMZ = temozolomide; MGMT = O6-methylguanine-DNA methyltransferase.
The addition of the vascular endothelial growth factor (VEGF)-targeting agent bevacizumab to the standard of care in patients with newly diagnosed glioblastoma resulted in prolonged PFS but not OS [34–36] and was therefore not approved in this situation.
Tumour-treating fields
Tumour-treating fields (TTFields) have emerged as a new treatment modality for glioblastoma patients. TTFields are low-intensity, intermediate-frequency (200 kHz) alternating electrical fields, which are applied to the tumour region using specific transducer arrays. Administration of TTFields in patients with recurrent glioblastoma, most of them with multiple prior lines of therapy, did not result in superior outcome compared with the control arm, where the best available chemotherapy according to the treating physician’s choice was allowed [37]. In contrast, the addition of TTFields to maintenance temozolomide in patients with newly diagnosed glioblastoma prolonged PFS and OS compared with standard treatment alone [38]. Treatment with TTFields is usually well tolerated except for skin reactions [39]. Subgroups of patients with preferential benefit from TTFields have not been identified. Controversies regarding this treatment within the neuro-oncology community concern the unclear mode of action in vivo and the discrepancy between efficacy in the front-line setting versus the recurrent setting.
Treatment at recurrence
For patients with recurrent glioma, treatment is less standardised. The benefit of re-resection at recurrence remains contested because of the lack of evidence from prospective trials. It is most likely limited to patients with focal tumour recurrence who are candidates for a gross total resection. Re-irradiation may be administered in selected patients with small tumours, but its efficacy has not been assessed in a randomised trial. Furthermore, several systemic therapy options are available and in use [40]. Patients who initially only received radiotherapy or alkylating chemotherapy should be treated with the therapeutic modality not used so far. Although patients with lower-grade tumours typically present with a good performance status at recurrence, the situation may be different in glioblastoma where only a fraction of patients qualify for further treatment [41]. Systemic treatment options at recurrence comprise alkylating agents including primarily nitrosoureas such as lomustine, particularly in patients with MGMT-methylated tumours [42, 43]. However, access to lomustine has become more difficult as it is not licenced in Switzerland, in contrast to carmustine, an analogue nitrosourea formulation that is administered intravenously and less well studied in the context of glioblastoma. Re-exposure to temozolomide is less frequently used today than some years ago [44]. A positive trial compared temozolomide with procarbazine [45], but this trial predated the introduction of temozolomide into the first-line setting. Treatment options for patients with MGMT-unmethylated tumours thus remain limited.
Bevacizumab was approved in Switzerland for patients with recurrent glioblastoma in 2009. Similarly to the situation in the newly diagnosed setting, the addition of bevacizumab to lomustine in patients with recurrent glioblastoma prolonged PFS but not OS [43]. In line with these data, a Swiss population-based analysis did not reveal prolonged survival of glioblastoma patients after the introduction of bevacizumab [41]. However, the drug remains a useful option in patients with symptomatic tumours who experience a clinical benefit due to relief of the mass effect.
Supportive therapy
Glioma patients frequently suffer from clinical symptoms related to therapeutic interventions or directly to the tumour, such as surrounding oedema. Steroids such as dexamethasone or prednisone have been used for decades to reduce peritumoural oedema and thereby alleviate neurological symptoms. They should always be used at the lowest effective dose and tapering should be considered as soon as clinically justified [46]. Many glioma patients are affected by seizures requiring treatment with anti-epileptic drugs. Drug interactions,such as with chemotherapeutic agents, should be considered. There is no indication for primary prophylactic antiseizure medication in patients with gliomas who never had a seizure [47]. Furthermore, glioma patients must be monitored for the occurrence of venous thromboembolic events, which should be adequately treated according to local guidelines as in other patients unless there are clear-cut contraindications. Supportive measures are the major therapeutic focus in patients who present in poor performance status, unable to undergo radiotherapy or chemotherapy [48].
Further developments and outlook
The role of re-resection in patients with recurrent glioblastoma shall be clarified in the ongoing ReSurge trial (NCT02394626). Furthermore, immunotherapy has been considered an attractive treatment option for glioma patients. However, so far, no immunotherapeutic agent has been shown to prolong the survival of glioma patients. This includes the EGFRvIII-targeting peptide vaccine rindopepimut, which was explored in patients with newly diagnosed glioblastoma and the PD-1 inhibitor nivolumab, which was assessed in patients with recurrent glioblastoma [49, 50]. Furthermore, the combination of nivolumab and radiotherapy was not superior to temozolomide-based radiotherapy in patients with newly diagnosed MGMT-unmethylated glioblastoma (CheckMate 498 [NCT02617589], press release). Nivolumab is currently also being explored in the CheckMate 548 trial (NCT02667587), in patients with newly diagnosed glioblastoma with MGMT promoter methylation. The antibody-drug conjugate depatuxizumab-mafodotin was assessed in patients with recurrent EGFR-amplified glioblastoma. Although the primary endpoint of the trial was not met, a long-term analysis suggested that the drug may be active [51]. Yet, according to a press release, the addition of depatuxizumab-mafodotin to the standard of care in patients with newly diagnosed glioblastoma harboring an EGFR amplification did not prolong overall survival (NCT02573324). Marizomib, a brain-penetrant pan-proteasome inhibitor is currently examined in patients with newly diagnosed glioblastoma in a randomised phase III trial (EORTC 1709, NCT03345095). Furthermore, molecular alterations such as BRAFV600E mutations, which are found in a subset of gliomas, will be used more frequently for targeted therapeutic strategies in the future [52, 53].
References
1
Weller
M
,
van den Bent
M
,
Tonn
JC
,
Stupp
R
,
Preusser
M
,
Cohen-Jonathan-Moyal
E
, et al.; European Association for Neuro-Oncology (EANO) Task Force on Gliomas. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017
b;18(6):e315–29. doi:.https://doi.org/10.1016/S1470-2045(17)30194-8
2
Hartmann
C
,
Meyer
J
,
Balss
J
,
Capper
D
,
Mueller
W
,
Christians
A
, et al.
Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118(4):469–74. doi:.https://doi.org/10.1007/s00401-009-0561-9
3
Louis
DN
,
Perry
A
,
Reifenberger
G
,
von Deimling
A
,
Figarella-Branger
D
,
Cavenee
WK
, et al.
The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–20. doi:.https://doi.org/10.1007/s00401-016-1545-1
4
Meyronet
D
,
Esteban-Mader
M
,
Bonnet
C
,
Joly
MO
,
Uro-Coste
E
,
Amiel-Benouaich
A
, et al.
Characteristics of H3 K27M-mutant gliomas in adults. Neuro-oncol. 2017;19(8):1127–34. doi:.https://doi.org/10.1093/neuonc/now274
5
Brat
DJ
,
Aldape
K
,
Colman
H
,
Holland
EC
,
Louis
DN
,
Jenkins
RB
, et al.
cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018;136(5):805–10. doi:.https://doi.org/10.1007/s00401-018-1913-0
6
Shirahata
M
,
Ono
T
,
Stichel
D
,
Schrimpf
D
,
Reuss
DE
,
Sahm
F
, et al.
Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018;136(1):153–66. doi:.https://doi.org/10.1007/s00401-018-1849-4
7
Capper
D
,
Jones
DTW
,
Sill
M
,
Hovestadt
V
,
Schrimpf
D
,
Sturm
D
, et al.
DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–74. doi:.https://doi.org/10.1038/nature26000
8
Wen
PY
,
Macdonald
DR
,
Reardon
DA
,
Cloughesy
TF
,
Sorensen
AG
,
Galanis
E
, et al.
Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–72. doi:.https://doi.org/10.1200/JCO.2009.26.3541
9
Albert
NL
,
Weller
M
,
Suchorska
B
,
Galldiks
N
,
Soffietti
R
,
Kim
MM
, et al.
Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-oncol. 2016;18(9):1199–208. doi:.https://doi.org/10.1093/neuonc/now058
10
Robinson
C
,
Kleinschmidt-DeMasters
BK
. IDH1-Mutation in Diffuse Gliomas in Persons Age 55 Years and Over. J Neuropathol Exp Neurol. 2017;76(2):151–4. doi:.https://doi.org/10.1093/jnen/nlw112
11
Wiestler
B
,
Capper
D
,
Holland-Letz
T
,
Korshunov
A
,
von Deimling
A
,
Pfister
SM
, et al.
ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta Neuropathol. 2013;126(3):443–51. doi:.https://doi.org/10.1007/s00401-013-1156-z
12
Cocco
E
,
Scaltriti
M
,
Drilon
A
. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15(12):731–47. doi:.https://doi.org/10.1038/s41571-018-0113-0
13
Nayak
L
,
DeAngelis
LM
,
Brandes
AA
,
Peereboom
DM
,
Galanis
E
,
Lin
NU
, et al.
The Neurologic Assessment in Neuro-Oncology (NANO) scale: a tool to assess neurologic function for integration into the Response Assessment in Neuro-Oncology (RANO) criteria. Neuro-oncol. 2017;19(5):625–35. doi:.https://doi.org/10.1093/neuonc/nox029
14
Stummer
W
,
Pichlmeier
U
,
Meinel
T
,
Wiestler
OD
,
Zanella
F
,
Reulen
HJ
; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. doi:.https://doi.org/10.1016/S1470-2045(06)70665-9
15
Senft
C
,
Bink
A
,
Franz
K
,
Vatter
H
,
Gasser
T
,
Seifert
V
. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 2011;12(11):997–1003. doi:.https://doi.org/10.1016/S1470-2045(11)70196-6
16
Walker
MD
,
Strike
TA
,
Sheline
GE
. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5(10):1725–31. doi:.https://doi.org/10.1016/0360-3016(79)90553-4
17
van den Bent
MJ
,
Afra
D
,
de Witte
O
,
Ben Hassel
M
,
Schraub
S
,
Hoang-Xuan
K
, et al., EORTC Radiotherapy and Brain Tumor Groups and the UK Medical Research Council. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366(9490):985–90. doi:.https://doi.org/10.1016/S0140-6736(05)67070-5
18
Roa
W
,
Brasher
PM
,
Bauman
G
,
Anthes
M
,
Bruera
E
,
Chan
A
, et al.
Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–8. doi:.https://doi.org/10.1200/JCO.2004.06.082
19
Brada
M
,
Sharpe
G
,
Rajan
B
,
Britton
J
,
Wilkins
PR
,
Guerrero
D
, et al.
Modifying radical radiotherapy in high grade gliomas; shortening the treatment time through acceleration. Int J Radiat Oncol Biol Phys. 1999;43(2):287–92. doi:.https://doi.org/10.1016/S0360-3016(98)00390-3
20
Chan
JL
,
Lee
SW
,
Fraass
BA
,
Normolle
DP
,
Greenberg
HS
,
Junck
LR
, et al.
Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol. 2002;20(6):1635–42. doi:.https://doi.org/10.1200/JCO.2002.20.6.1635
21
Karim
AB
,
Maat
B
,
Hatlevoll
R
,
Menten
J
,
Rutten
EH
,
Thomas
DG
, et al.
A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996;36(3):549–56. doi:.https://doi.org/10.1016/S0360-3016(96)00352-5
22
Shaw
E
,
Arusell
R
,
Scheithauer
B
,
O’Fallon
J
,
O’Neill
B
,
Dinapoli
R
, et al.
Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20(9):2267–76. doi:.https://doi.org/10.1200/JCO.2002.09.126
23
Malmström
A
,
Grønberg
BH
,
Marosi
C
,
Stupp
R
,
Frappaz
D
,
Schultz
H
, et al.; Nordic Clinical Brain Tumour Study Group (NCBTSG). Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–26. doi:.https://doi.org/10.1016/S1470-2045(12)70265-6
24
Wick
W
,
Platten
M
,
Meisner
C
,
Felsberg
J
,
Tabatabai
G
,
Simon
M
, et al.; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–15. doi:.https://doi.org/10.1016/S1470-2045(12)70164-X
25
Buckner
JC
,
Shaw
EG
,
Pugh
SL
,
Chakravarti
A
,
Gilbert
MR
,
Barger
GR
, et al.
Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N Engl J Med. 2016;374(14):1344–55. doi:.https://doi.org/10.1056/NEJMoa1500925
26
Baumert
BG
,
Hegi
ME
,
van den Bent
MJ
,
von Deimling
A
,
Gorlia
T
,
Hoang-Xuan
K
, et al.
Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17(11):1521–32. doi:.https://doi.org/10.1016/S1470-2045(16)30313-8
27
Cairncross
G
,
Wang
M
,
Shaw
E
,
Jenkins
R
,
Brachman
D
,
Buckner
J
, et al.
Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–43. doi:.https://doi.org/10.1200/JCO.2012.43.2674
28
van den Bent
MJ
,
Brandes
AA
,
Taphoorn
MJ
,
Kros
JM
,
Kouwenhoven
MC
,
Delattre
JY
, et al.
Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–50. doi:.https://doi.org/10.1200/JCO.2012.43.2229
29
van den Bent
MJ
,
Baumert
B
,
Erridge
SC
,
Vogelbaum
MA
,
Nowak
AK
,
Sanson
M
, et al.
Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 2017;390(10103):1645–53. doi:.https://doi.org/10.1016/S0140-6736(17)31442-3
30
Stupp
R
,
Mason
WP
,
van den Bent
MJ
,
Weller
M
,
Fisher
B
,
Taphoorn
MJ
, et al.; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. doi:.https://doi.org/10.1056/NEJMoa043330
31
Perry
JR
,
Laperriere
N
,
O’Callaghan
CJ
,
Brandes
AA
,
Menten
J
,
Phillips
C
, et al.; Trial Investigators. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N Engl J Med. 2017;376(11):1027–37. doi:.https://doi.org/10.1056/NEJMoa1611977
32
Hegi
ME
,
Diserens
AC
,
Gorlia
T
,
Hamou
MF
,
de Tribolet
N
,
Weller
M
, et al.
MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi:.https://doi.org/10.1056/NEJMoa043331
33
Herrlinger
U
,
Tzaridis
T
,
Mack
F
,
Steinbach
JP
,
Schlegel
U
,
Sabel
M
, et al.; Neurooncology Working Group of the German Cancer Society. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet. 2019;393(10172):678–88. doi:.https://doi.org/10.1016/S0140-6736(18)31791-4
34
Chinot
OL
,
Wick
W
,
Mason
W
,
Henriksson
R
,
Saran
F
,
Nishikawa
R
, et al.
Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–22. doi:.https://doi.org/10.1056/NEJMoa1308345
35
Gilbert
MR
,
Dignam
JJ
,
Armstrong
TS
,
Wefel
JS
,
Blumenthal
DT
,
Vogelbaum
MA
, et al.
A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. doi:.https://doi.org/10.1056/NEJMoa1308573
36
Wirsching
HG
,
Tabatabai
G
,
Roelcke
U
,
Hottinger
AF
,
Jörger
F
,
Schmid
A
, et al.
Bevacizumab plus hypofractionated radiotherapy versus radiotherapy alone in elderly patients with glioblastoma: the randomized, open-label, phase II ARTE trial. Ann Oncol. 2018;29(6):1423–30. doi:.https://doi.org/10.1093/annonc/mdy120
37
Stupp
R
,
Wong
ET
,
Kanner
AA
,
Steinberg
D
,
Engelhard
H
,
Heidecke
V
, et al.
NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48(14):2192–202. doi:.https://doi.org/10.1016/j.ejca.2012.04.011
38
Stupp
R
,
Taillibert
S
,
Kanner
A
,
Read
W
,
Steinberg
D
,
Lhermitte
B
, et al.
Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA. 2017;318(23):2306–16. doi:.https://doi.org/10.1001/jama.2017.18718
39
Taphoorn
MJB
,
Dirven
L
,
Kanner
AA
,
Lavy-Shahaf
G
,
Weinberg
U
,
Taillibert
S
, et al.
Influence of Treatment With Tumor-Treating Fields on Health-Related Quality of Life of Patients With Newly Diagnosed Glioblastoma: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018;4(4):495–504. doi:.https://doi.org/10.1001/jamaoncol.2017.5082
40
Hundsberger
T
,
Hottinger
AF
,
Roelcke
U
,
Roth
P
,
Migliorini
D
,
Dietrich
PY
, et al.
Patterns of care in recurrent glioblastoma in Switzerland: a multicentre national approach based on diagnostic nodes. J Neurooncol. 2016;126(1):175–83. doi:.https://doi.org/10.1007/s11060-015-1957-0
41
Gramatzki
D
,
Roth
P
,
Rushing
EJ
,
Weller
J
,
Andratschke
N
,
Hofer
S
, et al.
Bevacizumab may improve quality of life, but not overall survival in glioblastoma: an epidemiological study. Ann Oncol. 2018;29(6):1431–6. doi:.https://doi.org/10.1093/annonc/mdy106
42
Taal
W
,
Oosterkamp
HM
,
Walenkamp
AM
,
Dubbink
HJ
,
Beerepoot
LV
,
Hanse
MC
, et al.
Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–53. doi:.https://doi.org/10.1016/S1470-2045(14)70314-6
43
Wick
W
,
Gorlia
T
,
Bendszus
M
,
Taphoorn
M
,
Sahm
F
,
Harting
I
, et al.
Lomustine and Bevacizumab in Progressive Glioblastoma. N Engl J Med. 2017;377(20):1954–63. doi:.https://doi.org/10.1056/NEJMoa1707358
44
Weller
M
,
Tabatabai
G
,
Kästner
B
,
Felsberg
J
,
Steinbach
JP
,
Wick
A
, et al.; DIRECTOR Study Group. MGMT Promoter Methylation Is a Strong Prognostic Biomarker for Benefit from Dose-Intensified Temozolomide Rechallenge in Progressive Glioblastoma: The DIRECTOR Trial. Clin Cancer Res. 2015;21(9):2057–64. doi:.https://doi.org/10.1158/1078-0432.CCR-14-2737
45
Yung
WK
,
Albright
RE
,
Olson
J
,
Fredericks
R
,
Fink
K
,
Prados
MD
, et al.
A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83(5):588–93. doi:.https://doi.org/10.1054/bjoc.2000.1316
46
Roth
P
,
Wick
W
,
Weller
M
. Steroids in neurooncology: actions, indications, side-effects. Curr Opin Neurol. 2010;23(6):597–602. doi:.https://doi.org/10.1097/WCO.0b013e32833e5a5d
47
Weller
M
,
Stupp
R
,
Wick
W
. Epilepsy meets cancer: when, why, and what to do about it?
Lancet Oncol. 2012;13(9):e375–82. doi:.https://doi.org/10.1016/S1470-2045(12)70266-8
48
Pace
A
,
Dirven
L
,
Koekkoek
JAF
,
Golla
H
,
Fleming
J
,
Rudà
R
, et al.; European Association of Neuro-Oncology palliative care task force. European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol. 2017;18(6):e330–40. doi:.https://doi.org/10.1016/S1470-2045(17)30345-5
49
Reardon
DA
,
Brandes
AA
,
Omuro
A
,
Mulholland
P
,
Lim
M
,
Wick
A
, et al.
Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020 May 21. doi:.https://doi.org/10.1001/jamaoncol.2020.1024
50
Weller
M
,
Butowski
N
,
Tran
DD
,
Recht
LD
,
Lim
M
,
Hirte
H
, et al.; ACT IV trial investigators. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017
a;18(10):1373–85. doi:.https://doi.org/10.1016/S1470-2045(17)30517-X
51
van den Bent
MJ
,
French
P
,
Juan Sepulveda
M
,
Walenkamp
A
,
Frenel
J-S
,
Franceschi
E
, et al.
Two-year results of the INTELLANCE 2/EORTC trial 1410 randomized-phase II study on Depatux-M alone, Depatux-M combined with temozolomide (TMZ) and either TMZ or lomustine in reucrrent EGFR amplified glioblastoma. Neuro-oncol. 2018;20(suppl_6):vi20. doi:.https://doi.org/10.1093/neuonc/noy148.072
52
Kaley
T
,
Touat
M
,
Subbiah
V
,
Hollebecque
A
,
Rodon
J
,
Lockhart
AC
, et al.
BRAF Inhibition in BRAF
V600-Mutant Gliomas: Results From the VE-BASKET Study. J Clin Oncol. 2018;36(35):3477–84. doi:.https://doi.org/10.1200/JCO.2018.78.9990
53
Le Rhun
E
,
Preusser
M
,
Roth
P
,
Reardon
DA
,
van den Bent
M
,
Wen
P
, et al.
Molecular targeted therapy of glioblastoma. Cancer Treat Rev. 2019;80:101896. doi:.https://doi.org/10.1016/j.ctrv.2019.101896