Copeptin-based diagnosis of diabetes insipidus
DOI: https://doi.org/10.4414/smw.2020.20237
Julie
Refardtab, Mirjam
Christ-Crainab
aDepartments of Endocrinology, Diabetology and Metabolism, University Hospital Basel, Switzerland
bUniversity of Basel, Switzerland
Summary
Polyuria-polydipsia syndrome consists of the three main entities: central or nephrogenic diabetes insipidus and primary polydipsia. Reliable distinction between these diagnoses is essential as treatment differs substantially, with the wrong treatment potentially leading to serious complications. Past diagnostic measures using the classical water deprivation test had several pitfalls and clinicians were often left with uncertainity concerning the diagnosis.
With the establishment of copeptin, a stable and reliable surrogate marker for arginine vasopressin, diagnosis of the polyuria-polydipsia syndrome has been newly evaluated. Whereas unstimulated basal copeptin measurement reliably diagnoses nephrogenic diabetes insipidus, two new tests using stimulated copeptin cutoff levels showed a high diagnostic accuracy in differentiating central diabetes insipidus from primary polydipsia. For the hypertonic saline infusion test, osmotic stimulation via the induction of hypernatraemia is used. This makes the test highly reliable and superior to the classical water deprivation test, but also requires close supervision and the availability of rapid sodium measurements to guarantee the safety of the test. Alternatively, arginine infusion can be used to stimulate copeptin release, opening the doors for an even shorter and safer diagnostic test. The test protocols of the two tests are provided and a new copeptin-based diagnostic algorithm is proposed to reliably differentiate between the different entities. Furthermore, the role of copeptin as a predictive marker for the development of diabetes insipidus following surgical procedures in the sellar region is described.
Introduction
Polyuria-polydipsia syndrome – characterised by a high (>50 ml per kg body weight per 24 hours) output of hypotonic urine accompanied by increased fluid intake of more than 3 litres a day [1] – is a clinical picture that challenges specialists from internal medicine, endocrinology and nephrology alike. Whereas osmotic diuresis, as in uncontrolled diabetes mellitus, is a straight forward diagnosis, the differential diagnostic challenge of hypotonic polyuria lies in the distinction between diabetes insipidus (DI) on the one hand, and primary polydipsia (PP) on the other. Diabetes insipidus must then be further differentiated into central or nephrogenic origin. The underlying cause of central DI is deficient synthesis or inadequate secretion of arginine vasopressin (AVP) upon osmotic stimulation. This deficiency is usually acquired from disorders causing a disruption in the neurohypophysis (details in table 1), but also several genetic mutations of the AVP gene and other mutations have been described [2]. Nephrogenic DI, on the other hand, is characterised by decreased renal sensitivity to AVP. Although there are congenital forms due to mutations of the type-2 AVP receptor (AVPR2) gene or aquaporin 2 (AQP2) water channel gene [3], it is more often caused by adverse effects of drugs such as lithium or electrolyte disorders such as hypercalcaemia or hypokalaemia (table 1). Central or nephrogenic DI can appear as complete or partial forms, which makes it further challenging to differentiate them from PP. PP is characterised by excessive fluid intake and consecutive polyuria despite adequate AVP secretion and renal response to it. The chronic polyuria gradually decreases the concentrating ability of the kidneys, which explains the difficulties in differentiating it from DI [4]. However, differentiation between these entities is crucial, as treatment differs substantially and a wrong diagnosis can lead to serious compliations such as water intoxication [5]. Despite the importance of a reliable diagnostic measure, the water deprivation test, which is considered as the current gold standard, has a low diagnostic accuracy [1, 6, 7]. With the establishment of copeptin measurement, a stable and reliable surrogate marker for AVP, diagnosis of the polyuria-polydipsia syndrome has been re-evaluated and improved. This overview will first cover the different entities of polyuria-polydipsia syndrome and then focuse on copeptin and its role in the differential diagnosis of diabetes insipidus in adult patients.
Table 1 Characteristics of the different entities of the polyuria-polydipsia syndrome.
|
Polyuria-polydipsia entity and its defect:
|
Acquired causes
|
Hereditary causes
|
Characteristics
|
|
Central diabetes insipidus
|
| AVP deficiency |
Trauma
Neoplasia
Vascular
Granulomatous
Infectious
Inflammatory
Toxic
Idiopathic |
AVP mutations
WFS1 mutations
PCSK1 mutations
X-linked recessive mutations |
Persistent symptoms cave: no polydipsia in patients with osmoreceptor dysfunction (adipsic DI)
Loss of pituitary bright spot
Pituitary stalk enlargement
Family history |
|
Nephrogenic diabetes insipidus
|
| Reduced renal sensitivity to AVP |
Drug induced (lithium)
Hypercalcaemia
Hypokalaemia
Infiltrating lesions
Vascular disorders |
AVPR2 mutation
AQP2 mutation |
Medication history
Family history |
|
Primary polydipsia
|
| Excessive fluid intake despite adequat AVP secretion and renal sensitivity |
Osmoreceptor dysfunction
Psychosis / compulsive disorder
Idiopathic
Fluid intake above normal thirst threshold |
|
Gradual onset |
|
Gestational diabetes insipidus
|
| Increased AVP metabolism through placental vasopressinase |
Pregnancy
(subclinical central DI) |
|
Personal history of pregnancy induced polyuria
Twin pregnancy |
Polyuria-polydipsia syndrome
Polyuria-polydipsia syndrome is divided into central and nephrogenic DI and PP.
With a prevalence of around 1:25,000 [8] DI is a rare disease. It has a similar prevalence among males and females and can manifest at any age. Whereas central DI is characterised by a complete (complete central DI) or partial (partial central DI) deficiency of AVP secretion upon osmotic stimulation from the pituitary [9, 10], nephrogenic diabetes insipidus results from AVPresistance of the kidneys [3], both leading to hypotonic polyuria with compensatory polydipsia. Numerous conditions affecting the hypothalamic-neurohypophyseal system can lead to central DI, the most common being trauma resulting from surgery, tumours or other infiltrative diseases [11, 12] (table 1). Genetic forms (mutations in the AVP genes, X-linked recessive mutations, mutations in the WFS-1 and PCSK1 genes) have also been described and are known as familial central DI [2, 13]. Another rare form of DI is gestational DI, which is characterised by increased AVP metabolism due to the placental enzyme vasopressinase [14, 15]. In some patients there is an underlying mild partial central DI, which becomes apparent only during pregnancy, whereas others have a normal function of the neurohypophysis but high levels of the placental vasopressinase, mainly due to twin pregnancy [16, 17]. Disorders that affect not only AVP secretion but also thirst perception are the most challenging forms of central DI to treat [18]. As these patients fail to compensate the polyuria with polydipsia, they usually present with severe hypernatraemia and their daily fluid intake has to be closely monitored [19, 20].
Lack of AQP2-mediated water reabsorption in the collecting duct is the main cause for nephrogenic diabetes insipidus. This is most frequently secondarily caused by certain drugs, such as lithium or electrolyte disorders, but also occurs as the result of gene-mutations in the key proteins vasopressin V2 receptor or AQP2 (table 1) [3].
PP is the third player in the polyuria-polydipsia syndrome. It is characterised by excessive fluid intake despite physiological secretion of AVP and adequate renal response to it. In chronic PP, long-term increased water diuresis blunts the gradient of the renal medullary concentration (so-called washout phenomenon) and, owing to the suppressed endogenous AVP, leads to a downregulation of the AQP2 channels in the proximal tubule and in the collecting duct. Those changes mimic diabetes insipidus, making any diagnostic evaluation of the urine osmolality and urinary output difficult [21]. Although severe forms of PP have been described in psychiatric patients, it is nowadays also increasingly observed in health-conscious persons who exhibit fluid intake above the normal thirst threshold.
Copeptin
Located in the posterior pituitary and hypothalamic magnocellular cells, AVP is the main regulating hormone of body fluid homeostasis. It promotes water reabsorption via the V2 receptors in the kidneys, with increase of plasma osmolality being the main stimulus and hypovolaemia the main non-osmotic stimulus. AVP measurement could therefore theoretically be helpful for the differential diagnosis of diabetes insipidus. However, because of complex preanalytical requirements that make measurement difficult and time consuming with only a few reliable assays available, it failed to enter clinical practice [22].
Copeptin derives from the precursor protein pre-pro-vasopressin together with AVP and neurophysin II and was detected in 1972 in the posterior pituitary of pigs [23, 24] (fig 1). It mirrors AVP concentrations [25], but unlike AVP it is stable and can be easily measured with commercially available assays. The two CE certified assays currently available are the original manual sandwich immunoluminometric assay (LIA) [26] and its successor the automated immunofluorescent assay (KRYPTOR platform). Other commercially available copeptin assays are mostly enzyme-linked immunosorbent assays, which are not approved for diagnostic purposes. Further advantages of copeptin measurement are that it is stable at rome temperature for 7 days, that only a small sample volume is needed (50 μl of serum or plasma), that there are no preanalytical procedures and that results are usually available within 0.5−2.5 hours.
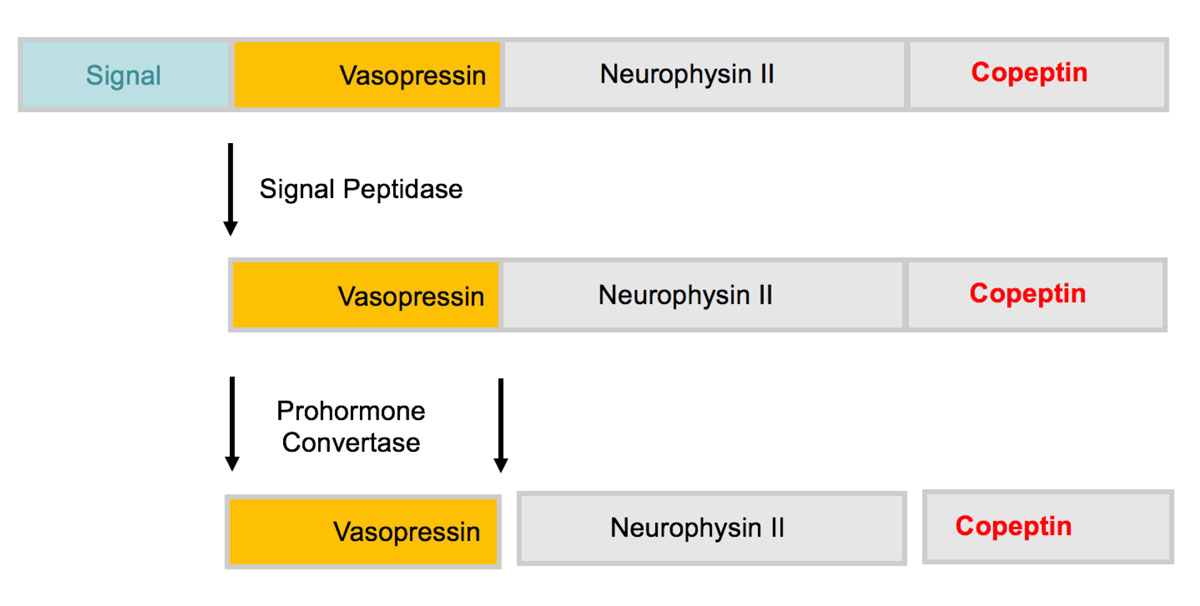
Figure 1 Structure of prepro-vasopressin-neurophysin II. The prohormone is packaged into neurosecretory granules of magnocellular neurons. During axonal transport of the granules from the hypothalamus to the posterior pituitary, enzymatic cleavage of the prohormone generates the final products: Vasopressin, neurophysin II and the COOH-terminal glycoprotein copeptin.
Beyond its function as a reliable AVP surrogate marker [26, 27], the physiological function of copeptin is largely unknown. Involvement of copeptin as a prolactin-releasing factor [28, 29], a role in the folding of the AVP precursor [30], aswell as involvement in granule sorting and regulation of AVP secretion [31] and its interaction with the calnexin-calreticulin system [32, 33] have been proposed but await further confirmation. The elimination pathway of copeptin has also not been clarified yet; renal clearance seems likely as copeptin levels showed an inverse correlation with the glomerular filtration rate in patients with impaired kidney function [34].
Copeptin is stored in the same neurosecretory granules as AVP, leading to co-secretion upon increase in plasma osmolality and volume depletion, and rapid suppression upon fluid intake (oral water load or infusion) [25, 35, 36,]. Interestingly, it has been shown that copeptin levels significantly decrease after oral fluid intake of as little as 200–300 ml [37], which must be taken into account for the interpretation of the values in clinical practice.
An additional function of copeptin has been found as an unspecific stress marker for several acute diseases such as myocardial infarction, pneumonia and ischaemic stroke [38–40]. On top of the reaction to somatic stress, some studies also showed a correlation of copeptin levels with psychological stress [41, 42]. With these stimuli in mind, it is important to avoid any emotional or physical stress before taking blood samples for basal copeptin analysis.
The normal range of plasma copeptin levels is from 1.0 to 13.8 pmol/l with higher median levels in men than in women according to two large physiological studies in healthy volunteers [26, 43]. The difference in gender is poorly understood, also because no significant changes during the menstrual cycle were observed [44]. Importantly, the difference in gender is seen only at baseline, but not at osmotically stimulated levels [26]. Further studies showed no circadial copeptin variations [45, 46] or changes with age.
The role of copeptin in the differential diagnosis of diabetes insipidus
Differential diagnosis according to clinical, laboratory or radiological findings
Since the lead symptoms of both DI and PP are increased thirst, polyuria and polydipsia, they are not helpful for their discrimination [47]. Nocturia and a sudden onset of symptoms are described to be more typical in patients with central DI; however this mainly depends on the underlying cause as familial forms or onset after irradiation of the pituitary, for example, manifest gradually (table 1). Psychiatric diagnoses such as dependency disorders and depression, on the other hand, have been described to be more prominent in PP patients [48], but a recent prospective study with 156 patients with polyuria-polydipsia syndrome showed a similar rate (around 30%) of psychiatric disorders in PP and DI patients [47]. The same study showed that plasma sodium and osmolality levels were surprisingly similar between patient groups at baseline since DI patients with free access to water rarely develop hypernatraemia.
The pituitary bright spot – an area of hyperintensity in the posterior pituitary possibly resulting from stored AVP in neurosecretory granules – on magnetic resonance imaging (MRI) has been described to be pathognomonic for central DI patients [49, 50]. However, subsequent larger studies reported an age-related absence of the bright spot not only in over half of healthy subjects [51], but also in some patients with congenital nephrogenic DI [52]. Furthermore, a prospective evaluation of 92 patients with polyuria-polydipsia syndrome showed its absence in 70% of patients with central DI as well as 39% of patients with PP [47]. In addition, several cases of central DI patients with a persistent bright spot have been reported [53, 54], which could be due to an early disease stage but also a reflection of oxytocin rather than AVP stores. Accordingly, the presence or absence of the pituitary bright spot on MRI does not qualify as the only diagnostic distinction between the entities. Although the second characteristic radiological finding, a thickened pituitary stalk, is also not specific for central DI [47, 55], its combination with absence of the bright spot should lead to a thorough evaluation for pituitary or hypothalamic diseases [56].
Differential diagnosis according to the water deprivation test
For many years, the indirect water deprivation test described by Miller et al. [10] was considered the gold standard for the differential diagnosis of polyuria-polydipsia syndrome. The evaluation of the water deprivation test is based on two main criteria: First, AVP activity is assessed indirectly by measuring urine concentration capacity over a period of 16 hours of water deprivation. Second, change of urine osmolality upon injection of desmopressin (a synthetic AVP variant) is evaluated. The different entities are then diagnosed as follows: patients in whom urinary osmolality remains below 300 mosm/kg upon water deprivation are diagnosed as complete DI, with central DI diagnosed in patients whose urinary osmolality increases by more than 50% upon desmopressin injection and nephrogenic DI in patients below this cutoff. Urinary concentration in patients with partial central DI and PP is expected to increase to 300−800 mosm/kg, with a further increase in osmolality of more than 9% in partial central DI patients and less than 9% in PP patients after desmopressin injection. Although this evaluation feels intuitive, it is important to note that these cutoffs were derived from a single study involving only 36 patients with post-hoc assessment that has never been prospectively validated [5, 10]. Given the reduction of the renal medullary concentration gradient in PP patients, which makes it especially difficult to differentiate them from partial DI patients, it is not surprising that the diagnostic accuracy of the water deprivation test was only around 70% and especially poor in the diagnosis of PP patients in two prospective evaluations [7, 47]. In addition to these diagnostic difficulties, some patients with central DI may have a higher urinary concentration ability than expected due to compensatory increase in AVPR2 gene expression [57]. Also, nephrogenic DI patients can show only partial resistance to AVP leading to a picture similar to that of partial central DI.
Differential diagnosis using copeptin measurement
Hypertonic saline infusion test
A logical way to overcome the limitations of the indirect water deprivation test would be the direct measurement of AVP. Promising first data with AVP measurements upon osmotic stimulation indeed showed levels below the calculated normal range for central DI patients, and levels were above this for nephrogenic DI patients and in the area for PP patients [58]. However, the direct AVP measurement failed to enter clinical practice because of the technical limitations of the AVP assay described above [26, 59], as well as the disappointing diagnostic accuracy of commercially available AVP assays [5, 7].
With the establishment of copeptin measurement as a reliable surrogate marker for AVP concentrations [27], direct testing was re-evaluated. The first promising observation from two prospective studies showed that a basal copeptin level equal to or above 21.4 pmol/l without prior thirsting diagnoses nephrogenic DI with a 100% sensitivity and specificity, rendering further tests redundant in these patients [7, 60]. Unfortunately, basal copeptin concentrations are not useful for distinguishing patients with central DI from PP as they show a large overlap [47, 60]. However, data for osmotically stimulated (increased plasma sodium >147 mmol/l) copeptin values showed promising results [60], which were recently confirmed in the so-far largest prospective study including 156 patients with DI or PP [47]. To achieve sufficient osmotic stimulation, patients received a bolus of 250 ml followed by a body-weight adapted hypertonic (3%) saline infusion with the aim to increase plasma sodium levels to 150 mmol/l, at which time copeptin was measured (see detailed test description in fig. 2). Using the previously defined copeptin cutoff level of >4.9 pmol/l, patients with PP were distinguished from patients with central DI with a high diagnostic accuracy of 97% (93% sensitivity and 100% specificity, fig. 3) [47]. Meanwhile, the water deprivation test had a diagnostic accuracy of only 77% (86% sensitivity and 70% specificity), which was further decreased when copeptin levels at the end of the test were included into the diagnosis.This finding underlines the limitations of the water deprivation test, as it fails to induce a sufficient osmotic stimulus despite the prolonged fluid withdrawal [47]. Further advantages of the hypertonic saline stimulation test is its short duration of 2−3 hours which makes it possible in the out-patient setting: patients often have to be hospitalised for the water deprivation test. Also, although adverse symptoms were more frequent and more pronounced during the hypertonic saline infusion test, patients preferred it to the water deprivation test [47]. Despite these advantages it must be mentioned that the correct execution of the hypertonic saline test is crucial for its safety. Clinicans performing the test must have access to rapid sodium measurements (e.g., via venous blood gas analysis) and closly monitor sodium level increase to prevent osmotic overstimulation (details in fig. 2).
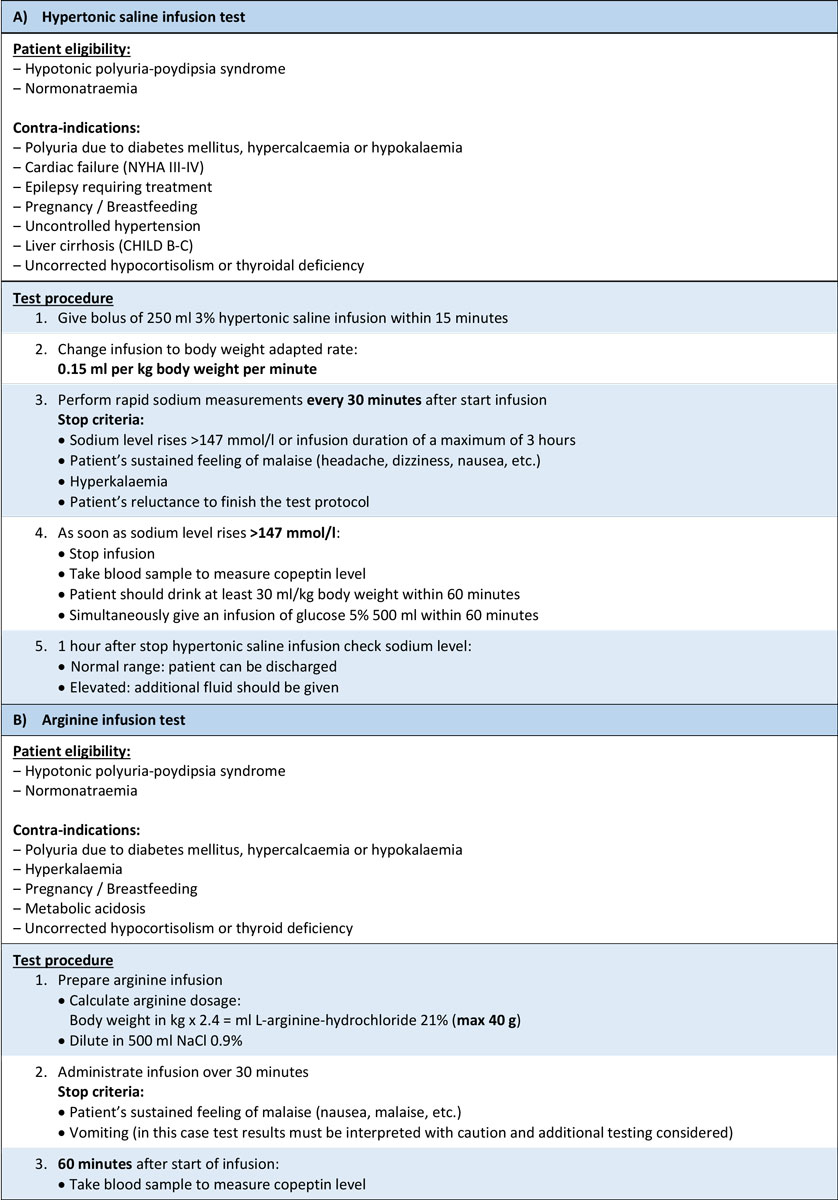
Figure 2 Test protocols for (A) the hypertonic saline infusion test according to Fenske et al [47] and (B) the arginine infusion test according to Winzeler et al. [61] for the differential diagnosis of diabetes insipidus in adult patients.
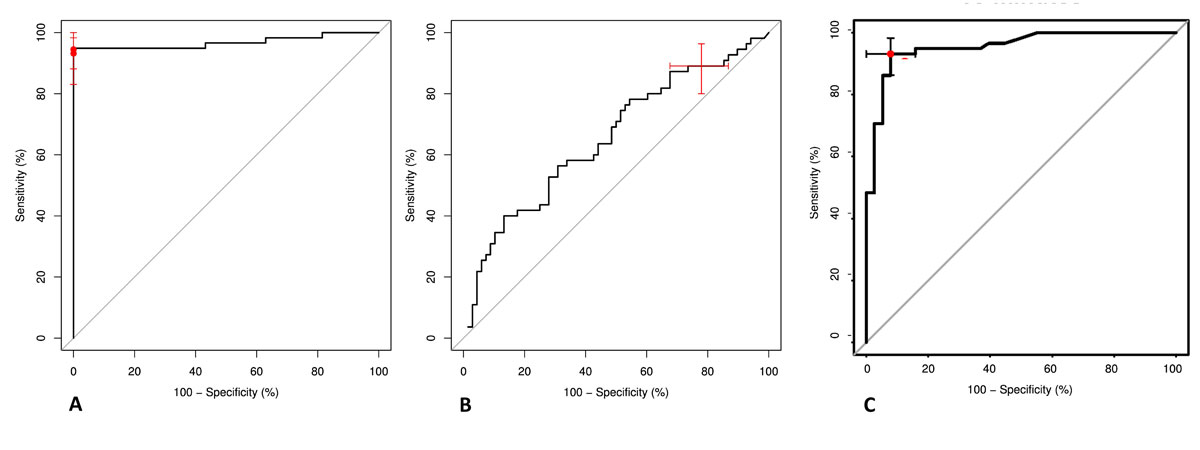
Figure 3 Receiver operating characteristic (ROC) curves for the hypertonic saline infusion test, the indirect water deprivation test and the arginine infusion test
(A) ROC curve for hypertonic saline stimulated copeptin levels for discriminating patients with primary polydipsia from patients with central diabetes insipidus. ROC area under the curve = 0.968 (95% confidence interval [CI] 0.931−1.00). Copeptin cut-off levels 4.9 pmol/l (predefined) and 6.5 pmol/l (post-hoc derived) are indicated. Data according to Fenske et al [47].
(B) ROC curve for change in urine osmolality before and after injection of desmopressin during the water deprivation test for discriminating patients with primary polydipsia from patients with central diabetes insipidus. ROC area under the curve = 0.654 (95% CI 0.556−0.753). 95% CIs are indicated for the cut-off 9%. Data according to Fenske et al [47].
(C) ROC curve for arginine infusion-stimulated copeptin levels for discriminating patients with primary polydipsia from patients with central diabetes insipidus. ROC area under the curve = 0.95 (95% CI 0.91−0.99). Copeptin cut-off level 3.8 pmol/l is indicated. Data according to Winzeler et al [61].
Arginine infusion test
To overcome the limitations of the hypertonic saline infusion test, alternative ways of stimulating the posterior pituitary were sought. Arginine infusion is known as a stimulator of the anterior pituitary [62, 63] and is used as a standard test in the evaluation of suspected growth hormone deficiency [64–66]. Recent data showed that arginine is also a potent stimulator of the posterior pituitary [61]. The study was divided into a physiological and diagnostic part, evaluating the effect of arginine infusion in healthy volunteers and in 96 patients with polyuria-polydipsia syndrome. Arginine infusion (detailed test protocol in fig. 2) lead to a median copeptin increase from 5.2 pmol/l (interquartile range 3.3−10.9) to 9.8 pmol/ll (6.4−19.6) in the healthy volunteers. Post-hoc evaluation of arginine stimulation in patients showed that a copeptin level of 3.8 pmol/l 60 minutes after start of the infusion had a diagnostic accuracy of 93% to distinguish between central DI and PP [61] (fig. 3). The most common adverse effect was mild nausea, which occurred in 48% of the patients. Two patients were excluded from the main analysis because of vomiting, as vomiting can be a strong stimulator for AVP/copeptin release. If severe nausea or vomiting occurs during arginine infusion, test results can be used only if copeptin concentrations remain low. In all other cases a confirmatory test is recommended [61].
In a post-hoc head-to-head comparison of the 60 patients who underwent the hypertonic saline infusion and the arginine infusion test, hypertonic saline-stimulated copeptin had a higher diagnostic accuracy of 100% compared with 93% for arginine-stimulated copeptin to differentiate patients with central DI from patients with PP [61]. This can be best explained by a stronger copeptin stimulation via hyperosmolality. The advantage of the arginine infusion test compared with the hypertonic saline stimulation test is its better tolerability, the even shorter duration of 1 hour and the lack of constant laboratory monitoring. A prospective, randomised, multicentre study is currently ongoing to validate the arginine-stimulated copeptin cutoff and compare its diagnostic accuracy with the hypertonic saline-stimulated copeptin cutoff (NCT03572166).
In summary, current data showed that copeptin-based diagnostic tests reliably differentiate between the different entities of the polyuria-polydipsia syndrome and have the potential to become the new gold standard tests. Of the two stimulation tests, the arginine infusion test would be preferable owing to its simple and safe test procedure, and would be an attractive test especially in children, for whom the hypertonic saline test is contraindicated. However, the head-to-head comparison between the two tests and the validation of the proposed copeptin cutoff level has not yet been completed. Meanwhile, a stepwise approach for the diagnostic evaluation of diabetes insipidus is recommended (fig. 4).
Copeptin as a predicitive marker for diabetes insipidus
While diagnostic evaluation for polyuria-polydipsia syndrome usually takes place in the out-patient setting, the use of copeptin has also been evaluated as a predictive marker for the development of central DI following surgical procedures in the sellar region. Postoperative central DI results from trauma induced by surgical manipulation of the pituitary and can be transient or permanent. Its timely diagnosis in the early postoperative phase can be challenging and may lead to severe hypernatraemia if not treated adequately [67]. Accordingly, a predictive marker for development of central DI would be useful and the use of copeptin for this role has been evaluated in two prospective trials. The larger one − involving 205 patients undergoing pituitary surgery − showed, that a copeptin value <2.5 pmol/l within the first 12 hours after surgery had a positive predictive value for central DI of 81%, whereas a copeptin value >30 pmol/l had a negative predictive value of 95% [68]. The second study involving 66 patients undergoing pituitary surgery used copeptin values 1 hour after extubation to predict the occurrence of central DI [69]. Here, a copeptin value of ≤12.8 pmol/l indicated patients at risk for central DI and a value of ≥4.2 pmol/l excluded permanent forms. Together, the available data can help to identify patients at risk for postoperative central DI as well as to stratify the risk of transient and permanent forms.
Conclusion
In conclusion, copeptin is a stable and reliable surrogate parameter of AVP and can be measured easily in serum or plasma. Copeptin-based diagnosis clearly simplifies the differential diagnosis of polyuria-polydipsia syndrome. High basal levels unequivocally indicate nephrogenic DI, whereas stimulated copeptin levels differentiate between central DI and PP with a high sensitivitiy and specificity. With its superior diagnostic accuracy, shorter test duration and higher patient acceptance, the hypertonic saline infusion test should replace the indirect water deprivation test as the gold standard test for the assessment of polyuria-polydipsia syndrome. However, it is important that the hypertonic saline stimulation test is executed according to the validated test protocol, including close monitoring of sodium levels to prevent overstimulation. The use of arginine-stimulated copeptin values is an even simpler and safer diagnostic test. Since the copeptin cutoff levels are currently being validated, confirmation with the hypertonic saline infusion test is recommended in unclear cases.
In addition to differentiating between the different entities of polyuria-polydipsia syndrome, copeptin can also be used as a predictive marker for the development of postoperative central DI after pituitary surgery.
References
1
Robertson
GL
. Diabetes insipidus. Endocrinol Metab Clin North Am. 1995;24(3):549–72. doi:.https://doi.org/10.1016/S0889-8529(18)30031-8
2
Babey
M
,
Kopp
P
,
Robertson
GL
. Familial forms of diabetes insipidus: clinical and molecular characteristics. Nat Rev Endocrinol. 2011;7(12):701–14. doi:.https://doi.org/10.1038/nrendo.2011.100
3
Bockenhauer
D
,
Bichet
DG
. Pathophysiology, diagnosis and management of nephrogenic diabetes insipidus. Nat Rev Nephrol. 2015;11(10):576–88. doi:.https://doi.org/10.1038/nrneph.2015.89
4
Cadnapaphornchai
MA
,
Summer
SN
,
Falk
S
,
Thurman
JM
,
Knepper
MA
,
Schrier
RW
. Effect of primary polydipsia on aquaporin and sodium transporter abundance. Am J Physiol Renal Physiol. 2003;285(5):F965–71. doi:.https://doi.org/10.1152/ajprenal.00085.2003
5
Fenske
W
,
Allolio
B
. Clinical review: Current state and future perspectives in the diagnosis of diabetes insipidus: a clinical review. J Clin Endocrinol Metab. 2012;97(10):3426–37. doi:.https://doi.org/10.1210/jc.2012-1981
6
Carter
AC
,
Robbins
J
. The use of hypertonic saline infusions in the differential diagnosis of diabetes insipidus and psychogenic polydipsia. J Clin Endocrinol Metab. 1947;7(11):753–66. doi:.https://doi.org/10.1210/jcem-7-11-753
7
Fenske
W
,
Quinkler
M
,
Lorenz
D
,
Zopf
K
,
Haagen
U
,
Papassotiriou
J
, et al.
Copeptin in the differential diagnosis of the polydipsia-polyuria syndrome--revisiting the direct and indirect water deprivation tests. J Clin Endocrinol Metab. 2011;96(5):1506–15. doi:.https://doi.org/10.1210/jc.2010-2345
8
Di Iorgi
N
,
Napoli
F
,
Allegri
AE
,
Olivieri
I
,
Bertelli
E
,
Gallizia
A
, et al.
Diabetes insipidus--diagnosis and management. Horm Res Paediatr. 2012;77(2):69–84. doi:.https://doi.org/10.1159/000336333
9
Robertson
GL
. The regulation of vasopressin function in health and disease. Recent Prog Horm Res. 1976;33:333–85.
10
Miller
M
,
Dalakos
T
,
Moses
AM
,
Fellerman
H
,
Streeten
DH
. Recognition of partial defects in antidiuretic hormone secretion. Ann Intern Med. 1970;73(5):721–9. doi:.https://doi.org/10.7326/0003-4819-73-5-721
11
Robertson
GL
. Differential diagnosis of polyuria. Annu Rev Med. 1988;39(1):425–42. doi:.https://doi.org/10.1146/annurev.me.39.020188.002233
12
Verbalis
JG
. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab. 2003;17(4):471–503. doi:.https://doi.org/10.1016/S1521-690X(03)00049-6
13
Birk
J
,
Friberg
MA
,
Prescianotto-Baschong
C
,
Spiess
M
,
Rutishauser
J
. Dominant pro-vasopressin mutants that cause diabetes insipidus form disulfide-linked fibrillar aggregates in the endoplasmic reticulum. J Cell Sci. 2009;122(21):3994–4002. doi:.https://doi.org/10.1242/jcs.051136
14
Barron
WM
,
Cohen
LH
,
Ulland
LA
,
Lassiter
WE
,
Fulghum
EM
,
Emmanouel
D
, et al.
Transient vasopressin-resistant diabetes insipidus of pregnancy. N Engl J Med. 1984;310(7):442–4. doi:.https://doi.org/10.1056/NEJM198402163100707
15
Durr
JA
,
Hoggard
JG
,
Hunt
JM
,
Schrier
RW
. Diabetes insipidus in pregnancy associated with abnormally high circulating vasopressinase activity. N Engl J Med. 1987;316(17):1070–4. doi:.https://doi.org/10.1056/NEJM198704233161707
16
Iwasaki
Y
,
Oiso
Y
,
Kondo
K
,
Takagi
S
,
Takatsuki
K
,
Hasegawa
H
, et al.
Aggravation of subclinical diabetes insipidus during pregnancy. N Engl J Med. 1991;324(8):522–6. doi:.https://doi.org/10.1056/NEJM199102213240803
17
Hashimoto
M
,
Ogura
T
,
Otsuka
F
,
Yamauchi
T
,
Mimura
Y
,
Hayakawa
N
, et al.
Manifestation of subclinical diabetes insipidus due to pituitary tumor during pregnancy. Endocr J. 1996;43(5):577–83. doi:.https://doi.org/10.1507/endocrj.43.577
18
Hiyama
TY
,
Utsunomiya
AN
,
Matsumoto
M
,
Fujikawa
A
,
Lin
CH
,
Hara
K
, et al.
Adipsic hypernatremia without hypothalamic lesions accompanied by autoantibodies to subfornical organ. Brain Pathol. 2017;27(3):323–31. doi:.https://doi.org/10.1111/bpa.12409
19
Christ-Crain
M
,
Fenske
W
. Copeptin in the diagnosis of vasopressin-dependent disorders of fluid homeostasis. Nat Rev Endocrinol. 2016;12(3):168–76. doi:.https://doi.org/10.1038/nrendo.2015.224
20
Thompson
CJ
,
Baylis
PH
. Thirst in diabetes insipidus: clinical relevance of quantitative assessment. Q J Med. 1987;65(246):853–62.
21
Epstein
FH
,
Kleeman
CR
,
Hendrikx
A
. The influence of bodily hydration on the renal concentrating process. J Clin Invest. 1957;36(5):629–34. doi:.https://doi.org/10.1172/JCI103462
22
Kluge
M
,
Riedl
S
,
Erhart-Hofmann
B
,
Hartmann
J
,
Waldhauser
F
. Improved extraction procedure and RIA for determination of arginine8-vasopressin in plasma: role of premeasurement sample treatment and reference values in children. Clin Chem. 1999;45(1):98–103. doi:.https://doi.org/10.1093/clinchem/45.1.98
23
Holwerda
DA
. A glycopeptide from the posterior lobe of pig pituitaries. I. Isolation and characterization. Eur J Biochem. 1972;28(3):334–9. doi:.https://doi.org/10.1111/j.1432-1033.1972.tb01918.x
24
Levy
B
,
Chauvet
MT
,
Chauvet
J
,
Acher
R
. Ontogeny of bovine neurohypophysial hormone precursors. II. Foetal copeptin, the third domain of the vasopressin precursor. Int J Pept Protein Res. 1986;27(3):320–4. doi:.https://doi.org/10.1111/j.1399-3011.1986.tb01827.x
25
Balanescu
S
,
Kopp
P
,
Gaskill
MB
,
Morgenthaler
NG
,
Schindler
C
,
Rutishauser
J
. Correlation of plasma copeptin and vasopressin concentrations in hypo-, iso-, and hyperosmolar States. J Clin Endocrinol Metab. 2011;96(4):1046–52. doi:.https://doi.org/10.1210/jc.2010-2499
26
Morgenthaler
NG
,
Struck
J
,
Alonso
C
,
Bergmann
A
. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52(1):112–9. doi:.https://doi.org/10.1373/clinchem.2005.060038
27
Fenske
WK
,
Schnyder
I
,
Koch
G
,
Walti
C
,
Pfister
M
,
Kopp
P
, et al.
Release and Decay Kinetics of Copeptin vs AVP in Response to Osmotic Alterations in Healthy Volunteers. J Clin Endocrinol Metab. 2018;103(2):505–13. doi:.https://doi.org/10.1210/jc.2017-01891
28
Nagy
G
,
Mulchahey
JJ
,
Smyth
DG
,
Neill
JD
. The glycopeptide moiety of vasopressin-neurophysin precursor is neurohypophysial prolactin releasing factor. Biochem Biophys Res Commun. 1988;151(1):524–9. doi:.https://doi.org/10.1016/0006-291X(88)90625-0
29
Hyde
JF
,
North
WG
,
Ben-Jonathan
N
. The vasopressin-associated glycopeptide is not a prolactin-releasing factor: studies with lactating Brattleboro rats. Endocrinology. 1989;125(1):35–40. doi:.https://doi.org/10.1210/endo-125-1-35
30
Barat
C
,
Simpson
L
,
Breslow
E
. Properties of human vasopressin precursor constructs: inefficient monomer folding in the absence of copeptin as a potential contributor to diabetes insipidus. Biochemistry. 2004;43(25):8191–203. doi:.https://doi.org/10.1021/bi0400094
31
Beuret
N
,
Hasler
F
,
Prescianotto-Baschong
C
,
Birk
J
,
Rutishauser
J
,
Spiess
M
. Amyloid-like aggregation of provasopressin in diabetes insipidus and secretory granule sorting. BMC Biol. 2017;15(1):5. doi:.https://doi.org/10.1186/s12915-017-0347-9
32
Parodi
AJ
. Protein glucosylation and its role in protein folding. Annu Rev Biochem. 2000;69(1):69–93. doi:.https://doi.org/10.1146/annurev.biochem.69.1.69
33
Schrag
JD
,
Procopio
DO
,
Cygler
M
,
Thomas
DY
,
Bergeron
JJ
. Lectin control of protein folding and sorting in the secretory pathway. Trends Biochem Sci. 2003;28(1):49–57. doi:.https://doi.org/10.1016/S0968-0004(02)00004-X
34
Roussel
R
,
Fezeu
L
,
Marre
M
,
Velho
G
,
Fumeron
F
,
Jungers
P
, et al.
Comparison between copeptin and vasopressin in a population from the community and in people with chronic kidney disease. J Clin Endocrinol Metab. 2014;99(12):4656–63. doi:.https://doi.org/10.1210/jc.2014-2295
35
Morgenthaler
NG
,
Müller
B
,
Struck
J
,
Bergmann
A
,
Redl
H
,
Christ-Crain
M
. Copeptin, a stable peptide of the arginine vasopressin precursor, is elevated in hemorrhagic and septic shock. Shock. 2007;28(2):219–26. doi:.https://doi.org/10.1097/SHK.0b013e318033e5da
36
Szinnai
G
,
Morgenthaler
NG
,
Berneis
K
,
Struck
J
,
Müller
B
,
Keller
U
, et al.
Changes in plasma copeptin, the c-terminal portion of arginine vasopressin during water deprivation and excess in healthy subjects. J Clin Endocrinol Metab. 2007;92(10):3973–8. doi:.https://doi.org/10.1210/jc.2007-0232
37
Walti
C
,
Siegenthaler
J
,
Christ-Crain
M
. Copeptin levels are independent of ingested nutrient type after standardised meal administration--the CoMEAL study. Biomarkers. 2014;19(7):557–62. doi:.https://doi.org/10.3109/1354750X.2014.940504
38
Katan
M
,
Fluri
F
,
Morgenthaler
NG
,
Schuetz
P
,
Zweifel
C
,
Bingisser
R
, et al.
Copeptin: a novel, independent prognostic marker in patients with ischemic stroke. Ann Neurol. 2009;66(6):799–808. doi:.https://doi.org/10.1002/ana.21783
39
Reichlin
T
,
Hochholzer
W
,
Stelzig
C
,
Laule
K
,
Freidank
H
,
Morgenthaler
NG
, et al.
Incremental value of copeptin for rapid rule out of acute myocardial infarction. J Am Coll Cardiol. 2009;54(1):60–8. doi:.https://doi.org/10.1016/j.jacc.2009.01.076
40
Katan
M
,
Christ-Crain
M
. The stress hormone copeptin: a new prognostic biomarker in acute illness. Swiss Med Wkly. 2010;140:w13101. doi:.https://doi.org/10.4414/smw.2010.13101
41
Maeder
MT
,
Staub
D
,
Brutsche
MH
,
Arenja
N
,
Socrates
T
,
Reiter
M
, et al.
Copeptin response to clinical maximal exercise tests. Clin Chem. 2010;56(4):674–6. doi:.https://doi.org/10.1373/clinchem.2009.136309
42
Hew-Butler
T
,
Hoffman
MD
,
Stuempfle
KJ
,
Rogers
IR
,
Morgenthaler
NG
,
Verbalis
JG
. Changes in copeptin and bioactive vasopressin in runners with and without hyponatremia. Clin J Sport Med. 2011;21(3):211–7. doi:.https://doi.org/10.1097/JSM.0b013e31821a62c2
43
Bhandari
SS
,
Loke
I
,
Davies
JE
,
Squire
IB
,
Struck
J
,
Ng
LL
. Gender and renal function influence plasma levels of copeptin in healthy individuals. Clin Sci (Lond). 2009;116(3):257–63. doi:.https://doi.org/10.1042/CS20080140
44
Puder
JJ
,
Blum
CA
,
Mueller
B
,
De Geyter
Ch
,
Dye
L
,
Keller
U
. Menstrual cycle symptoms are associated with changes in low-grade inflammation. Eur J Clin Invest. 2006;36(1):58–64. doi:.https://doi.org/10.1111/j.1365-2362.2006.01591.x
45
Darzy
KH
,
Dixit
KC
,
Shalet
SM
,
Morgenthaler
NG
,
Brabant
G
. Circadian secretion pattern of copeptin, the C-terminal vasopressin precursor fragment. Clin Chem. 2010;56(7):1190–1. doi:.https://doi.org/10.1373/clinchem.2009.141689
46Beglinger S, Drewe J, Christ-Crain M. The circadian rhythm of Copeptin, the C-terminal portion of Arginin Vasopressin. Poster Presentation, SGED Congress Nov 17-18, 2016, Bern, Switzerland 2016.
47
Fenske
W
,
Refardt
J
,
Chifu
I
,
Schnyder
I
,
Winzeler
B
,
Drummond
J
, et al.
A Copeptin-Based Approach in the Diagnosis of Diabetes Insipidus. N Engl J Med. 2018;379(5):428–39. doi:.https://doi.org/10.1056/NEJMoa1803760
48
Sailer
CO
,
Winzeler
B
,
Nigro
N
,
Suter-Widmer
I
,
Arici
B
,
Bally
M
, et al.
Characteristics and outcomes of patients with profound hyponatraemia due to primary polydipsia. Clin Endocrinol (Oxf). 2017;87(5):492–9. doi:.https://doi.org/10.1111/cen.13384
49
Arslan
A
,
Karaarslan
E
,
Dinçer
A
. High intensity signal of the posterior pituitary. A study with horizontal direction of frequency-encoding and fat suppression MR techniques. Acta Radiol. 1999;40(2):142–5. doi:.https://doi.org/10.3109/02841859909177729
50
Moses
AM
,
Clayton
B
,
Hochhauser
L
. Use of T1-weighted MR imaging to differentiate between primary polydipsia and central diabetes insipidus. AJNR Am J Neuroradiol. 1992;13(5):1273–7.
51
Côté
M
,
Salzman
KL
,
Sorour
M
,
Couldwell
WT
. Normal dimensions of the posterior pituitary bright spot on magnetic resonance imaging. J Neurosurg. 2014;120(2):357–62. doi:.https://doi.org/10.3171/2013.11.JNS131320
52
Ranadive
SA
,
Ersoy
B
,
Favre
H
,
Cheung
CC
,
Rosenthal
SM
,
Miller
WL
, et al.
Identification, characterization and rescue of a novel vasopressin-2 receptor mutation causing nephrogenic diabetes insipidus. Clin Endocrinol (Oxf). 2009;71(3):388–93. doi:.https://doi.org/10.1111/j.1365-2265.2008.03513.x
53
Maghnie
M
,
Cosi
G
,
Genovese
E
,
Manca-Bitti
ML
,
Cohen
A
,
Zecca
S
, et al.
Central diabetes insipidus in children and young adults. N Engl J Med. 2000;343(14):998–1007. doi:.https://doi.org/10.1056/NEJM200010053431403
54
Hannon
M
,
Orr
C
,
Moran
C
, et al.
Anterior Hypopituitarism is Rare and Autoimmune Disease is Common in Adults with Idiopathic Central Diabetes Insipidus. Clin Endocrinol (Oxf). 2012;76(5):725–8. doi:.https://doi.org/10.1111/j.1365-2265.2011.04270.x
55
Leger
J
,
Velasquez
A
,
Garel
C
,
Hassan
M
,
Czernichow
P
. Thickened pituitary stalk on magnetic resonance imaging in children with central diabetes insipidus. J Clin Endocrinol Metab. 1999;84(6):1954–60. doi:.https://doi.org/10.1210/jc.84.6.1954
56JG. V. Disorders of water balance. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu ASL, Brenner BM, eds Brenner and Rector’s The Kidney, 9th ed Chap 15. Philadelphia: Saunders; 2011. Pp 552–69
57
Block
LH
,
Furrer
J
,
Locher
RA
,
Siegenthaler
W
,
Vetter
W
. Veränderte Gewebsempfindlichkeit gegenüber Vasopressin bei hereditärem hypothalamischen Diabetes insipidus [Changes in tissue sensitivity to vasopressin in hereditary hypothalamic diabetes insipidus]. Klin Wochenschr. 1981;59(15):831–6. German. doi:.https://doi.org/10.1007/BF01721052
58
Zerbe
RL
,
Robertson
GL
. A comparison of plasma vasopressin measurements with a standard indirect test in the differential diagnosis of polyuria. N Engl J Med. 1981;305(26):1539–46. doi:.https://doi.org/10.1056/NEJM198112243052601
59
Robertson
GL
,
Mahr
EA
,
Athar
S
,
Sinha
T
. Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J Clin Invest. 1973;52(9):2340–52. doi:.https://doi.org/10.1172/JCI107423
60
Timper
K
,
Fenske
W
,
Kühn
F
,
Frech
N
,
Arici
B
,
Rutishauser
J
, et al.
Diagnostic Accuracy of Copeptin in the Differential Diagnosis of the Polyuria-polydipsia Syndrome: A Prospective Multicenter Study. J Clin Endocrinol Metab. 2015;100(6):2268–74. doi:.https://doi.org/10.1210/jc.2014-4507
61
Winzeler
B
,
Cesana-Nigro
N
,
Refardt
J
,
Vogt
DR
,
Imber
C
,
Morin
B
, et al.
Arginine-stimulated copeptin measurements in the differential diagnosis of diabetes insipidus: a prospective diagnostic study. Lancet. 2019;394(10198):587–95. doi:.https://doi.org/10.1016/S0140-6736(19)31255-3
62
Merimee
TJ
,
Rabinowitz
D
,
Fineberg
SE
. Arginine-initiated release of human growth hormone. Factors modifying the response in normal man. N Engl J Med. 1969;280(26):1434–8. doi:.https://doi.org/10.1056/NEJM196906262802603
63
Nair
NP
,
Lal
S
,
Thavundayil
JX
,
Isaac
I
,
Eugenio
H
,
Achim
A
, et al.
Effect of normal aging on the prolactin response to graded doses of sulpiride and to arginine. Prog Neuropsychopharmacol Biol Psychiatry. 1985;9(5-6):633–7. doi:.https://doi.org/10.1016/0278-5846(85)90031-4
64
Alba-Roth
J
,
Müller
OA
,
Schopohl
J
,
von Werder
K
. Arginine stimulates growth hormone secretion by suppressing endogenous somatostatin secretion. J Clin Endocrinol Metab. 1988;67(6):1186–9. doi:.https://doi.org/10.1210/jcem-67-6-1186
65
Ghigo
E
,
Bellone
J
,
Aimaretti
G
,
Bellone
S
,
Loche
S
,
Cappa
M
, et al.
Reliability of provocative tests to assess growth hormone secretory status. Study in 472 normally growing children. J Clin Endocrinol Metab. 1996;81(9):3323–7.
66
Maghnie
M
,
Cavigioli
F
,
Tinelli
C
,
Autelli
M
,
Aricò
M
,
Aimaretti
G
, et al.
GHRH plus arginine in the diagnosis of acquired GH deficiency of childhood-onset. J Clin Endocrinol Metab. 2002;87(6):2740–4. doi:.https://doi.org/10.1210/jcem.87.6.8546
67
Christ-Crain
M
,
Bichet
DG
,
Fenske
WK
,
Goldman
MB
,
Rittig
S
,
Verbalis
JG
, et al.
Diabetes insipidus. Nat Rev Dis Primers. 2019;5(1):54. doi:.https://doi.org/10.1038/s41572-019-0103-2
68
Winzeler
B
,
Zweifel
C
,
Nigro
N
,
Arici
B
,
Bally
M
,
Schuetz
P
, et al.
Postoperative Copeptin Concentration Predicts Diabetes Insipidus After Pituitary Surgery. J Clin Endocrinol Metab. 2015;100(6):2275–82. doi:.https://doi.org/10.1210/jc.2014-4527
69
Berton
AM
,
Gatti
F
,
Penner
F
,
Varaldo
E
,
Prencipe
N
,
Rumbolo
F
, et al.
Early copeptin determination allows prompt diagnosis of post-neurosurgical central diabetes insipidus. Neuroendocrinology. 2019. doi:.https://doi.org/10.1159/000503145



