Management of neurosurgical implant-associated infections
DOI: https://doi.org/10.4414/smw.2020.20208
Anna
Conena, Andreas
Raabeb, Karl
Schallerc, Christoph A
Fuxa, Peter
Vajkoczyd, Andrej
Trampuze
aDepartment of Infectious Diseases and Hospital Hygiene, Kantonsspital Aarau, Switzerland
bDepartment of Neurosurgery, Inselspital Bern, Switzerland
cDepartment of Neurosurgery, University Hospital Geneva, Switzerland
dCharité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Department
of Neurosurgery, Berlin, Germany
eCharité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Centre for
Musculoskeletal Surgery, Berlin, Germany
Summary
With the technical developments in neurosurgery, increasing numbers of neurosurgical implants are used in an increasingly aged population of patients with several comorbidities. Consequently, the number of neurosurgical implant-associated infections is continuously raising, resulting in significant morbidity and mortality, including disfiguring skull deformities and lack of brain protection. In this article we review infections associated with craniotomy, cranioplasty, neurostimulators, internal cerebrospinal fluid shunts, and external ventricular and lumbar cerebrospinal fluid drainages.
In all implant-associated infections biofilms are involved, which are difficult to eradicate. A low number of microorganisms is sufficient to form a biofilm on the implant surface. In most infections, microorganisms of the skin flora are involved. Microorganisms reach the implant during surgery or immediately thereafter as a result of wound healing disturbances. In about two thirds of patients, implant-associated infections manifest early (within the first month after surgery), whereas the remaining infections present later as a result of low-grade infections or by direct extension from adjacent infections (per continuitatem) to the implants due to soft tissue damage. Except for ventriculo-atrial cerebrospinal fluid shunts, neurosurgical implants are rarely infected by the haematogenous route.
In this article we review established and clinically validated concepts for the management of biofilm-associated infections in orthopaedic and trauma surgery, which can be extrapolated to other surgical disciplines that use implants. However, the evidence for the success of this extrapolation to neurosurgical patients is sparse and has not been evaluated in large patient populations. For favourable outcome, an optimised microbiological diagnosis including sonication of removed implants and prolonged incubation of cultures is required. Furthermore, a combined surgical and antimicrobial management strategy is needed. Surgery includes an appropriate debridement with or without implant exchange or removal, depending on the age of the biofilm and the soft tissue condition. Antimicrobial treatment includes a prolonged biofilm-active therapy, typically for 4–12 weeks. This concept is attractive, because in selected patients, implants can be retained or exchanged in a one-stage surgical procedure, which improves not only quality of life, but also decreases morbidity because every additional neurosurgical intervention can lead to secondary complications, including intracerebral bleeding or ischemia.
Introduction
An increasing number of implant insertions are observed in all surgical disciplines, including neurosurgery. Indications are degenerative and age-related, but also ischaemic, neoplastic and trauma-related health problems. In 2004, an estimated 450,000 neurosurgical implants were inserted in the United States [1]. Implant-associated infections occur in about 3–15%, which is 0.3–12% for craniotomies, 1–24.4% for craniectomies, 1–6% for neurostimulators, 4–17% for cerebrospinal fluid (CSF) shunts, 2–22% for external ventricular CSF drainages (EVD) and 5% for external lumbar CSF drainages (ELD) [1, 2]. These infections can be devastating for the patient and are associated with increased morbidity and mortality, as usually more than one revision operation is needed and the patient is left with unaesthetic skull deformities without brain protection in between; in addition pain, movement disorders and depressive symptoms can deteriorate [3, 4]. Therefore, the management of neurosurgical implant-associated infections is of high importance.
Diagnosis of neurosurgical implant-associated infections is challenging [5]. Clinical signs and symptoms are often nonspecific, insensitive and difficult to interpret in the context of neurologically disabled patients after intracerebral bleeding or trauma and in patients with impaired consciousness due to the treatment with sedative drugs. The same is true for laboratory tests including CSF parameters. A thoughtful approach to the patient is necessary, which includes observation of the dynamic of the clinical evolution and laboratory tests, and considers as well the time interval from surgery and the presence of other nosocomial infectious foci [2].
Implant surfaces are highly susceptible to bacterial colonisation and within a few minutes a biofilm is formed [6, 7]. Biofilms complicate microbiological diagnosis and treatment of implant-associated infections, because bacteria in biofilms live in a dormant state and are not planktonic in tissue or CSF [8, 9]. Most neurosurgical implants are infected exogenously by contamination with microorganisms of the skin or mucosal flora during surgery or in the first days postoperatively during wound healing disturbance. Therefore, more than two thirds of patients present within the first month of surgery [6, 7, 10, 11]. Haematogenous seeding from a distant infectious focus is rare, and almost exclusively found in ventriculo-atrial (VA) shunts owing to their intravascular position.
Standardised approaches to the management of neurosurgical implant-associated infections are lacking. The extrapolation of well-established concepts from orthopaedic and trauma surgery dealing with implant-associated infections seems attractive and logical, as these have been clinically validated and proven successful [12–14]. Microbiological diagnosis can be optimised by sonication of removed implants and prolonged incubation of cultures for up to 14 days [15, 16]. Interdisciplinary treatment consists of a combined surgical and antimicrobial approach. Surgery always includes debridement and either implant retention, exchange or removal. In addition, a prolonged antimicrobial biofilm-active treatment is needed [17]. It is important to note that in neurosurgery removal or exchange of implants and extensive debridement are not always feasible because of secondary damage to the central nervous system (CNS), and that antimicrobial penetration across the blood-brain barrier is critical.
This review aims to discuss diagnostic and treatment challenges in neurosurgical implant-associated infections and to extrapolate interdisciplinary management concepts from implant-associated infections in orthopaedic and trauma surgery, where the concept is established and clinically validated. However, the evidence for the success of this extrapolation to neurosurgical patients is sparse and has not been evaluated in large patient populations. Infections associated with craniotomy, cranioplasty, neurostimulators, internal CSF shunts (ventriculo-peritoneal [VP] and VA shunts), as well as EVD and ELD will be covered.
Classification of neurosurgical implant-associated infections
Implant-associated infections are classified according to the time-point of manifestation after implantation and according to the acuity of infection, because the two parameters (together with the detected microorganism) define infection management [10, 17, 18]. The classification includes acute and chronic infections (table 1). Acute infections present early, typically within 6 weeks of implantation. Patients usually have fever and signs of local inflammation with pain, heat, swelling, redness and a putrid wound discharge. Chronic infections, on the other hand, present later, often months after implantation. Patients usually have discrete signs of local inflammation, but may present with persisting wound discharge, fistula or implant on view. In acute infections an immature biofilm is present, therefore eradication of infection can be achieved by implant debridement and retention, and a 12-week biofilm-active treatment, whereas in chronic infections a mature biofilm is present, which cannot be eradicated without implant removal or exchange [9, 14, 19–22]. EVD and ELD are non-permanent implants, therefore this classification does not apply to them. They usually can be removed if an infection occurs and therefore no biofilm-active therapy is needed.
Table 1 Classification of implant-associated infections according to the time of occurrence.
| |
Acute infection*
|
Chronic infection
|
| Time-point |
≤6 weeks after implantation |
>6 weeks after implantation |
| Biofilm |
Immature |
Mature |
| Treatment concept |
Debridement and implant retention |
Removal or exchange of implant in a one- or two-stage procedure |
Diagnostic concepts for neurosurgical implant-associated infections
Sonication of the removed implants and prolonged incubation of cultures for up to 14 days became the standard of care, as most low-virulent pathogens require 7–14 days of cultivation [15, 16]. However, with longer sample cultivation the risk of contamination increases, and the microbiological results need to be interpreted accordingly. Specificity of sonication in orthopaedic surgery is high (up to 99%) and sensitivity is significantly higher than for tissue cultures, because biofilms are detached from the implant surface and cultured (sensitivity 80–90% vs 60% in tissue cultures). This is especially true for patients with antimicrobial pretreatment (sensitivity 75 vs 45%) [15]. As biofilms are patchily distributed in the periimplant tissue, several (ideally three to five) independent tissue samples are recommended. In culture-negative infections, molecular diagnostic testing such as polymerase chain reaction tests or next-generation sequencing may identify the pathogen.
In neurosurgery, sonication has been proven useful for osteosynthesis (as used for craniotomy fixation), cranioplasties, CSF shunts and EVD tips [23–26]. Prinz et al. sonicated VP shunts in 22 patients and compared them with 13 patients with conventional culture only [26]. Sonication detected the pathogen in all cases, whereas conventional culture in only 61% (p = 0.018). When pathogen detection was analysed by method, sonication was positive in all in whom it was performed, and culture in only 22 of 35 patients (60%, p <0.001). For patients with antimicrobial pretreatment (n = 18), sonication again was positive in all 12 patients, but conventional culture only in 3 of 6 patients (50%, p = 0.005), underlining the high diagnostic impact of sonication. The same was true for EVD-associated infections in a smaller study by Jost et al. [25]. In 14 patients sonication of the EVD tip was performed and had a higher detection rate than conventional culture of ventricular CSF (64 vs 14%; p <0.05), which can be explained by the biofilm concept, where microorganisms attach to the implant surface and are not planktonic in the CSF. Nevertheless, contamination is still possible if the EVD tip is not properly collected (need for disinfection of cutaneous exit site before pulling out the catheter, collection of the distal 2–3 cm of the drainage). Five patients had a positive sonication culture, but a negative CSF culture and the findings were initially rated as contamination, but two of them suffered a meningitis episode a few days after EVD removal.
Treatment concepts for neurosurgical implant-associated infections
In permanent neurosurgical implants (i.e., except EVD and ELD), the treatment concepts as described in orthopaedic and trauma surgery can be extrapolated, although the evidence for this extrapolation is sparse and not validated in large neurosurgical patient populations [17, 18]. To achieve high treatment success, an interdisciplinary management is needed including surgery and biofilm-active therapy. Surgical debridement is always necessary to remove the necrotic tissue and to mechanically reduce the pathogen load in established biofilms. The implant management depends on the acuity and time of infection after device implantation, and includes debridement and retention in acute infections and one- or two-stage exchange or implant removal in chronic infections (fig. 1). In addition, it is of utmost importance to have adequate postoperative soft tissue coverage of the implant to avoid secondary implant colonisation with skin pathogens. Thereafter, a biofilm-active therapy is given for usually 4–12 weeks (1–2 weeks intravenous and 3–10 weeks oral treatment), depending on the duration of the implant-free interval and the intraoperative culture results. A drug holiday (antibiotic-free period before reimplantation) is not recommended since neurosurgical patients are often in urgent need of the implant not only to avoid disfiguring skull deformities and lack of brain protection, but also to decrease the risk of the development of a sinking flap syndrome with progressive neurological deterioration. In addition, in extrapolation from the orthopaedic literature, in periprosthetic joint infections it was demonstrated that the outcome was better with continuous antibiotic therapy than with an antibiotic-free interval before reimplantation [27]. The rationale for continuous antibiotic therapy is that there is no diagnostic test that can accurately exclude persistent infection at the time of reimplantation and any viable organism can cause a relapse of infection. Therefore, treatment after reimplantation should be continued to complete a full treatment course for implant-associated infections. Treatment failure in neurosurgical implant-associated infections is a devastating situation and must be absolutely avoided. Bactericidal antimicrobial drugs with penetration into the CSF should be used if needed (tables 2, 3 and 4
) [17, 18, 28].
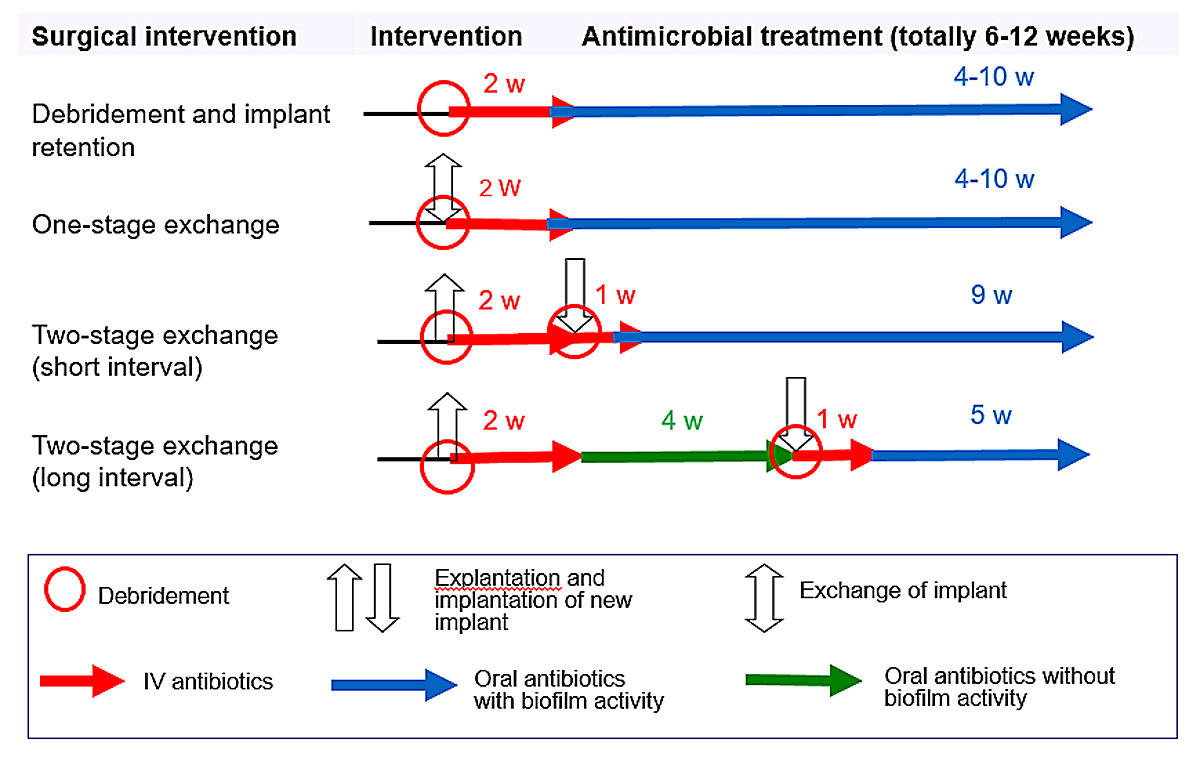
Figure 1
Treatment algorithm for implant-associated infections. Criteria for implant debridement and retention: acute infection (≤6 weeks postoperative), adequate soft tissue coverage, no implant dysfunction, availability of biofilm-active treatment, absence of life-threatening infection (e.g., brain abscess or meningitis). Antibiotics with biofilm activity: rifampin for staphylococci and Cutibacterium spp.; fluoroquinolones for gram-negative bacilli.
IV = intravenous; w = week(s)
Table 2 Empirical intravenous treatment for intra- and extradural infections (local susceptibility data and patient’s renal function and body weight must be taken into account).
|
Localisation
|
First choice
|
Alternative
|
Extradural infection
(craniotomy- or cranioplasty-associated infections, epidural empyema) |
Amoxicillin/clavulanic acid 3 × 2.2 g* OR
Ampicillin/sulbactam 3 × 3 g |
Cefuroxime 3 × 1.5 g |
| – With implants in place |
PLUS vancomycin†‡ 2 × 15 mg/kg |
PLUS daptomycin‡ 1 × 8 mg/kg |
Intradural infection
(subdural empyema, meningitis, brain abscess) |
Cefepime 3 × 2 g |
Meropenem 3 × 2 g |
| – With implants in place |
PLUS vancomycin†‡ 2 × 15 mg/kg |
PLUS vancomycin†‡ 2 × 15 mg/kg |
Table 3 Targeted intravenous treatment for intra- and extradural infections (patient’s renal function and body weight must be taken into account). Rifampin treatment is indicated only if an implant is involved.
|
Pathogen
|
Extradural infection
|
Intradural infection
|
|
Staphylococcus spp. |
Methicillin-susceptible |
Flucloxacillin 4 × 2 g
PLUS consider
fosfomycin* 3 × 5 g |
Flucloxacillin 6 × 2 g
PLUS consider
fosfomycin* 3 × 8 g |
| Allergy to penicillin (none type 1) |
Cefazolin 3 × 2g
PLUS consider
fosfomycin* 3 × 5 g |
Cefepime 3 × 2 g OR
meropenem 3 × 2 g
for both PLUS consider
fosfomycin* 3 × 8 g |
| Methicillin-resistant or allergy to penicillin (type 1) |
Vancomycin† 2 × 15 mg/kg OR
daptomycin 1 × 8 mg/kg
for both PLUS consider
fosfomycin* 3 × 5 g |
Cotrimoxazole 3 × 1920 mg p.o. OR
vancomycin† 2 × 15 mg/kg
for both PLUS consider
fosfomycin* 3 × 8 g |
| For all staphylococcal infections with implants in place |
PLUS rifampin‡ 2 × 450 mg p.o. |
PLUS rifampin‡ 2 × 600 mg p.o. |
|
Streptococcus spp. |
Penicillin G 4 × 5 mio. IU OR
ceftriaxone 1 × 2 g |
Penicillin G 4 × 5 mio. IU OR
ceftriaxone 2 × 2 g |
|
Enterococcus faecalis
|
Penicillin-susceptible |
Ampicillin 4 × 2 g
PLUS
gentamicin§ 1 × 3 mg/kg OR
ceftriaxone 2 × 2 g |
Ampicillin 6 × 2 g
PLUS
gentamicin§ 1 × 3 mg/kg OR
ceftriaxone 2 × 2 g |
| Penicillin-resistant |
Vancomycin† 2 × 15 mg/kg
PLUS
gentamicin§ 1 × 3 mg/kg |
Vancomycin† 2 × 15 mg/kg
PLUS
gentamicin§ 1 × 3 mg/kg |
|
Enterococcus faecium
|
Penicillin-susceptible |
Ampicillin 4 × 2g
PLUS
gentamicin§ 1 × 3 mg/kg |
Ampicillin 6 × 2 g
PLUS
gentamicin§ 1 × 3 mg/kg |
| Penicillin-resistant |
Vancomycin† 2 × 15 mg/kg
PLUS
gentamicin§ 1 × 3 mg/kg |
Vancomycin† 2 × 15 mg/kg
PLUS
gentamicin§ 1 × 3 mg/kg |
|
Cutibacterium spp. |
Penicillin G 4 × 5 mio. IU OR
ceftriaxone 1 × 2 g |
Penicillin G 4 x 5 mio. IU OR
Ceftriaxone 2 × 2g |
| – With implants in place |
PLUS rifampin‡ 2 × 450 mg p.o. |
PLUS rifampin‡ 2 × 600 mg p.o. |
| Enterobacteriaceae |
Ceftriaxone 1 × 2 g OR
amoxicillin/clavulanic acid 3 × 2.2 g¶ OR
ampicillin/sulbactam 3 × 3 g |
Ceftriaxone 2 × 2 g |
Non-fermenters
(e.g., Pseudomonas aeruginosa, Acinetobacter spp.) |
Ceftazidime 3 × 2 g OR
cefepime 3 × 2 g OR
piperacillin/tazobactam 3–4 × 4.5 g OR
meropenem 3 × 2 g |
Ceftazidime 3 × 2 g OR
cefepime 3 × 2 g OR
meropenem 3 × 2 g |
| Culture-negative infections |
Amoxicillin/clavulanic acid 3 × 2.2 g¶
OR
ampicillin/sulbactam 3 × 3 g
both PLUS
vancomycin† 2 × 15 mg/kg |
Ceftriaxone 2 × 2 g
PLUS
vancomycin† 2 × 15 mg/kg |
| – With implants in place |
PLUS rifampin‡ 2 × 450 mg p.o. |
PLUS rifampin‡ 2 × 600 mg p.o. |
Table 4 Targeted oral treatment for intra- or extradural infections (patient’s renal function and body weight must be taken into account). Rifampin treatment is only indicated if an implant is involved.
|
Pathogen
|
Extradural infection
|
Intradural infection
|
|
Staphylococcus spp. |
Cotrimoxazole 3 × 960 mg OR
clindamycin 3 × 600 mg OR
doxycycline* 2 × 100 mg |
Cotrimoxazole 3 × 1920 mg OR
doxycycline* 2 × 100 mg |
| – With implants in place |
Levofloxacin 2 × 500 mg OR
cotrimoxazole 3 × 960 mg OR
doxycycline* 2 × 100 mg OR
fusidic acid 3 × 500 mg
all PLUS
rifampin† 2 × 450 mg |
Cotrimoxazole 3 × 1920 mg OR
moxifloxacin 1 × 4 00 mg OR
doxycycline* 2 × 100 mg
all PLUS
rifampin† 2 × 600 mg |
|
Streptococcus spp. |
Amoxicillin 3 × 1 g OR
doxycycline* 2 × 100 mg OR
clindamycin 3 × 600 mg |
Prolonged i.v. treatment (e.g., outpatient treatment with ceftriaxone 1 × 2 g i.v.), then:
moxifloxacin 1 × 400 mg |
|
Enterococcus spp. |
Penicillin-susceptible |
Amoxicillin 3 × 1 g OR
doxycycline 2 × 100 mg |
Prolonged i.v. treatment, then:
linezolid 2 × 600 mg OR
doxycycline 2 × 100 mg |
| Penicillin-resistant |
Linezolid 2 × 600 mg OR
doxycycline 2 × 100 mg |
Prolonged i.v. treatment, then:
Linezolid 2 × 600 mg OR
doxycycline 2 × 100 mg |
|
Cutibacterium spp. |
Amoxicillin 3 × 1 g OR
levofloxacin 2 × 500 mg OR
doxycycline* 2 × 100 mg OR
clindamycin 3 × 600 mg |
Moxifloxacin 1 × 400 mg OR
doxycycline* 2 × 100 mg |
| – With implants in place |
all PLUS rifampin† 2 × 450 mg |
all PLUS rifampin† 2 × 600 mg |
| Enterobacteriaceae |
Ciprofloxacin 2 × 750 mg OR
cotrimoxazole 3 × 960 mg |
Prolonged i.v. treatment, then:
cotrimoxazole 3 × 1920 mg OR
ciprofloxacin 2 × 750 mg |
| – With implants in place |
Ciprofloxacin 2 × 750 mg |
Ciprofloxacin 2 × 750 mg |
| Non-fermenters (e.g. P. aeruginosa, Acinetobacter spp.) |
Ciprofloxacin 2 × 750 mg |
Prolonged i.v. treatment, then:
ciprofloxacin 2 × 750 mg |
| – With implants in place |
Ciprofloxacin 2 × 750mg |
Ciprofloxacin 2 × 750 mg |
| Culture-negative infection |
Levofloxacin 2 x 500 mg PLUS
clindamycin 3 x 600 mg
OR
levofloxacin 2 x 500 mg PLUS
cotrimoxazole 3 x 960 mg |
Prolonged i.v. treatment, then:
cotrimoxazole 3 x 1920 mg PLUS
moxifloxacin 1 x 400 mg |
| – With implants in place |
Levofloxacin 2 × 500 mg PLUS
rifampin† 2 × 450 mg |
Moxifloxacin 1 × 400mg PLUS
rifampin† 2 × 600 mg |
Biofilm-active treatment against gram-positive pathogens (staphylococci and Cutibacterium spp.) relies on bactericidal rifampin combinations; against gram-negative bacilli it relies on fluoroquinolones. Rifampin (reaching 56% of plasma levels in CSF) should not be administered as a single antibiotic since resistance rapidly emerges; therefore it has to be combined with an antibiotic with similarly good CSF penetration, such as cotrimoxazole (40–50% of plasma levels in CSF), levofloxacin (30–50% of plasma levels in CSF), moxifloxacin (>50% of plasma levels in CSF) and doxycycline (26% of plasma levels in CSF) [29]. In CSF shunt-associated infections, emergence of rifampin resistance has been observed when coagulase-negative staphylococci were treated with intravenous vancomycin, which has insufficient CSF penetration and therefore results in a rifampin monotherapy in CSF, leading to treatment failure [10]. For ciprofloxacin, CSF levels reach about 26% of plasma levels [22, 28–30].
Intravenous fosfomycin can be considered for the treatment of CNS infections because of its activity against most relevant pathogens, synergy with other antibiotics and sufficient penetration across the blood-brain barrier in both noninflamed (27%) and inflamed meninges (50–70%) [31]. In a prospective, multicentre study (NCT01173575) including patients with severe bacterial infections from 12 intensive care units in Germany and Austria, a subgroup of patients treated with fosfomycin was analysed [32]. Most patients had severe CNS infections (22%), followed by pneumonia (15%), bone and joint infections (11%), abdominal infections (11%) and bacteraemia (11%). The overall clinical success was favourable in 81.3% (148/182) of cases, and in 84.8% (39/46) of patients with ≥1 multidrug resistant pathogen. These data suggest that intravenous fosfomycin is an effective and safe combination partner for the treatment of severe bacterial infections.
Intraventricular antimicrobial treatment is not routinely recommended [16]. However, it can be considered in patients with a poor response to systemic therapy or in the case of multidrug resistant difficult-to-eradicate pathogens. The main advantage is that the blood-brain barrier is bypassed and high intraventricular concentrations can be reached, but evidence for this treatment modality, especially dosing recommendations are sparse.
In selected patients, implant retention is possible. Prerequisites for implant retention are acute infections with immature biofilms (≤6 weeks), availability of biofilm-active treatments and adequate soft tissue coverage of the implant [17]. Prolonged biofilm-active treatment for 12 weeks eradicates the biofilm thereafter. In chronic infections (>6 weeks) implant removal or exchange in a one- or two-stage procedure with only a short implant-free interval of 14 days is recommended to remove the mature biofilm. This surgical strategy is followed by usually 12 weeks of biofilm-active treatment. If intraoperative culture results are negative in the case of a two-stage implant exchange, a shorter treatment duration of 4–6 weeks is possible. Despite negative intraoperative culture results during reimplantation, which usually is performed under antimicrobial treatment, treatment continuation is suggested, as culture results under antimicrobial treatment might be false negative. And an infection relapse can be associated with devastating complications for the patient, if, for example, another surgery is needed.
A difficult-to-treat implant-associated infection is present if the causative pathogen is not susceptible to biofilm-active therapy (e.g., rifampin-resistant staphylococci, quinolone-resistant gram-negative bacilli and Candida spp.). Eradication of infection is only possible with implant removal and antimicrobial treatment without any implant in place for 1–6 weeks, depending on the pathogen and the infection site as described below. Preferably, antibiotic treatment should be continued until reimplantation to avoid any viable microorganism to regrow.
If implant removal is not feasible, long-term antimicrobial suppression therapy can be an alternative, but this, however, causes patient discomfort and might be associated with adverse effects. According to the susceptibility pattern, cotrimoxazole and doxycycline are alternatives for intradural suppression treatment, with the addition of clindamycin if blood-brain barrier penetration is not necessary in the case of extradural infections. Streptococcal implant-associated infections on orthopaedic implants have been shown to be difficult to eradicate, therefore a suppression treatment for at least 1 year, possibly also longer if well tolerated, is necessary [33].
Specific infections
Craniotomy- and cranioplasty-associated infections
To reach intracranial structures for brain biopsies, abscess or haemorrhage drainage, clipping of vascular malformations or skull base tumour surgery, a bone flap is removed and re-fixated with titanium plates or clamps. This procedure is called craniotomy. If a larger bone flap is removed and not immediately replaced in decompression surgery after trauma, malignant cerebral infarction or intracerebral bleeding or in case of an infected bone flap, the procedure is called craniectomy. The removed bone flap can be cryopreserved and reinserted at a later stage, which is called autologous cranioplasty. In cases of skull trauma, chronic bone flap infection or bone flap resorption, where the bone flap is not reusable owing to multiple fragments, mature biofilm or aseptic bone necrosis, a synthetic cranioplasty is used (polyether ether ketone [PEEK], poly methyl methacrylate [PMMA] or titanium) [34, 35]. Risk factors for infection are diabetes, presence of an EVD, CSF leakage, intracerebral haematoma and tumour surgery [36–41]. Craniotomy- and cranioplasty-associated infections usually manifest early after surgery (within the first month) and most commonly are caused by pathogens of the skin flora, namely staphylococci [36–39, 42–44]. Clinical presentation includes wound healing problems and purulent wound drainage, but also fever, headache, seizures or focal neurological deficits [42, 43, 45]. The infection rate is lower for craniotomies (0.3–12%) than for cranioplasties (1–24.4%) [2]. For cranioplasties, the infection rate is independent from the material (autologous vs synthetic) [35, 46–48]. In a recent cohort study there was an association of very early cranioplasty (within 14 days of craniectomy) with infection, but there is no other evidence that delayed cranioplasty is associated with a lower infection risk [35, 46, 49, 50]. Nevertheless, most cranioplasties are performed with a delay of 7.3 months on average (range 1–40 months), leaving the patient with a disfiguring skull deformity without brain protection, with disturbed CSF circulation and the possibility of a sinking flap syndrome with progressive neurological deterioration [3, 46]. For this reason, an earlier cranioplasty should be considered.
As superficial and deep compartments are not anatomically separated early after surgery and because of the thin layer of soft tissue covering craniotomies and cranioplasties, all infections should be considered deep wound infections and therefore craniotomy- or cranioplasty-associated. Current clinical practice usually includes removal of the infected implant or bone flap and delayed cranioplasty once infection is eradicated. A new concept, extrapolated from implant-associated infections in orthopaedic surgery, allows surgical debridement and implant retention in acute infections in selected patients where biofilm-active therapy is available and soft tissue coverage is sufficient, or a one- or two-stage implant exchange with only a short implant-free interval of 2 weeks (fig. 2) [17]. Afterwards, the patient is treated for 12 weeks with a biofilm-active therapy (tables 2, 3 and 4
) [17]. With this concept the patient stays for only a short time, if at all, with a disfiguring skull deformity without brain protection, improving quality of life. This concept has recently been shown to be effective in neurosurgical patient populations [43, 44, 51]. Of 12 patients with an autologous craniotomy-associated infection and resection craniectomy, in 10 (83%) immediate titanium cranioplasty with the administration of intravenous (range 3–8 weeks) and oral (range 0–16 weeks) antibiotic treatment was successful [44]. In another study, debridement and bone flap retention were shown to be effective in 10 of 11 patients (91%) together with intravenous (range 2–6 weeks) and oral (range 0–6 weeks) antibiotic treatment [51]. These small studies confirm that implant retention or immediate exchange are alternatives to removal. Although a suboptimal antibiotic treatment was used in most patients (no biofilm-active treatment), successful outcome was observed. Owing to the small patient numbers, one can only conclude that surgical debridement is obviously a main pier in the treatment concept “debridement and implant retention” and contributes to the high treatment success in combination with the biofilm-active treatment. In a larger cohort, an infection-free survival of 87% during a 12-month follow-up was achieved by applying the concept suggested here, including optimised biofilm-active therapy [43]. In univariate analysis, inadequate antimicrobial therapy (no biofilm-active treatment for the defined treatment period) was associated with treatment failure.
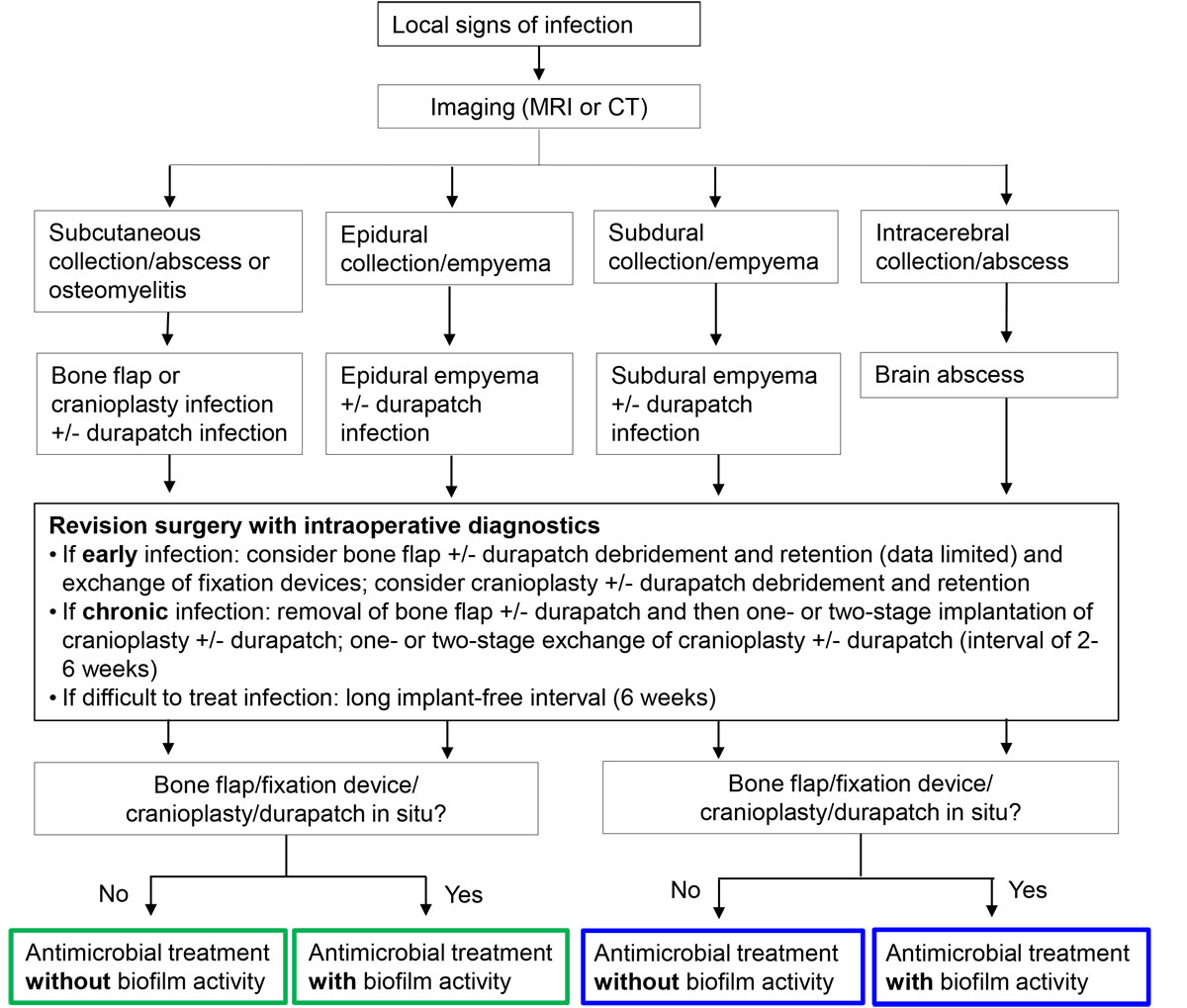
Figure 2
Management algorithm for craniotomy- and cranioplasty-associated infections. Antibiotics with biofilm activity: rifampin for staphylococci and Cutibacterium spp.; fluoroquinolones for gram-negative bacilli.
MRI = magnet resonance imaging; CT = computed tomography
Blue frame = intradural treatment; green frame = extradural treatment
Neurostimulator-associated infections (deep brain and spinal cord stimulators)
Deep brain stimulators are being increasingly used in movement disorders, such as Parkinson’s disease or dystonia, whereas spinal cord stimulators are particularly used to treat chronic back pain. A stimulator system includes leads and electrodes, which are placed in the brain (deep brain stimulators) and the spinal epidural space (spinal cord stimulators). Wires connect the leads and electrodes with the generator, which is placed in the subcutaneous tissue of the chest for deep brain stimulators or the abdomen for spinal cord stimulators (fig. 3). Infections occur at the generator pocket in 31–85% (so-called pocket infection), but may also affect wires (15–69%), whereas leads and electrodes with brain or epidural abscesses are rarely involved; the predominant pathogens are staphylococci and other members of the skin flora [52–55]. The infection rate for deep brain stimulators lays between 4.5% and 6.5%, with the majority presenting within 3 months of implantation [52, 54–58]. For spinal cord stimulators an infection rate of 5% is found, with 38% manifesting as early implant-associated infections [53, 59–61].
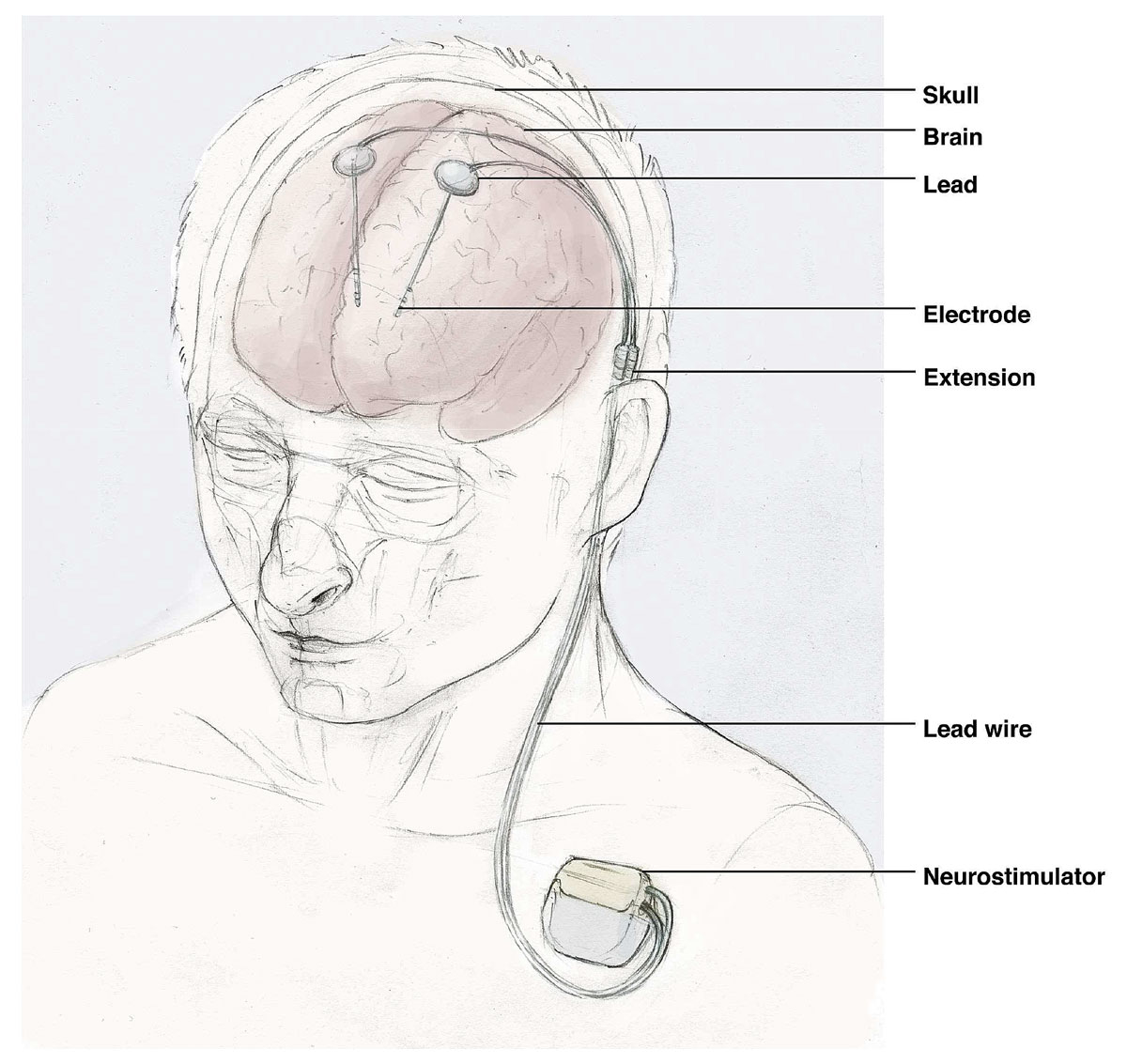
Figure 3 Illustration of a deep brain stimulator.
In cases of infection, device removal or a lead/electrode-sparing procedure with partial explantation of the device and reimplantation weeks after the infection is cured is recommended by several authors. However, this procedure is associated with significant morbidity and inconvenience for the patient, as multiple (brain) surgeries are necessary, and symptoms recur during the implant-free interval. In addition, high healthcare costs due to the lost stimulator system can be expected [52, 54]. Therefore, our suggestion includes again extrapolating the treatment algorithm from orthopaedic surgery, which allows the implant to be retained or only partially exchanged in selected patients. Prerequisites for this strategy are acute infections, availability of biofilm-active therapy and adequate soft tissue coverage of the implant. Thereafter, a 12-week biofilm-active therapy is followed (tables 2, 3 and 4
) [17]. Depending on the infected part of the neurostimulator, we suggest the following procedures (fig. 4). In acute pocket infections, generators can be debrided and retained if biofilm-active therapy is available. Preferably, the implantation site of the generator should be changed because of the usually poor soft tissue condition in infection. Leads, electrodes and wires can be retained. If there is an extracranial or spinal wire infection, wires should be changed as far as possible, but generator, leads and electrodes can be retained. The antimicrobial treatment and its duration depend on the infected part of the stimulator and the surgical strategy chosen. In the case of a difficult-to-treat infection, where no biofilm-active therapy is available, long-term antimicrobial suppression therapy is an alternative, especially if device removal is deemed impossible. Device removal is mandatory in the event of life-threatening infections including lead- and electrode-associated brain or epidural abscesses, or meningitis.
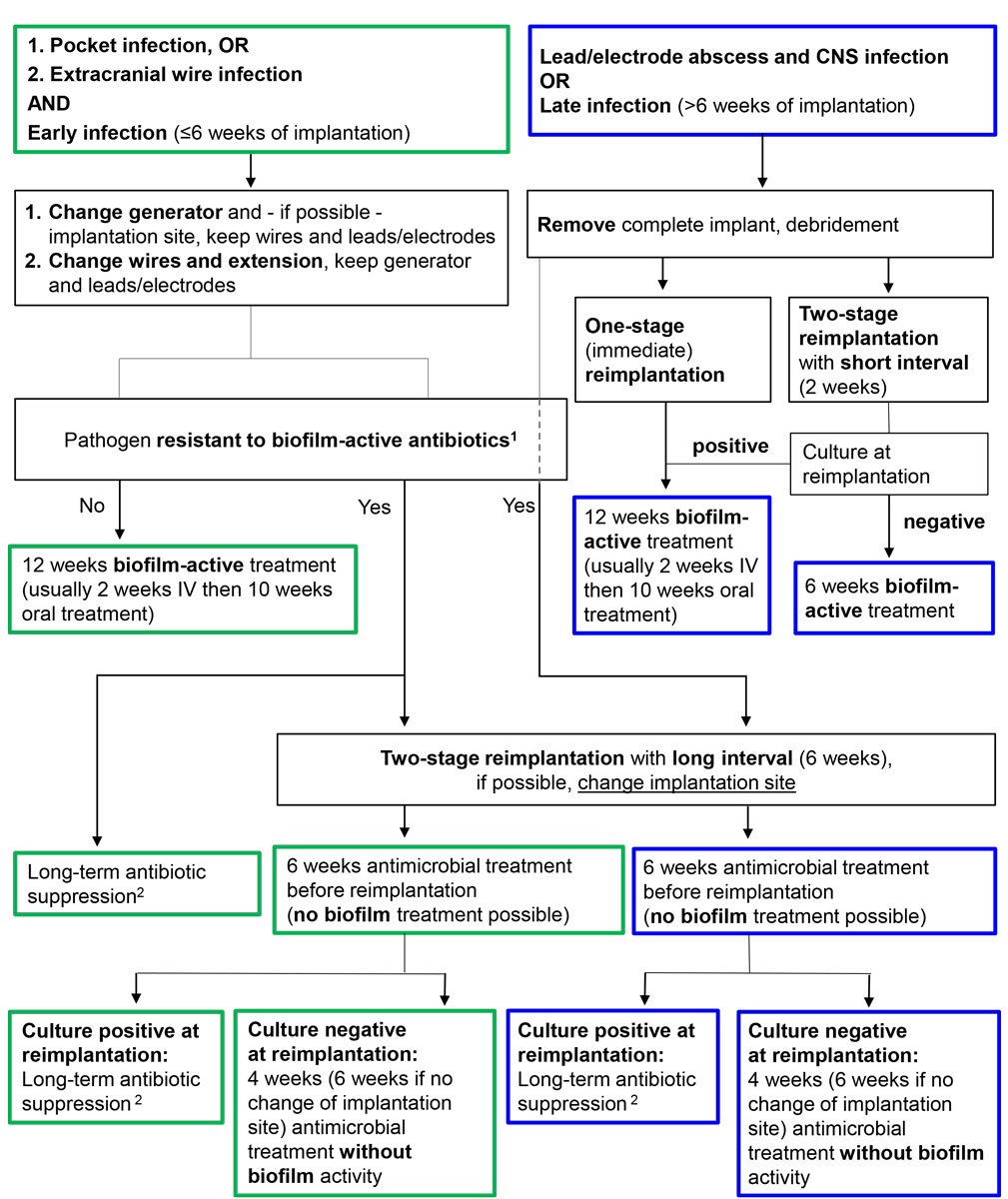
Figure 4
Management algorithm for neurostimulator-associated infections. Antibiotics with biofilm activity: rifampin for staphylococci and Cutibacterium spp.; fluoroquinolones for gram-negative bacilli.
CNS = central nervous system; IV = intravenous
Blue frame = intradural treatment; green frame = extradural treatment
1 Rifampin-resistant staphylococci, quinolone-resistant gram-negative bacilli and Candida spp.
2 Antibiotics for long-term antimicrobial suppression treatment are cotrimoxazole and doxycycline for intradural infections and cotrimoxazole, doxycycline and clindamycin for extradural infections
Ventriculo-peritoneal and ventriculo-atrial cerebrospinal fluid shunt-associated infections
VP and VA shunt systems drain CSF in patients with chronic hydrocephalus from the cerebral ventricles to the peritoneal cavity or right cardiac atrium, respectively. In both CSF shunt systems the infection rate is similar at 4–16%, but complications are more common and usually more severe in VA shunts [2]. The latter require more surgical revisions and cause high morbidity in cases of distal shunt disconnection, intracardiac thrombus formation and shunt migration, which may result in myocardial perforation [62]. There are many risk factors for CSF shunt-associated infections, including previous CSF shunt infection, postoperative CSF leakage, revision surgery, concomitant EVD and the use of a neuroendoscope [63, 64]. CSF shunt systems coated with antibiotics (e.g., clindamycin and rifampin) have been recently shown to decrease infection rate [65]. However, the risk of emergence of antimicrobial resistance by using local antibiotics is of concern, particularly for rifampin, as resistance may develop rapidly as a consequence of point mutation. This risk has been observed in rifampin-soaked vascular grafts in vitro [66]. Rifampin is the only biofilm-active antibiotic against staphylococci and development of resistance means that eradication of biofilm infection is impossible. Combination of rifampin with clindamycin usually does not prevent development of resistance as many staphylococci are resistant to clindamycin.
Patients with CSF shunt-associated infections usually present early within the first month of CSF shunt placement and the dominant pathogens are staphylococci and Cutibacterium spp. [10, 67]. Patients often present with few or no clinical signs and symptoms [10, 67]. Clinical presentation differs between VP and VA shunt-associated infections, especially if the distal shunt part is infected [10, 67]. If the distal VP shunt part is infected, patients may present with abdominal pain or discomfort due to peritonitis, intraabdominal pseudocysts or intestinal shunt perforation; if the distal VA shunt part is infected, stigmata of right-sided endocarditis can be present including continuous bacteraemia and septic lung emboli. In long-lasting VA shunt-associated low-grade infection, shunt nephritis, an immune complex-mediated glomerulonephritis, is a rare complication [10, 68–70]. In case of a low-grade infection of the proximal shunt part, shunt dysfunction with progressive signs of hydrocephalus, namely headache, vomiting and seizures, may be the only hint for infection. In high-grade infections, on the other hand, patients can present with acute meningitis or brain abscess, which represent emergency indications for CSF shunt removal. Fever, although the most common clinical finding, is present in only 78% [10, 67]. As clinical signs and symptoms often are non-specific, CSF analysis is of utmost importance. According to current literature, CSF from shunt valve puncture has a higher diagnostic yield than CSF from lumbar puncture or ventricular CSF: the microbiological yield was 68–91% in shunt valve puncture, compared with 8–45% in lumbar and 20–70% in ventricular CSF [10, 67]. The same is true for the CSF cell count, which is elevated in only 80% overall, but is definitively higher in CSF from shunt valve and lumbar puncture, with a median of 484 cells/µl and 573 cells/µl, respectively, compared with ventricular CSF with a median of 8 cells/µl [10]. These findings again can be explained by the biofilm concept: microorganisms and leucocytes are close around biofilms on the shunt valve, which is supported by the high positivity rate of shunt tip cultures of 49–78% (which for sure is underestimated, as in both studies sonication of the implants was not available). Other explanations for these CSF findings could be differences in CSF circulation in patients with hydrocephalus, but also the retrospective study design where a sampling and treatment bias cannot be excluded [10, 67]. In VA shunt-associated infections, blood cultures are an important diagnostic tool with positivity rates >80% due to the intravascular location of the distal shunt part [10].
Published studies on treatment modalities are very contradictory. An older randomised study by James et al. of 50 patients with CSF shunt-associated infections compared shunt removal, one-stage shunt exchange and shunt retention. Antibiotic treatment consisted of 2 weeks intraventricular and 3 weeks intravenous application of antibiotics without activity against biofilms [71]. High cure rates with shunt removal (95%) and one-stage shunt exchange (88%) contrasted with a high failure rate (64%) for shunt retention, underlining the need for surgery to cure implant-associated infections. In a retrospective study of 78 adult patients with CSF shunt-associated infections, 81% received a combined surgical and antimicrobial treatment – including shunt removal (47%) and a one- (10%) or two-stage (23%) shunt exchange with or without an intercurrent EVD [10]. The overall cure rate in those patients was high at 98%. Interestingly, the 19% of patients without surgery showed a cure rate of 87%, which contrasts with James’ study and might be explained by the optimised antimicrobial therapy with biofilm activity in the more recent study [10]. In another retrospective analysis the surgical treatment concept included CSF shunt removal (28%) and one- (22%) or two-stage (43%) shunt exchange, whereas the strategy of antibiotics alone has progressively been abandoned over time (only 7%) [67]. The cure rate overall was lower at 70% (17% with antibiotics alone, 31% with one-stage and 89% with two-stage exchange, 83% with shunt removal), and might be explained by the fact that many patients with staphylococcal infections did not receive biofilm-active treatment with rifampin, and also by the strategy of distal shunt externalisation with secondary infection of the externalised shunt in five patients. Nevertheless, the only risk factor for treatment failure in this study was CSF shunt retention (odds ratio 46.04, 95% confidence interval 5.30–399.88) [67].
Based on these studies, showing that either CSF shunt retention in acute infections or one-stage shunt exchange in chronic infections where biofilm-active therapy is available are valuable treatment alternatives with lower morbidity for the patient, the following treatment algorithm is suggested (fig. 5). If the CSF shunt is retained or exchanged in a one-stage procedure, 12 weeks of biofilm-active therapy is administered. It must be emphasised that CSF shunt retention is not possible in cases of acute ventriculitis or meningitis, brain abscess, shunt dysfunction, skin erosion over the shunt system or gut perforation. If the CSF shunt is removed or exchanged in a two-stage procedure, the duration of treatment and implant-free interval is guided by the intraoperative culture results and the pathogen. If the CSF shunt is removed, one can expect CSF infection to be eradicated after 5–7 days for coagulase-negative staphylococci and Cutibacterium spp., after 14 days for Staphylococcus aureus, Streptococcus spp., Enterococcus spp. and culture-negative infections, and after 21 days in the case of gram-negative bacilli [16]. If intraoperative cultures during CSF shunt replacement are still positive, a 12-week biofilm-active treatment is followed; in the case of negative intraoperative culture results, 4–6 weeks of treatment is recommended to eradicate any remaining microorganisms (tables 2, 3 and 4
). In cases where a difficult-to-treat pathogen is isolated with a new CSF shunt in place, a long-term antimicrobial suppression therapy is indicated (cotrimoxazole or doxycycline), if shunt removal is not an option. As treatment failures occurred in patients with CSF shunt-associated infections due to coagulase-negative staphylococci treated with intravenous vancomycin and oral rifampin according to the susceptibility testing, one can speculate that insufficient vancomycin CSF penetration leads to rifampin monotherapy with development of resistance, although additional subtherapeutic vancomycin blood levels cannot be excluded owing to the retrospective design of the study [10]. Therefore, optimised treatment combinations with CSF penetration in intradural infections are warranted. For VA shunt-associated endocarditis prolonged intravenous treatment might be adequate [72].
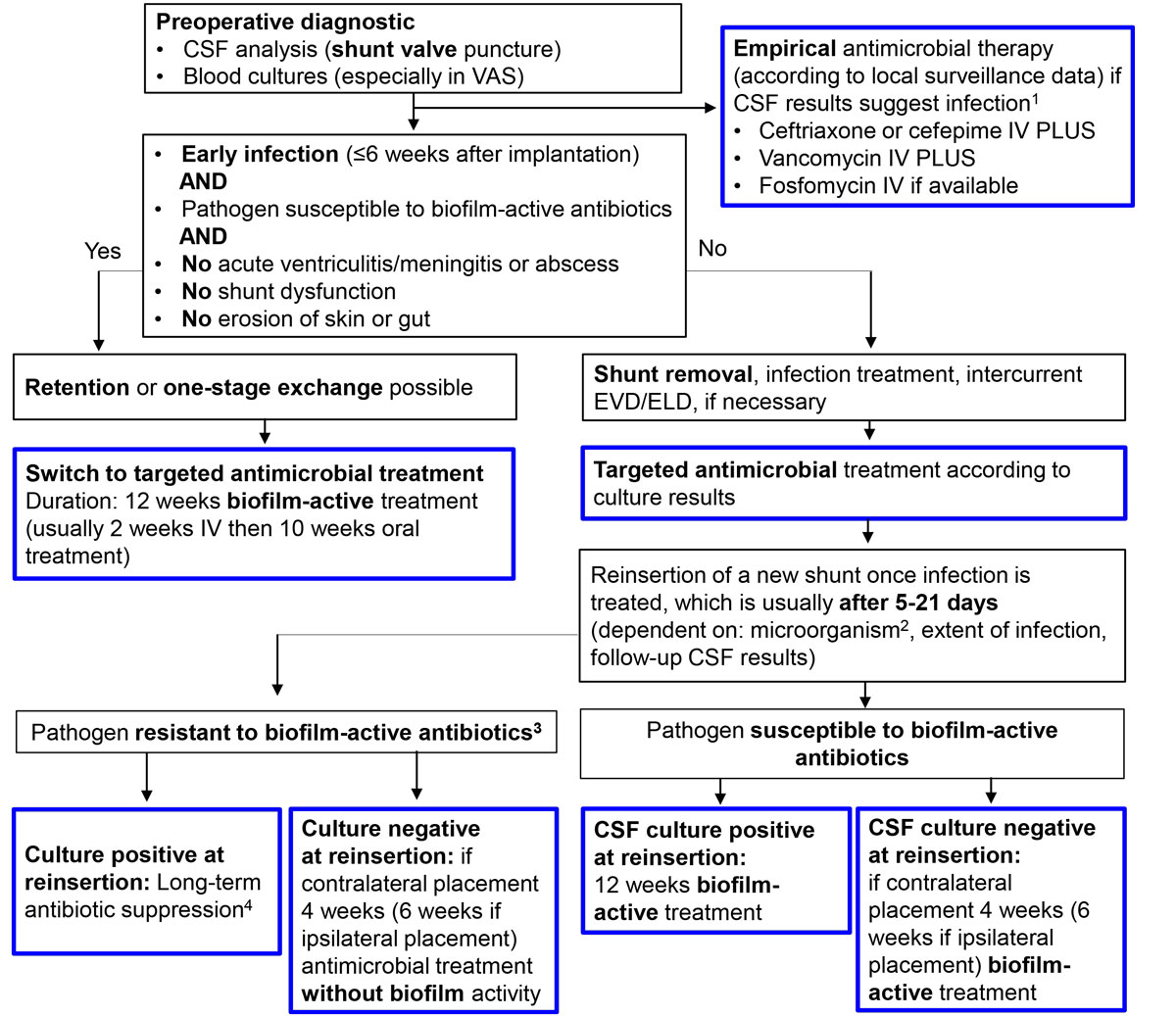
Figure 5 Management algorithm for CSF shunt-associated infections.
CSF = cerebrospinal fluid; EVD/ELD = external ventricular/lumbar drainage; IV = intravenous
Blue frame = intradural treatment
1 CSF leucocyte count > 5x106 cells/µl, with predominance of granulocytes, CSF lactate > 1.9 mmol/l, CSF total protein > 0.45 g/l, glucose CSF/blood ratio < 0.5
2 For low-virulent microorganisms, including coagulase-negative staphylococci and Cutibacterium spp., reimplantation after 5–7 days; for S. aureus, Streptococcus spp., Enterococcus spp. and culture-negative infections 14 days; for Enterobacteriaceae and P. aeruginosa 21 days [16]
3 Rifampin-resistant staphylococci, quinolone-resistant gram-negative bacilli and Candida spp.
4 Antibiotics for long-term antimicrobial suppression treatment are cotrimoxazole and doxycycline
Infections associated with external ventricular and lumbar cerebrospinal fluid drainages
EVD and ELD are temporary implants mostly used to treat acute hydrocephalus after intracranial bleeding or severe craniocerebral injury. Because of the temporary placement, management of EVD- and ELD-associated infections differs from that of CSF shunt-associated infections, as no biofilm-active treatment is necessary. However, EVD- and ELD-associated infections can be challenging to manage, especially if the patient is dependent on an external CSF drainage and needs a permanent CSF shunt, which often must be placed timely and at the time when the infected EVD or ELD is removed. Up to 44% of patients with an EVD or ELD need a CSF shunt [73]. EVD- and ELD-associated infections occur in 2–22%, with an average infection rate of 8% [2]. The most common pathogens are staphylococci and other members of the skin flora such as Cutibacterium spp., but polymicrobial infections also occur [73, 74]. Risk factors for infection are cranial fracture with CSF leakage, duration of CSF catheterisation (≥8 days), repetitive CSF sampling from EVD or EVD irrigation [75–78]. Whether the EVD was placed in the operating room or at the bedside in an intensive care unit or emergency department, and whether a silver-coated EVD or ELD was used did not have an impact on the infection rate; a tunnelled EVD, however, was associated with a lower rate of infection [38, 74, 78–80]. We absolutely discourage the use of rifampin-coated EVDs and ELDs, which expose pathogens in the CSF to rifampin monotherapy and select for rifampin-resistant bacteria (mainly coagulase-negative staphylococci) [81]. This will prevent cure of an ensuing CSF shunt-associated infection, as no biofilm-active therapy is available.
The diagnosis of EVD- or ELD-associated infections is challenging. Clinical signs and symptoms, as well as results of CSF analyses, overlap with those of the underlying disease including CSF haemorrhage causing a sterile inflammation [5]. In addition, signs of CNS infection can be masked by the low level of consciousness due to the underlying condition, as well as by the treatment with sedative drugs. Therefore, a high level of clinical suspicion is needed. Remarkably, 23% of patients presented with meningitis only within 10 days after EVD had been removed [25, 73]. In a retrospective study with 39 patients, clinical and CSF analyses were compared at three different time-points, the time of EVD insertion, 48 hours before occurrence of EVD-associated infection and at the time of CSF culture-positive infection [82]. The only significant indicators for infection were a higher incidence of fever, an increased respiratory rate and a decreased mental state; CSF parameters did not differ. In another study with 48 patients, clinical and CSF parameters were compared between the time-points of EVD insertion and EVD-associated infection: fever was more common in EVD-associated infection (79 vs 15%), as were headache, vomiting and neck stiffness (31 vs 6%), and an increased CSF cell count (175 vs 46 cells/µl) [73]. As there is a lack of more sensitive and specific criteria, EVD-associated infection is mostly diagnosed on the basis of CSF pleocytosis and the presence of fever, resulting in a significantly higher rate of postulated than microbiologically proven infections, which is considered the gold standard [83]. Some studies found an increased cell index and CSF lactate (at a cut-off of 4 mmol/l) to be predictive for EVD-associated infections [84–87]. The cell index is calculated as the ratios of white blood cell count and red blood cell count in the CSF and the blood. Lunardi’s study showed, that a cut-off point of 2.9 was indicative for infection, as was a 4.33-fold increase of the cell index over time [86].
Based on the above-mentioned findings, we suggest the following management. The exclusion of an alternative nosocomial infection focus is mandatory in every febrile patient with an EVD or ELD. If the suspicion for an EVD- or ELD-associated infection is high and the CSF analysis compatible with infection (CSF leucocyte count >300 cells/µl or increasing cell index, lactate >2.1 mmol/l, decreased glucose CSF/blood ratio <0.5), CSF should be sampled for microbiological culture and empirical antimicrobial treatment started, for example with intravenous vancomycin plus either ceftriaxone, cefepime or ceftazidime according to local surveillance data (table 2). Intraventricular antimicrobial therapy is of no proven benefit if the patient responds to intravenous treatment [16]. As EVD and ELD are intercurrent implants only, use of rifampin as biofilm-active therapy is strongly discouraged. Rifampin might be needed later if a permanent CSF shunt is inserted and becomes infected. According to the causative pathogen, treatment duration is 7 days in the case of low-virulent pathogens such as coagulase-negative staphylococci and Cutibacterium spp., 14 days in the case of S. aureus, Streptococcus spp., Enterococcus spp. and culture-negative infections, and 21 days if gram-negative bacilli are isolated (table 3) [16]. EVD and ELD manipulations should be restricted as much as possible, as they are associated with an increased risk of infection; accordingly, daily CSF sampling and prophylactic EVD or ELD exchange are not recommended [16, 88, 89]. But in the event of a high-grade infection (S. aureus, gram-negative bacilli or Candida spp.), or of an inadequate response to therapy, the EVD or ELD should be changed, if still needed.
Conclusions
With the technical developments in neurosurgery, increasing numbers of neurosurgical implants are used and therefore device-associated infections in neurosurgery are becoming more and more relevant. There are few validated diagnostic and treatment approaches, and most recommendations are extrapolated from other implant-associated infections, although the evidence for the success of this extrapolation to neurosurgical patients is sparse and has not been evaluated in large patient populations. As biofilms are involved, management is challenging and should follow well-known and clinically validated interdisciplinary concepts from orthopaedic or trauma surgery, where high cure rates of >90% are achieved. Interdisciplinarity is crucial for successful outcome. Sonication of the removed implants and prolonged culture incubation significantly improve microbiological diagnosis. Treatment includes surgery, with a thorough debridement and stage-specific implant management, i.e., debridement and retention in acute infections, or a one- or two-stage implant exchange in chronic infections. In addition, 4–12 weeks of a biofilm-active treatment is usually needed. Cure without implant removal or with a one-stage exchange are therefore new treatment options, which decrease morbidity, especially as removal of neurosurgical implants is often difficult or impossible owing to the risk of damaging brain tissue or causing intracerebral bleeding. With this strategy, the number of operations can be reduced and the quality of patients’ lives improved. With less invasive surgical approaches, antimicrobial treatment needs to be optimised using biofilm-active therapies. If the intradural compartment is infected, special attention to antibiotic penetration across the blood-brain barrier is needed.
Acknowledgment
We thank Lucius Fekonja from the Department of Neurosurgery, Charité – Universitätsmedizin Berlin, Germany, for the design of the illustration of a deep brain stimulator (figure 3).
References
1
Darouiche
RO
. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–9. doi:.https://doi.org/10.1056/NEJMra035415
2
Martin
RM
,
Zimmermann
LL
,
Huynh
M
,
Polage
CR
. Diagnostic approach to health care- and device-associated central nervous system infections. J Clin Microbiol. 2018;56(11):e00861-18. doi:.https://doi.org/10.1128/JCM.00861-18
3
Di Rienzo
A
,
Colasanti
R
,
Gladi
M
,
Pompucci
A
,
Della Costanza
M
,
Paracino
R
, et al.
Sinking flap syndrome revisited: the who, when and why. Neurosurg Rev. 2020;43(1):323–35. doi:.https://doi.org/10.1007/s10143-019-01148-7
4
Vinchon
M
,
Dhellemmes
P
. Cerebrospinal fluid shunt infection: risk factors and long-term follow-up. Childs Nerv Syst. 2006;22(7):692–7. doi:.https://doi.org/10.1007/s00381-005-0037-8
5
Zarrouk
V
,
Vassor
I
,
Bert
F
,
Bouccara
D
,
Kalamarides
M
,
Bendersky
N
, et al.
Evaluation of the management of postoperative aseptic meningitis. Clin Infect Dis. 2007;44(12):1555–9. doi:.https://doi.org/10.1086/518169
6
Zimmerli
W
,
Waldvogel
FA
,
Vaudaux
P
,
Nydegger
UE
. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982;146(4):487–97. doi:.https://doi.org/10.1093/infdis/146.4.487
7
James
RC
,
MacLeod
CJ
. Induction of staphylococcal infections in mice with small inocula introduced on sutures. Br J Exp Pathol. 1961;42:266–77.
8
Costerton
JW
,
Stewart
PS
,
Greenberg
EP
. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–22. doi:.https://doi.org/10.1126/science.284.5418.1318
9
Bjarnsholt
T
,
Ciofu
O
,
Molin
S
,
Givskov
M
,
Høiby
N
. Applying insights from biofilm biology to drug development - can a new approach be developed?
Nat Rev Drug Discov. 2013;12(10):791–808. doi:.https://doi.org/10.1038/nrd4000
10
Conen
A
,
Walti
LN
,
Merlo
A
,
Fluckiger
U
,
Battegay
M
,
Trampuz
A
. Characteristics and treatment outcome of cerebrospinal fluid shunt-associated infections in adults: a retrospective analysis over an 11-year period. Clin Infect Dis. 2008;47(1):73–82. doi:.https://doi.org/10.1086/588298
11
Korinek
AM
,
Baugnon
T
,
Golmard
JL
,
van Effenterre
R
,
Coriat
P
,
Puybasset
L
. Risk factors for adult nosocomial meningitis after craniotomy: role of antibiotic prophylaxis. Neurosurgery. 2008;62(Suppl 2):126–33. doi:.https://doi.org/10.1227/01.neu.0000316256.44349.b1
12
Karczewski
D
,
Winkler
T
,
Renz
N
,
Trampuz
A
,
Lieb
E
,
Perka
C
, et al.
A standardized interdisciplinary algorithm for the treatment of prosthetic joint infections. Bone Joint J. 2019;101-B(2):132–9. doi:.https://doi.org/10.1302/0301-620X.101B2.BJJ-2018-1056.R1
13
Sendi
P
,
Lötscher
PO
,
Kessler
B
,
Graber
P
,
Zimmerli
W
,
Clauss
M
. Debridement and implant retention in the management of hip periprosthetic joint infection: outcomes following guided and rapid treatment at a single centre. Bone Joint J. 2017;99-B(3):330–6. doi:.https://doi.org/10.1302/0301-620X.99B3.BJJ-2016-0609.R1
14
Tschudin-Sutter
S
,
Frei
R
,
Dangel
M
,
Jakob
M
,
Balmelli
C
,
Schaefer
DJ
, et al.
Validation of a treatment algorithm for orthopaedic implant-related infections with device-retention-results from a prospective observational cohort study. Clin Microbiol Infect. 2016;22(5):457.e1–9. doi:.https://doi.org/10.1016/j.cmi.2016.01.004
15
Trampuz
A
,
Piper
KE
,
Jacobson
MJ
,
Hanssen
AD
,
Unni
KK
,
Osmon
DR
, et al.
Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357(7):654–63. doi:.https://doi.org/10.1056/NEJMoa061588
16
Tunkel
AR
,
Hasbun
R
,
Bhimraj
A
,
Byers
K
,
Kaplan
SL
,
Scheld
WM
, et al.
2017 Infectious Diseases Society of America’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. 2017;64(6):e34–65. doi:.https://doi.org/10.1093/cid/ciw861
17
Zimmerli
W
,
Trampuz
A
,
Ochsner
PE
. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645–54. doi:.https://doi.org/10.1056/NEJMra040181
18
Kleber
C
,
Schaser
KD
,
Trampuz
A
. Komplikationsmanagement bei infizierter Osteosynthese [Complication management of infected osteosynthesis: Therapy algorithm for peri-implant infections]. Chirurg. 2015;86(10):925–34. German. doi:.https://doi.org/10.1007/s00104-015-0073-1
19
Furustrand Tafin
U
,
Corvec
S
,
Betrisey
B
,
Zimmerli
W
,
Trampuz
A
. Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2012;56(4):1885–91. doi:.https://doi.org/10.1128/AAC.05552-11
20
Furustrand Tafin
U
,
Majic
I
,
Zalila Belkhodja
C
,
Betrisey
B
,
Corvec
S
,
Zimmerli
W
, et al.
Gentamicin improves the activities of daptomycin and vancomycin against Enterococcus faecalis in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2011;55(10):4821–7. doi:.https://doi.org/10.1128/AAC.00141-11
21
John
AK
,
Baldoni
D
,
Haschke
M
,
Rentsch
K
,
Schaerli
P
,
Zimmerli
W
, et al.
Efficacy of daptomycin in implant-associated infection due to methicillin-resistant Staphylococcus aureus: importance of combination with rifampin. Antimicrob Agents Chemother. 2009;53(7):2719–24. doi:.https://doi.org/10.1128/AAC.00047-09
22
Zimmerli
W
,
Widmer
AF
,
Blatter
M
,
Frei
R
,
Ochsner
PE
; Foreign-Body Infection (FBI) Study Group. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. JAMA. 1998;279(19):1537–41. doi:.https://doi.org/10.1001/jama.279.19.1537
23
Yano
MH
,
Klautau
GB
,
da Silva
CB
,
Nigro
S
,
Avanzi
O
,
Mercadante
MT
, et al.
Improved diagnosis of infection associated with osteosynthesis by use of sonication of fracture fixation implants. J Clin Microbiol. 2014;52(12):4176–82. doi:.https://doi.org/10.1128/JCM.02140-14
24
Thomaidis
PC
,
Pantazatou
A
,
Kamariotis
S
,
Vlachos
K
,
Roussos
G
,
Panagiotou
P
, et al.
Sonication assisted microbiological diagnosis of implant-related infection caused by Prevotella disiens and Staphylococcus epidermidis in a patient with cranioplasty. BMC Res Notes. 2015;8(1):307. doi:.https://doi.org/10.1186/s13104-015-1274-x
25
Jost
GF
,
Wasner
M
,
Taub
E
,
Walti
L
,
Mariani
L
,
Trampuz
A
. Sonication of catheter tips for improved detection of microorganisms on external ventricular drains and ventriculo-peritoneal shunts. J Clin Neurosci. 2014;21(4):578–82. doi:.https://doi.org/10.1016/j.jocn.2013.05.025
26
Prinz
V
,
Bayerl
S
,
Renz
N
,
Trampuz
A
,
Vajkoczy
P
,
Finger
T
. Sonication improves pathogen detection in ventriculoperitoneal shunt-associated infections. Neurosurgery. 2019;85(4):516–23. doi:.https://doi.org/10.1093/neuros/nyy383
27
Ascione
T
,
Balato
G
,
Mariconda
M
,
Rotondo
R
,
Baldini
A
,
Pagliano
P
. Continuous antibiotic therapy can reduce recurrence of prosthetic joint infection in patients undergoing 2-stage exchange. J Arthroplasty. 2019;34(4):704–9. doi:.https://doi.org/10.1016/j.arth.2018.12.017
28
Nau
R
,
Sörgel
F
,
Eiffert
H
. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858–83. doi:.https://doi.org/10.1128/CMR.00007-10
29Gilbert D, Chambers H, Eliopoulos G, Saag M, Pavia A, eds. Sanford Guide to Antimicrobial Therapy. Sperryville, USA: Antimicrobial Therapy; 2019.
30
Widmer
AF
,
Frei
R
,
Rajacic
Z
,
Zimmerli
W
. Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J Infect Dis. 1990;162(1):96–102. doi:.https://doi.org/10.1093/infdis/162.1.96
31
Pfausler
B
,
Spiss
H
,
Dittrich
P
,
Zeitlinger
M
,
Schmutzhard
E
,
Joukhadar
C
. Concentrations of fosfomycin in the cerebrospinal fluid of neurointensive care patients with ventriculostomy-associated ventriculitis. J Antimicrob Chemother. 2004;53(5):848–52. doi:.https://doi.org/10.1093/jac/dkh158
32
Putensen
C
,
Ellger
B
,
Sakka
SG
,
Weyland
A
,
Schmidt
K
,
Zoller
M
, et al.
Current clinical use of intravenous fosfomycin in ICU patients in two European countries. Infection. 2019;47(5):827–36. doi:.https://doi.org/10.1007/s15010-019-01323-4
33
Renz
N
,
Rakow
A
,
Müller
M
,
Perka
C
,
Trampuz
A
. Long-term antimicrobial suppression prevents treatment failure of streptococcal periprosthetic joint infection. J Infect. 2019;79(3):236–44. doi:.https://doi.org/10.1016/j.jinf.2019.06.015
34
Moreira-Gonzalez
A
,
Jackson
IT
,
Miyawaki
T
,
Barakat
K
,
DiNick
V
. Clinical outcome in cranioplasty: critical review in long-term follow-up. J Craniofac Surg. 2003;14(2):144–53. doi:.https://doi.org/10.1097/00001665-200303000-00003
35
Yadla
S
,
Campbell
PG
,
Chitale
R
,
Maltenfort
MG
,
Jabbour
P
,
Sharan
AD
. Effect of early surgery, material, and method of flap preservation on cranioplasty infections: a systematic review. Neurosurgery. 2011;68(4):1124–9, discussion 1130. doi:.https://doi.org/10.1227/NEU.0b013e31820a5470
36
Buchanan
IA
,
Donoho
DA
,
Patel
A
,
Lin
M
,
Wen
T
,
Ding
L
, et al.
Predictors of surgical site infection after nonemergent craniotomy: A nationwide readmission database analysis. World Neurosurg. 2018;120:e440–52. doi:.https://doi.org/10.1016/j.wneu.2018.08.102
37
Shi
ZH
,
Xu
M
,
Wang
YZ
,
Luo
XY
,
Chen
GQ
,
Wang
X
, et al.
Post-craniotomy intracranial infection in patients with brain tumors: a retrospective analysis of 5723 consecutive patients. Br J Neurosurg. 2017;31(1):5–9. doi:.https://doi.org/10.1080/02688697.2016.1253827
38
Strahm
C
,
Albrich
WC
,
Zdravkovic
V
,
Schöbi
B
,
Hildebrandt
G
,
Schlegel
M
. Infection rate after cranial neurosurgical procedures: A prospective single-center study. World Neurosurg. 2018;111:e277–85. doi:.https://doi.org/10.1016/j.wneu.2017.12.062
39
Sneh-Arbib
O
,
Shiferstein
A
,
Dagan
N
,
Fein
S
,
Telem
L
,
Muchtar
E
, et al.
Surgical site infections following craniotomy focusing on possible post-operative acquisition of infection: prospective cohort study. Eur J Clin Microbiol Infect Dis. 2013;32(12):1511–6. doi:.https://doi.org/10.1007/s10096-013-1904-y
40
Lieber
BA
,
Appelboom
G
,
Taylor
BE
,
Lowy
FD
,
Bruce
EM
,
Sonabend
AM
, et al.
Preoperative chemotherapy and corticosteroids: independent predictors of cranial surgical-site infections. J Neurosurg. 2016;125(1):187–95. doi:.https://doi.org/10.3171/2015.4.JNS142719
41
Rubeli
SL
,
D’Alonzo
D
,
Mueller
B
,
Bartlomé
N
,
Fankhauser
H
,
Bucheli
E
, et al.
Implementation of an infection prevention bundle is associated with reduced surgical site infections in cranial neurosurgery. Neurosurg Focus. 2019;47(2):E3. doi:.https://doi.org/10.3171/2019.5.FOCUS19272
42
Dashti
SR
,
Baharvahdat
H
,
Spetzler
RF
,
Sauvageau
E
,
Chang
SW
,
Stiefel
MF
, et al.
Operative intracranial infection following craniotomy. Neurosurg Focus. 2008;24(6):E10. doi:.https://doi.org/10.3171/FOC/2008/24/6/E10
43
Renz
N
,
Özdirik
B
,
Finger
T
,
Vajkoczy
P
,
Trampuz
A
. Infections after cranial neurosurgery: Prospective cohort of 103 episodes treated according to a standardized algorithm. World Neurosurg. 2018;116:e491–9. doi:.https://doi.org/10.1016/j.wneu.2018.05.017
44
Kshettry
VR
,
Hardy
S
,
Weil
RJ
,
Angelov
L
,
Barnett
GH
. Immediate titanium cranioplasty after debridement and craniectomy for postcraniotomy surgical site infection. Neurosurgery. 2012;70(1, Suppl Operative):8–14, discussion 14–5.
45
Lange
N
,
Berndt
M
,
Jörger
AK
,
Wagner
A
,
Lummel
N
,
Ryang
YM
, et al.
Clinical characteristics and course of postoperative brain abscess. World Neurosurg. 2018;120:e675–83. doi:.https://doi.org/10.1016/j.wneu.2018.08.143
46
Riordan
MA
,
Simpson
VM
,
Hall
WA
. Analysis of factors contributing to infections after cranioplasty: A single-institution retrospective chart review. World Neurosurg. 2016;87:207–13. doi:.https://doi.org/10.1016/j.wneu.2015.11.070
47
Sundseth
J
,
Sundseth
A
,
Berg-Johnsen
J
,
Sorteberg
W
,
Lindegaard
KF
. Cranioplasty with autologous cryopreserved bone after decompressive craniectomy: complications and risk factors for developing surgical site infection. Acta Neurochir (Wien). 2014;156(4):805–11, discussion 811. doi:.https://doi.org/10.1007/s00701-013-1992-6
48
Vince
GH
,
Kraschl
J
,
Rauter
H
,
Stein
M
,
Grossauer
S
,
Uhl
E
. Comparison between autologous bone grafts and acrylic (PMMA) implants - A retrospective analysis of 286 cranioplasty procedures. J Clin Neurosci. 2019;61:205–9. doi:.https://doi.org/10.1016/j.jocn.2018.10.017
49
Morton
RP
,
Abecassis
IJ
,
Hanson
JF
,
Barber
J
,
Nerva
JD
,
Emerson
SN
, et al.
Predictors of infection after 754 cranioplasty operations and the value of intraoperative cultures for cryopreserved bone flaps. J Neurosurg. 2016;125(3):766–70. doi:.https://doi.org/10.3171/2015.8.JNS151390
50
Quah
BL
,
Low
HL
,
Wilson
MH
,
Bimpis
A
,
Nga
VDW
,
Lwin
S
, et al.
Is there an optimal time for performing cranioplasties? Results from a prospective multinational study. World Neurosurg. 2016;94:13–7. doi:.https://doi.org/10.1016/j.wneu.2016.06.081
51
Wallace
DJ
,
McGinity
MJ
,
Floyd
JR, 2nd
. Bone flap salvage in acute surgical site infection after craniotomy for tumor resection. Neurosurg Rev. 2018;41(4):1071–7. doi:.https://doi.org/10.1007/s10143-018-0955-z
52
Abode-Iyamah
KO
,
Chiang
HY
,
Woodroffe
RW
,
Park
B
,
Jareczek
FJ
,
Nagahama
Y
, et al.
Deep brain stimulation hardware-related infections: 10-year experience at a single institution. J Neurosurg. 2019;130:629–38. doi:.https://doi.org/10.3171/2017.9.JNS1780
53
Follett
KA
,
Boortz-Marx
RL
,
Drake
JM
,
DuPen
S
,
Schneider
SJ
,
Turner
MS
, et al.
Prevention and management of intrathecal drug delivery and spinal cord stimulation system infections. Anesthesiology. 2004;100(6):1582–94. doi:.https://doi.org/10.1097/00000542-200406000-00034
54
Bjerknes
S
,
Skogseid
IM
,
Sæhle
T
,
Dietrichs
E
,
Toft
M
. Surgical site infections after deep brain stimulation surgery: frequency, characteristics and management in a 10-year period. PLoS One. 2014;9(8):e105288. doi:.https://doi.org/10.1371/journal.pone.0105288
55
Jitkritsadakul
O
,
Bhidayasiri
R
,
Kalia
SK
,
Hodaie
M
,
Lozano
AM
,
Fasano
A
. Systematic review of hardware-related complications of Deep Brain Stimulation: Do new indications pose an increased risk?
Brain Stimul. 2017;10(5):967–76. doi:.https://doi.org/10.1016/j.brs.2017.07.003
56
Sillay
KA
,
Larson
PS
,
Starr
PA
. Deep brain stimulator hardware-related infections: incidence and management in a large series. Neurosurgery. 2008;62(2):360–6, discussion 366–7. doi:.https://doi.org/10.1227/01.neu.0000316002.03765.33
57
Bhatia
S
,
Zhang
K
,
Oh
M
,
Angle
C
,
Whiting
D
. Infections and hardware salvage after deep brain stimulation surgery: a single-center study and review of the literature. Stereotact Funct Neurosurg. 2010;88(3):147–55. doi:.https://doi.org/10.1159/000303528
58
Hardaway
FA
,
Raslan
AM
,
Burchiel
KJ
. Deep brain stimulation-related infections: Analysis of rates, timing, and seasonality. Neurosurgery. 2018;83(3):540–7. doi:.https://doi.org/10.1093/neuros/nyx505
59
Shamji
MF
,
Westwick
HJ
,
Heary
RF
. Complications related to the use of spinal cord stimulation for managing persistent postoperative neuropathic pain after lumbar spinal surgery. Neurosurg Focus. 2015;39(4):E15. doi:.https://doi.org/10.3171/2015.7.FOCUS15260
60
Cameron
T
. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg. 2004;100(3, Suppl Spine):254–67.
61
Kumar
K
,
Wilson
JR
,
Taylor
RS
,
Gupta
S
. Complications of spinal cord stimulation, suggestions to improve outcome, and financial impact. J Neurosurg Spine. 2006;5(3):191–203. doi:.https://doi.org/10.3171/spi.2006.5.3.191
62
Borgbjerg
BM
,
Gjerris
F
,
Albeck
MJ
,
Hauerberg
J
,
Børgesen
SV
. A comparison between ventriculo-peritoneal and ventriculo-atrial cerebrospinal fluid shunts in relation to rate of revision and durability. Acta Neurochir (Wien). 1998;140(5):459–64, discussion 465. doi:.https://doi.org/10.1007/s007010050125
63
McGirt
MJ
,
Zaas
A
,
Fuchs
HE
,
George
TM
,
Kaye
K
,
Sexton
DJ
. Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin Infect Dis. 2003;36(7):858–62. doi:.https://doi.org/10.1086/368191
64
Kulkarni
AV
,
Drake
JM
,
Lamberti-Pasculli
M
. Cerebrospinal fluid shunt infection: a prospective study of risk factors. J Neurosurg. 2001;94(2):195–201. doi:.https://doi.org/10.3171/jns.2001.94.2.0195
65
Mallucci
CL
,
Jenkinson
MD
,
Conroy
EJ
,
Hartley
JC
,
Brown
M
,
Dalton
J
, et al.; BASICS Study collaborators. Antibiotic or silver versus standard ventriculoperitoneal shunts (BASICS): a multicentre, single-blinded, randomised trial and economic evaluation. Lancet. 2019;394(10208):1530–9. doi:.https://doi.org/10.1016/S0140-6736(19)31603-4
66
Berard
X
,
Puges
M
,
Pinaquy
JB
,
Cazanave
C
,
Stecken
L
,
Bordenave
L
, et al.
In vitro evidence of improved antimicrobial efficacy of silver and triclosan containing vascular grafts compared with rifampicin soaked grafts. Eur J Vasc Endovasc Surg. 2019;57(3):424–32. doi:.https://doi.org/10.1016/j.ejvs.2018.08.053
67
Pelegrín
I
,
Lora-Tamayo
J
,
Gómez-Junyent
J
,
Sabé
N
,
García-Somoza
D
,
Gabarrós
A
, et al.
Management of ventriculoperitoneal shunt infections in adults: Analysis of risk factors associated with treatment failure. Clin Infect Dis. 2017;64(8):989–97. doi:.https://doi.org/10.1093/cid/cix005
68
Burström
G
,
Andresen
M
,
Bartek
J, Jr
,
Fytagoridis
A
. Subacute bacterial endocarditis and subsequent shunt nephritis from ventriculoatrial shunting 14 years after shunt implantation. BMJ Case Rep. 2014;2014(jun24 1):bcr2014204655. doi:.https://doi.org/10.1136/bcr-2014-204655
69
Sacar
S
,
Turgut
H
,
Toprak
S
,
Cirak
B
,
Coskun
E
,
Yilmaz
O
, et al.
A retrospective study of central nervous system shunt infections diagnosed in a university hospital during a 4-year period. BMC Infect Dis. 2006;6(1):43. doi:.https://doi.org/10.1186/1471-2334-6-43
70
von der Brelie
C
,
Simon
A
,
Gröner
A
,
Molitor
E
,
Simon
M
. Evaluation of an institutional guideline for the treatment of cerebrospinal fluid shunt-associated infections. Acta Neurochir (Wien). 2012;154(9):1691–7. doi:.https://doi.org/10.1007/s00701-012-1329-x
71
James
HE
,
Walsh
JW
,
Wilson
HD
,
Connor
JD
. The management of cerebrospinal fluid shunt infections: a clinical experience. Acta Neurochir (Wien). 1981;59(3-4):157–66. doi:.https://doi.org/10.1007/BF01406345
72
Habib
G
,
Lancellotti
P
,
Antunes
MJ
,
Bongiorni
MG
,
Casalta
JP
,
Del Zotti
F
, et al.; ESC Scientific Document Group. 2015 ESC Guidelines for the management of infective endocarditis: The task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075–128. doi:.https://doi.org/10.1093/eurheartj/ehv319
73
Walti
LN
,
Conen
A
,
Coward
J
,
Jost
GF
,
Trampuz
A
. Characteristics of infections associated with external ventricular drains of cerebrospinal fluid. J Infect. 2013;66(5):424–31. doi:.https://doi.org/10.1016/j.jinf.2012.12.010
74
Zhou
YJ
,
Wu
JN
,
Chen
LJ
,
Zhao
HY
. Comparison of infection rate with tunneled vs standard external ventricular drainage: A prospective, randomized controlled trial. Clin Neurol Neurosurg. 2019;184:105416. doi:.https://doi.org/10.1016/j.clineuro.2019.105416
75
Arabi
Y
,
Memish
ZA
,
Balkhy
HH
,
Francis
C
,
Ferayan
A
,
Al Shimemeri
A
, et al.
Ventriculostomy-associated infections: incidence and risk factors. Am J Infect Control. 2005;33(3):137–43. doi:.https://doi.org/10.1016/j.ajic.2004.11.008
76
Lyke
KE
,
Obasanjo
OO
,
Williams
MA
,
O’Brien
M
,
Chotani
R
,
Perl
TM
. Ventriculitis complicating use of intraventricular catheters in adult neurosurgical patients. Clin Infect Dis. 2001;33(12):2028–33. doi:.https://doi.org/10.1086/324492
77
Jamjoom
AAB
,
Joannides
AJ
,
Poon
MT
,
Chari
A
,
Zaben
M
,
Abdulla
MAH
, et al.; British Neurosurgical Trainee Research Collaborative. Prospective, multicentre study of external ventricular drainage-related infections in the UK and Ireland. J Neurol Neurosurg Psychiatry. 2018;89(2):120–6. doi:.https://doi.org/10.1136/jnnp-2017-316415
78
Nilsson
A
,
Uvelius
E
,
Cederberg
D
,
Kronvall
E
. Silver-coated ventriculostomy catheters do not reduce rates of clinically diagnosed ventriculitis. World Neurosurg. 2018;117:e411–6. doi:.https://doi.org/10.1016/j.wneu.2018.06.045
79
Hoffman
H
,
Jalal
MS
,
Chin
LS
. The incidence of meningitis in patients with traumatic brain injury undergoing external ventricular drain placement: A nationwide inpatient sample analysis. Neurocrit Care. 2019;30(3):666–74. doi:.https://doi.org/10.1007/s12028-018-0656-z
80
Kohli
G
,
Singh
R
,
Herschman
Y
,
Mammis
A
. Infection incidence associated with external ventriculostomy placement: A comparison of outcomes in the emergency department, intensive care unit, and operating room. World Neurosurg. 2018;110:e135–40. doi:.https://doi.org/10.1016/j.wneu.2017.10.129
81
Klimo
P, Jr
,
Thompson
CJ
,
Baird
LC
,
Flannery
AM
; Pediatric Hydrocephalus Systematic Review and Evidence-Based Guidelines Task Force. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 7: Antibiotic-impregnated shunt systems versus conventional shunts in children: a systematic review and meta-analysis. J Neurosurg Pediatr. 2014;14(Suppl 1):53–9. doi:.https://doi.org/10.3171/2014.7.PEDS14327
82
Wiegand
J
,
Hickson
L
,
Merz
TM
. Indicators of external ventricular drainage-related infections--a retrospective observational study. Acta Neurochir (Wien). 2016;158(3):595–601, discussion 601. doi:.https://doi.org/10.1007/s00701-016-2709-4
83
Berger-Estilita
J
,
Passer
M
,
Giles
M
,
Wiegand
J
,
Merz
TM
. Modalities and accuracy of diagnosis of external ventricular drainage-related infections: a prospective multicentre observational cohort study. Acta Neurochir (Wien). 2018;160(10):2039–47. doi:.https://doi.org/10.1007/s00701-018-3643-4
84
Grille
P
,
Verga
F
,
Biestro
A
. Diagnosis of ventriculostomy-related infection: Is cerebrospinal fluid lactate measurement a useful tool?
J Clin Neurosci. 2017;45:243–7. doi:.https://doi.org/10.1016/j.jocn.2017.07.031
85
Leib
SL
,
Boscacci
R
,
Gratzl
O
,
Zimmerli
W
. Predictive value of cerebrospinal fluid (CSF) lactate level versus CSF/blood glucose ratio for the diagnosis of bacterial meningitis following neurosurgery. Clin Infect Dis. 1999;29(1):69–74. doi:.https://doi.org/10.1086/520184
86
Lunardi
LW
,
Zimmer
ER
,
Dos Santos
SC
,
Merzoni
J
,
Portela
LV
,
Stefani
MA
. Cell index in the diagnosis of external ventricular drain-related infections. World Neurosurg. 2017;106:504–8. doi:.https://doi.org/10.1016/j.wneu.2017.07.012
87
Pfausler
B
,
Beer
R
,
Engelhardt
K
,
Kemmler
G
,
Mohsenipour
I
,
Schmutzhard
E
. Cell index--a new parameter for the early diagnosis of ventriculostomy (external ventricular drainage)-related ventriculitis in patients with intraventricular hemorrhage?
Acta Neurochir (Wien). 2004;146(5):477–81. doi:.https://doi.org/10.1007/s00701-004-0258-8
88
Schade
RP
,
Schinkel
J
,
Roelandse
FW
,
Geskus
RB
,
Visser
LG
,
van Dijk
JM
, et al.
Lack of value of routine analysis of cerebrospinal fluid for prediction and diagnosis of external drainage-related bacterial meningitis. J Neurosurg. 2006;104(1):101–8. doi:.https://doi.org/10.3171/jns.2006.104.1.101
89
Hader
WJ
,
Steinbok
P
. The value of routine cultures of the cerebrospinal fluid in patients with external ventricular drains. Neurosurgery. 2000;46(5):1149–53, discussion 1153–5. doi:.https://doi.org/10.1097/00006123-200005000-00025




