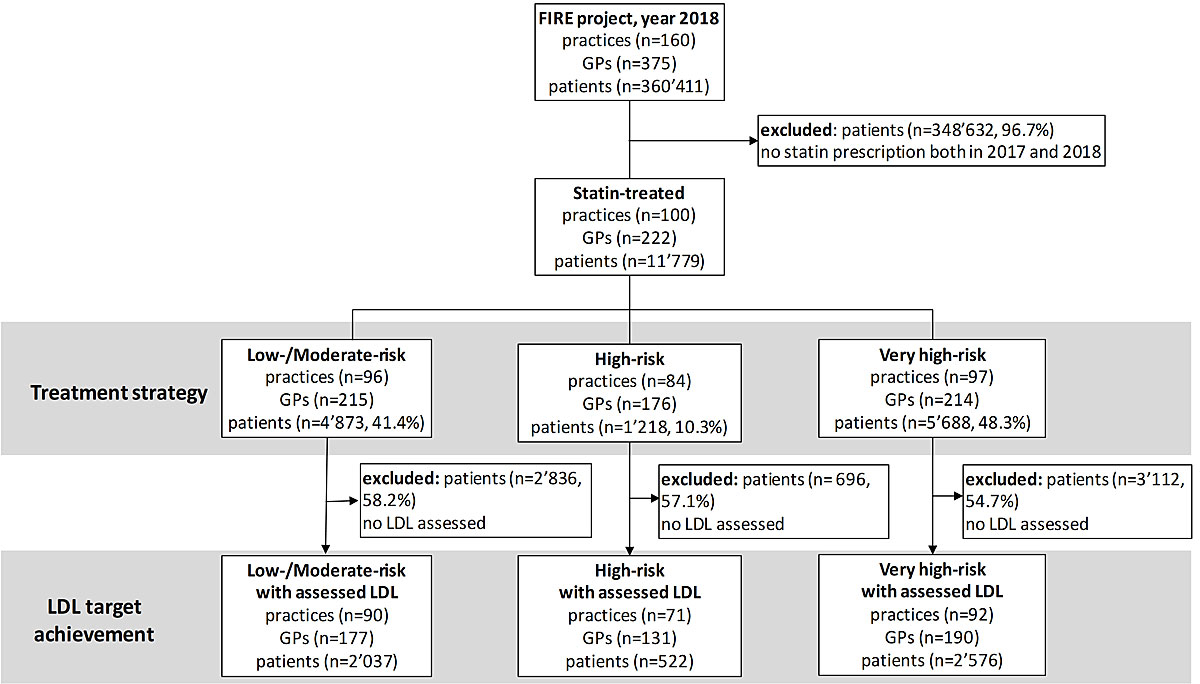
Figure 1 Flowchart.
DOI: https://doi.org/10.4414/smw.2020.20244
Cardiovascular disease (CVD) is the leading cause of global life years lost [1]. Low-density lipoprotein (LDL) is among the most important and reversible risk factors for CVD, and causality is backed by genetic, epidemiological and clinical studies [2]. A large meta-analysis reported a 19% reduction in coronary mortality per mmol/l reduction in LDL [3]. Several drugs for lowering LDL levels are available, and among these, statins have consistently been shown to reduce CVD mortality in primary and secondary prevention and are the recommended first choice among lipid-lowering agents [4–6]. Patients with previous cardiovascular (CV) events are at a very high risk of subsequent adverse CV events and benefit most from statins (secondary prevention) [7]. In primary prevention, benefits depend on the magnitude of CV risk. CV risk is determined by a plethora of risk factors such as LDL, age, gender and others [8]. To facilitate CV risk estimation and thereby eligibility for statin treatment, predictive instruments have been developed that provide prognostic probabilities for CV events [9].
In 2016, the European Society of Cardiology (ESC) published guidelines covering indications for treatment with statins, encompassing both primary and secondary prevention [10]. The guidelines recommend using a classification scheme with four risk categories, ranging from “low” to “very high”, to classify patients. The guidelines then support the determination of an appropriate treatment strategy by specifying risk-dependent target levels for LDL following the “treat to target” approach [11]. In this latter specification, the ESC guidelines differ from the guidelines published by the American College of Cardiology (ACC) in 2013 [12]. The ACC guidelines explicitly refrain from specifying LDL target levels depending on CV risk. Instead, they give specific recommendations for statin treatment intensity.
International guidelines thus contain important differences in treatment strategies, and little is known about the treatment strategies followed in general practice and the actual achievement rates of LDL targets. Such measures, however, are important surrogates for treatment costs and treatment outcomes. Therefore, in this study, we aimed to determine statin-related treatment strategies and LDL target achievement, stratified by CV risk category, in patients treated by Swiss general practitioners (GPs).
We performed a retrospective observational study in the year 2018 using Swiss general practice data from the FIRE (Family Medicine ICPC-Research using Electronic Medical Records) project [13]. Since the project began, more than 540 GPs have exported anonymised clinical routine data from their electronic medical records to the FIRE project. We included all patients with a statin prescription in 2018 who had already been prescribed a statin in 2017 (fig. 1).

Figure 1 Flowchart.
The Local Ethics Committee of the Canton of Zurich waived approval, because the project is outside the scope of the law on human research (BASEC-Nr. Req-2017-00797).
We extracted practice-, GP- and patient-level data. From the practice data we used practice type (single vs. group) and urbanity level (urban vs. non-urban). From the GP data we used gender and year of birth. From the patient data we extracted gender; year of birth; the presence of relevant morbidities (atherosclerotic CVD [ASCVD], moderate or severe chronic kidney disease (CKD), diabetes, hypertension; for definition see appendix 1); morbidity-defined CV risk category (low-/moderate-risk, high-risk or very high-risk) based on the 2016 ESC guidelines [10] (for the classification scheme see appendix 1); number of consultations in 2018; last statin prescription in 2018 (product, daily dose); number of lipid level measurements in 2018; and values of last lipid measurement (LDL, high-density lipoprotein (HDL), total cholesterol, triglycerides) in 2018. Statin treatment intensity was calculated from the product and the daily dose according to the 2013 ACC/AHA guidelines [12], as specified in the appendix 1.
We used R software (version 3.5.0) to perform the data analysis [14]. We reported the data as counts and proportions (n and %) or medians and interquartile ranges (IQR). For group comparisons, we applied a Kruskal-Wallis rank sum test, a chi-square test or Fisher’s exact test. To investigate determinants of LDL target achievement, we applied patient-level multilevel logistic regression with GP random effects. Variables of interest were: GP age and gender and patient age, gender and CV risk category. We selected the best model based on the likelihood ratio test. This resulted in the omission of GP age and gender, the inclusion of standardised patient age as a linear feature, and adding interaction effects between the fixed effects variables that were ultimately included (patient gender, age and risk category). For better interpretability of the results, we used the R-package multcomp to calculate estimates for multiple comparisons from the various interaction terms. Missing data was left unchanged. If it was below 5% we extrapolated results to the total population. Otherwise, missing counts and/or proportions were declared. We reported p-values and 95% confidence intervals (CI).
We observed 11,779 patients who were treated with statins by 222 GPs in 100 practices. The median patient age was 71 (IQR = 62-78) and 60.3% (n = 7,103) of them were male. Their median LDL level was 2.3 mmol/l (IQR = 1.8–3.0, 56.4% missing), their median HDL level was 1.4 mmol/l (IQR = 1.1–1.7, 54.0% missing), their median cholesterol level was 4.4 mmol/l (IQR = 3.8–5.3, 47.6% missing), and their median triglyceride level was 1.4 mmol/l (IQR = 1.0–2.1, 54.8% missing). The distribution of the patients’ LDL levels by CV risk category is shown in figure 2. Graphical distributions of HDL, total cholesterol and triglycerides are shown in the appendix 1. Hypertension was present in 74.4% (n = 8768) of the patients, diabetes in 32.4% (n = 3816), previous ASCVD in 22.2% (n = 2616), moderate CKD in 22.6% (n = 2,660) and severe CKD in 3.6% (n = 416). With very high CV risk were 48.3% (n = 5688) of the patients, 10.3% (n = 1218) were at high risk and 41.4% (n = 4,873) were at low/moderate risk. The patient characteristics are shown stratified by CV risk in table 1. Of the GPs, 64.4% (n = 143) were male and their median age was 51 years (IQR = 43–59); 91.9% (n = 204) worked in group practices and 72.5% (n = 161) in urban areas.
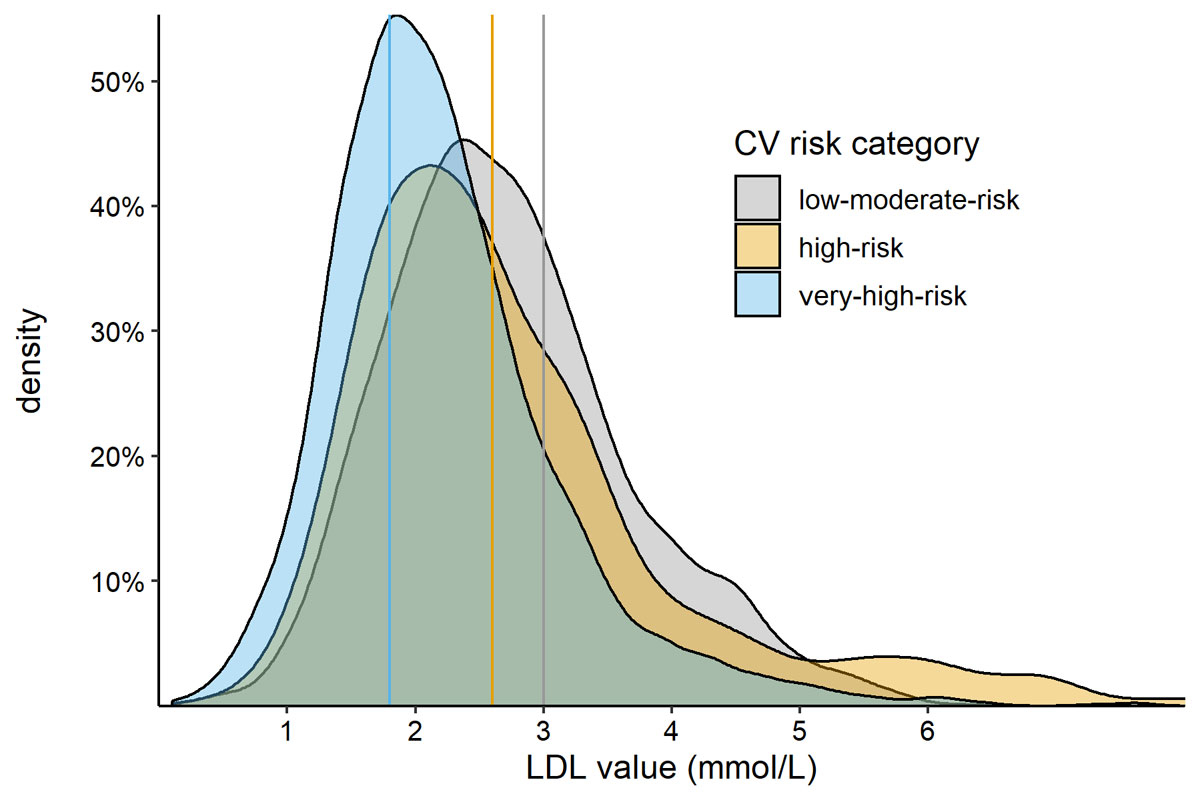
Figure 2 Distribution of patients’ LDL levels by cardiovascular risk. Areas under the curve represent all patients with a measurement (100%), the height of the curve represents the estimated percentage of patients with the corresponding LDL value (Kernel density estimation) for the different CV risk categories. Guideline recommended target values for the respective CV risk category (low/moderate <3.0 mmol/l, high <2.6 mmol/l, very high <1.8 mmol/l) are indicated by colour-coded vertical lines. Upper limit for data visualisation was 8 mmol/l.
Table 1 Patient characteristics by cardiovascular risk.
|
Low/moderate risk
(n = 4873, 41.4%) |
High risk
(n = 1218, 10.3%) |
Very high risk
(n = 5688, 48.3%) |
p-value | |
|---|---|---|---|---|
| Median age (IQR) | 68 (60–75) | 76 (71–83) | 71 (63–79) | <0.001* |
| % male | 58.5 | 46.7 | 64.8 | <0.001† |
| Median lipid levels (IQR) in mmol/l | ||||
| LDL | 2.6 (2.1–3.3) | 2.5 (1.9–3.2) | 2.1 (1.6–2.6) | <0.001* |
| HDL | 1.5 (1.2–1.8) | 1.5 (1.2–1.8) | 1.3 (1.1–1.5) | <0.001* |
| Total cholesterol | 4.8 (4.1–5.6) | 4.7 (3.9–5.7) | 4.1 (3.5–4.9) | <0.001* |
| Triglycerides | 1.4 (1.0–1.9) | 1.5 (1.1–2.0) | 1.5 (1.1–2.2) | <0.001* |
| Morbidities | ||||
| % with previous ASCVD | 0.0 | 0.0 | 46.0 | <0.001† |
| % with severe CKD | 0.0 | 0.0 | 7.4 | <0.001† |
| % with moderate CKD | 0.0 | 91.1 | 27.3 | <0.001† |
| % with diabetes | 0.0 | 2.0 | 66.7 | <0.001† |
| % with hypertension | 62.4 | 78.0 | 84.0 | <0.001† |
IQR = interquartile range, LDL = low-density lipoprotein, HDL = high-density lipoprotein, ASCVD = atherosclerotic cardiovascular disease, CKD = chronic kidney disease Test applied for group comparisons: * Kruskal-Wallis rank sum test, † chi-square test Very high risk: previous atherosclerotic cardiovascular disease, diabetes mellitus with target organ damage or with a major risk factor, or severe chronic kidney disease. High risk: diabetes mellitus without risk factors or target organ damage, single risk factors (cholesterol >8 mmol/l or blood pressure >180/110 mm Hg), or moderate chronic kidney disease. Low/moderate risk: the remaining patients.
Patients had a median of 10 (IQR = 5–17) consultations per year and 53.9% (n = 6349) had at least one lipid measurement. Statin treatment intensity was low for 4.4.% (n = 522), moderate for 50.6% (n = 5955) and high for 39.0% (n = 4588) of the patients (7% missing). A combination treatment of statin with other lipid modifying agents was prescribed to 8.9% (n = 1,047) of the patients. The most commonly prescribed statins were atorvastatin (56.3%, n = 6,634), rosuvastatin (22.6%, n = 2,667) and simvastatin (12.5%, n = 1,472). Treatment strategies stratified by CV risk are shown in table 2.
Table 2 Treatment strategy by cardiovascular risk.
|
Low/moderate-risk
(n = 4873, 41.4%) |
High risk
(n = 1218, 10.3%) |
Very high risk
(n = 5688, 48.3%) |
p-value | |
|---|---|---|---|---|
| Median number of consultations (IQR) | 8 (4–14) | 11 (6–19) | 11 (6–19) | <0.001* |
| Number of lipid measurements | ||||
| % with none | 49.8 | 46.2 | 42.8 | <0.001† |
| % with 1 | 38.7 | 39.6 | 41.5 | 0.012† |
| % with 2 | 8.6 | 10.0 | 10.8 | 0.001† |
| % with more than 2 | 2.8 | 4.2 | 4.8 | <0.001† |
| Treatment intensity | ||||
| % low | 5.1 | 5.6 | 3.6 | <0.001† |
| % moderate | 53.1 | 58.7 | 46.6 | <0.001† |
| % high | 34.5 | 30.0 | 44.7 | <0.001† |
| % missing | 8.0 | 7.0 | 6.0 | <0.001† |
| Combination treatment with other LMA | 8.1 | 7.6 | 9.8 | 0.002† |
| Statin product (generic name) | ||||
| % atorvastatin | 55.2 | 53.0 | 58.0 | 0.001† |
| % fluvastatin | 0.7 | 1.0 | 0.8 | 0.630† |
| % pitavastatin | 0.4 | 0.4 | 0.3 | 0.366‡ |
| % pravastatin | 7.6 | 9.8 | 6.8 | 0.002† |
| % rosuvastatin | 23.9 | 21.8 | 21.7 | 0.025† |
| % simvastatin | 12.2 | 14.0 | 12.4 | 0.256† |
IQR = interquartile range, LMA = lipid modifying agents Test applied for group comparisons: * Kruskal-Wallis rank sum test, † chi-square test, ‡ Fisher’s exact test Very high risk: previous atherosclerotic cardiovascular disease, diabetes mellitus with target organ damage or with a major risk factor, or severe chronic kidney disease. High risk: diabetes mellitus without risk factors or target organ damage, single risk factors (cholesterol >8 mmol/l or blood pressure >180/110 mm Hg), or moderate chronic kidney disease. Low/moderate risk: the remaining patients. Treatment intensity was calculated from product and daily dose according to the 2014 ACC/AHA guidelines [11] as specified in appendix 1.
Of all the statin-treated patients, 43.6% (n = 5135) had an LDL measurement in 2018 and could thus be analysed for LDL target achievement. In total, 49.6% (n = 2549) of these patients reached their recommended LDL target value. Target achievement was 65.7% (n = 1338) for low-/moderate-risk patients (n = 2037), 56.3% (n = 294) for high-risk patients (n = 522) and 35.6% (n = 917) for very high-risk patients (n = 2576, p <0.001).
Multivariable logistic regression analysis also suggested that target achievement decreased with increasing CV risk (high risk vs low/moderate risk: OR = 0.58, CI = 0.42–0.80, p <0.001; very high risk vs low/moderate risk: 0.30, CI = 0.24–0.37, p = 0.008; very high-risk vs high risk: 0.52, CI = 0.38–0.73, p = 0.001). Moreover, target achievement rates were higher for male than for female patients across all CV risk categories, particularly among low/moderate risk (OR = 2.35, CI = 1.90–2.90, p <0.001) and high-risk (OR = 2.38, CI = 1.56–3.63, p = 0.001) groups. Additionally, in male patients at low/moderate risk, age was positively associated with LDL target achievement (OR = 1.56, CI = 1.36–1.79, p <0.001). The complete regression analysis, as well as estimates computed from multiple comparisons, can be found in the appendix 1. A visual representation of the empirical and predicted target achievement rates by age, CV risk category and gender is shown in figure 3.
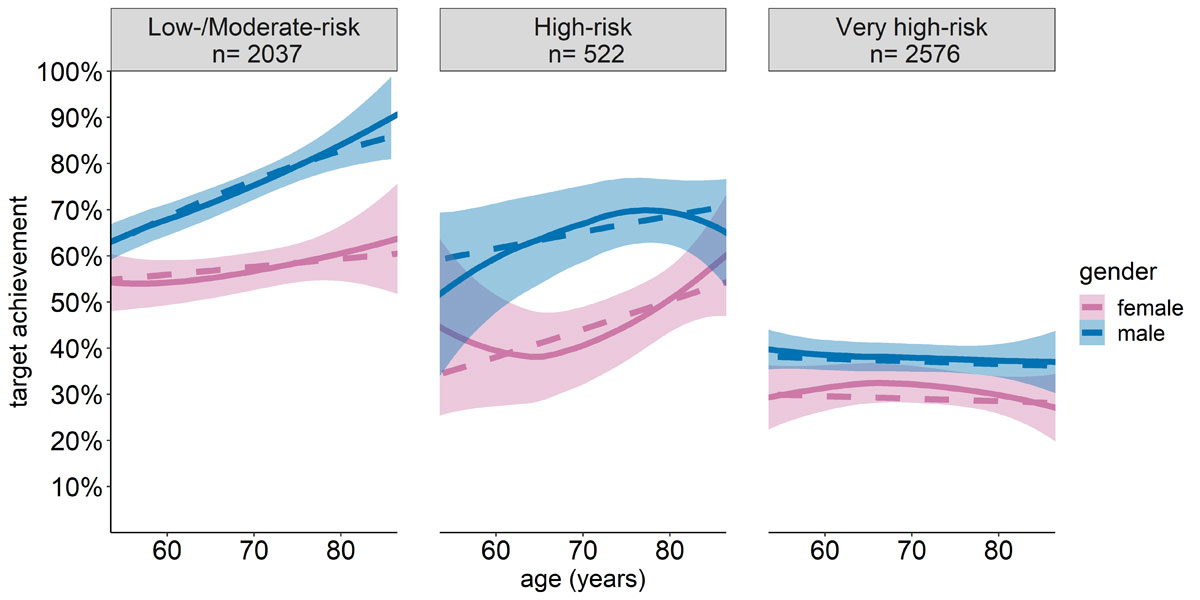
Figure 3 LDL target achievement by cardiovascular risk, age and gender. Target achievement plotted with respect to patient age, stratified by CV risk category and gender. Solid lines and CI represent empirical data, while dashed lines represent the predicted values based on the regression analysis. Data visualisation was restricted to ages 55–85 years due to limited sample sizes at the tails of the distributions.
Of 11,779 patients treated with statins, 59% were at a high or very high risk of fatal CVD. Only 39% of all patients received high-intensity statin therapy and only 44% had their LDL levels measured. LDL targets were achieved by only a third of patients with very high CV risk, and by two thirds of patients with low/moderate CV risk. Target achievement was higher in male patients across all risk categories. Our findings suggest that European and national guidelines [15] are not thoroughly implemented.
The European and American guidelines agree that high-intensity statin treatment is indicated for patients with very high CV risk [10, 12]. In our study, however, we found that a minority of these patients actually received high-intensity statin treatment. Underuse of high-intensity statin treatment is common in general practice [16–18], and several reasons may explain this finding. Statin-related side-effects led to the discontinuation of treatment in 10% of patients [19], and Deshpande et al. observed poorer patient adherence to high-intensity statin regimens when compared to lower intensity regimens [20]. Such patient-related treatment limitations may decrease the implementation of high-intensity statins, but they scarcely explain all of the under-treatment we observed.
Similarly to statin treatment, there appears to be a considerable gap in annual LDL measurements, which were only performed in about half of the patients with very high CV risk. Again, previous studies have made similar observations [16]. One reason for not measuring LDL levels is the impossibility of further increasing the treatment intensity because of side effects or because the maximum intensity has already been reached. Also, some GPs may follow a “fire and forget” treatment strategy, in which statins are prescribed strictly in accordance with the CV risk and no LDL targets are followed, making lipid monitoring less important [12].
Our study revealed multiple interesting findings concerning LDL target achievement. In patients with very high CV risk, an achievement rate of 35% is rather high compared to the rates found by other studies from a range of different countries [16, 21–24]. Nevertheless, this rate still indicates room for further improvement. Potential reasons for not achieving the recommended treatment target values are the underuse of high-intensity statins and low measurement rates. Since ESC and national guidelines do not recommend a “fire and forget” approach, it seems unlikely that a major proportion of Swiss GPs actually follow this strategy.
Interestingly, and in accordance with previous studies, we found a systematic under-achievement in CV prevention in female compared to male patients [25–28]. This difference was especially pronounced in LDL target achievement for patients with low/moderate to high risk. Guidelines, however, do not provide gender-specific target values, and statins are equally effective in male and female patients [29]. Bairey Merz et al. have shown that beliefs and attitudes are barriers to the CV risk management of women, and suggest these barriers should be targeted with specific campaigns to close the gender gap [30].
The main limitation of this study was that we only used morbidities for our risk classification, without considering prognostic probabilities based on the parameter values of age, hypertension and baseline LDL, which might be used by GPs in practice. Thus, there was a risk of potential underestimation in our study, meaning we may have evaluated some patients as being in too low a risk category for LDL target achievement. Consequently, achievement rates in the low/moderate risk category may be overestimated. This is especially the case for elderly male patients because with increasing age, risk estimation instruments will estimate higher risks for men compared to women. However, overestimation of risk and therefore false-positive classification of high- and very high-risk patients was unlikely. Thus, our results are most valid in those risk categories for which patients have the largest potential to benefit from statin treatment. In addition, it should be recognised that target achievement was derived from the subgroup with available LDL measurements, and the “true” target achievement of the total population remains unknown in this study, as in real life. Ultimately, it must be acknowledged that the reasons for insufficient statin therapy remain unclear, and complete guideline adherence is never possible due to individual patient factors such as the appearance of side effects or other contraindications, which we did not assess in this study.
Half of the patients treated with statins in Swiss general practice were at very high risk of fatal CV events. The majority of patients did not receive high-intensity statin treatment, and only a third of patients with very high CV risk achieved LDL target values. Moreover, there was a gender gap in LDL target achievement disadvantaging female patients. Results from this study suggest that current treatment may warrant reconsideration in a large proportion of patients, especially in light of the new 2019 ESC guidelines recommending even lower LDL target levels.
The appendix is available in a separate file at https://smw.ch/article/doi/smw.2020.2044.
Our thanks go to Fabio Valeri for statistical consulting and the FIRE study group of general practitioners for their participation in the project.
This study was supported by a grant from the Swiss Medical Board.
The authors declare that they have no relevant conflicts of interest regarding this study.
1 Roth GA , Abate D , Abate KH , Abay SM , Abbafati C , Abbasi N , et al.; GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–88. doi:.https://doi.org/10.1016/S0140-6736(18)32203-7
2 Ference BA , Ginsberg HN , Graham I , Ray KK , Packard CJ , Bruckert E , et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–72. doi:.https://doi.org/10.1093/eurheartj/ehx144
3 Baigent C , Keech A , Kearney PM , Blackwell L , Buck G , Pollicino C , et al., Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–78. doi:.https://doi.org/10.1016/S0140-6736(05)67394-1
4 Tramacere I , Boncoraglio GB , Banzi R , Del Giovane C , Kwag KH , Squizzato A , et al. Comparison of statins for secondary prevention in patients with ischemic stroke or transient ischemic attack: a systematic review and network meta-analysis. BMC Med. 2019;17(1):67. doi:.https://doi.org/10.1186/s12916-019-1298-5
5 Taylor F , Huffman MD , Macedo AF , Moore TH , Burke M , Davey Smith G , et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816. doi:.https://doi.org/10.1002/14651858.CD004816.pub5
6 Ward S , Lloyd Jones M , Pandor A , Holmes M , Ara R , Ryan A , et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess. 2007;11(14):1–160, iii–iv. doi:.https://doi.org/10.3310/hta11140
7 Vrecer M , Turk S , Drinovec J , Mrhar A . Use of statins in primary and secondary prevention of coronary heart disease and ischemic stroke. Meta-analysis of randomized trials. Int J Clin Pharmacol Ther. 2003;41(12):567–77. doi:.https://doi.org/10.5414/CPP41567
8 Byrne P , Cullinan J , Smith A , Smith SM . Statins for the primary prevention of cardiovascular disease: an overview of systematic reviews. BMJ Open. 2019;9(4):e023085. doi:.https://doi.org/10.1136/bmjopen-2018-023085
9 Damen JA , Hooft L , Schuit E , Debray TP , Collins GS , Tzoulaki I , et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416. doi:.https://doi.org/10.1136/bmj.i2416
10 Catapano AL , Graham I , De Backer G , Wiklund O , Chapman MJ , Drexel H , et al.; ESC Scientific Document Group. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J. 2016;37(39):2999–3058. doi:.https://doi.org/10.1093/eurheartj/ehw272
11 Shepherd J . Resource management in prevention of coronary heart disease: optimising prescription of lipid-lowering drugs. Lancet. 2002;359(9325):2271–3. doi:.https://doi.org/10.1016/S0140-6736(02)09299-1
12 Stone NJ , Robinson JG , Lichtenstein AH , Bairey Merz CN , Blum CB , Eckel RH , et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, 25 Pt B):2889–934. doi:. Correction in: J Am Coll Cardiol. 2014;63(25, 25 Pt B):3024–5 https://doi.org/10.1016/j.jacc.2013.11.002
13 Chmiel C , Bhend H , Senn O , Zoller M , Rosemann T ; FIRE study-group. The FIRE project: a milestone for research in primary care in Switzerland. Swiss Med Wkly. 2011;140:w13142.
14R Core Team. R: A language and environment for statistical computing. In: Computing RFfS, editor. Vienna, Austria2018.
15 Swiss Atherosclerosis Association (AGLA). Available from: https://www.agla.ch/.
16 Danese MD , Sidelnikov E , Kutikova L . The prevalence, low-density lipoprotein cholesterol levels, and treatment of patients at very high risk of cardiovascular events in the United Kingdom: a cross-sectional study. Curr Med Res Opin. 2018;34(8):1441–7. doi:.https://doi.org/10.1080/03007995.2018.1463211
17 Rodriguez F , Maron DJ , Knowles JW , Virani SS , Lin S , Heidenreich PA . Association Between Intensity of Statin Therapy and Mortality in Patients With Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2017;2(1):47–54. doi:.https://doi.org/10.1001/jamacardio.2016.4052
18 Yang Z , Edwards D , Massou E , Saunders CL , Brayne C , Mant J . Statin use and high-dose statin use after ischemic stroke in the UK: a retrospective cohort study. Clin Epidemiol. 2019;11:495–508. doi:.https://doi.org/10.2147/CLEP.S201983
19 Zhang H , Plutzky J , Skentzos S , Morrison F , Mar P , Shubina M , et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158(7):526–34. doi:.https://doi.org/10.7326/0003-4819-158-7-201304020-00004
20 Deshpande S , Quek RGW , Forbes CA , de Kock S , Kleijnen J , Gandra SR , et al. A systematic review to assess adherence and persistence with statins. Curr Med Res Opin. 2017;33(4):769–78. doi:.https://doi.org/10.1080/03007995.2017.1281109
21 Presta V , Figliuzzi I , Miceli F , Coluccia R , Fogacci F , Cicero AFG , et al.; EFFECTUS Steering Committee. Achievement of low density lipoprotein (LDL) cholesterol targets in primary and secondary prevention: Analysis of a large real practice database in Italy. Atherosclerosis. 2019;285:40–8. doi:.https://doi.org/10.1016/j.atherosclerosis.2019.03.017
22 Arca M , Ansell D , Averna M , Fanelli F , Gorcyca K , Iorga SR , et al. Statin utilization and lipid goal attainment in high or very-high cardiovascular risk patients: Insights from Italian general practice. Atherosclerosis. 2018;271:120–7. doi:.https://doi.org/10.1016/j.atherosclerosis.2018.02.024
23 Ferrières J , Gorcyca K , Iorga ŞR , Ansell D , Steen DL . Lipid-lowering Therapy and Goal Achievement in High-risk Patients From French General Practice. Clin Ther. 2018;40(9):1484–1495.e22. doi:.https://doi.org/10.1016/j.clinthera.2018.07.008
24 März W , Dippel F-W , Theobald K , Gorcyca K , Iorga ŞR , Ansell D . Utilization of lipid-modifying therapy and low-density lipoprotein cholesterol goal attainment in patients at high and very-high cardiovascular risk: Real-world evidence from Germany. Atherosclerosis. 2018;268:99–107. doi:.https://doi.org/10.1016/j.atherosclerosis.2017.11.020
25 Rodriguez F , Olufade TO , Ramey DR , Friedman HS , Navaratnam P , Heithoff K , et al. Gender Disparities in Lipid-Lowering Therapy in Cardiovascular Disease: Insights from a Managed Care Population. J Womens Health (Larchmt). 2016;25(7):697–706. doi:.https://doi.org/10.1089/jwh.2015.5282
26 Santos RD , Waters DD , Tarasenko L , Messig M , Jukema JW , Ferrières J , et al.; L-TAP 2 Investigators. Low- and high-density lipoprotein cholesterol goal attainment in dyslipidemic women: The Lipid Treatment Assessment Project (L-TAP) 2. Am Heart J. 2009;158(5):860–6. doi:.https://doi.org/10.1016/j.ahj.2009.08.009
27 Moreno-Arellano S , Delgado-de-Mendoza J , Santi-Cano MJ . Sex disparity persists in the prevention of cardiovascular disease in women on statin therapy compared to that in men. Nutr Metab Cardiovasc Dis. 2018;28(8):810–5. doi:.https://doi.org/10.1016/j.numecd.2018.03.012
28 Schoen MW , Tabak RG , Salas J , Scherrer JF , Buckhold FR . Comparison of Adherence to Guideline-Based Cholesterol Treatment Goals in Men Versus Women. Am J Cardiol. 2016;117(1):48–53. doi:.https://doi.org/10.1016/j.amjcard.2015.10.007
29 Fulcher J , O’Connell R , Voysey M , Emberson J , Blackwell L , Mihaylova B , et al., Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385(9976):1397–405. doi:.https://doi.org/10.1016/S0140-6736(14)61368-4
30 Bairey Merz CN , Andersen H , Sprague E , Burns A , Keida M , Walsh MN , et al. Knowledge, Attitudes, and Beliefs Regarding Cardiovascular Disease in Women: The Women’s Heart Alliance. J Am Coll Cardiol. 2017;70(2):123–32. doi:. Correction in: J Am Coll Cardiol. 2017;70(8)https://doi.org/10.1016/j.jacc.2017.05.024
This study was supported by a grant from the Swiss Medical Board.
The authors declare that they have no relevant conflicts of interest regarding this study.