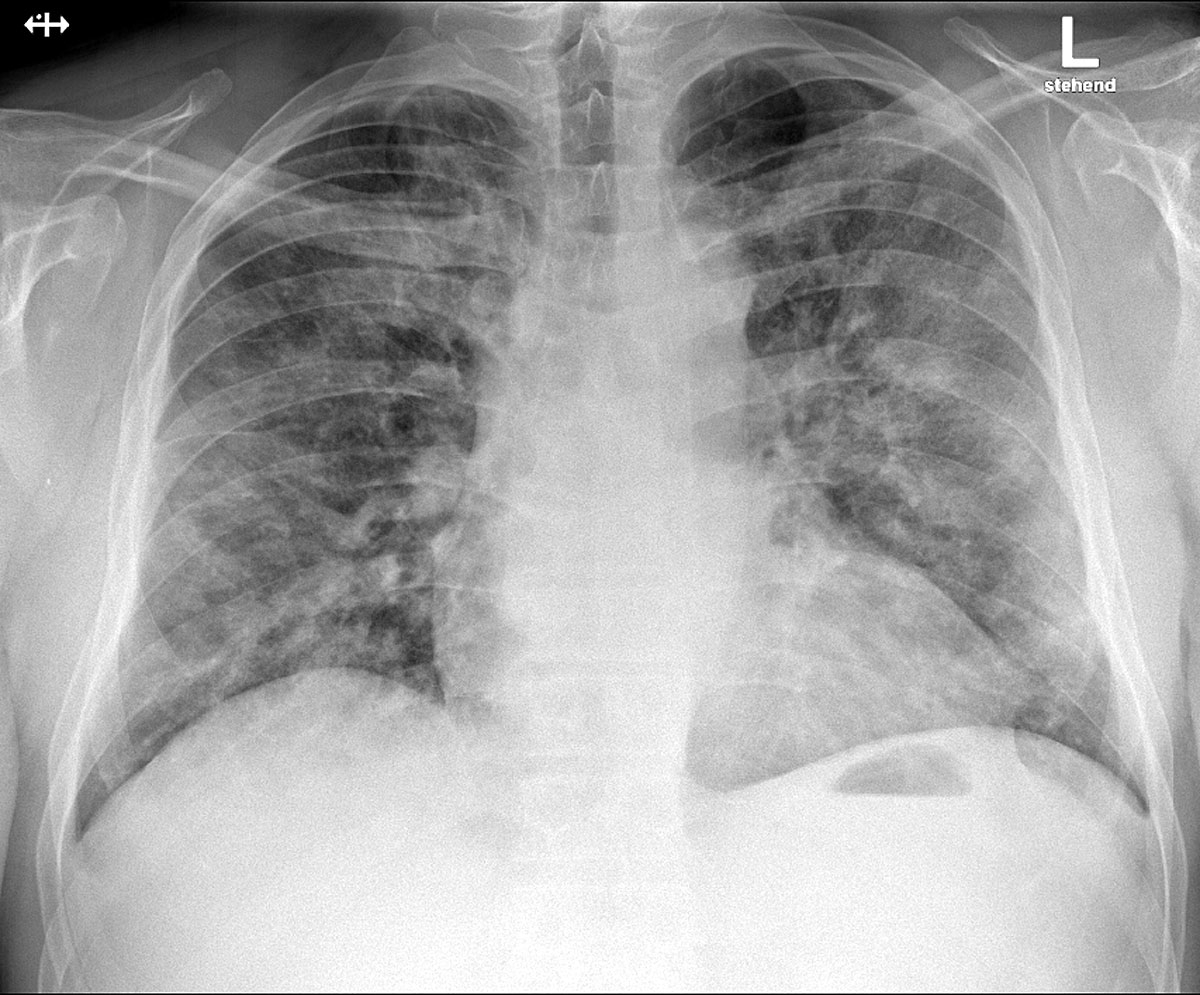
Figure 1 Posteroanterior chest X-ray at admission, demonstrating bilateral patchy perihilar opacities consistent with a pneumonic infiltration.
DOI: https://doi.org/10.4414/smw.2020.20293
coronavirus disease 2019
C-reactive protein
computed tomography
disseminated intravascular coagulation
intensive care unit
real-time polymerase chain reaction
severe acute respiratory syndrome coronavirus 2
Sepsis-induced Coagulopathy score
The emergence of SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) as a global phenomenon has presented clinicians around the world with multiple challenges. The difficult task of reorganising healthcare on a large scale to deal with a rapidly changing situation has been matched by the everyday clinical challenge of treating the varied effects of a novel viral infection.
Thromboembolic events are recognised complications of viral infection, but the diagnosis of an acute pulmonary thrombotic complication in the context of COVID-19 (coronavirus disease 2019) can be challenging because of the similarities of presentation, logistical considerations of diagnosis in a patient isolated for infection control reasons, and the effects of cognitive errors in diagnostic reasoning. Emerging and cumulative international data from clinical observations [1–4] continue to contribute to the understanding of the underlying pathophysiology and point towards a distinctive coagulopathy associated with severe cases of COVID-19.
We present the case of a patient who was diagnosed with a pulmonary thrombotic complication during inpatient care for COVID-19. We explore the diagnostic and monitoring considerations for patients with COVID-19 with worsening dyspnoea and elevated D-dimers, along with cognitive considerations when managing the complications of an emerging disease process.
A 55-year-old male patient presented to our emergency department in the week following the federal implementation of social distancing measures in Switzerland. He described a history of fever and dry cough over the previous 4 days, but reported no dyspnoea, pleuritic pain or typical angina pectoris symptoms. Although he described a generalised malaise, he had been able to continue working in his moderately physically demanding profession.
The patient’s previous medical history was limited to hypertension, diagnosed 20 years previously, treated with a once daily combination tablet of verapamil 180 mg and trandolapril 2 mg. The patient was a non-smoker and had no contact with a known case of COVID-19 or travel history to an area of high prevalence. The prevalence of positive real-time polymerase chain reaction (RT-PCR) test for SARS-CoV-2 in our local administrative authority on the day of admission was 42/100,000.
Clinical examination on admission in the emergency department revealed bilateral reduced breath sounds at the posterior lung bases without wheeze or rales. No tenderness or peritonitic signs were present on abdominal examination. Examination of the oral cavity and cervical, submandibular and supraclavicular recesses was unremarkable. Blood pressure was 136/79 mm Hg and the pulse regular with a rate of 59/min. Peripheral oxygen saturation under room air was 95% and tympanic temperature was 36.5°C.
A chest radiograph was performed in the emergency department which demonstrated bilateral patchy perihilar opacities consistent with a pneumonic infiltration, with no evidence of pleural effusions or cardiomegaly (fig. 1). Arterial blood gas measurements revealed type 1 respiratory failure with a compensated respiratory alkalosis on room air (pH 7.44, pCO2 34.5 mm Hg, pO2 72.6 mm Hg, bicarbonate 22.9 mmol/l, base excess −0.7 mmol/l). Full blood count showed no anaemia (haemoglobin 142 g/l), but a slight lymphopenia (leucocytes 4.5 ×109/l, lymphocytes 1.0 ×109/l). Thrombocyte count was normal (194 ×109/L). A chemistry panel showed a raised C-reactive protein (CRP) of 52 mg/l and lactate dehydrogenase (LDH) of 260 U/l with no other significant abnormalities. A nasopharyngeal swab was taken on admission (in keeping with national guidelines recommending testing of all inpatients with suspected respiratory infection) and reported positive for SARS-CoV-2 by RT-PCR. In the initial phase of hospitalisation CRP rose to a peak of 233.4 mg/l on day 5, with ferritin peaking at 2125 µg/l on day 6. Serial measurements of the transaminases peaked on day 5 with an aspartate aminotransferase (ASAT) of 134 U/l and alanine aminotransferase (ALAT) of 119 U/l, and sank below twice the upper limit of normal by day 10. Following the emergence of literature [5] observing a prognostic association, on day 5 we assayed D-dimer, which was elevated (1.33 mg/l).

Figure 1 Posteroanterior chest X-ray at admission, demonstrating bilateral patchy perihilar opacities consistent with a pneumonic infiltration.
On day 10, following a respiratory deterioration requiring oxygenation with 15 l/min delivered via a face mask with reservoir, the patient was transferred to our intensive care unit (ICU). After an initial early peak, CRP had at this point fallen to 79.5 mg/l although D-dimer levels were now 6.62 mg/l with a fibrinogen of 5.9 g/l. Given the respiratory deterioration, despite apparent improvement in laboratory markers of inflammation, we elected to perform a computed tomography (CT) examination of the thorax with a pulmonary angiogram to assess for parenchymal complications of infection (such as abscess, empyema) and rule out a macrovascular thromboembolism. This examination demonstrated filling defects in the left upper lobe, right upper lobe and right middle lobe pulmonary arteries extending to the segmental arteries consistent with bilateral paracentral pulmonary embolism, and in addition widespread peripheral pneumonic infiltrates consistent with the diagnosis of COVID-19 [6] in all lobes (fig. 2).
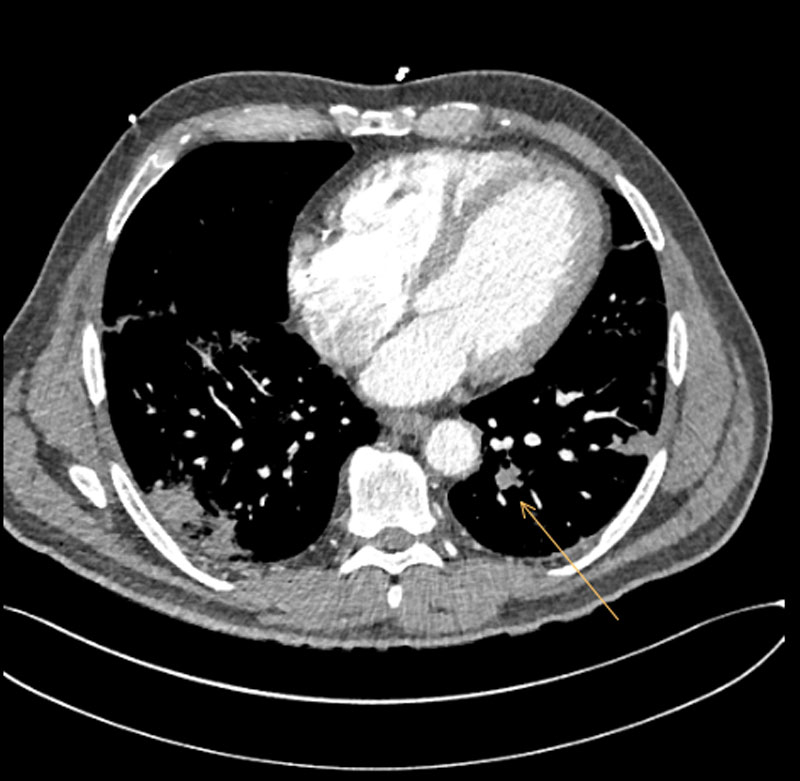
Figure 2 Computed tomography pulmonary angiogram demonstrating pulmonary embolism 10 days after admission.
To further investigate a secondary or acquired coagulopathy, we performed a thrombophilia screen which demonstrated a raised factor VIII:C (278%) and raised von Willebrand factor activity and antigen (233% and 254%, respectively). Antithrombin (101%), activated partial thromboplastin time (aPTT; 35.8 sec), protein C (chromogenic 109% and coagulometric method 80%) and free protein S antigen (64%) were normal. Lupus anticoagulans, anti-cardiolipin and anti-β2-glycoprotein I antibodies (IgG and IgM) were negative.
The patient's usual therapy with a combination tablet of verapamil and trandolapril was stopped on admission due to the admitting team’s concerns about potential haemodynamic instability. Antibiotic therapy with doxycycline was initiated on admission as an empirical therapy to cover for atypical bacterial infection. Following the positive diagnosis of COVID-19 with typical radiological findings and RT-PCR on day 2, hydroxychloroquine was started per os (three 12-hourly doses of 400 mg followed by 200 mg twice daily, which was continued for 10 days) in accordance with contemporaneous regional practice. The patient received daily thromboembolism prophylaxis with subcutaneous 5000 IU dalteparin from admission onwards, in keeping with our inpatient protocol and interim national guidance [7]. Following an increasing oxygen requirement on day 5, the antibiotic therapy was changed to intravenous amoxicillin/clavulanic acid with the intention of broader empirical treatment of a possible bacterial superinfection. On day 10, after diagnosing bilateral pulmonary embolism, we began a subcutaneous and weight-adjusted therapeutic anticoagulation with 10,000 IU dalteparin in the morning and 7,500 IU in the evening. The twice-daily administration was chosen in order to avoid high peak concentrations and possible lung alveolar haemorrhage.
Following the diagnosis of a pulmonary embolism, and due to increasing oxygen requirements with impending respiratory exhaustion, the patient was intubated and required positive pressure ventilation for 48 hours. Peak laboratory values for D-dimers and erythrocyte sedimentation rate occurred at day 14 after the onset of symptoms and were 15.74 mg/l and 92 mm/h, respectively. After weaning, the patient was transferred to a peripheral ward for further inpatient observation. Therapeutic anticoagulation with low molecular weight heparin was uneventful; no haemorrhagic complication occurred. There were no further indications of progressive multiorgan dysfunction, haemodynamic instability or neurological sequelae. The patient was discharged into the community with no ongoing oxygen requirements and a therapeutic dose of rivaroxaban. Values for D-dimers and erythrocyte sedimentation rate normalised 6 weeks after the onset of symptoms (<0.15 mg/l and 17 mm/h, respectively).
D-dimer elevation over 1 mg/l at admission has been associated with increased odds of in-hospital death in COVID-19 (odds ratio 18.42), with a significant difference in elevation between survivors and nonsurvivors 7 days after admission [5]. More recent cohorts of patients with COVID-19 have been also described, with evidence pointing to an unusually high prevalence of arterial and venous thromboembolic events. An early cohort of 81 Chinese patients with severe COVID-19 were described as having a 25% crude prevalence of venous thromboembolism [8]. A Dutch 184-patient ICU cohort with COVID-19, receiving pharmacological thromboprophylaxis according to local hospital protocols, had a cumulative incidence of venous and arterial thrombotic complications of 43% after adjustment for competing risk of death [9, 10]. Sixty-four clinically relevant thrombotic complications were diagnosed in a French ICU cohort of 150 patients with COVID-19 [3], and a further mixed inpatient cohort of Dutch patients had a 42% cumulative incidence of thrombotic complications in a competing risk model [11]. In the first 12 consecutive COVID-19 autopsies performed in Hamburg, 7 patients were found to have previously undiagnosed deep venous thrombosis [4], and pulmonary embolism was the direct cause of death in 4 of these patients. Histological analysis revealed a common phenomenon of microthrombosis and thrombosis of the small pulmonary vessels.
The objective evidence of filling defects in the pulmonary arterial macrovasculature in our case confirms the presence of a thrombotic obstruction, and published evidence from autopsies extend this phenomenon to the pulmonary microvasculature [4]. An initial, and conventional interpretation, would be to consider the pulmonary embolism as a thromboembolic complication of a sepsis-associated coagulopathy, implied by temporal association between typical symptoms of COVID-19 and an acute thromboembolic event. It is common to see a concomitant rise of the D-dimers in many inflammatory and infectious pathologies, but the discrepant and continued evolution of CRP and D-dimer levels in our patient, despite the short in-vitro half-life of D-dimers (approximately 16 hours [12]) prompted the suspicion of an underlying coagulopathy.
At the time of diagnosis of pulmonary embolism in this patient, there were no signs of multi-organ failure, inflammatory markers had significantly fallen from an initial peak and appropriate thromboembolic chemoprophylaxis had been initiated from admission provoking suspicion of an alternative underlying process.
Screening of patients with severe COVID-19 for hyperinflammation has been proposed, with the conceptual proposal of selective cytokine blockade for patients with a clinical constellation suggestive of a secondary haemophagocytic lymphohistiocytosis (sHLH) [13]. sHLH has been observed in other viral infections, including SARS-CoV [14], and a macrophage activation syndrome-like immunopathology in COVID-19 has been proposed [15]. In this case, a peak HScore of 97 points with low serum triglycerides (peak 1.9 mmol/l, 10 days after initial symptoms), without the examination of bone marrow aspirate, suggested a low probability (<1%) of a typical haemophagocytic syndrome [16].
The presence of a progressive normochromic, normocytic anaemia during the hospitalisation together with a raised LDH and an acute pulmonary thrombotic complication prompted suspicion of a microangiopathic haemolytic anaemia. However, the microscopic examination of a peripheral blood smear showed a normal erythrocyte morphology and no schistocytes, which, together with the normal thrombocyte count, made a microangiopathic haemolytic anaemia unlikely.
Disseminated intravascular coagulation (DIC) was diagnosed in 16/183 patients (overt DIC with DIC score ≥5 points [17]) in an early description of inpatients with COVID-19 [18]. Interestingly the prevalence in this inpatient cohort was not replicated in an ICU cohort [3]. The raised fibrinogen in our patient (5.99–7.72 g/l during hospitalisation) and normal thrombocyte counts (194–423 ×109/l during hospitalisation) are unusual in the context of a consumptive coagulopathy and make this diagnosis unlikely. In a retrospective analysis of 449 patients with severe COVID-19, a difference in mortality with heparin was demonstrated in a subgroup of patients with a Sepsis-induced Coagulopathy score (SIC) ≥4 or D-dimers more than 6 times the upper limit of normal [19]. The DIC score at the time of pulmonary embolism diagnosis in our patient was 3 points, not suggestive of overt consumptive DIC [20], and suggesting a less pronounced coagulopathy than seen in the group that benefited from anticoagulation in the aforementioned cohort.
Von Willebrand factor is recognised as an acute phase protein, being released from endothelial cells in response to inflammation [21], but the high levels in this case, in the context of receding inflammatory parameters, are suggestive of an endothelial disruption, with release of von Willebrand factor from Weibel-Palade bodies. We have made this observation previously in a separate patient [1] and these findings have been replicated in a larger ICU cohort of patients with COVID-19 [3]. Interestingly, endothelial cells express angiotensin-converting enzyme 2, the receptor for SARS-CoV-2, thus possibly mediating endothelial activation [22], and histopathological evidence of direct viral infection of endothelial cells and diffuse endothelial inflammation has been demonstrated in an autopsy case series of patients with COVID-19 [2]. Perfusion abnormalities on dual-energy CT are suggestive of intrapulmonary shunting, further supporting the hypothesis of thrombosis of the small lung arteries [23].
Given the negative prognostic implications of a significantly raised D-dimers [5, 18, 24], and our experience with a macrovascular thrombotic complication with a disproportionate rise of von Willebrand factor and factor VIII:C, we suggest that it is possible to hypothesise a spectrum of secondarily acquired, prothrombotic coagulopathy mediated by the endothelial interaction with SARS-CoV-2 as a cause of mortality in a subset of patients with a complicated clinical course of COVID-19. Given this hypothesis, we suggest being cautious in order to avoid a framing effect, where a clinician is strongly influenced by the way a problem or clinical scenario is framed [25] (in this context framing severe COVID-19 as a purely viral pneumonia) and consider the possibility of clinical thrombotic complications. In this way confirmation bias, with a selective tendency to focus on data that appear to support a currently held hypothesis [26], leading to a self-fulfilling prophecy of rising D-dimers and nonsurvival can potentially be avoided.
The downstream implications of isolation strategies in emergency and inpatient care settings have been demonstrated to influence diagnostic strategies and prescribing patterns [27]. The hospital room of a symptomatic patient with COVID-19 returned positive RT-PCR swabs in 13 of 15 sites around the room [28], and nosocomial infection was suspected in 41% of an early 138 inpatient cohort of patients with COVID-19, demonstrating the need for meticulous in-hospital infection control measures [29]. When deciding about the individual imaging indication for a patient, the risk of environmental contamination during transfer to a CT suite may play a role in the clinical suspicion threshold required to order contrast CT imaging by inducing an omission bias (a tendency towards inaction, and the acceptance of events attributed to a disease process [25]). In this context the use of point-of-care ultrasound for compression sonography to assess for the presence of deep vein thrombosis at the point of care in ICU may be of value, in keeping with the European Society of Cardiology Guidelines, which consider this modality as a first-line investigation and further imaging obviated if the result is positive (evidence level 1A [30]).
Renal failure is also common in inpatients with COVID-19, with acute kidney injury prevalence in multiple international cohorts of between 22 and 28% [31–33]. The potential complication of contrast-precipitated renal dysfunction can play a role in the decision-making process of clinicians [34], presenting a further barrier to appropriate imaging where lung scintigraphy is not readily available.
The use of low-dose, non-contrast CT imaging of the thorax has been used in China as an initial diagnostic modality where the performance characteristics of RT-PCR were either unclear or insufficient to exclude disease [35], although this approach may be of limited value at very early stages of disease [36]. This aggressive early imaging strategy without intravenous contrast would also fail to identify the later macrovascular or microvascular complications suggested by the dynamic change in D-dimer levels in nonsurvivors, giving diagnostic momentum to the erroneous hypothesis of a pure and uncomplicated viral pneumonia.
International guidelines broadly support a conventional spectrum of thromboprophylaxis in hospitalised patients with COVID-19. Joint recommendations endorsed by the International Society on Thrombosis and Haemostasis and the European Society of Vascular Medicine [37], and similarly the French Society of Vascular Medicine [38], recommend a risk-stratified thromboprophylaxis for hospitalised patients with COVID-19 [37]. The American Society of Hematologists and the Italian Society on Thrombosis and Haemostasis extend this recommendation to all hospitalised COVID-19 patients [39, 40], with the Italian guidelines recommending intermediate-dose anticoagulation for patients with multiple risk factors for venous thromboembolism. The British Thoracic Society supports local protocols under the guidance of a haematologist and discusses a risk-stratification strategy based on D-dimers and treatment-dose anticoagulation for patients with D-dimers >3.0 mg/l [41],
Current local recommendations from the Swiss Society of Haematology advocate pharmacological thromboprophylaxis according to a risk stratification score, with regular monitoring of conventional haemostasis parameters, and consideration of intermediate or therapeutic dosing in specific situations, balanced against the risk of bleeding [7].
German recommendations from the Gesellschaft für Thrombose- und Hämostaseforschung stress the importance of evaluating thromboprophylaxis for outpatients in addition to compulsory thromboprophylaxis appropriate for high-risk situations in all COVID-19 inpatients in the absence of contraindications.
Individual authors of observational studies have advocated the use of higher levels of anticoagulation [9, 42], but national professional societies and international organisations have maintained relative restraint. The divergence in specific aspects of managing coagulation in COVID-19 is understandable given the limited amount of prospective therapeutic trial data.
Raised levels of D-dimers has been negatively associated with prognosis in inpatients with COVID-19, although the pathophysiological mechanism behind the rise in D-dimers in COVID-19 has not been comprehensively described. The diagnosis of macro- and microvascular thrombotic complications in COVID-19 is challenging and they are potentially underdiagnosed. Where clinical signs or raised clinical suspicion of thromboembolism are present in patients with COVID-19, the additional logistic complexity of imaging in an isolated patient should not deter definitive diagnostic tests to rule out a macrovascular complication of COVID-19.
In the absence of robust therapeutic trial data, we suggest monitoring the coagulation parameters (in particular D-dimers), particularly in the subset of patients with severe COVID-19 pneumonia and increasing oxygen requirements, or patients with COVID-19 in a critical condition.
In keeping with international guidelines, we support the recommendation of thromboembolic chemoprophylaxis for all inpatients with COVID-19 as a very minimum in the absence of strict contraindications, while recognising that the patient we describe, and patients in other ICU cohorts, developed pulmonary thrombotic complications while under standard thromboprophylaxis. We further suggest that higher, possibly therapeutic levels of anticoagulation might be mandatory for a further subset of patients with COVID-19, especially if a discrepant evolution of falling CRP and rising D-dimers is observed. Therapeutic levels of anticoagulation are obligatory where new evidence of a macrovascular thrombotic complication has been documented. More research to delineate the macro- and microvascular thrombotic complications of COVID-19, and the therapeutic implications for this patient group is required.
The patient has given informed consent to the use of his biological data in anonymised form.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Escher R , Breakey N , Lämmle B . Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. doi:.https://doi.org/10.1016/j.thromres.2020.04.014
2 Varga Z , Flammer AJ , Steiger P , Haberecker M , Andermatt R , Zinkernagel AS , et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–8. doi:.https://doi.org/10.1016/S0140-6736(20)30937-5
3 Helms J , Tacquard C , Severac F , Leonard-Lorant I , Ohana M , Delabranche X , et al.; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020 May 4. [Online ahead of print] doi:.https://doi.org/10.1007/s00134-020-06062-x
4 Wichmann D , Sperhake J-P , Lütgehetmann M , Steurer S , Edler C , Heinemann A , et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020 May 6;M20-2003. [Online ahead of print] doi:.https://doi.org/10.7326/M20-2003
5 Zhou F , Yu T , Du R , Fan G , Liu Y , Liu Z , et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. doi:.https://doi.org/10.1016/S0140-6736(20)30566-3
6 Guan W-J , Ni Z-Y , Hu Y , Liang W-H , Ou C-Q , He J-X , et al.; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. doi:.https://doi.org/10.1056/NEJMoa2002032
7 Casini A , Alberio L , Angelillo-Scherrer A , Fontana P , Gerber B , Graf L , et al. Thromboprophylaxis and laboratory monitoring for in-hospital patients with COVID-19 - a Swiss consensus statement by the Working Party Hemostasis. Swiss Med Wkly. 2020;150:w20247. doi:.https://doi.org/10.4414/smw.2020.20247
8 Cui S , Chen S , Li X , Liu S , Wang F . Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020 April 9;jth.14830. [Online ahead of print] doi:.https://doi.org/10.1111/jth.14830
9 Klok FA , Kruip MJHA , van der Meer NJM , Arbous MS , Gommers DAMPJ , Kant KM , et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 April 10;S0049-3848(20)30120-1. [Online ahead of print] doi:.https://doi.org/10.1016/j.thromres.2020.04.013
10 Klok FA , Kruip MJHA , van der Meer NJM , Arbous MS , Gommers D , Kant KM , et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020 Apr 30;S0049-3848(20)30157-2. [Online ahead of print] doi:.https://doi.org/10.1016/j.thromres.2020.04.041
11 Middeldorp S , Coppens M , van Haaps TF , Foppen M , Vlaar AP , Müller MCA , et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020 May 5;jth.14888. [Online ahead of print] doi:.https://doi.org/10.1111/jth.14888
12 Rühl H , Berens C , Winterhagen A , Müller J , Oldenburg J , Pötzsch B . Label-Free Kinetic Studies of Hemostasis-Related Biomarkers Including D-Dimer Using Autologous Serum Transfusion. PLoS One. 2015;10(12):e0145012. doi:.https://doi.org/10.1371/journal.pone.0145012
13 Mehta P , McAuley DF , Brown M , Sanchez E , Tattersall RS , Manson JJ ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–4. doi:.https://doi.org/10.1016/S0140-6736(20)30628-0
14 Cascio A , Pernice LM , Barberi G , Delfino D , Biondo C , Beninati C , et al. Secondary hemophagocytic lymphohistiocytosis in zoonoses. A systematic review. Eur Rev Med Pharmacol Sci. 2012;16(10):1324–37. doi:https://www.ncbi.nlm.nih.gov/pubmed/23104648
15 McGonagle D , O’Donnell JS , Sharif K , Emery P , Bridgewood C . Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. The Lancet Rheumatology. 2020 May 7. doi:.https://doi.org/10.1016/S2665-9913(20)30121-1
16 Debaugnies F , Mahadeb B , Ferster A , Meuleman N , Rozen L , Demulder A , et al. Performances of the H-Score for Diagnosis of Hemophagocytic Lymphohistiocytosis in Adult and Pediatric Patients. Am J Clin Pathol. 2016;145(6):862–70. doi:.https://doi.org/10.1093/ajcp/aqw076
17 Taylor FB, Jr , Toh CH , Hoots WK , Wada H , Levi M ; Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–30. doi:.https://doi.org/10.1055/s-0037-1616068
18 Tang N , Li D , Wang X , Sun Z . Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–7. doi:.https://doi.org/10.1111/jth.14768
19 Yin S , Huang M , Li D , Tang N . Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020 Apr 3. [Online ahead of print] doi:.https://doi.org/10.1007/s11239-020-02105-8
20 Gando S , Saitoh D , Ogura H , Fujishima S , Mayumi T , Araki T , et al.; Japanese Association for Acute Medicine Sepsis Registry Study Group. A multicenter, prospective validation study of the Japanese Association for Acute Medicine disseminated intravascular coagulation scoring system in patients with severe sepsis. Crit Care. 2013;17(3):R111. doi:.https://doi.org/10.1186/cc12783
21 Kawecki C , Lenting PJ , Denis CV . von Willebrand factor and inflammation. J Thromb Haemost. 2017;15(7):1285–94. doi:.https://doi.org/10.1111/jth.13696
22 Hamming I , Timens W , Bulthuis MLC , Lely AT , Navis G , van Goor H . Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–7. doi:.https://doi.org/10.1002/path.1570
23 Lang M , Som A , Mendoza DP , Flores EJ , Reid N , Carey D , et al. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis. 2020 Apr 30;S1473-3099(20)30367-4. [Online ahead of print] doi:.https://doi.org/10.1016/S1473-3099(20)30367-4
24 Han H , Yang L , Liu R , Liu F , Wu K-L , Li J , et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;0(0):3. Available at: http://www.degruyter.com/view/j/cclm.ahead-of-print/cclm-2020-0188/cclm-2020-0188.xml. doi:.https://doi.org/10.1515/cclm-2020-0188
25 Croskerry P . The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775–80. doi:.https://doi.org/10.1097/00001888-200308000-00003
26 Croskerry P . Achieving Quality in Clinical Decision Making: Cognitive Strategies and Detection of Bias. Acad Emerg Med. 2002;9(11):1184–204. doi:http://doi.wiley.com/10.1197/aemj.9.11.1184
27 Stelfox HT , Bates DW , Redelmeier DA . Safety of patients isolated for infection control. JAMA. 2003;290(14):1899–905. doi:.https://doi.org/10.1001/jama.290.14.1899
28 Ong SWX , Tan YK , Chia PY , Lee TH , Ng OT , Wong MSY , et al. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA. 2020;323(16):1610. doi:.https://doi.org/10.1001/jama.2020.3227
29 Wang D , Hu B , Hu C , Zhu F , Liu X , Zhang J , et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi:.https://doi.org/10.1001/jama.2020.1585
30 Konstantinides SV , Meyer G , Becattini C , Bueno H , Geersing G-J , Harjola V-P , et al.; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543–603. doi:.https://doi.org/10.1093/eurheartj/ehz405
31 Richardson S , Hirsch JS , Narasimhan M , Crawford JM , McGinn T , Davidson KW , et al.; and the Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020 Apr 22. [Online ahead of print] doi:.https://doi.org/10.1001/jama.2020.6775
32 Fanelli V , Fiorentino M , Cantaluppi V , Gesualdo L , Stallone G , Ronco C , et al. Acute kidney injury in SARS-CoV-2 infected patients. Crit Care. 2020;24(1):155. doi:.https://doi.org/10.1186/s13054-020-02872-z
33 Yang X , Yu Y , Xu J , Shu H , Xia J , Liu H , et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81. doi:.https://doi.org/10.1016/S2213-2600(20)30079-5
34 Hinson JS , Ehmann MR , Fine DM , Fishman EK , Toerper MF , Rothman RE , et al. Risk of Acute Kidney Injury After Intravenous Contrast Media Administration. Ann Emerg Med. 2017;69(5):577–586.e4. doi:.https://doi.org/10.1016/j.annemergmed.2016.11.021
35 Ai T , Yang Z , Hou H , Zhan C , Chen C , Lv W , et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;200642:200642. doi:.https://doi.org/10.1148/radiol.2020200642
36 Simpson S , Kay FU , Abbara S , Bhalla S , Chung JH , Chung M , et al. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. J Thorac Imaging. 2020;2(2):e200152. doi:.https://doi.org/10.1148/ryct.2020200152
37 Bikdeli B , Madhavan MV , Jimenez D , Chuich T , Dreyfus I , Driggin E , et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-up. J Am Coll Cardiol. 2020 Apr 15;S0735-1097(20)35008-7. doi:.https://doi.org/10.1016/j.jacc.2020.04.031
38Propositions de la Société Française de Médecine Vasculaire pour la prévention, le diagnostic et le traitement de la maladie thromboembolique veineuse des patients avec COVID 19 non hospitalisés [Internet]. [cited 2020 May 13]. Available from: https://www.portailvasculaire.fr/sites/default/files/docs/propositions_sfmv_covid_mtev.pdf
39COVID-19 and Coagulopathy - American Society of Hematology [Internet]. [cited 2020 May 13]. Available from: https://www.hematology.org/covid-19/covid-19-and-coagulopathy
40 Marietta M , Ageno W , Artoni A , De Candia E , Gresele P , Marchetti M , et al. COVID-19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET). Blood Transfus. 2020 Apr 8. [Online ahead of print] doi:.https://doi.org/10.2450/2020.0083-20
41BTS Guidance on Venous Thromboembolic Disease in patients with COVID-19 – British Thoracic Society [Internet]. [cited 2020 May 17]. Available from: https://www.brit-thoracic.org.uk/document-library/quality-improvement/covid-19/bts-guidance-on-venous-thromboembolic-disease-in-patients-with-covid-19/
42 Atallah B , Mallah SI , AlMahmeed W . Anticoagulation in COVID-19. Eur Heart J Cardiovasc Pharmacother. 2020 Apr 30;pvaa036. [Online ahead of print] doi:.https://doi.org/10.1093/ehjcvp/pvaa036
No financial support and no other potential conflict of interest relevant to this article was reported.