Is antibody-dependent enhancement playing a role in COVID-19 pathogenesis?
DOI: https://doi.org/10.4414/smw.2020.20249
Divisions of Gastroenterology and Hepatology and of Clinical Pathology, University Hospitals, Geneva, Switzerland
The pathogenesis of COVID-19 is currently believed to proceed via both directly cytotoxic and immune-mediated mechanisms [1]. An additional mechanism facilitating viral cell entry and subsequent damage may involve the so-called antibody-dependent enhancement (ADE). ADE is a very well-known cascade of events whereby viruses may infect susceptible cells via interaction between virions complexed with antibodies or complement components and, respectively, Fc or complement receptors, leading to the amplification of their replication [2] (fig. 1). This phenomenon is of enormous relevance not only for the understanding of viral pathogenesis, but also for developing antiviral strategies, notably vaccines.
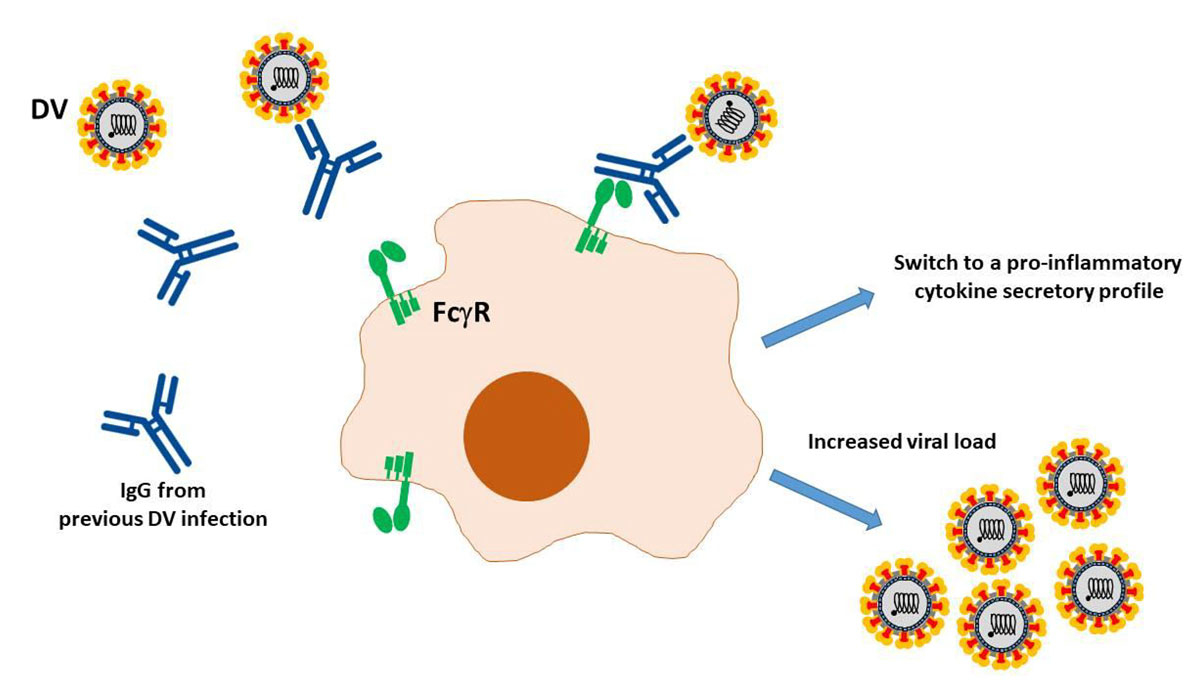
Figure 1 Schematic representation of antibody-dependent enhancement as reported for the Dengue virus (DV). Virions are recognised by heterotypic antibodies from a previous infection. Complexes bind then to the Fcγ receptor on the surface of macrophages, and internalized. DV replication leads to an increased viral load and a functional switch of macrophages towards a proinflammatory cytokine secretory profile.
Role of ADE in human infections: the example of Dengue
ADE was initially reported for a variety of members of the Flaviviridae family, and formally proven in an in vitro experimental model of West Nile fever virus infection by blocking the Fc receptors with anti-FcRIgG or their Fab fragments [3]. Subsequently, ADE was observed in vitro for an ever-growing number of human and animal viral infections, including the human immunodeficiency virus and the Ebola virus, although the clinical impact of these findings remains in most cases unclear [2]. At least one human infection, the Dengue fever, stands out, however, owing to its significant consequences on vaccination programmes. The Dengue virus is a flavivirus transmitted to humans by female mosquitoes of the Aedes type. Clinical manifestations include fever, headache, vomiting, arthromyalgias and skin rash. Severe forms are referred to as Dengue haemorrhagic fever and Dengue shock syndrome, mostly affecting the youth. The Dengue fever concerns tropical countries and is the most frequent human arbovirus disease worldwide, with 100 million new infections and 40,000 deaths annually [4]. There are four serotypes of Dengue virus, all eliciting protective immunity. However, although homotypic protection is long-lasting, cross-neutralising antibodies against different serotypes are short-lived and may last only up to 2 years [5]. In Dengue fever, reinfection with a different serotype runs a more severe course when the protective antibody titre wanes. Here, non-neutralising antibodies take over neutralising ones, bind to Dengue virions, and these complexes mediate the infection of phagocytic cells via interaction with the Fc receptor, in a typical ADE. In other words, heterotypic antibodies at subneutralising titres account for ADE in persons infected with a serotype of Dengue virus that is different from the first infection. Cross-reactive neutralising antibodies are associated with decreased odds of symptomatic secondary infection, and the higher the titre of such antibodies following the primary infection, the longer the delay to symptomatic secondary infection, as shown in a paediatric cohort from Nicaragua [6]. Indeed, in the same cohort, these same authors noticed a protection against all Dengue diseases when antibody titres were elevated, but at the same time the hazard of severe Dengue fever forms (both Dengue haemorrhagic fever and shock syndrome) increased by about 8 times in children with lower levels of antibodies [7]. In a more recent work, the authors showed also that the viral load at presentation and the odds of a severe course of disease were higher in persons with low/intermediate titres of antibodies elicited by a previous Dengue infection, and vice versa, confirming previous data from a children cohort from Thailand [8] and thus providing an additional, elegant supporting evidence of ADE in Dengue fever [9]. The most worrisome aspect of this phenomenon was observed during the Dengue vaccine development. Efficacy trials in Asia and Latin America led to the licensing of the first recombinant, live, attenuated, tetravalent Dengue vaccine in 2015 [10]. Its safety became a focus of scrutiny when follow-up data were published. The rate of hospitalisation for Dengue in year 3 for children who were 9 years old or younger was higher in vaccine recipients than among controls, although the numbers were small [11]. The likely explanation for these occurrences was that vaccination was mimicking a primary infection, and that waning of immunity may have exposed some children to the risk of ADE in the event of secondary infection. A post hoc analysis of efficacy trials, using an anti-nonstructural protein 1 immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) to distinguish antibodies elicited by wild-type infection from those following vaccination, showed that the vaccine was able to protect against severe Dengue those who had been exposed to the natural infection before vaccination, and that the risk of severe clinical outcome was increased among seronegative persons [12]. Based on this, a Strategic Advisor Group of Experts convened by World Health Organization (WHO) concluded that only Dengue seropositive persons should be vaccinated whenever Dengue control programmes are planned that include vaccination [10]. Furthermore, the vaccine is not indicated for children under the age of 9 years.
ADE in coronavirus infections
The feline infectious peritonitis virus (FIPV) is a highly virulent variant of feline coronavirus, an alphacoronavirus that is highly prevalent in both wild and domestic cats [13].
Immunisation against FIPV paradoxically increases the disease severity [14]. In vitro infection of macrophages by FIPV can be enhanced by non-neutralising monoclonal antibodies against the spike viral protein, and this phenomenon may occur even with highly diluted neutralising antibodies, whereas pretreatment with protein A prevents the enhancement [15]. In addition, as many as 50% of cats passively immunised with anti-FIPV antibodies develop peritonitis when challenged with the same FIPV serotype [16]. An attenuated virus vaccine is currently available in several countries for intranasal delivery, but its use is still deemed controversial by some experts, both in terms of safety and efficacy.
ADE has been reported also for a human coronavirus infection, severe acute respiratory syndrome (SARS). Antibodies elicited by a SARS-CoV vaccine [17] enhanced infection of B cell lines in spite of protective responses in the hamster model. The mechanism was later shown to be dependent on the expression of the Fcγ receptor II, and it is interesting to remark that virion cell uptake did not use the endosomal/lysosomal pathway exploited by the angiotensin 1 converting enzyme 2 (ACE2)-based mechanism [18]. These results were confirmed using a HL-CZ human promonocyte cell line. Here, infection with SARS-CoV was neutralised by concentrated antisera against the spike protein, but higher dilutions not only failed to prevent infection, but even facilitated it and induced higher levels of apoptosis. Conversely, anti-nucleocapsid antibodies did not exert any effect − they neither neutralised infection nor caused viral ADE. Again, HL-CZ cells were shown to express both ACE2 and Fcγ receptors [19]. Another troublesome FcγR-associated phenomenon observed in a macaque model of SARS is the skewing of the wound-healing response in lung-infiltrating macrophages towards a proinflammatory profile concomitant with the appearance of anti-spike IgG [20]. The same authors reported similar observations in patients deceased of SARS. Thus, the interaction with Fc receptors of anti-SARS-CoV antibodies complexed with virions may lead to both an enhancement of viral cell entry and replication, and a clinically impactful modulation of the local cytokine response.
Is there a role for ADE in COVID-19?
ADE has been proposed to account for the severity also of COVID-19 cases initially observed in China compared with other regions of the world [21]. In particular, it was suggested that prior infection with other coronaviruses, from the agents of the common cold to the SARS-CoV, may have primed COVID-19 patients, predisposing them to the development of severe disease once infected with SARS-CoV-2. Although severe cases of COVID-19 have later been reported from all over the world, the above hypothesis cannot be completed dismissed. Cross-reactivity of antibodies against the spike protein of SARS-CoV-2 and SARS-CoV is common, and some preliminary data claim that they seem to be rarely cross-neutralising [22]. Priming may also occur with other bat coronaviruses, on the assumption that the recent spillover has occurred previously, albeit in a clinically silent form, and appropriate serosurveys may address this point in the future. If occurring in COVID-19 patients, ADE may account for some severe outcomes occurring later during the natural course of the disease. The protean clinical features that seem to be associated with autopsy reports should probably be investigated, since the expression of Fc receptors is widespread in nonimmune cells, including intestinal epithelial, kidney and endothelial cells [23–26]. On the other hand, ADE may affect safety and efficacy of passive and active immunisation schedules. A recent work reported the development of neutralising antibodies in most patients recovered from mild COVID-19 [27]: since patients with progression to a severe course were not studied, it was impossible to establish an association between this humoral response and disease. The authors cautioned that the variability of neutralising antibody development may raise a concern about their role on disease progression. Nonetheless, the use of convalescent plasma has been encouraged and reported recently in a prospective study of 10 patients with severe COVID-19 [28]. In this uncontrolled trial, the administration of plasma containing high titres of SARS-CoV-2 neutralising antibodies was shown to be effective on several clinical, biochemical and radiological parameters, in parallel with a prompt viral suppression. Importantly, no severe adverse effects were observed. Wider implementation of this approach should however be conducted with caution. On the other hand, the need for rapid development of a COVID-19 vaccine has been literally met with a worldwide race among dozens of research teams [29]. However, it has been stressed that a hasty development of such vaccines may be risky [30], and that only rigorous research can lead to a safe and effective management of the current pandemic.
References
1
Jin
Y
Yang
H
Ji
W
Wu
W
Chen
S
Zhang
W
Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12(4):372
. [doi:.].https://doi.org/10.3390/v12040372
2
Takada
A
Kawaoka
Y
. Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev Med Virol. 2003;13(6):387–98
. [doi:.].https://doi.org/10.1002/rmv.405
3
Peiris
JS
Gordon
S
Unkeless
JC
Porterfield
JS
. Monoclonal anti-Fc receptor IgG blocks antibody enhancement of viral replication in macrophages. Nature. 1981;289(5794):189–91
. [doi:.].https://doi.org/10.1038/289189a0
4
Roth
GA
Abate
D
Abate
KH
Abay
SM
Abbafati
C
Abbasi
N
GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–88
. [doi:.].https://doi.org/10.1016/S0140-6736(18)32203-7
5
Montoya
M
Gresh
L
Mercado
JC
Williams
KL
Vargas
MJ
Gutierrez
G
Symptomatic versus inapparent outcome in repeat dengue virus infections is influenced by the time interval between infections and study year. PLoS Negl Trop Dis. 2013;7(8):e2357
. [doi:.].https://doi.org/10.1371/journal.pntd.0002357
6
Katzelnick
LC
Montoya
M
Gresh
L
Balmaseda
A
Harris
E
. Neutralizing antibody titers against dengue virus correlate with protection from symptomatic infection in a longitudinal cohort. Proc Natl Acad Sci USA. 2016;113(3):728–33
. [doi:.].https://doi.org/10.1073/pnas.1522136113
7
Katzelnick
LC
Gresh
L
Halloran
ME
Mercado
JC
Kuan
G
Gordon
A
Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358(6365):929–32
. [doi:.].https://doi.org/10.1126/science.aan6836
8
Endy
TP
Nisalak
A
Chunsuttitwat
S
Vaughn
DW
Green
S
Ennis
FA
Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis. 2004;189(6):990–1000
. [doi:.].https://doi.org/10.1086/382280
9Waggoner JJ, Katzelnick LC, Burger-Calderon R, Gallini J, Moore RH, Kuan G, et al. Antibody-dependent enhancement of severe disease is mediated by serum viral load in pediatric Dengue virus infections. J Infect Dis. 2020;jiz618. Published online April 1, 2020.
10who.int. [Internet]. Immunization, Vaccines and Biologicals. Questions and Answers on Dengue Vaccines [cited 2020 Apr 9]. Available from: https://www.who.int/immunization/research/development.
11
Hadinegoro
SR
Arredondo-García
JL
Capeding
MR
Deseda
C
Chotpitayasunondh
T
Dietze
R
CYD-TDV Dengue Vaccine Working Group
. Efficacy and long-term safety of a Dengue vaccine in regions of endemic disease. N Engl J Med. 2015;373(13):1195–206
. [doi:.].https://doi.org/10.1056/NEJMoa1506223
12
Sridhar
S
Luedtke
A
Langevin
E
Zhu
M
Bonaparte
M
Machabert
T
Effect of Dengue serostatus on Dengue vaccine safety and efficacy. N Engl J Med. 2018;379(4):327–40
. [doi:.].https://doi.org/10.1056/NEJMoa1800820
13
Vennema
H
Poland
A
Foley
J
Pedersen
NC
. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virology. 1998;243(1):150–7
. [doi:.].https://doi.org/10.1006/viro.1998.9045
14
Vennema
H
de Groot
RJ
Harbour
DA
Dalderup
M
Gruffydd-Jones
T
Horzinek
MC
Early death after feline infectious peritonitis virus challenge due to recombinant vaccinia virus immunization. J Virol. 1990;64(3):1407–9
. [doi:.].https://doi.org/10.1128/JVI.64.3.1407-1409.1990
15
Hohdatsu
T
Nakamura
M
Ishizuka
Y
Yamada
H
Koyama
H
. A study on the mechanism of antibody-dependent enhancement of feline infectious peritonitis virus infection in feline macrophages by monoclonal antibodies. Arch Virol. 1991;120(3-4):207–17
. [doi:.].https://doi.org/10.1007/BF01310476
16
Takano
T
Yamada
S
Doki
T
Hohdatsu
T
. Pathogenesis of oral type I feline infectious peritonitis virus (FIPV) infection: Antibody-dependent enhancement infection of cats with type I FIPV via the oral route. J Vet Med Sci. 2019;81(6):911–5
. [doi:.].https://doi.org/10.1292/jvms.18-0702
17
Kam
YW
Kien
F
Roberts
A
Cheung
YC
Lamirande
EW
Vogel
L
Antibodies against trimeric S glycoprotein protect hamsters against SARS-CoV challenge despite their capacity to mediate FcgammaRII-dependent entry into B cells in vitro. Vaccine. 2007;25(4):729–40
. [doi:.].https://doi.org/10.1016/j.vaccine.2006.08.011
18
Jaume
M
Yip
MS
Cheung
CY
Leung
HL
Li
PH
Kien
F
Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcγR pathway. J Virol. 2011;85(20):10582–97
. [doi:.].https://doi.org/10.1128/JVI.00671-11
19
Wang
SF
Tseng
SP
Yen
CH
Yang
JY
Tsao
CH
Shen
CW
Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 2014;451(2):208–14
. [doi:.].https://doi.org/10.1016/j.bbrc.2014.07.090
20
Liu
L
Wei
Q
Lin
Q
Fang
J
Wang
H
Kwok
H
Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4(4):e123158
. [doi:.].https://doi.org/10.1172/jci.insight.123158
21
Tetro
JA
. Is COVID-19 receiving ADE from other coronaviruses?
Microbes Infect. 2020;22(2):72–3
. [doi:.].https://doi.org/10.1016/j.micinf.2020.02.006
22Lv N, Wu NC, Tsang OTY, Yuan M, Perera RAPM, Leung WS, et al. Cross-reactive antibody response between SARS-CoV-2 and SARS-CoV infections. BioRxiv 2020.03.15.993097 [Preprint]. 2020 [posted 2020 March 17, cited 2020 April 9]. Available from: https://www.biorxiv.org/content/10.1101/2020.03.15.993097v1
23
Israel
EJ
Simister
N
Freiberg
E
Caplan
A
Walker
WA
. Immunoglobulin G binding sites on the human foetal intestine: a possible mechanism for the passive transfer of immunity from mother to infant. Immunology. 1993;79(1):77–81.
24
Dickinson
BL
Badizadegan
K
Wu
Z
Ahouse
JC
Zhu
X
Simister
NE
Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999;104(7):903–11
. [doi:.].https://doi.org/10.1172/JCI6968
25
Haymann
JP
Levraud
JP
Bouet
S
Kappes
V
Hagège
J
Nguyen
G
Characterization and localization of the neonatal Fc receptor in adult human kidney. J Am Soc Nephrol. 2000;11(4):632–9.
26
Borvak
J
Richardson
J
Medesan
C
Antohe
F
Radu
C
Simionescu
M
Functional expression of the MHC class I-related receptor, FcRn, in endothelial cells of mice. Int Immunol. 1998;10(9):1289–98
. [doi:.].https://doi.org/10.1093/intimm/10.9.1289
27Wu F, Wang A, Liu M, Wang Q, Chen J, Xia S, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. MedRxiv 2020.03.30.20047365 [Preprint]. 2020 [posted 2020 April 6, cited 2020 April 9]. Available at https://www.medrxiv.org/content/10.1101/2020.03.30.20047365v1
28Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020;202004168. Published online April 6, 2020. doi:https://doi.org/10.1073/pnas.2004168117.
29Subramanian S. 'It’s a razor’s edge we’re walking': inside the race to develop a coronavirus vaccine. The Guardian. 2020 Mar 27.
30
Jiang
S
. Don’t rush to deploy COVID-19 vaccines and drugs without sufficient safety guarantees. Nature. 2020;579(7799):321
. [doi:.].https://doi.org/10.1038/d41586-020-00751-9
