Prevalence of tuberculosis in migrant children in Switzerland and relevance of current screening guidelines
DOI: https://doi.org/10.4414/smw.2020.20253
Myriam
Boukamela, Yves
Fougèreb, Mario
Gehric, Joan Carles
Surisc, Isabelle
Rochatd, Damien
Milettoc, Spyridoula
Kyrillic, Athina
Fourikic, Pierre Alex
Crisinelb
aService of Internal Medicine, Department of Medicine,
bUnit of Paediatric Infectious Diseases and Vaccinology, Woman-Mother-Child Department,
cService of Paediatric, Woman-Mother-Child Department,
dPaediatric Pulmonology Unit, Woman-Mother-Child Department,
Prevalence of tuberculosis in migrant children in Switzerland and relevance of current screening guidelines
Summary
AIMS
Since 2016, Swiss guidelines recommend screening of all migrant children <5 years of age for tuberculosis (TB) and to screen older children only if they have risk factors for tuberculosis. Our goals were to describe the epidemiology of latent tuberculosis in migrant children at the Lausanne University Hospital, to identify determinants of latent tuberculosis and tuberculosis disease, and to evaluate the risk of a false-positive tuberculin skin test when using a positivity limit of 5 mm.
METHODS
Newly arrived migrant children 0–18 years of age were prospectively enrolled from 31 August 2015 to 31 August 2017. Every migrant child was assessed for the risk of tuberculosis exposure and tuberculosis disease and was administered a tuberculin skin test. A tuberculosis-spot test was performed in children ≥5 years of age when the tuberculin skin test was positive. Children with clinical and/or radiological signs of tuberculosis disease were further investigated. Children ≥5 years of age with a positive tuberculosis-spot test and children <5 years of age with a positive tuberculin skin test, without clinico-radiological signs of tuberculosis disease received a diagnosis of latent tuberculosis. A false-positive tuberculin skin test result was diagnosed in children ≥5 years of age when the tuberculosis-spot test was negative. Potential determinants of tuberculosis (latent tuberculosis and tuberculosis disease) and of false-positive tuberculin skin tests were identified. Student’s t-test or the Kruskal-Wallis test were used for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. All variables with a p-value <0.05 were included in a multivariate logistic regression model.
RESULTS
Two hundred and fifty-three patients were eligible for the study. The median age of the patients was 8.1 years (interquartile range [IQR] 4.5–12.8) and 104 (41%) were female. Twenty-four percent of the patients (62/253) came from a country with a moderate–high incidence of tuberculosis disease (≥80 cases per 100,000 individuals). Twenty-eight patients (11%) had positive tuberculin skin tests, and tuberculosis was confirmed in 17 (6.7%) of these patients (16 with latent tuberculosis and 1 with tuberculosis disease). On multivariate analysis, moderate–high incidence of tuberculosis disease in the country of origin (adjusted odds ratio [aOR] 18.8, 95% confidence interval [CI] 5.1–68.6; p <0.001), older age (aOR 1.1, 95% CI 1.0–1.3; p = 0.025), and contact with a tuberculosis disease patient (aOR 8, 95% CI 1.8–36.2; p = 0.007) were associated with a diagnosis of tuberculosis. Among the 23 children over 5 years of age who had a positive tuberculin skin test with measurement available, a measure between 5–9 mm was more frequent in case of a false-positive tuberculin skin test (5/9, 56% vs 0/14, 0%, p = 0.002). BCG vaccination was the only predictor of a false-positive tuberculin skin test (p = 0.03).
CONCLUSION
Screening migrant children ≥5 years of age for tuberculosis could confer a public health benefit even in the absence of other risk factors. The limit of tuberculin skin test positivity could be raised from ≥5 mm to ≥10 mm to decrease the rate of false-positive results. A national assessment of migrant children between the ages of 5 and 15 should be carried out to confirm our findings.
Introduction
Despite a global downward trend, tuberculosis (TB) remains one of the deadliest communicable diseases. The World Health Organization (WHO) reported 10 million new tuberculosis disease cases in 2017, and 10% of the cases were children with tuberculosis disease [1]. It was estimated that tuberculosis caused 1.3 million deaths among human immunodeficience virus (HIV)-negative people and 300,000 additional deaths among HIV-positive people in 2017 [1].
In Switzerland, approximately 550 people are diagnosed with tuberculosis each year, and the majority of them were born in a foreign country with a high prevalence of tuberculosis and/or were of foreign origin [2]. In 2018, Switzerland recorded 70 cases of tuberculosis disease in patients <18 years of age and 13 of the patients were <5 years of age. Since 2016, Swiss guidelines recommend that all migrant children <5 years of age are screened for tuberculosis and that older children are screened for tuberculosis only if they have risk factors for tuberculosis [3]. Children <5 years of age are at much higher risk of developing severe forms of tuberculosis disease, especially tuberculous meningitis and milliary tuberculosis. However, older children are at higher risk of having been exposed to tuberculosis [4]. The absence of screening of the older children for tuberculosis may have public health implications because they are at risk of developing tuberculosis disease later on, but this risk is difficult to assess because there are no data on the prevalence of latent tuberculosis infection (LTBI) among migrant children in Switzerland.
Our main objective was to describe the epidemiology of latent tuberculosis infection in migrant children followed up at the Lausanne University Hospital. Our secondary objectives were to identify determinants of latent tuberculosis infection and tuberculosis disease, and to evaluate the risk of a false-positive Mantoux tuberculin skin test with a positivity limit of 5 mm.
Methods
Design, setting and population
We conducted a prospective single-centre cohort study in a tertiary care hospital. Lausanne University Hospital is the reference centre for children seeking asylum in our region. More than 90% of migrant children in the district of Lausanne and Western Lausanne are followed up in our hospital. Based on our estimates, this represents about 40% and 3% of asylum seekers located in the Canton of Vaud and in Switzerland, respectively. Newly arrived migrant children and adolescents who were born abroad and arrived in Switzerland less than one year ago and who were between 0 and 18 years of age were approached by paediatric residents between 31 August 2015 and 31 Augus 2017 for participation in the study. They were enrolled on a consecutive basis. Primary exclusion criteria were refusal to participate, screening before the medical assessment and tuberculosis disease diagnosed before the assessment. Parents or legal guardians and adolescents who agreed to participate provided written informed consent. Patients were secondarily excluded in the event of absence of a tuberculin skin test or lack of tuberculin skin test interpretation.
Past medical and migratory history obtained from medical charts and interviews were recorded on a standardised case-report form. Paediatric residents filled in the questionnaire with the patients and their parents. An interpreter translated when patients and their parents did not speak French fluently. This study was approved by the local institutional ethics committee (protocol number 290/15) and conducted in accordance with the principles of the Declaration of Helsinki, the standards of Good Clinical Practice and Swiss regulatory requirements.
Study procedure and definitions
Procedure
Every migrant child was assessed for their risk of tuberculosis exposure and tuberculosis disease based on a thorough review of their past and current medical history and a complete physical examination. Presence of a bacillus Calmette-Guérin (BCG) vaccine scar, an indicator of vaccination against tuberculosis, was also noted. A tuberculin skin test using purified protein derivate (PPD-RT 23, 2 TU from SSI, Copenhagen, Denmark) was performed on every child. The tuberculin skin test was read after 48–72 hours, and according to Swiss tuberculosis guidelines [3], a tuberculin skin test ≥5 mm was considered positive. A chest X-ray was performed for all children with a positive tuberculin skin test and was interpreted firstly by an attending paediatrician and secondly by a paediatric radiologist in doubtful cases. When X-ray results and/or clinical evaluation showed findings compatible with tuberculosis disease, children were investigated further to confirm or exclude the diagnosis. Children ≥5 years of age with a positive tuberculin skin test but no clinico-radiological indication of tuberculosis disease were prescribed a TB-spot test (TB-SPOT.TB®, Oxford Immunotec North America).
All children diagnosed with latent tuberculosis infection received either monotherapy with isoniazid (INH) for 9 months or bitherapy with INH and rifampicin (RIF) for 3 months, which are the recommended treatments for latent tuberculosis infection [5].
Definitions
Latent tuberculosis infection: children <5 years of age: positive tuberculin skin test but no clinico-radiological indication of tuberculosis disease; children ≥5 years of age: positive tuberculin skin test and positive TB-spot test but no clinicradiological indication of tuberculosis disease
False-positive tuberculin skin test (no latent tuberculosis infection): children ≥5 years of age with a tuberculin skin test ≥ 5 mm and a negative TB-spot test.
Tuberculosis disease: clinico-radiological signs of tuberculosis disease with no other potential aetiology with or without a positive tuberculin skin test and/or TB-spot test and, with or without documentation (PCR, culture, or microscopy) of the presence of the Mycobacterium tuberculosis complex.
Moderate-high incidence of tuberculosis disease: ≥80,000 cases per 100,000 individuals per year.
Outcomes
Our main outcome measure was diagnosis of latent tuberculosis infection or tuberculosis disease. Our secondary outcome measure was a false-positive tuberculin skin test. Gender, age, BCG vaccination, tuberculosis disease contact, migration time, urban residency, tuberculosis disease incidence and exposure to armed conflicts were considered potential risk factors for the above outcomes.
Statistical analysis
No sample size calculation was made, as our main objective was to describe the epidemiology of latent tuberculosis infection in migrant children. We estimated that we would be able to recruit about 250 patients, including around 20 patients with latent tuberculosis infection, in a 2-year period. Demographic information, including gender, region of origin, exposure to moderate–high tuberculosis disease incidence, age, and migratory information, including duration and exposure to conflict, were compared using the Student’s t-test or the Kruskal-Wallis test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. All variables with a p-value <0.05 were included in a multivariate logistic regression model and adjusted odds ratios (aORs) and 95% confidence intervals (CIs) were calculated. All tests were two-tailed, and a p-value ≤0.05 was considered statistically significant. Statistical analyses were computed using Stata software (Stata/IC 11.2 for Mac; StataCorp, Lakeway, TX).
Results
Population
Three hundred and seventy-five newly arrived migrant children were seen in our hospital from 31 August 2015 to 31 August 2017 and were eligible for inclusion. However, 122 children were not included because of paediatric residents’ lack of awareness or overwork (n = 87), they were not screened for tuberculosis (n = 16), parental refusal (n = 4), tuberculosis disease was diagnosed before the first consultation (n = 3), the tuberculin skin test was not read (n = 2), the tuberculin skin test was out of stock (n = 9), and tuberculosis screening occurred before the first consultation (n = 1; fig. 1). There were no differences in terms of age, gender, and WHO region of origin between included and excluded patients (data not shown).
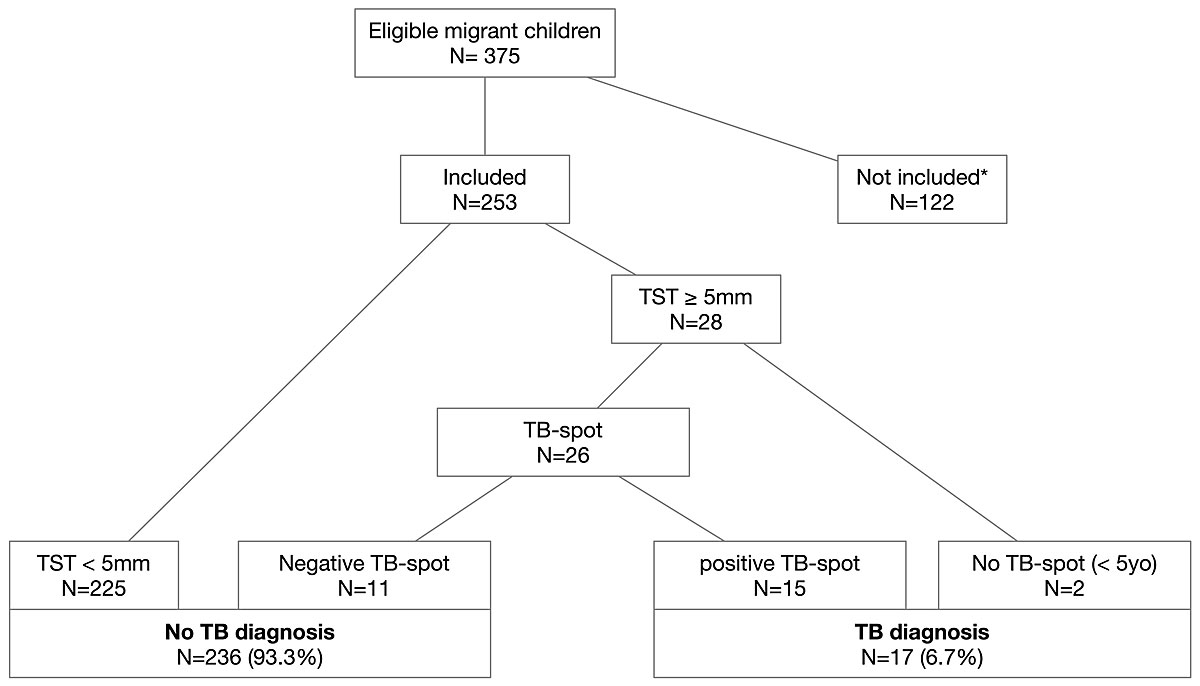
Figure 1 Description of the population.
TB = tuberculosis; TBD = tuberculosis disease; TST = tuberculin skin test; * no TB screening (n = 16), parental refusal (n = 4), tuberculosis disease diagnosed before the assessment (n = 3), oblivion/lack of time (n = 87), lack of tuberculin skin test interpretation (n = 2), sold out of tuberculin skin tests (n = 9), screening before the medical assessment (n = 1).
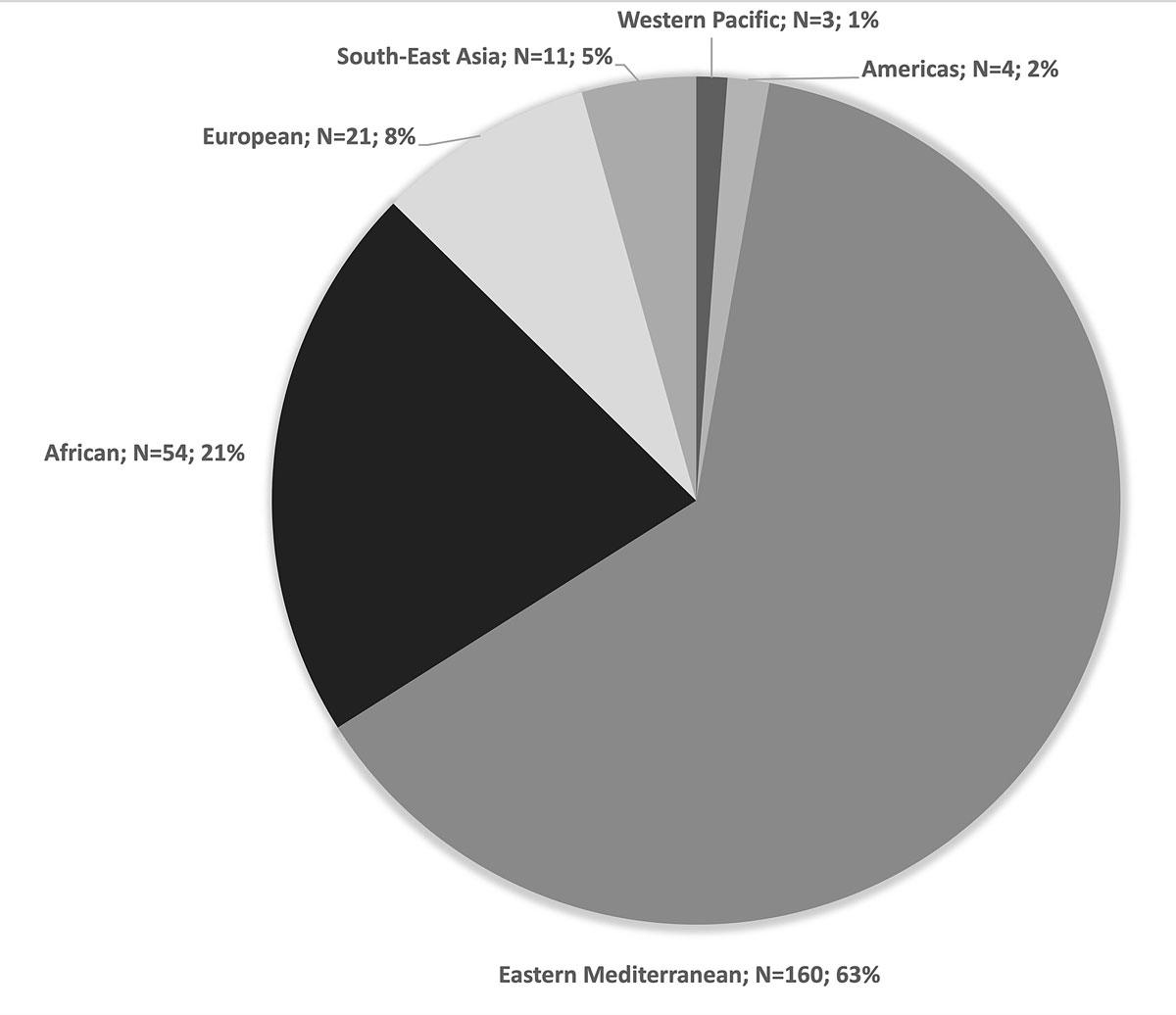
Two hundred and fifty-three patients were included. The median age of the enrolled patients was 8.1 years (interquartile range [IQR] 4.5–12.8) and 104 (41%) were female (table 1). The majority of the patients were born in the WHO Eastern Mediterranean region (n = 160, 63.2%; fig. 2). Seventy-eight percent of the patients (198/253) were living in urban areas in their country of origin. Two hundred and twenty children (87%) reported exposure to armed conflicts. The median duration of migration was 1 month (IQR 1–7) and the median number of transit countries before arriving in Switzerland was 4 (IQR 2–6). Seventy-two patients (28%) had evidence of BCG vaccination (scar or positive history) (table 1).
Table 1 Baseline characteristics of the population.
|
Characteristic
|
Total N = 253
|
| Female gender, n (%) |
104 (41%) |
| Median age, years (IQR) |
8.1 (7.5–14) |
| tuberculosis disease contact, n (%) |
9 (4%) |
| Median migration time, months (IQR) |
1 (1–7) |
| Urban residency, n (%) |
198 (78%) |
| Moderate–high tuberculosis disease incidence*, n (%) |
62 (24%) |
| Exposure to armed conflicts, n (%) |
220 (87%) |
| BCG vaccination, n (%) |
72 (28%) |
Twenty-eight patients (11%) had a tuberculin skin test ≥5 mm. Three of the patients were <5 years of age and had no evidence of tuberculosis. One of the patients was diagnosed with tuberculosis disease; he was fifteen years and nine months old, came from Eritrea, and had clinical and radiological evidence of tuberculosis. Microbiological work up (culture and PCR from bronchoalveolar lavage and bronchial aspiration) revealed positive results for the M. tuberculosis complex. The TB-spot test was prescribed for 25 patients ≥5 years of age and for one patient aged <5 years of age, and 15 (15/26, 58%) of the patients, including the patient with tuberculosis disease, had positive TB-spot tests. Thus, overall, 17 out of 253 patients (6.7%) received a diagnosis of tuberculosis. Sixteen of the patients, including three patients <5 years of age, were diagnosed with latent tuberculosis infection, and one patient was diagnosed with tuberculosis disease. False-positive tuberculin skin tests were found in 11 patients (11/253, 4%) (fig. 1 and table 2). On comparing tuberculin skin test measurements for patients ≥ 5 years of age with true-positive and false-positive tuberculin skin test results, we found that a higher proportion of patients with a false-positive tuberculin skin test result had a measure between 5 and 9 mm in comparison with patients with a true-positive result, who had all a measurement of more than 9 mm (5/9, 56% vs 0/14, 0%, p = 0.002). It should be noted that tuberculin skin test measurements were not retrievable for two patients positive for the tuberculin skin test and negative for the TB-spot test. No patient with a negative tuberculin skin test result developed signs of tuberculosis disease during the study.
Table 2 Tuberculin skin test results and TB-spot results in children aged ≥5 years*
|
TST results
|
Negative TB-spot results
|
Positive TB-spot results
|
|
Negative TST results
|
n = 152, TB-spot not done |
|
Positive TST results†
|
False-positive: n = 11 |
True-positive: n = 15*
|
Determinants of tuberculosis diagnosis
Univariate analysis
Tuberculosis patients were significantly older (median of 12.1 years, IQR 9.1–15.9) than non-tuberculosis patients (median of 7.8 years, IQR 4.5–12.3, p = 0.019; table 3). The median duration of migration was longer in tuberculosis patients (8 months, IQR 2–26 vs 1 month, IQR 1–6; p = 0.0017), and the country of origin differed between the tuberculosis and non-tuberculosis groups. Eighty-two percent (14/17) of the tuberculosis patients were born in a country with a moderate–high incidence of tuberculosis disease, whereas only 20% (48/236) of the non-tuberculosis patients were born in a country with a moderate–high incidence of tuberculosis disease (p <0.001). Overall, 23% (14/62) of patients from a country with moderate–high incidence of tuberculosis disease were in the tuberculosis group. Finally, more tuberculosis patients (3/17, 17%) than non-tuberculosis patients (6/236, 3%) reported contact with tuberculosis disease patients (p = 0.017). There were no differences between the groups with regards to gender, area of residency, exposure to armed conflict, or BCG vaccination (table 3).
Table 3 Predictors of tuberculosis diagnosis (univariate and multivariate analysis).
|
Characteristics
|
Univariate analysis
|
Multivariate analysis†
|
Tuberculosis*
(n = 17)
|
No tuberculosis
(n = 236)
|
p-value
|
Odds ratio
(95% CI)
|
p-value
|
| Female gender, n (%) |
4 (24%) |
100 (42%) |
0.2 |
|
|
| Median age, years IQR) |
12.1 (9.1–15.9) |
7.8 (4.5–12.3) |
0.019
|
1.1 (1.0–1.3) |
0.025
|
| BCG vaccination, n (%) |
7 (41%) |
65 (27.5%) |
0.27 |
|
|
| Tuberculosis disease contact, n (%) |
3 (17%) |
6 (3%) |
0.017
|
8 (1.8–36.2) |
0.007
|
| Median migration time, months (IQR) |
8 (2-26) |
1 (1-6) |
0.0017
|
1.02 (0.99–1.05) |
0.087 |
| Urban residency, n (%) |
13 (76%) |
185 (78%) |
1 |
|
|
| Moderate–high tuberculosis disease incidence‡, n (%) |
14 (82%) |
48 (20%) |
<0.001
|
18.8 (5.1–68.6) |
<0.001
|
| Exposure to armed conflicts, n (%) |
14 (82%) |
206 (87%) |
0.56 |
|
|
Multivariate analysis
Moderate–high incidence of tuberculosis disease (OR 18.8, 95% CI 5.1–68.6), age (OR per 1 year increase 1.1, 95% CI 1–1.3) and contact with tuberculosis disease patients (OR 8, 95% CI 1.8–36.2) were significant independent variables associated with a tuberculosis diagnosis, whereas duration of migration was not associated with a tuberculosis diagnosis (table 3).
Determinants of false-positive tuberculin skin tests in non-tuberculosis patients (older than 5 years of age)
Among non-tuberculosis patients ≥5 years of age, 11 out of 163 patients (7%) had false-positive tuberculin skin tests (positive tuberculin skin tests with a negative tuberculosis-spot tests). Five of these patients had tuberculin skin tests between 5 and 9 mm, four had tuberculin skin tests ≥10 mm, and the tuberculin skin test measurements were not retrievable for two of the patients (table 2). BCG vaccination was the only variable associated significantly with a false-positive tuberculin skin test (table 4). Thus no multivariate analysis was realised. Fifty-five per cent of patients with a false-positive tuberculin skin test (6/11) were vaccinated with BCG, whereas only 24% (37/152) of the patients with a negative tuberculin skin test were vaccinated with BCG (p = 0.03). Overall, 6 out of 43 BCG-vaccinated, non-tuberculosis patients (14%) had false-positive tuberculin skin tests, 4 of the patients had tuberculin skin tests between 5 and 9 mm, 1 patient had a tuberculin skin test of 10 mm, and the tuberculin skin test measurement for 1 of the patients was not retrievable.
Table 4 Predictors of false-positive tuberculin skin tests results (negative TB-spot tests) in non-tuberculosis children ≥5 years of age (univariate analysis).
|
Characteristics
|
Total
(n = 163)
|
False-positive TST
(n = 11)
|
True-negative TST
(n = 152)
|
p-value
|
| Female gender, n (%) |
61 (37%) |
3 (27%) |
58 (38%) |
0.48 |
| Median age, years (IQR) |
10.4 (7.5–14) |
11.8 (9.4–14) |
10.2 (7.4–14) |
0.25 |
| Tuberculosis disease contact, n (%) |
4 (2%) |
1 (9%) |
3 (2%) |
0.14 |
| Median migration time, months (IQR) |
1 (1–8) |
1 (0–6) |
1 (1–8) |
0.5 |
| Urban residency, n (%) |
130 (84%) |
11 (100%) |
119 (83%) |
0.14 |
Moderate–high tuberculosis disease incidence*, n
(%) |
34 (21%) |
3 (27%) |
31 (20%) |
0.59 |
| Exposure to armed conflicts, n (%) |
143 (88%) |
8 (73%) |
135 (89%) |
0.12 |
| BCG vaccination, n (%) |
43 (26%) |
6 (55%) |
37 (24%) |
0.03
|
Discussion
We found a prevalence of tuberculosis of 6.7% in our population of 253 migrant children, and only 1 patient (0.4%) was diagnosed with tuberculosis disease. The majority of the tuberculosis patients (82%) were ≥5 years of age and most (82%) came from a country with a moderate–high incidence of tuberculosis. We also found that the false-positive tuberculin skin tests in non-tuberculosis patients ≥5 years of age could mostly be explained by BCG vaccination.
Studies from other high-income countries showed comparable prevalences of tuberculosis in migrant children. Data from the United States [6–8] and Italy [9] showed latent tuberculosis infection prevalences of 5–7% based on interferon gamma release assay (IGRA) results. Although the countries of origin of the migrant children in the studies from the United States and Italy may differ from the countries of origin of the children in our population, the association between latent tuberculosis infection prevalence and countries of origin with moderate–high incidence of tuberculosis were similar. Although there is no consensus regarding the incidence of tuberculosis in the country of origin, which varies from 20–500 per 100,000 individuals, that warrants screening, most national policies consider tuberculosis incidence as a risk factor for latent tuberculosis infection [10, 11].
We found a higher risk of tuberculosis in older patients, which is not surprising and can be explained by a greater opportunity for exposure to tuberculosis and, mostly in the adult population, by immunosenescence. A study conducted in the United States showed that the risk of tuberculosis disease in recent entrants (less than 2 years) is low in children aged <5 years of age (25–30 children out of 100,000). In contrast, the incidence in adolescents raises up to 50–70 out of 100,000. The risk was highest in people over 60 years of age (incidence of >150 out of 100,000 individuals) [4]. Likewise, Usdin et al. showed that the risk of latent tuberculosis infection was 2.2–3.3 times higher in patients 21–35 years of age than in students 15–20 years of age [12].
Swiss guidelines published in 2016 [3] recommended screening all children <5 years of age even in the absence of clear risk factors for tuberculosis disease and screening children ≥5 years of age only if they had contact with an index case, if they were immunosuppressed or if they presented with symptoms associated with tuberculosis. Our results demonstrate that all children coming from countries with a moderate–high incidence of tuberculosis should be screened regardless of age. In our study, only 3/17 patients with latent tuberculosis infection were <5 years of age. Of the 14 patients ≥5 years of age, only 2 were exposed to index cases and only the patient with active tuberculosis presented with symptoms of tuberculosis disease, including weight loss. If we had followed the Swiss guidelines [3], we might have missed diagnosing 65% (11/17) of our patients with latent tuberculosis infection and these missed diagnoses may not have been inconsequential in terms of public health. For example, a study from the United States showed that the screening of every new migrant would prevent 6–26 new cases of tuberculosis disease for every 3000 migrants [13]. Furthermore, in terms of health costs, treating latent tuberculosis infection is cheaper and easier than treating tuberculosis disease [10, 14]. Moreover, as reported by the WHO, latent tuberculosis infection cases are responsible for the majority of the tuberculosis disease cases in low tuberculosis incidence countries and contribute significantly to the transmission of tuberculosis in high incidence countries. Thus screening for and treating latent tuberculosis infection are the main goals of the WHO plan to contain tuberculosis [15]. It is very important to implement screening for latent tuberculosis infection early in the migration process. Indeed, the majority of tuberculosis cases occur within 2 years after arrival in the host country [4]. The 2017 report of the Swiss Paediatric Surveillance Unit (SPSU) analysed 29 paediatric cases of tuberculosis that were diagnosed in 2017. Forty-five percent of these children were born abroad and 81% of the children born in Switzerland had at least 1 parent who was born abroad. Among the children born abroad, the diagnosis of tuberculosis was made within 9 months of their arrival in Switzerland [16].
Our study also showed an association between a history of contact with tuberculosis patients and tuberculosis diagnoses, which indicates that systematic screening as proposed in numerous guidelines is justified [3, 10]. Although the use of a tuberculin skin test limit ≥5 mm regardless of age, origin and risk factors may diminish the risk of false-negative results, we believe that a higher threshold should be used to screen migrant children ≥5 years of age. As proposed in guidelines from other countries, and to reduce the risk of false-positive tuberculin skin tests without significantly increasing the rate of false-negative results, the limit for a positive tuberculin skin test could be raised to 10 mm in children ≥5 years of age regardless of BCG vaccination status if there are no other risk factors, such as contact with a tuberculosis patient and immunosuppression [17, 18].
Seven percent of non-tuberculosis patients had false-positive tuberculin skin tests and in more than half of the cases, this was explained by BCG vaccination. In a meta-analysis of articles published from 1967–2005, Farhat et al. reported a false-positive rate of 8.5% for tuberculin skin tests ≥10 mm in patients vaccinated as infants [19]. We found a higher false-positive rate of 14% among BCG-vaccinated patients, which can be explained by our use of a positivity threshold ≥5 mm because four out of five of our patients had tuberculin skin test measurements between 5 and 9 mm. The fact that only one of our vaccinated patients had a tuberculin skin test ≥10 mm can be explained by the fact that they were >9 years of age. Farhat et al. showed that the false-positive rate fell to only 1% if the tuberculin skin test was performed ≥10 years after BCG vaccination [19]. The false-positive results in non-BCG vaccinated patients could be explained by contact with atypical mycobacteria. In the Farhat study, the rate of cross-reactivity with atypical mycobacteria ranged from 0.1–2.3%, depending on the geographic region [19]. Also, some children may have been vaccinated with BCG, but did not report the vaccination and did not have a vaccine scar to indicate vaccination.
Our study has some limitations. First, the number of patients with latent tuberculosis infection or tuberculosis disease was low, and this may have impacted our ability to identify other determinants of tuberculosis. Moreover, a significant number of eligible patients were not included (122/375 patients). However, we compared the excluded and included populations and confirmed that they did not differ. Second, our results may not be generalisable to other settings. Indeed, we conducted a single-centre study and our population may not be representative of other populations in Switzerland or elsewhere, because migratory flows might be different or may change over time. We think however that our population is representative of the asylum seekers in the Canton de Vaud, as we estimate it to represent a large proportion of all migrant children in this canton (about 40%). We also expect that the link between the incidence rate of tuberculosis disease and migration will remain regardless of the setting. Third, our estimate of the rate of BCG vaccination may be inaccurate because some patients may not have vaccine scars and their vaccination histories may not be accurate. Fourth, we didn’t prescribe a TB-spot to patients who had a negative tuberculin skin test. We cannot rule out that we had false-negative tuberculin skin tests. Even if no patient with a negative tuberculin skin test developed symptoms suggestive of tuberculosis during our study, we cannot completely rule out the risk of a false-negative tuberculin skin test. Finally, it is impossible to definitively conclude that the Mantoux tuberculin skin test results for the children <5 years of age were really positive results because two out of the three positive tuberculin skin test results were between 5 and 9 mm. In the future, we may test the reliability of the interferon test to detect tuberculosis in young children [20].
Conclusion
This study showed a prevalence of tuberculosis of 23% in children who migrated from countries with a moderate–high incidence of tuberculosis disease, and most of these children were ≥5 years of age. Thus, it could be a benefit to public health to screen these children in addition to children <5 years of age even in the absence of other risk factors, such as contact with a tuberculosis disease patient. Also, to reduce the rate of false-positive results, the tuberculin skin test limit could be raised to 10 mm in children ≥5 years of age. As these results are based on a small cohort in a unique area of Switzerland, a national assessment of migrant children between the ages of 5 and 15 should be carried out to confirm our findings.
Acknowledgments
The foundation of the Childhood Hospital of Lausanne (foundation de l’Hôpital de l’Enfance) who financially supported this study, all caretakers who contributed to the study, and parents and patients.
References
1World Health Organization. Global tuberculosis report 2018 [Internet]. World Health Organization, editor. 2018. 174 p. Available from: https://www.who.int/tb/publications/global_report/gtbr2018_main_text_30Oct2018.pdf? ua=1
2Office fédérale de la santé publique OFSP. Stratégie nationale de lutte contre la tuberculose 2012–2017 [Internet]. Office fédérale de la santé publique OFSP. 2012. 23 p. Available from: www.bag.admin.ch
3Bernhard-Stirnemann S, Büttcher M, Heininger U, Relly C, Trück J, Wagner N, et al. Mémento pour le diagnostic et la prévention de maladies infectieuses et la mise à jour des vaccinations auprès d’enfants et adolescents migrants en Suisse, asymptomatiques. Paediatrica. 2016??:11–8.
4
Cain
KP
,
Benoit
SR
,
Winston
CA
,
Mac Kenzie
WR
. Tuberculosis among foreign-born persons in the United States. JAMA. 2008;300(4):405–12. doi:.https://doi.org/10.1001/jama.300.4.405
5Federal Office of Public Health. Tuberculosis in Switzerland, guidance for healthcare professionals, Manual of Tuberculosis - revised version, Federal Office of Public Health. March 2019
6Wang Z, Wang Z. Latent Tuberculosis Infection among Immigrant and Refugee Children Aged 2-14 Years Who Arrived in the United States in 2008-2012 [Internet]. Georgia State University; 2015. pp. 1–42. Available from: https://scholarworks.gsu.edu/iph_theses/411
7
Grinsdale
JA
,
Islam
S
,
Tran
OC
,
Ho
CS
,
Kawamura
LM
,
Higashi
JM
. Interferon-Gamma Release Assays and Pediatric Public Health Tuberculosis Screening: The San Francisco Program Experience 2005 to 2008. J Pediatric Infect Dis Soc. 2016;5(2):122–30. doi:.https://doi.org/10.1093/jpids/piu119
8
Howley
MM
,
Painter
JA
,
Katz
DJ
,
Graviss
EA
,
Reves
R
,
Beavers
SF
, et al.; Tuberculosis Epidemiologic Studies Consortium. Evaluation of QuantiFERON-TB gold in-tube and tuberculin skin tests among immigrant children being screened for latent tuberculosis infection. Pediatr Infect Dis J. 2015;34(1):35–9. doi:.https://doi.org/10.1097/INF.0000000000000494
9
Garazzino
S
,
Galli
L
,
Chiappini
E
,
Pinon
M
,
Bergamini
BM
,
Cazzato
S
, et al.; SITIP IGRA Study Group. Performance of interferon-γ release assay for the diagnosis of active or latent tuberculosis in children in the first 2 years of age: a multicenter study of the Italian Society of Pediatric Infectious Diseases. Pediatr Infect Dis J. 2014;33(9):e226–31. doi:.https://doi.org/10.1097/INF.0000000000000353
10Adams LV, Starke JR. Latent tuberculosis infection in children. In: Reyn von CF, Edwards MS, editors. UpToDate [Internet]. Waltham, MA: UpToDate. Available from: https://uptodate.com (accessed on 2018 October 23)
11
Pareek
M
,
Baussano
I
,
Abubakar
I
,
Dye
C
,
Lalvani
A
. Evaluation of immigrant tuberculosis screening in industrialized countries. Emerg Infect Dis. 2012;18(9):1422–9. doi:.https://doi.org/10.3201/eid1809.120128
12
Usdin
M
,
Dedicoat
M
,
Gajraj
R
,
Harrison
P
,
Kaur
H
,
Duffield
K
, et al.
Latent tuberculous screening of recent migrants attending language classes: a cohort study and cost analysis. Int J Tuberc Lung Dis. 2017;21(2):175–80. doi:.https://doi.org/10.5588/ijtld.16.0398
13
Porco
TC
,
Lewis
B
,
Marseille
E
,
Grinsdale
J
,
Flood
JM
,
Royce
SE
. Cost-effectiveness of tuberculosis evaluation and treatment of newly-arrived immigrants. BMC Public Health. 2006;6(1):157. doi:.https://doi.org/10.1186/1471-2458-6-157
14Adams LV, Starke JR. Tuberculosis disease in children. In: Reyn von CF, Edwards MS, editors. UpToDate. Waltham, MA: UpToDate. (accessed on 2018 October 23)
15World Health Organization. The End TB Strategy [Internet]. World Health Organization, editor. 2015 [cited 2019 Apr 24]. pp. 1–30. Available from: https://www.who.int/tb/strategy/End_TB_Strategy.pdf?ua=1
16Rudin C, Bernet V, Posfay-Barbe KM, Bolt I, Laubscher B, Simonetti G, et al. SPSU Swiss paediatric surveillance unit, annual report 2017. 2018;49:10–25. Available from: https://www.bag.admin.ch/bag/fr/home/das-bag/publikationen/periodika/bag-bulletin.html
17
Lewinsohn
DM
,
Leonard
MK
,
LoBue
PA
,
Cohn
DL
,
Daley
CL
,
Desmond
E
, et al.
Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Infect Dis. 2017;64(2):e1–33. doi:.https://doi.org/10.1093/cid/ciw694
18Harries AD, Maher D, Graham SM, Graham S. TB/VIH. World Health Organization. Organisation mondiale de la Santé; 2005. 220 p.
19
Farhat
M
,
Greenaway
C
,
Pai
M
,
Menzies
D
. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria?
Int J Tuberc Lung Dis. 2006;10(11):1192–204.
20
Yun
KW
,
Kim
YK
,
Kim
HR
,
Lee
MK
,
Lim
IS
. Usefulness of interferon-γ release assay for the diagnosis of latent tuberculosis infection in young children. Korean J Pediatr. 2016;59(6):256–61. doi:.https://doi.org/10.3345/kjp.2016.59.6.256