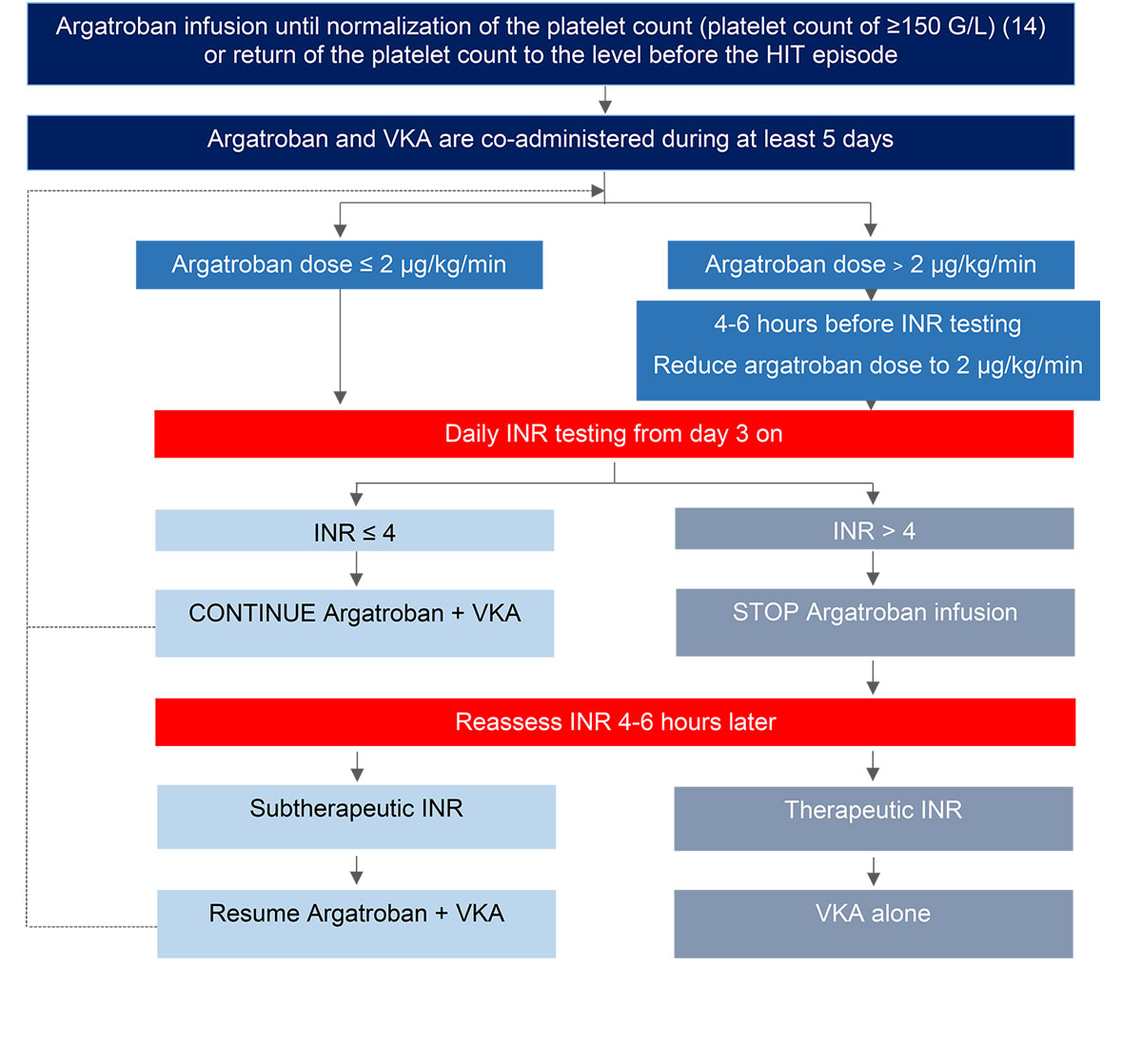
Figure 1 Proposed algorithm for transitioning patients from argatroban to vitamin K antagonists (VKA) [15, 41, 42].
DOI: https://doi.org/10.4414/smw.2020.20210
Heparin-induced thrombocytopenia (HIT) is an immune-mediated severe adverse drug effect that occurs in 0.1–5% of heparin-treated patients. HIT is defined by the concomitant presence of acquired thrombocytopenia (platelet count below 100 G/l or a relative drop of ≥30%) and HIT antibodies (HIT-Abs). HIT-Abs are immunoglobulin G (IgG) antibodies directed against a multimeric complex of a patient’s chemokine, most often platelet factor 4 (PF4), and the drug, heparin or other polyanionic macromolecules. Not all HIT-Abs induce thrombocytopenia. Only the so-called functional HIT-Abs lead to thrombocytopenia and the subsequent generation of thrombin. Thrombocytopenia in HIT, caused by platelet consumption, is typically moderate (platelet count around 60 G/l). Hypercoagulability is caused by platelet, monocyte, neutrophil and endothelial cell activation, resulting in concomitant activation of coagulation. As both platelets and coagulation become activated in HIT, the resulting hypercoagulable state affects both the venous and the arterial vascular beds [1, 2].
HIT diagnosis requires assessment of pretest probability utilising a validated score. Based on the result of the score, and the result of a quantitative HIT-Abs immunoassay, post-test probabilities are determined, permitting HIT diagnosis. The HIT-Abs enzyme-linked immunosorbent assay (ELISA) tests are usually highly sensitive, but not very specific, diagnostic tests. There are differences between immunoassays for HIT, and only some of them have at the same time high sensitivity and high specificity [3]. Imperfect specificity makes it necessary that positive immunoassay results are confirmed by functional tests, such as heparin-induced platelet aggregation or serotonin release assays. Recent publications [4–6] have demonstrated that the Bayesian combination of pretest clinical likelihood with the magnitude of an immunoassay for HIT-Abs can predict the outcome of the functional assay, so that additional confirmatory platelet-activation assays may become unnecessary, for example for patients with high pretest clinical probability and high HIT-Abs titres [7].
Evidence-based management of acute HIT currently involves two main aspects: cessation of heparin exposure and inhibition of coagulation with an anticoagulant other than heparin. Recently, the use of high-dose intravenous immunoglobulin has been reported to rapidly inhibit HIT-Abs-induced platelet activation [8, 9]. This therapeutic approach might constitute an important adjunct treatment to anticoagulation in patients with HIT [8, 9]. Efficient treatment of HIT will lead to the normalisation of the platelet count (typically within 5–7 days) and prompt mitigation of the hypercoagulable state [10, 11].
The aim of this article is confined to the appropriate use of anticoagulants and does not include general management of HIT. Several drugs can be considered for the anticoagulant treatment of HIT. Anticoagulant monitoring, management of drug-induced adverse events particularly bleeding, and dosing schedules in the presence of renal or hepatic dysfunction and other selected clinical situations represent challenges to the clinician treating HIT patients. Moreover, the fact that not all registered anticoagulants are approved for HIT in Switzerland further complicates the management of HIT. Lepirudin, licensed for HIT treatment, was withdrawn from the market worldwide.
Once the acute phase of HIT has been successfully treated, transitioning of parenteral anticoagulant treatment to a direct oral anticoagulant (DOAC) or a vitamin K antagonist (VKA) can be considered. DOACs are preferred over VKAs for stable patients with an average bleeding risk after platelet count recovery [12, 13]. Prerequisite for transitioning to oral anticoagulant is the normalisation of the platelet count (platelet count ≥150 G/l [14]) / return of the platelet count to the level before the HIT episode. VKA treatment should be started only when both of criteria are met. These precautionary measures aim to minimise the risk of coumarin-associated microthrombosis syndromes (venous limb gangrene, skin necrosis). Alternative anticoagulation can be stopped once two subsequent international normalised ratio (INR) measurements, performed at least 24 hours apart, are in the therapeutic range. Administration of low doses of a VKA without a loading dose is recommended.
The Working Party Hemostasis (WPH) of the Swiss Society of Hematology proposes a guideline for the treatment of HIT based on current available evidence, international guidelines and consensus expert opinion. The paper presents all available treatment modalities in Switzerland, allowing direct implementation. Multiple rather than single recommendations are presented, allowing a tailored strategy according to physicians’ preferences and experience. It is in the context of a complex disease with relevant treatment risks, associated with potentially high treatment costs and limited resources, that the WPH has prepared a guideline for the treatment of HIT in Switzerland. In addition to other current guidelines, including those of the American Society of Hematology 2018 [12], we provide information on dosing schedules of anticoagulants for HIT patients in different clinical conditions.
The pharmacokinetic/pharmacodynamics properties of all anticoagulants in use for HIT treatment are summarised in table 1 and discussed in alphabetical order.
Table 1 Properties of anticoagulants used for the treatment of heparin-induced thrombocytopenia.
|
Argatroban
(Argatra®) |
Bivalirudin
(Angiox®) |
Danaparoid
(Orgaran®) |
Fondaparinux
(Arixtra®) |
Rivaroxaban*
(Xarelto®) |
|
|---|---|---|---|---|---|
| Mode of action | Anti-factor IIa (thrombin) | Anti-factor IIa (thrombin) | Anti-factor Xa AT-mediated |
Anti-factor Xa AT-mediated |
Anti-factor Xa |
| Route of application | i.v. | i.v. | i.v./s.c. | s.c. | p.o. |
| Predominant clearance | Hepatobiliary | Proteolytic inactivation (80%) and renal (20%) | Renal | Renal | Hepatobiliary (67%), renal (33%) |
| Elimination half-life | 40–50 minutes | 25 minutes | 19–25 hours | 17–21 hours | 7–11 hours |
| Hepatic metabolism | Yes | No | No | No | Yes |
| Monitoring preference | Anti-factor IIa assay (± aPTT) |
Anti-factor IIa assay (± aPTT) |
Anti-factor Xa assay | Anti-factor Xa assay† | Anti-factor Xa assay† |
| Significant influence on routine coagulation tests | Yes | Yes | None | None | Yes |
aPTT = activated partial thromboplastin time; AT = antithrombin; i.v. = intravenous; s.c. = subcutaneous; p.o. = per os * Limited available data supporting the use of rivaroxaban in HIT, for details see text † Monitoring is not generally required but may be recommended in special situations, such renal insufficiency
Molecule: Argatroban is a small (molecular weight 527 Daltons), direct, selective and reversible inhibitor of the thrombin active site, which is synthetically derived from L-arginine (structurally unrelated to heparin) [17]. Argatroban inhibits all thrombin-catalysed steps in the coagulation system (fibrin formation, activation of factors V, VIII, and XIII, and of protein C, and thrombin-induced platelet aggregation). It is not inactivated by PF4 and does not cross-react with heparin-induced antibodies. Argatroban does not require antithrombin for its anticoagulant activity (hence “direct”).
Distribution: Predominantly in the extracellular fluid; 20–54% is reversibly bound to human serum proteins [18].
Metabolism: Hepatic.
Excretion: Hepatobiliary clearance.
Half-life: 40–50 minutes.
The WPH recommends the use of argatroban as a continuous intravenous infusion (table 2), with an initial bolus only in a restricted number of specific indications (table 3). In the absence of organ failure, the dose recommended in the manufacturer’s prescribing information is 2 μg/kg/min. However, several publications indicate that this dose is too high and that an initial dose of 1 μg/kg/min is adequate for patients without organ function impairment [22, 29, 30]. In critically ill patients with cardiac failure or other organ dysfunctions, it is suggested to begin with an even lower dose (0.5 μg/kg/min or less). Indeed, there may be a four-fold clearance decrease of the drug, especially when hepatic impairment is present. In patients with moderate hepatic dysfunction (bilirubin >25.5 μmol/l, alanine aminotransferase [ALAT] >3 times the upper limit of the reference range) a starting dose of 0.25 μg/kg/min is suggested [31]. Table 2 summarises the recommended dosing schedules of argatroban [23, 24].
Table 2 Initial dose of argatroban in various clinical situations.
| Situation | Initial dose |
|---|---|
| Acute HIT (with or without thromboembolism) | 1.0 µg/kg/min (standard dose) |
| Acute HIT with life-threatening thromboembolism | 2.0 µg/kg/min (monitoring in intensive care mandatory) |
| Critically ill patients | 0.5 µg/kg/min or other doses according to the nomogram (table 3) |
| Moderate liver insufficiency | 0.25 µg/kg/min |
| Severe liver insufficiency | Argatroban contraindicated (prescribe fondaparinux or bivalirudin instead) |
| Children with normal liver function | 0.75 µg/kg/min |
| Children with reduced liver function | 0.25 µg/kg/min |
| HIT = heparin-induced thrombocytopenia | |
Table 3 Dosing schedules of anticoagulants in patients with heparin-induced thrombocytopenia.
| Indication | Anticoagulant | ||||
|---|---|---|---|---|---|
| Argatroban | Bivalirudin | Danaparoid | Fondaparinux | DOACs* | |
| Prevention of VTE in patients with a history of HIT |
Bolus: No Injection: 750 U s.c. 2–3 ×/d [19, 20] |
Bolus: No Injection: 2.5 mg s.c. 1 ×/d [21] |
Rivaroxaban: 1 × 10 mg/d orally Apixaban: 2 × 2.5 mg/d [15] |
||
| Acute HIT (with or without VTE) |
Bolus: No Continuous infusion: 0.5–1 µg/kg/min (max. 10 µg/kg/min) Adjust to aPTT of 1.5–3.0 × patient baseline and/or TT (in-house target) Adjust to anti-factor IIa activity of 0.4–1.0 (max 1.5) µg/ml [22–25] |
Bolus: No Continuous infusion: Cr Cl >60ml/min 0.06 mg/kg/h Cr Cl 30−60ml/min 0.04 mg/kg/h Cr Cl 15−30ml/min 0.02 mg/kg/h Cr Cl <15ml/min contraindicated† Adjust to aPTT of 1.5–2.5 × patient baseline Adjust to anti-factor IIa activity of 1.0 µg/ml, range 0.5–1.5 µg/ml‡ Dose reduction in cases of renal or hepatic dysfunction as described above or according to literature [26, 27] |
i.v. schedule
Bolus: <60 kg: 1500 U 60–75 kg: 2250 U 75–90 kg: 3000 U >90 kg: 3750 U Continuous infusion: 400 U/h for 4 h followed by 300 U/h for 4 h followed by 150–200 U/h depending on the renal function Adjust to anti-factor Xa activity 0.5–0.8 IU/ml [19, 20] s.c. schedule Low dose scheme: i.v. loading dose of 750 U (≤90 kg) or 1250 U (>90 kg), followed by 750 U 3 ×/d (≤90 kg) or 1250 U 3 ×/d (>90 kg) s.c. High dose scheme: i.v. loading dose of 2000 U followed by 2000 U 2 ×/d s.c. 1500–2250 U i.v. 2 ×/d (i.v. schedule during the acute phase of HIT) Adjust to anti-factor Xa activity 0.5–0.8 U/ml [19, 20] |
s.c. schedule (daily)
Bolus: No Injection: ≥50 ≤100 kg: 7.5 mg <50 kg: 5 mg >100kg: 10 mg Creatinine clearance 30–50 ml/min: use with caution Creatinine clearance <30 ml/min: contraindicated [21, 28] |
|
| Treatment of ATE |
Bolus: No Continuous infusion: 0.5−1 µg/kg/min (max. 10 µg/kg/min) Adjust to aPTT of 1.5−3.0 × patient baseline Adjust to anti-factor IIa activity of 0.4−1.5 µg/ml [23, 24] |
Bolus: No Continuous infusion: See above Adjust to aPTT of 1.5−2.5 × patient baseline Adjust to anti-factor IIa activity of 1.0 µg/ml, range 0.5−1.5 µg/ml‡ Dose reduction in the case of renal or hepatic dysfunction [26, 27] |
i.v. schedule
Bolus: <60 kg: 1500 U 60−75 kg: 2250 U 75−90 kg: 3000 U >90 kg: 3750 U Continuous infusion: 400 U/h for 4 h followed by 300 U/h for 4 h followed by 150−200 U/h Adjust to anti-factor Xa activity of 0.5−0.8 U/ml [19, 20, 49] s.c. schedule Bolus: No Injection: 1500−2250 U s.c. 2 × daily (i.v. during the acute phase of HIT) Adjust to anti-factor Xa activity of 0.5−0.8 U/ml [19, 20, 49] |
Bolus: Optional (i.v.) Injection: 2.5 mg/d s.c. [21] |
− |
| Treatment of unstable angina / MI | − |
Bolus: No Continuous infusion: see above Adjust to aPTT 1.5−2.5 × patient baseline Adjust to anti-factor IIa activity of 1.0 µg/l, range 0.5−1.5 µg/ml‡ Dose reduction in the case of renal or hepatic dysfunction [47] |
− |
Bolus: Optional (i.v.) Injection: 2.5 mg/d s.c. [15] |
− |
| Stroke prevention and prophylaxis of systemic embolism in non-valvular atrial fibrillation | − | − | − | − | DOACs according to labelling [15] |
| Catheter patency | − | − | 750 U in 50 ml NaCl 0.9%; 5−10 ml/catheter or more when necessary [74] | − | − |
| Intermittent haemodialysis (alternating days) |
Bolus: Optional 250 µg/kg i.v. Continuous infusion: 2 µg/kg/min i.v. If no bolus, start continuous infusion 4 h before dialysis Adjust to aPTT of 1.5−3.0 patient baseline Adjust to anti-factor IIa activity of 0.4−1.5 µg/ml [32] |
− |
Bolus: No Injection: 3750 U (2250 U if weight <55 kg) i.v. prior to 1st and 2nd dialyses, 3000 U (2000 U if weight <55 kg) i.v. prior to 3rd dialysis For subsequent dialyses, adjust according to predialysis anti-factor Xa: If anti-factor Xa activity <0.3 U/ml, give 3000 U i.v. Adjust to anti-factor Xa during dialysis: 0.5−0.8 U/ml [15, 74] |
− | − |
| Continuous renal replacement therapy |
Bolus: No Continuous infusion: 0.5−1 µg/kg/min depending on liver function Adjust to aPTT of 1.5−3.0 × patient baseline Adjust to anti-IIa activity of 0.4−1.5 µg/ml [75, 76] |
Bolus: No Continuous infusion: 0.006−0.04 mg/kg/h Adjust to aPTT of 1.5−2.5 × patient baseline [26] Adjust to anti-factor IIa activity of 1.0 µg/ml, range 0.5−1.5 µg/ml‡ |
Bolus: 2250 U i.v. Continuous infusion: 400 U/h for 4 h (600 U/h max.) followed by 300 U/h for 4 h, followed by 150−400 U/h aiming for anti-factor Xa activity 0.5−0.8 IU/ml [74] |
− | − |
| Peripheral vascular surgery |
Bolus:
350 µg/kg (in 3−5 min) Continuous infusion: 25 µg/kg/min Adjust continuous infusion dose to ACT 300−500 sec, checked 5−10 min after the initial bolus. If necessary, additional bolus of 150 µg/kg [77] |
− |
Preoperative
Bolus: <60 kg: 1500 U 60−75 kg: 2250 U 75−90 kg: 3000 U >90 kg: 3750 U Peroperative rinsing: 750 U in 250 ml NaCl 0.9%, using up to 50 ml Postoperative (≥ 6 h after surgery) Low risk patients: 750 U 3 × day s.c. High risk patients: 150−200 U/h i.v. [74] |
− | − |
| PCI |
Bolus:
350 µg/kg (in 3−5 min) without GP IIb/IIIa inhibitor 250−350 µg/kg with GP IIb/IIIa inhibitor Continuous infusion: 25 µg/kg/min without GP IIb/IIIa inhibitor 15 µg/kg/min with GP IIb/IIIa inhibitor Adjust continuous infusion dose to ACT 300−500 sec, checked 5−10 min after the initial bolus. If necessary, additional bolus of 150 µg/kg Removal arterial introducer: ≥2 h after drug interruption and check for ACT 160 sec [78, 79] |
Bolus:
0.75 mg/kg aiming for ACT >225 sec; second bolus optional (0.3 mg/kg) Continuous infusion: 1.75 mg/kg/h during PCI, max 4 h Adjust infusion to 1.4 mg/kg/min if creatinine clearance is 30-59 ml/min Contraindicated if creatinine clearance is <30 ml/min Removal arterial introducer ≥2 h after perfusion arrest [15] |
Preprocedure
Bolus: <60 kg: 1500 U 60−75 kg: 2250 U 75−90 kg: 3000 U >90 kg: 3750 U Postprocedure Continuous infusion: 150−200 U/h for 1−2 days Adjust to anti-factor Xa activity of 0.5−0.8 U/ml [74] |
− | − |
| Intra-aortic balloon pump |
Bolus: No Continuous infusion: 0.5−1 µg/kg/min depending on liver function Adjust to aPTT 1.5−3.0 × patient baseline <100 sec Adjust to anti-factor IIa activity of 0.4−1.5 µg/ml [80] |
− |
Preprocedure
Bolus: <60 kg: 1500 U 60−75 kg: 2250 U 75−90 kg: 3000 U >90 kg: 3750 U Postprocedure Continuous infusion: 150−200 U/h i.v. until removal of balloon pump Adjust to anti-factor Xa activity of 0.5−0.8 U/ml [74] |
− | − |
| Extracorporeal membrane oxygenation |
Bolus: No Continuous infusion: 0.2 µg/kg/min Adjust to aPTT 50−60 sec Adjust to anti-factor IIa activity 0.4−1.5 µg/ml [81] |
− |
Bolus: No Continuous infusion: 400 U/h Adjust to anti-Xa activity 0.6−0.8 U/ml (monitoring 2× daily) [82] |
− | − |
| Off-pump coronary artery bypass | − |
Bolus: 0.75 mg/kg Continuous infusion: 1.75 mg/kg/h aiming for ACT >300 sec (stop infusion approx. 20 min before end of grafting) Increase of perfusion rate by 0.25 mg/kg/h when ACT <300 sec [83–85]. |
− | − | − |
| CPB | − |
Bolus: 1 mg/kg Continuous perfusion: 2.5 mg/kg/h In pump circuit: 50 mg Adjust to ACT 2.5 × patient baseline If ACT <2.5 patient baseline: bolus 0.25 mg/kg and increase of perfusion of 0.25 mg/kg/h Stop infusion approx. 15 min before end of CPB Extracorporeal elimination: enhanced by the use of modified haemofiltration (Minntech Hemocor HPH 700 membrane) Management of circuit after CPB: bolus of 50 mg followed by a continuous infusion of 50 mg/h. Process blood with a cell saver before reinfusion to avoid overdosage. Cell saver: contains sodium citrate [48, 86, 87] |
− | − | − |
ACT = activated clotting time; ATE = arterial thromboembolism; CBP = cardiopulmonary bypass; d = day; GP = glycoprotein; HIT = heparin-induced thrombocytopenia; h = hour; i.v. = intravenous; IU = international unit; MI = myocardial infarction; min = minute; PCI = percutaneous coronary intervention; s.c. = subcutaneous; sec = second; U = unit; VTE = venous thromboembolism * Note: prophylaxis only as authorised by Swissmedic (major orthopaedic surgery). Currently there are not enough data supporting the use of direct oral anticoagulents as a first line therapy in HIT. † Depending on the type of renal replacement system (hemofiltration/haemodialysis) utilised, bivalirudin can be used in the context of severe renal insufficiency. ‡ The target has to be evaluated in the routine laboratory with the local reagents/coagulometer combination.
Renal insufficiency: No dosage adjustment is necessary in patients with renal insufficiency. Clearance of argatroban by high-flux membranes is considered clinically insignificant (approximately 20% of the drug is cleared through dialysis) [32, 33].
Liver insufficiency: Liver insufficiency is associated with decreased clearance and an increased elimination half-life of argatroban. Argatroban is contraindicated in severe liver insufficiency [33].
Pregnancy: Administer during pregnancy only if absolutely necessary and if no alternative exists. It is not known whether the drug is excreted into breast milk [15, 34–36].
Geriatrics: The effectivity of argatroban does not seem to be affected by age [33]. However, there are insufficient data to determine if its absorption, distribution and elimination are different in elderly patients (≥65 years).
Paediatrics: Limited evidence is available. Dose recommendations are found in the Swiss compendium [15]. Argatroban clearance is decreased in seriously ill paediatric patients.
Argatroban monitoring is detailed in table 4. Target ranges for the various tests used have not been established in an outcome-based setting. Argatroban increases thrombin-based coagulation times (prothrombin time [PT] and the PT-derived international normalised ratio [INR] expression; activated partial thromboplastin time (aPTT); thrombin time (TT); activated clotting time [ACT]) in a concentration-dependent manner. Daily anti-factor IIa testing (by a commercially calibrated diluted TT) is the preferred monitoring modality, followed by aPTT monitoring. Anti-factor IIa and/or aPTT monitoring is scheduled before and 2 hours after the start of the argatroban infusion (steady-state levels of drug and anticoagulant effect are expected after five half-lives), 2 hours after each dose adjustment and then daily. The proposed target argatroban concentration is 0.4–1.5 μg/ml [22, 25]. The aPTT is aimed at 1.5−3.0 times the patient’s baseline (provided baseline aPTT was within the reference range) without exceeding 100 sec with most reagents; the precise target range depends very much on the local reagent/coagulometer combination and has to be established in-house. Importantly, there is a flattening of the concentration response relationship, so that over-anticoagulation is difficult to detect [37, 38].
Table 4 Argatroban monitoring with aPTT and/or anti-IIa activity.
|
aPTT*
(sec.) |
Argatroban dose |
Next check†
(h) |
Anti-IIa activity
(µg/ml) |
|---|---|---|---|
| <35 | +20% | 2 | <0.3 |
| 35−45‡ | +10% | 2 | 0.3−0.4‡ |
| 46−70 | No change | 4−12; (in steady-state: 12−24 h) |
0.4−1.5 |
| 71−90 | −10% | 2 | >1.5 |
| >90 | −20% and Stop for 1−2 h |
2 |
aPTT = activated partial thromboplastin time; anti-IIa activity = anti-activated factor II (or thrombin) activity * Aim: 1.5 to 3.0 × patient baseline aPTT, the given aPTT values are valid only if the patient baseline aPTT is within the normal range; † daily anti-IIa activity in addition to aPTT and, as well as daily kidney and liver function tests; ‡ prophylactic dose: aPTT 35−45 sec., Anti-IIa activity argatroban: 0.3-0.4 µg/ml.
Of note, determination of the fibrinogen concentration can be affected by the administration of argatroban. Depending on the assay employed, argatroban may cause an underestimation of the fibrinogen concentration, particularly at supra-therapeutic argatroban plasma levels [39, 40].
Co-administration of a VKA and argatroban has a combined effect on INR, prolonging INR beyond the value produced by a VKA alone. The INR should be measured daily while argatroban and VKA are co-administered. The transition is considered moderately complicated and the initial target INR has to be set to at least 4; after two INR values in the desired therapeutic range 24 hours apart, argatroban can be stopped; the INR has to be reassessed 4–6 hours later [15, 41, 42], now targeting an INR of 2–3. See also the Introduction section regarding conditions necessary prior to transitioning to VKA, and figure 1.

Figure 1 Proposed algorithm for transitioning patients from argatroban to vitamin K antagonists (VKA) [15, 41, 42].
Caution is recommended in:
No specific antidote to argatroban is available. However, argatroban’s relatively short half-life reduces the need for an antidote agent.
No specific recommendations are defined. Coagulation parameters typically return to baseline within 2–4 hours after discontinuation of the drug (short half-life in the absence of hepatic dysfunction and third space). Symptomatic and supportive therapy, as well a local haemostasis, should be provided. Approximately 20% of argatroban can be cleared through dialysis.
Drug interactions: No drug interactions have been identified so far.
Molecule: Bivalirudin is a small (molecular weight 2180 Daltons), direct, selective, reversible and bivalent thrombin inhibitor. The bivalent binding properties contribute to bivalirudin’s high affinity and high specificity for thrombin. Bivalirudin does not require antithrombin for its anticoagulant activity (hence “direct”).
Distribution: Plasma and extracellular fluid; no protein binding apart from thrombin.
Metabolism: Proteolytic inactivation (80%); renal excretion (20%).
Excretion: Hepatobiliary and renal clearance.
Half-life: 25 minutes.
The WPH recommends the use of bivalirudin in acute HIT patients in the setting of urgent coronary angioplasty – PCI (see references [44, 45] for dosing) and as an option in HIT patients requiring cardiac surgery. Bivalirudin has been evaluated in HIT patients outside the context of cardiac surgery and PCI [25–27]. The WPH suggests the use of bivalirudin in HIT patients in an intensive care setting. Bivalirudin is administered as a continuous intravenous infusion without a bolus. Table 3 summarises the WPH recommended dosing schedules of bivalirudin in HIT patients [2, 25–27].
Renal insufficiency: No dosage adjustment is necessary in patients with mild renal insufficiency. In the case of moderate or severe renal insufficiency or in the presence of chronic renal replacement therapy, dosing should be adapted. Patients should be monitored by assessing aPTT and/or bivalirudin plasma concentration measured as anti-factor IIa activity (see “Monitoring”).
Liver insufficiency: No dosage adjustment is necessary in patients with hepatic dysfunction.
Pregnancy: Insufficient evidence. It is not known whether the drug is excreted into breast milk [34–36].
Geriatrics: The effectivity of bivalirudin does not seem to be affected by age. However, there are insufficient data to determine if its absorption, distribution, and elimination are different in elderly patients (≥65 years).
Paediatrics: Insufficient evidence.
Bivalirudin prolongs thrombin-based coagulation tests (PT, aPTT, TT, ACT) in a concentration-dependent manner. Daily bivalirudin concentration assessment by anti-factor IIa testing is the preferred monitoring modality, followed by aPTT monitoring. Anti-factor IIa and/or aPTT monitoring should be started before and repeated 3 hours after the start of the bivalirudin infusion (steady-state levels of drug and anticoagulant effect are typically attained after five half-lives), 3 hours after each dose adjustment and then daily.
Target ranges for the various tests have not been established in an outcome-based setting. In the experience of the authors, a target concentration of 1.0 µg/ml with a range of 0.5–1.5 µg/ml for the anti-factor IIa test is a practicable target range [25]. The 1.5 to 2.5 fold increase of basal aPTT is defined as a target in the prescribing information (provided aPTT is within the reference range at baseline); however, every laboratory should establish in-house target ranges with its own reagent/coagulometer combination [25]. In the eyes of the WPH members, aPTT and ACT should also be locally calibrated based on a validated anti-factor IIa whenever possible [25]. A single centre retrospective study comparing argatroban with bivalirudin showed that HIT patients receiving bivalirudin reached the aPTT-based target range more rapidly than patients treated with argatroban [46]. Whether or not this results in a clinical benefit has not been determined.
PT and INR are dose dependently influenced by bivalirudin. This aspect needs to be considered during the transition to a VKA (see also paragraph in the Introduction regarding conditions necessary prior to transitioning to a VKA). In our experience, a therapeutic bivalirudin concentration increases the INR by about 1.0 [25]. Therefore, we suggest aiming for an INR of 3–4 for two consecutive days when bivalirudin and VKA are co-administered and to check the INR 3–6 hours after stopping bivalirudin.
Caution is recommended in patients with increased risk of bleeding, such as patients with uncontrolled hypertension, inherited or acquired bleeding disorders, gastrointestinal lesions, or immediately following lumbar puncture, spinal anaesthesia or major surgery. Caution is also recommended in patients with any other form of anticoagulation or with antiplatelet therapy. Bivalirudin can easily diffuse into the extravascular space (third compartment) when this is increased owing to overhydration.
In patients with an elevated baseline aPTT, it is especially important to monitor bivalirudin using drug levels (a validated anti-factor IIa assay) rather than using the aPTT [43].
No specific antidote for bivalirudin is available. However, bivalirudin’s short half-life reduces the need for an antidote.
No specific recommendations has been defined. Anticoagulation parameters typically return to baseline within two to four half-lives after discontinuation of the drug. Symptomatic and supportive therapy should be provided. Bivalirudin can be cleared through dialysis, haemofiltration and plasmapheresis [44, 45].
Drug interactions: No drug interactions have been reported so far.
Concomitant medications: The following medications should not be infused over the same intravenous line: alteplase, amiodarone, amphotericin B, diazepam, dobutamine, haloperidol, labetalol, vancomycin (see references [44, 45] for a complete list).
Overdose: Because of low protein binding, overdosing can be treated by use of haemodialysis, haemofiltration and plasmapheresis. Elimination may vary according to the type of filter membrane [47, 48].
Molecule: Danaparoid sodium is a small heparinoid consisting of glycosaminoglycans, with a mean molecular weight of 5500 Daltons. Danaparoid catalyses the antithrombin-mediated inactivation of factor Xa, leading to inhibition of thrombin generation. Factor IIa (thrombin) is inactivated to a much lesser extent (anti-factor Xa : anti-factor IIa ratio = 20:1) than in the presence of unfractionated heparin or low molecular weight heparins.
Distribution: The distribution volume of anti-factor Xa activity has been estimated to be 8–9 L.
Metabolism: No evidence of hepatic metabolism.
Excretion: Predominantly renal clearance.
Half-life: 19–25 hours.
The WPH recommends the use of danaparoid in HIT patients as an intravenous infusion, with an initial weight-adapted bolus followed by an accelerated initial infusion and maintenance infusion, for example, as indicated in the Swiss compendium [15]. If intravenous infusion is not desirable, danaparoid may be administered subcutaneously. Table 3 summarises dosing schedules of danaparoid [19, 20, 49].
Renal Insufficiency: Because of its predominantly renal excretion, elimination of danaparoid (measured as anti-factor Xa activity) is prolonged in renal insufficiency. Careful monitoring of anti-factor Xa activity is recommended in this case.
Liver insufficiency: No evidence available.
Pregnancy: Limited evidence supports the use of danaparoid during pregnancy and breast-feeding (no or no significant anti-factor Xa activity in cord blood or breast milk), but a benefit-risk assessment is recommended [34–36].
Geriatrics: The effectivity of danaparoid does not seem to be affected by age. However, there are insufficient data to determine if its absorption, distribution, and elimination are different in elderly patients (≥65 years).
Paediatrics: Limited evidence is available. Dose recommendations are found in the Swiss compendium [15].
As a result of its predominant anti-factor Xa activity, danaparoid has little or no effect on routine coagulation assays such as PT, aPTT or TT. The preferred monitoring modality is an anti-factor Xa activity assay, calibrated for danaparoid. The therapeutic plasma anti-factor Xa range is 0.5–0.7 U/ml 5 to 10 minutes after bolus, up to 1.0 U/ml during the adjustment phase in the first hours of administration, and 0.5–0.8 U/ml during maintenance infusion [19, 20, 49].
Transition to a VKA is considered straightforward, as PT and INR are not affected by danaparoid. Therefore, we suggest aiming for an INR within the therapeutic range on two consecutive days when danaparoid and VKA are co-administered before stopping danaparoid.
Caution is recommended in patients displaying cross-reactivity between danaparoid and HIT-Abs. Cross-reactivity is observed in approximately 5% of patients, in whom discontinuation of danaparoid is recommended to prevent treatment failure. Routine testing is not recommended but platelet counts should be monitored initially. Caution is also recommended in patients with hypersensitivity to sulphites.
No specific antidote for danaparoid is available. Andexanet alfa (a potential direct antagonist) is not yet available in Switzerland.
Discontinuation of danaparoid followed by symptomatic and supportive treatment should be provided to the bleeding patient. Plasma exchange can be considered if conventional treatment is not sufficient. Protamine sulphate partially neutralises anti-factor Xa activity and can be co-administered safely; however, there is no evidence that it is effective in controlling severe bleeding during danaparoid treatment.
Drug interactions: Limited evidence that oral chlorthalidone increases the distribution volume of anti-factor IIa activity, and decreases the clearance and distribution volume of anti-factor Xa activity [15].
Although the use of fondaparinux is off-label both in acute HIT complicated by thrombosis and in acute HIT without thrombosis, there are several publications reporting its usefulness, even as initial anticoagulant in acute HIT [50, 51].
Molecule: Fondaparinux is a pentasaccharide (molecular weight 11,728 Daltons). It strongly binds to antithrombin and thereby enhances inactivation of factor Xa without interaction with factor IIa or platelets. Compared with heparins, fondaparinux has a lower affinity to PF4 and does not induce its immunogenic conformational changes [52].
Distribution: Blood; no significant plasma protein binding.
Metabolism: No evidence of hepatic metabolism.
Excretion: Renal clearance.
Half-life: 17–21 hours.
The WPH recommends the use of fondaparinux in selected HIT patients as a subcutaneous injection without bolus. Since there is no immunogenic conformational change of PF4, no cross-reactivity is expected, but a few cases of fondaparinux-associated thrombocytopenia resembling HIT have been reported [53]. Table 3 summarises dosing schedules of fondaparinux [21, 28, 54–56].
Renal insufficiency: In the presence of impaired renal function, caution is warranted because of possible drug accumulation. Consider dose reduction according to the Swiss compendium [15]. Contraindicated when creatinine clearance is <30 ml/min, and caution is necessary during prolonged treatment when creatinine clearance is between 30 and 50 ml/min.
Liver insufficiency: Use with caution in severe liver insufficiency owing to elevated haemorrhagic risk.
Pregnancy: Limited information on the use of fondaparinux in pregnant and breast-feeding women is available. Therefore, it should be administered with caution during pregnancy and breast-feeding [34–36].
Geriatrics: The effectivity of fondaparinux does not seem to be affected by age.
Paediatrics: Limited supportive evidence on the use of fondaparinux in children is available. A possible approach is to start treatment of DVT with a daily dosage between 0.1 and 0.15 mg per kg body weight subcutaneously and adjust dosage if peak drug concentration is below 0.6 or above 1.5 μg/ml.
Fondaparinux used in licensed indications does not require monitoring. If used in subpopulations or special situations, knowledge of the plasma concentration can be helpful for safety reasons. Chromogenic assay of anti-factor Xa activity calibrated with fondaparinux produces reliable results. Recommended time for measurement of plasma concentrations is 3–5 hours after application (target peak plasma concentration: 1.0–1.5 µg/ml) or before next application (target trough level: 0.4–0.6 µg/ml) [57–59]. Fondaparinux has no impact on routine coagulation tests.
PT and INR are not influenced by fondaparinux. Therefore, we suggest aiming for an INR within the therapeutic range for two consecutive days when fondaparinux and VKA are co-administered before stopping fondaparinux.
No specific antidote for fondaparinux is available. Andexanet alfa (a direct antagonist) is not yet available in Switzerland.
Recombinant factor VIIa is able to normalise prolonged PT and aPTT, as well as changes in thrombin generation seen during treatment with fondaparinux [60, 61]. In HIT patients caution is advocated with the use of all haemostatic agents because of the increased thrombotic risk. Moreover, prothrombin complex concentrate (PCC) usually contain low amounts of heparin that may re-activate HIT.
Drug interactions: No drug interactions have been reported in the literature.
DOACs are increasingly used worldwide. However, the clinical data supporting their application in patients with HIT is even more limited than the data collected with the other anticoagulants mentioned above. No randomised controlled trials are available and even large cohort studies are lacking. Nevertheless, because of their ease of use and favourable characteristics, DOACs have been used for anticoagulant treatment of acute HIT, though only a few case series and small cohort studies are available.
In a prospective cohort study, Linkins and co-workers enrolled 22 patients with suspected HIT (including 12 patients with definite HIT) and treated them with rivaroxaban 15 mg twice daily (followed by 20 mg; after an initial course of fondaparinux in six patients) [62]. Platelet recovery was observed in all patients but one. One recurrent thromboembolic event occurred, and one patient required limb amputation. Sharifi and co-workers reported on 22 retrospectively identified patients with suspected HIT, who were treated with dabigatran (150 mg twice daily), rivaroxaban (20 mg once daily) or apixaban (5 mg twice daily) following an initial course of argatroban [63]. No thromboembolic or bleeding events occurred, but six patients died for various reasons. Warkentin et al. reported in a small series of patients the experience in Hamilton (Canada) and performed a review of the literature concluding that DOACs, in particular rivaroxaban, seem to be safe and effective for the treatment of acute HIT [13]. Davis and co-workers retrospectively observed 12 patients with suspected HIT (definite HIT in four cases), who were treated with apixaban (2.5–10 mg twice daily) or rivaroxaban (15 mg twice daily) [64]. Besides these studies, 54 case reports are available (reviewed in [65]).
Even though these reports are encouraging, the WPH members emphasise the need for caution regarding the use of DOACs as the initial anticoagulant in HIT patients, as supporting clinical evidence is currently limited. DOACs may be considered when switching to oral anticoagulation if there are neither renal insufficiency nor liver impairment. Of the DOACs, we would give preference to rivaroxaban, given that the most experience is available for this DOAC [12, 66]. Moreover, because current literature for the use of DOACs in this setting remains limited, DOACs should not be the first option when an arterial thrombotic event occurred during HIT [12, 66].
Finally, DOACs should not be used during pregnancy and breast feeding [15, 34–36].
Currently there are only limited data regarding the use of rivaroxaban in HIT, and data on efficacy and safety of this drug in HIT are still limited.
Molecule: Rivaroxaban is a small synthetic molecule with a molecular weight of 436 Daltons, which strongly binds to and inhibits factor Xa.
Distribution: Plasma; plasma protein binding (albumin) of 92–95%.
Metabolism: Two thirds of the dose is metabolised in the liver. Half of the metabolised drug is excreted via the kidneys as inactive components, and the other half via the hepatobiliary route.
Excretion: One third of the dose is directly excreted via the kidneys as unchanged active compound.
Half-life: 5–9 hours, in elderly patients 11–13 hours.
Table 1 summarises the pharmacokinetic properties of rivaroxaban and other anticoagulants.
No specific antidote for rivaroxaban is currently available in Switzerland. Andexanet alfa is an antidote to factor Xa antagonists approved by the FDA under the name Andexxa [67] and has been conditionally authorised by the European Medicines Agency (EMA) under the name Ondexxya [68]. The approval of Swissmedic is still pending. In order to avoid absorption of rivaroxaban in the event of an overdose, the use of activated charcoal may be considered where tablets have been taken within the previous 8 hours [69]. Absorption of rivaroxaban is subject to saturation: the rivaroxaban concentration in plasma only increases negligibly at oral doses >50 mg (ceiling effect) [70].
In animal models, recombinant factor VIIa as well as PCC were able to stop bleeding in patients treated with DOACs. Moreover, PCC was able to normalise prolonged PT and aPTT ex vivo, induced by ingestion of rivaroxaban by healthy subjects. In addition, a number of case reports exist, with successful treatment of rivaroxaban-associated bleedings with PCC at a dose of 25 U/kg [71, 72].
Drug interactions: rivaroxaban is metabolised via cytochrome P450 (CYP) 3A4, CYP2J2 and CYP-independent biotransformation processes. Furthermore, in vitro studies indicate the drug to be a substrate of P-glycoprotein. For the respective possible interactions, see the Swiss compendium [15].
Our recommendations integrate multiple guidelines regarding the anticoagulation of patients with HIT [1, 12, 25, 34, 73]. They propose that argatroban, bivalirudin, danaparoid, fondaparinux and DOACs are potentially suitable anticoagulants for the treatment of patients with HIT in Switzerland and provide guidance on selecting anticoagulants for individual patients with HIT and special conditions. Our recommendations also provide information regarding adequate drug dosage and monitoring in special clinical situations.
The limitations of our recommendations are inherent to the (very) low level of the evidence that we identified for most aspects related to the anticoagulants used to treat patients with HIT.
In conclusion, we hope that our recommendations will support Swiss clinicians treating HIT patients with anticoagulants in different clinical conditions.
AC has received grants from CSL Behring and NovoNordisk and non-financial support from Bayer and Shire. LA received grants/research support from: Bayer, CSL-Behring, Novartis, NovoNordisk, Roche, Shire-Takeda, and Sobi; support for the CHUV hemophilia nurses programme from: CSL-Behring, Bayer, NovoNordisk, Octapharma, Roche, Shire, and Sobi; honoraria for participating in scientific advisory boards: Bayer, Boehringer Ingelheim, Daiichi Sankyo, NovoNordisk, OrPha Swiss, Pfizer, Roche, Shire/Baxalta, Sobi; honoraria as consultant/speaker: Bayer, Sanofi-Genzyme, Siemens. J-DS received lecture and advisory honoraria from: Alexion, Bayer, BMS-Pfizer, Sanofi, Shire-Takeda, Siemens Diagnostics. All other authors report no relevant conflict of interest.
1 Linkins LA , Dans AL , Moores LK , Bona R , Davidson BL , Schulman S , et al. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2, Suppl):e495S–530S. doi:.https://doi.org/10.1378/chest.11-2303
2 Warkentin TE , Anderson JA . How I treat patients with a history of heparin-induced thrombocytopenia. Blood. 2016;128(3):348–59. doi:.https://doi.org/10.1182/blood-2016-01-635003
3 Nagler M , Bachmann LM , ten Cate H , ten Cate-Hoek A . Diagnostic value of immunoassays for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2016;127(5):546–57. doi:.https://doi.org/10.1182/blood-2015-07-661215
4 Leroux D , Hezard N , Lebreton A , Bauters A , Suchon P , de Maistre E , et al. Prospective evaluation of a rapid nanoparticle-based lateral flow immunoassay (STic Expert(®) HIT) for the diagnosis of heparin-induced thrombocytopenia. Br J Haematol. 2014;166(5):774–82. doi:.https://doi.org/10.1111/bjh.12939
5 Linkins LA , Bates SM , Lee AY , Heddle NM , Wang G , Warkentin TE . Combination of 4Ts score and PF4/H-PaGIA for diagnosis and management of heparin-induced thrombocytopenia: prospective cohort study. Blood. 2015;126(5):597–603. doi:.https://doi.org/10.1182/blood-2014-12-618165
6 Nellen V , Sulzer I , Barizzi G , Lämmle B , Alberio L . Rapid exclusion or confirmation of heparin-induced thrombocytopenia: a single-center experience with 1,291 patients. Haematologica. 2012;97(1):89–97. doi:.https://doi.org/10.3324/haematol.2011.048074
7 Arepally GM . Heparin-induced thrombocytopenia. Blood. 2017;129(21):2864–72. doi:.https://doi.org/10.1182/blood-2016-11-709873
8 Warkentin TE . High-dose intravenous immunoglobulin for the treatment and prevention of heparin-induced thrombocytopenia: a review. Expert Rev Hematol. 2019;12(8):685–98. doi:.https://doi.org/10.1080/17474086.2019.1636645
9 Warkentin TE , Climans TH , Morin PA . Intravenous Immune Globulin to Prevent Heparin-Induced Thrombocytopenia. N Engl J Med. 2018;378(19):1845–8. doi:.https://doi.org/10.1056/NEJMc1801799
10 Pon TK , Mahajan A , Rosenberg A , Amin A , Shah D , Jenkins I , et al. Platelet response to direct thrombin inhibitor or fondaparinux treatment in patients with suspected heparin-induced thrombocytopenia. J Thromb Thrombolysis. 2018;45(4):536–42. doi:.https://doi.org/10.1007/s11239-018-1646-x
11 Warkentin TE , Kelton JG . Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med. 2001;344(17):1286–92. doi:.https://doi.org/10.1056/NEJM200104263441704
12 Cuker A , Arepally GM , Chong BH , Cines DB , Greinacher A , Gruel Y , et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360–92. doi:.https://doi.org/10.1182/bloodadvances.2018024489
13 Warkentin TE , Pai M , Linkins LA . Direct oral anticoagulants for treatment of HIT: update of Hamilton experience and literature review. Blood. 2017;130(9):1104–13. doi:.https://doi.org/10.1182/blood-2017-04-778993
14 Greinacher A . Heparin-Induced Thrombocytopenia. N Engl J Med. 2015;373(3):252–61. doi:.https://doi.org/10.1056/NEJMcp1411910
16 https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm.
17Borbe H, Müller J. Argatra Produktmonographie. Deutschland GmH: Mitsubishi Pharma; 2013.
18 Tatsuno J , Komatsu T , Iida S . Pharmacokinetic studies of argatroban (MD-805): Protein binding and blood cell binding. Jap Pharmacol Ther. 1986;14(suppl 5):243–9.
19 de Valk HW , Banga JD , Wester JW , Brouwer CB , van Hessen MW , Meuwissen OJ , et al. Comparing subcutaneous danaparoid with intravenous unfractionated heparin for the treatment of venous thromboembolism. A randomized controlled trial. Ann Intern Med. 1995;123(1):1–9. doi:.https://doi.org/10.7326/0003-4819-123-1-199507010-00001
20 Ibbotson T , Perry CM . Danaparoid: a review of its use in thromboembolic and coagulation disorders. Drugs. 2002;62(15):2283–314. doi:.https://doi.org/10.2165/00003495-200262150-00016
21 Nagler M , Haslauer M , Wuillemin WA . Fondaparinux - data on efficacy and safety in special situations. Thromb Res. 2012;129(4):407–17. doi:.https://doi.org/10.1016/j.thromres.2011.10.037
22 Shepherd MF , Jacobsen JM , Rosborough TK . Argatroban therapy using enzymatic anti-factor IIa monitoring. Ann Pharmacother. 2011;45(3):422–3. doi:.https://doi.org/10.1345/aph.1P274
23 Lewis BE , Wallis DE , Berkowitz SD , Matthai WH , Fareed J , Walenga JM , et al.; ARG-911 Study Investigators. Argatroban anticoagulant therapy in patients with heparin-induced thrombocytopenia. Circulation. 2001;103(14):1838–43. doi:.https://doi.org/10.1161/01.CIR.103.14.1838
24 Lewis BE , Wallis DE , Leya F , Hursting MJ , Kelton JG ; Argatroban-915 Investigators. Argatroban anticoagulation in patients with heparin-induced thrombocytopenia. Arch Intern Med. 2003;163(15):1849–56. doi:.https://doi.org/10.1001/archinte.163.15.1849
25 Colucci G , Nagler M , Klaus N , Conte T , Giabbani E , Alberio L . Practical guidelines for Argatroban and Bivalirudin in patients with Heparin-induced Thrombocytopenia. J Transl Sci. 2015;1(2):37–42. doi:.https://doi.org/10.15761/JTS.1000109
26 Kiser TH , Fish DN . Evaluation of bivalirudin treatment for heparin-induced thrombocytopenia in critically ill patients with hepatic and/or renal dysfunction. Pharmacotherapy. 2006;26(4):452–60. doi:.https://doi.org/10.1592/phco.26.4.452
27 Sciulli TM , Mauro VF . Pharmacology and clinical use of bivalirudin. Ann Pharmacother. 2002;36(6):1028–41. doi:.https://doi.org/10.1345/aph.1A197
28 Pistulli R , Oberle V , Figulla HR , Yilmaz A , Pfeifer R . Fondaparinux cross-reacts with heparin antibodies in vitro in a patient with fondaparinux-related thrombocytopenia. Blood Coagul Fibrinolysis. 2011;22(1):76–8. doi:.https://doi.org/10.1097/MBC.0b013e328340ff24
29 Bartholomew JR , Pietrangeli CE , Hursting MJ . Argatroban anticoagulation for heparin-induced thrombocytopenia in elderly patients. Drugs Aging. 2007;24(6):489–99. doi:.https://doi.org/10.2165/00002512-200724060-00005
30 Skrupky LP , Smith JR , Deal EN , Arnold H , Hollands JM , Martinez EJ , et al. Comparison of bivalirudin and argatroban for the management of heparin-induced thrombocytopenia. Pharmacotherapy. 2010;30(12):1229–38. doi:.https://doi.org/10.1592/phco.30.12.1229
31 Levine RL , Hursting MJ , McCollum D . Argatroban therapy in heparin-induced thrombocytopenia with hepatic dysfunction. Chest. 2006;129(5):1167–75. doi:.https://doi.org/10.1378/chest.129.5.1167
32 Murray PT , Reddy BV , Grossman EJ , Hammes MS , Trevino S , Ferrell J , et al. A prospective comparison of three argatroban treatment regimens during hemodialysis in end-stage renal disease. Kidney Int. 2004;66(6):2446–53. doi:.https://doi.org/10.1111/j.1523-1755.2004.66022.x
33 Swan SK , Hursting MJ . The pharmacokinetics and pharmacodynamics of argatroban: effects of age, gender, and hepatic or renal dysfunction. Pharmacotherapy. 2000;20(3):318–29. doi:.https://doi.org/10.1592/phco.20.4.318.34881
34 Bates SM , Greer IA , Pabinger I , Sofaer S , Hirsh J . Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6, Suppl):844S–86S. doi:.https://doi.org/10.1378/chest.08-0761
35 Fogerty AE . Challenges of Anticoagulation Therapy in Pregnancy. Curr Treat Options Cardiovasc Med. 2017;19(10):76. doi:.https://doi.org/10.1007/s11936-017-0575-x
36 Lindhoff-Last E , Bauersachs R . Heparin-induced thrombocytopenia-alternative anticoagulation in pregnancy and lactation. Semin Thromb Hemost. 2002;28(5):439–46. doi:.https://doi.org/10.1055/s-2002-35284
37Lewis B, Hursting M. Argatroban therapy in heparin-induced thrombocytopenia. In: Warkentin T, Greinacher A, editors. Heparin-induced thrombocytopenia. New York, Basel: Marcel Dekker; 2006.
38 Tardy-Poncet B , Nguyen P , Thiranos JC , Morange PE , Biron-Andréani C , Gruel Y , et al. Argatroban in the management of heparin-induced thrombocytopenia: a multicenter clinical trial. Crit Care. 2015;19(1):396. doi:.https://doi.org/10.1186/s13054-015-1109-0
39 Siegmund R , Boer K , Poeschel K , Wolf G , Deufel T , Kiehntopf M . Influence of direct thrombin inhibitor argatroban on coagulation assays in healthy individuals, patients under oral anticoagulation therapy and patients with liver dysfunction. Blood Coagul Fibrinolysis. 2008;19(4):288–93. doi:.https://doi.org/10.1097/MBC.0b013e3282fe73ec
40 Zhang L , Yang J , Zheng X , Fan Q , Zhang Z . Influences of argatroban on five fibrinogen assays. Int J Lab Hematol. 2017;39(6):641–4. doi:.https://doi.org/10.1111/ijlh.12719
41 Cuker A , Cines DB . How I treat heparin-induced thrombocytopenia. Blood. 2012;119(10):2209–18. doi:.https://doi.org/10.1182/blood-2011-11-376293
42 Sheth SB , DiCicco RA , Hursting MJ , Montague T , Jorkasky DK . Interpreting the International Normalized Ratio (INR) in individuals receiving argatroban and warfarin. Thromb Haemost. 2001;85(3):435–40. doi:.https://doi.org/10.1055/s-0037-1615601
43 Warkentin TE . Anticoagulant failure in coagulopathic patients: PTT confounding and other pitfalls. Expert Opin Drug Saf. 2014;13(1):25–43. doi:.https://doi.org/10.1517/14740338.2013.823946
44 https://ec.europa.eu/health/documents/community-register/2017/20170914138919/anx_138919_de.pdf.
45 https://www.accord-healthcare.de/sites/default/files/2018-06/FI_Bivalirudin_Stand%20102016.pdf.
46 Vo QA , Lin JK , Tong LM . Efficacy and safety of argatroban and bivalirudine in patients with suspected heparin-induced thrombocytopenia. Ann Pharmacother. 2015;49(2):178–84. doi:.https://doi.org/10.1177/1060028014562949
47Bartholomew JR. Bivalirudin for the treatment of heparin-induced thrombocytopenia. In: Warkentin TE, Greinacher A, editors. Heparin-induced thrombocytopenia. 4th edition. London, UK: Informa Healthcare; 2007. p. 409–39.
48 Koster A , Chew D , Gründel M , Bauer M , Kuppe H , Spiess BD . Bivalirudin monitored with the ecarin clotting time for anticoagulation during cardiopulmonary bypass. Anesth Analg. 2003;96(2):383–6. doi:.https://doi.org/10.1213/00000539-200302000-00015
49 Stiekema JC , Wijnand HP , Van Dinther TG , Moelker HC , Dawes J , Vinchenzo A , et al. Safety and pharmacokinetics of the low molecular weight heparinoid Org 10172 administered to healthy elderly volunteers. Br J Clin Pharmacol. 1989;27(1):39–48. doi:.https://doi.org/10.1111/j.1365-2125.1989.tb05333.x
50 Schindewolf M , Steindl J , Beyer-Westendorf J , Schellong S , Dohmen PM , Brachmann J , et al. Use of Fondaparinux Off-Label or Approved Anticoagulants for Management of Heparin-Induced Thrombocytopenia. J Am Coll Cardiol. 2017;70(21):2636–48. doi:.https://doi.org/10.1016/j.jacc.2017.09.1099
51 Linkins LA , Hu G , Warkentin TE . Systematic review of fondaparinux for heparin-induced thrombocytopenia: When there are no randomized controlled trials. Res Pract Thromb Haemost. 2018;2(4):678–83. doi:.https://doi.org/10.1002/rth2.12145
52 Kreimann M , Brandt S , Krauel K , Block S , Helm CA , Weitschies W , et al. Binding of anti-platelet factor 4/heparin antibodies depends on the thermodynamics of conformational changes in platelet factor 4. Blood. 2014;124(15):2442–9. doi:.https://doi.org/10.1182/blood-2014-03-559518
53 Warkentin TE . Fondaparinux: does it cause HIT? Can it treat HIT? Expert Rev Hematol. 2010;3(5):567–81. doi:.https://doi.org/10.1586/ehm.10.54
54 D’Angelo A , Valle PD , Fattorini A , Luciano C . Disappearance of anti-PF4/heparin antibodies under prolonged fondaparinux administration in a patient with DVT associated with LMWH-induced thrombocytopenia. Thromb Haemost. 2006;95(3):573–5. doi:.https://doi.org/10.1160/TH05-11-0722
55 Lobo B , Finch C , Howard A , Minhas S . Fondaparinux for the treatment of patients with acute heparin-induced thrombocytopenia. Thromb Haemost. 2008;99(1):208–14. doi:.https://doi.org/10.1160/TH07-04-0252
56 Warkentin TE , Cook RJ , Marder VJ , Sheppard JA , Moore JC , Eriksson BI , et al. Anti-platelet factor 4/heparin antibodies in orthopedic surgery patients receiving antithrombotic prophylaxis with fondaparinux or enoxaparin. Blood. 2005;106(12):3791–6. doi:.https://doi.org/10.1182/blood-2005-05-1938
57 www.pathology.med.umich.edu.
58 Depasse F , Gerotziafas GT , Busson J , Van Dreden P , Samama MM . Assessment of three chromogenic and one clotting assays for the measurement of synthetic pentasaccharide fondaparinux (Arixtra) anti-Xa activity. J Thromb Haemost. 2004;2(2):346–8. doi:.https://doi.org/10.1111/j.1538-7933.2004.0584a.x
59 Garcia DA , Baglin TP , Weitz JI , Samama MM . Parenteral anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2, Suppl):e24S–43S. doi:.https://doi.org/10.1378/chest.11-2291
60 Bijsterveld NR , Moons AH , Boekholdt SM , van Aken BE , Fennema H , Peters RJ , et al. Ability of recombinant factor VIIa to reverse the anticoagulant effect of the pentasaccharide fondaparinux in healthy volunteers. Circulation. 2002;106(20):2550–4. doi:.https://doi.org/10.1161/01.CIR.0000038501.87442.02
61 Bijsterveld NR , Vink R , van Aken BE , Fennema H , Peters RJ , Meijers JC , et al. Recombinant factor VIIa reverses the anticoagulant effect of the long-acting pentasaccharide idraparinux in healthy volunteers. Br J Haematol. 2004;124(5):653–8. doi:.https://doi.org/10.1111/j.1365-2141.2003.04811.x
62 Linkins LA , Warkentin TE , Pai M , Shivakumar S , Manji RA , Wells PS , et al. Rivaroxaban for treatment of suspected or confirmed heparin-induced thrombocytopenia study. J Thromb Haemost. 2016;14(6):1206–10. doi:.https://doi.org/10.1111/jth.13330
63 Sharifi M , Bay C , Vajo Z , Freeman W , Sharifi M , Schwartz F . New oral anticoagulants in the treatment of heparin-induced thrombocytopenia. Thromb Res. 2015;135(4):607–9. doi:.https://doi.org/10.1016/j.thromres.2015.01.009
64 Davis KA , Davis DO . Direct acting oral anticoagulants for the treatment of suspected heparin-induced thrombocytopenia. Eur J Haematol. 2017;99(4):332–5. doi:.https://doi.org/10.1111/ejh.12921
65 Shatzel JJ , Crapster-Pregont M , Deloughery TG . Non-vitamin K antagonist oral anticoagulants for heparin-induced thrombocytopenia. A systematic review of 54 reported cases. Thromb Haemost. 2016;116(2):397–400. doi:.https://doi.org/10.1160/TH16-02-0101
66 Barlow A , Barlow B , Reinaker T , Harris J . Potential Role of Direct Oral Anticoagulants in the Management of Heparin-induced Thrombocytopenia. Pharmacotherapy. 2019;39(8):837–53. doi:.https://doi.org/10.1002/phar.2298
67 Rogers KC , Finks SW . A New Option for Reversing the Anticoagulant Effect of Factor Xa Inhibitors: Andexanet Alfa (ANDEXXA). Am J Med. 2019;132(1):38–41. doi:.https://doi.org/10.1016/j.amjmed.2018.06.028
68Information for healthcare professionals for Ondexxyan http://www.ondexxya.eu/downloads/pdf/anx_144471_en.pdf.
69 Ollier E , Hodin S , Lanoiselée J , Escal J , Accassat S , De Magalhaes E , et al. Effect of Activated Charcoal on Rivaroxaban Complex Absorption. Clin Pharmacokinet. 2017;56(7):793–801. doi:.https://doi.org/10.1007/s40262-016-0485-1
70 Kubitza D , Becka M , Roth A , Mueck W . Dose-escalation study of the pharmacokinetics and pharmacodynamics of rivaroxaban in healthy elderly subjects. Curr Med Res Opin. 2008;24(10):2757–65. doi:.https://doi.org/10.1185/03007990802361499
71 Sauter TC , Eberle B , Wuillemin WA , Thiele T , Angelillo-Scherrer A , Exadaktylos AK , et al. How I manage patients with anticoagulation-associated bleeding or urgent surgery. Swiss Med Wkly. 2018;148:w14598. doi:.https://doi.org/10.4414/smw.2018.14598
72 Majeed A , Ågren A , Holmström M , Bruzelius M , Chaireti R , Odeberg J , et al. Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood. 2017;130(15):1706–12. doi:.https://doi.org/10.1182/blood-2017-05-782060
73 Joseph J , Rabbolini D , Enjeti AK , Favaloro E , Kopp MC , McRae S , et al. Diagnosis and management of heparin-induced thrombocytopenia: a consensus statement from the Thrombosis and Haemostasis Society of Australia and New Zealand HIT Writing Group. Med J Aust. 2019;210(11):509–16. doi:.https://doi.org/10.5694/mja2.50213
74Chong BH, Magnani HN. Danaparoid for the treatment of heparin-induced thrombocytopenia. In: Warkentin TE, Greinacher A, editors. Heparin-induced thrombocytopenia. 4th edition. London, UK: Informa Healthcare; 2007.
75Fischer KG. Hemodialysis in heparin-induced thrombocytopenia. In: Warkentin TE, Greinacher A, editors. Heparin-induced thrombocytopenia. 4th edition. London, UK: Informa Healthcare; 2007. p. 463–85.
76 Tang IY , Cox DS , Patel K , Reddy BV , Nahlik L , Trevino S , et al. Argatroban and renal replacement therapy in patients with heparin-induced thrombocytopenia. Ann Pharmacother. 2005;39(2):231–6. doi:.https://doi.org/10.1345/aph.1E480
77Lewis BE, Hursting MJ. Argatroban therapy in heparin-induced thrombocytopenia. In: Warkentin TE, Greinacher A, editors. Heparin-induced thrombocytopenia. 4th edition. Boca Raton, Florida: CRC Press; 2007. p. 379–408.
78 Jang IK , Lewis BE , Matthai WH, Jr , Kleiman NS . Argatroban anticoagulation in conjunction with glycoprotein IIb/IIIa inhibition in patients undergoing percutaneous coronary intervention: an open-label, nonrandomized pilot study. J Thromb Thrombolysis. 2004;18(1):31–7. doi:.https://doi.org/10.1007/s11239-004-0171-2
79 Lewis BE , Matthai WH, Jr , Cohen M , Moses JW , Hursting MJ , Leya F ; ARG-216/310/311 Study Investigators. Argatroban anticoagulation during percutaneous coronary intervention in patients with heparin-induced thrombocytopenia. Catheter Cardiovasc Interv. 2002;57(2):177–84. doi:.https://doi.org/10.1002/ccd.10276
80 Selleng K , Warkentin TE , Greinacher A . Heparin-induced thrombocytopenia in intensive care patients. Crit Care Med. 2007;35(4):1165–76. doi:.https://doi.org/10.1097/01.CCM.0000259538.02375.A5
81 Beiderlinden M , Treschan T , Görlinger K , Peters J . Argatroban in extracorporeal membrane oxygenation. Artif Organs. 2007;31(6):461–5. doi:.https://doi.org/10.1111/j.1525-1594.2007.00388.x
82 Kleinschmidt S , Stephan B , Pindur G , Bauer C . Argatroban: Pharmakologische Eigenschaften und anästhesiologische Aspekte [Argatroban: pharmacological properties and anaesthesiological aspects]. Anaesthesist. 2006;55(4):443–50. doi:.https://doi.org/10.1007/s00101-005-0962-7
83 Koster A , Spiess B , Jurmann M , Dyke CM , Smedira NG , Aronson S , et al. Bivalirudin provides rapid, effective, and reliable anticoagulation during off-pump coronary revascularization: results of the “EVOLUTION OFF” trial. Anesth Analg. 2006;103(3):540–4. doi:.https://doi.org/10.1213/01.ane.0000226098.95698.0f
84 Spiess BD . Update on heparin-induced thrombocytopenia and cardiovascular interventions. Semin Hematol. 2005;42(3, Suppl 3):S22–7. doi:.https://doi.org/10.1053/j.seminhematol.2005.05.014
85 Spiess BD , DeAnda A , McCarthy HL , Yeatman D , Katlaps G , Cooper C , et al. Case 1-2006: off-pump coronary artery bypass graft surgery anticoagulation with bivalirudin: a patient with heparin-induced thrombocytopenia syndrome type II and renal failure. J Cardiothorac Vasc Anesth. 2006;20(1):106–11. doi:.https://doi.org/10.1053/j.jvca.2005.11.011
86 Koster A , Dyke CM , Aldea G , Smedira NG , McCarthy HL, 2nd , Aronson S , et al. Bivalirudin during cardiopulmonary bypass in patients with previous or acute heparin-induced thrombocytopenia and heparin antibodies: results of the CHOOSE-ON trial. Ann Thorac Surg. 2007;83(2):572–7. doi:.https://doi.org/10.1016/j.athoracsur.2006.09.038
87 Vasquez JC , Vichiendilokkul A , Mahmood S , Baciewicz FA, Jr . Anticoagulation with bivalirudin during cardiopulmonary bypass in cardiac surgery. Ann Thorac Surg. 2002;74(6):2177–9. doi:.https://doi.org/10.1016/S0003-4975(02)04125-5
All authors contributed equally to this work.
AC has received grants from CSL Behring and NovoNordisk and non-financial support from Bayer and Shire. LA received grants/research support from: Bayer, CSL-Behring, Novartis, NovoNordisk, Roche, Shire-Takeda, and Sobi; support for the CHUV hemophilia nurses programme from: CSL-Behring, Bayer, NovoNordisk, Octapharma, Roche, Shire, and Sobi; honoraria for participating in scientific advisory boards: Bayer, Boehringer Ingelheim, Daiichi Sankyo, NovoNordisk, OrPha Swiss, Pfizer, Roche, Shire/Baxalta, Sobi; honoraria as consultant/speaker: Bayer, Sanofi-Genzyme, Siemens. J-DS received lecture and advisory honoraria from: Alexion, Bayer, BMS-Pfizer, Sanofi, Shire-Takeda, Siemens Diagnostics. All other authors report no relevant conflict of interest.