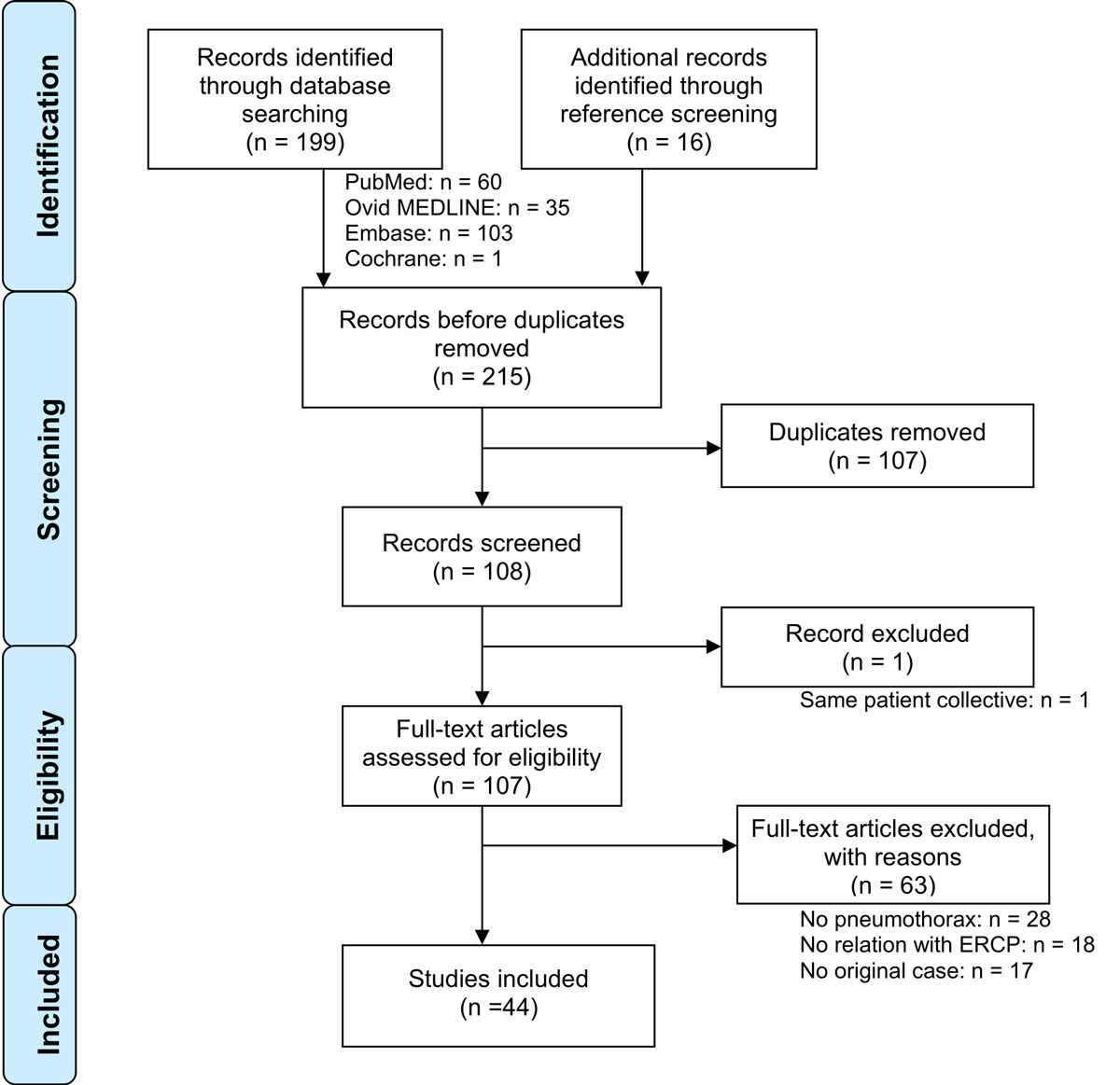
Figure 1 Flow chart of article selection, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [3]
DOI: https://doi.org/10.4414/smw.2020.20199
Since its introduction in 1968 [1], endoscopic retrograde cholangiopancreatography (ERCP) has played a pivotal role in the diagnosis and management of biliary obstructions, in which cases it is routinely performed. Patients are either managed in outpatient settings or require short hospitalisations [2]. However, life-threatening complications can occur, such as pancreatitis (1.30%), cholangitis (0.87%), haemorrhage (0.76%) and perforations (0.58%) [1].
A perforation that develops into a pneumothorax is a rare, but potentially lethal, complication. A previous prospective multicentre study evaluated early major complications after diagnostic and therapeutic ERCPs. That study identified only one pneumothorax out of 111 major complications in 2769 consecutive patients [1]. Scattered evidence and few recommendations are currently available on the diagnosis and management of this complication. We conducted a literature review to evaluate the incidence, diagnosis, and treatment recommendations for these events.
A systematic literature search was conducted in PubMed, Embase, Ovid MEDLINE and the Cochrane Library, with the following search terms in all fields and MeSH terms: “pneumothorax” and “ercp”; “pneumothorax” and “sphincterotomy”; or “pneumothorax” and “papillotomy”. We retrieved all articles published up to 23 September 2018. All available publications were evaluated. Then we screened the references of the selected articles for additional cases, by searching with the terms: “ercp”, “sphincterotomy”, or “papillotomy”; moreover, we applied the secondary search items: “air”, “gas”, “emphysema”, “pneumomediastinum”, “pneumothorax”, “pneumoretroperitoneum”, or “pneumoperitoneum”. The search strategy was validated by our hospital librarian.
All relevant articles were included in the review, according to the criteria of the initial search, including those retrieved from the iterative search through the references.
The reporting of the review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), with the 2009 PRISMA Checklist [3].
All original articles were eligible, when they reported cases of uni- or bilateral pneumothoraces after an attempted or successful ERCP.
The text and images of the reported cases were screened for air localisation and properties (e.g., side of pneumothorax, subcutaneous emphysema, pneumoperitoneum, pneumoretroperitoneum, pneumomediastinum and tension pneumothorax). The articles were also searched for demographic criteria (age and gender); underlying illness (e.g., biliary stricture, common bile duct stones [CBDS]); atypical anatomy near and distant from the peri-ampullary region (e.g., diverticulum, ulceration, tumour, surgical alterations); procedural steps and success (e.g., a precut papillotomy [PCP], a conventional endoscopic sphincterotomy [ES], cannulation, stent placement, stone extraction); probable cause of the pneumothorax (e.g., gastrointestinal perforations with localisation, endoscopy-independent causes); diagnostic tools utilised (fluoroscopy, transit study, conventional radiography, or computed tomography [CT]); treatment modalities (pleural drainage, antibiotic therapy, bowel rest or surgery); time to presentation of the pneumothorax; and the final outcome (hospital stay and/or reported death).
If a PCP was performed, any further mention of an ES was ignored. ERCP was defined as unsuccessful, when a CBD cannulation was not achieved.
The cause of the pneumothorax was defined as the most probable cause mentioned by the original authors. Gastrointestinal perforations were classified, based on elements from the text and images, according to a modified Stapfer classification [4] (table 1), with the localisation prioritised. Type I perforations included all endoscope-linked perforations in the gastrointestinal tract, except for those in the peri-ampullary area (in the direct vicinity of the papilla of Vater), which were classified as type II. Type III perforations included guide-wire-linked bile duct injuries and any air leakage, after a previously placed percutaneous transhepatic cholangiodrainage (PTCD) catheter. Type IV perforations included any perforations that were not documented on endoscopy, with imaging tools, or during surgical revision.
Table 1 Modified Stapfer classification [4].
| Type | Location of perforation | Typical cause |
|---|---|---|
| I | Gastrointestinal tract wall (classically, the medial or lateral duodenal wall) | Endoscope |
| II | Peri-ampullary area (in the immediate vicinity of the papilla of Vater) | Sphincterotome |
| III | Bile ducts | Guide-wire / Basket |
| IV | No proven perforation (retroperitoneal air alone) | Compressed air |
The time to presentation was defined as the time between the ERCP and the first described signs or symptoms of a pneumothorax (e.g., emphysema, dyspnoea, reduced chest sounds, cardiovascular instability). The time to presentation was classified qualitatively, as follows: immediate – onset during or in the first hour after the procedure; early – onset within 6 hours of the procedure; or late – later findings or discovery after the initial hospital discharge.
A tension pneumothorax was defined as cardiovascular instability (simultaneous hypotension and tachycardia); a mediastinal shift detected on imaging studies; or the performance of emergency decompression procedures.
Owing to the nature of the evidence (individual case reports, retrospective case series, reviews of case reports, retrospective cohorts), we conducted a quality assessment according to the tool proposed by Murad at al. [5] and displayed it in a fashion analogous to that suggested by Higgins et al. for systematic reviews of interventions [6].
Categorical variables are expressed as the frequency and percentage. Numerical data are expressed as the mean, median and range. Because of the nature of the review (a collection of individual cases), the limited number of cases and the rarity of this complication in prospective studies, further statistical analyses were not conducted.
A total of 164 articles were identified and screened. Only one original publication was found in the Cochrane Library. The PRISMA flow chart of the study selection procedure is shown in figure 1. The vast majority of the 44 included publications were in English [4, 7–46], 4 were in German [42, 47], Spanish [48] or Czech [49]. No publication was excluded for language reasons.

Figure 1 Flow chart of article selection, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [3]
The above-mentioned search results included all the cases described in the two included reviews [7, 40]. One record, a congress communication, was excluded since its patient collective was already analysed in another publication [8] included in our review.
Three articles included multiple cases [4, 32, 40]. In total, 49 separate cases were identified (table 2).
Table 2 Characteristics of case studies and reviews.
| Reference | Year | Patient | Patho. | Atypical anatomy | Procedure | Time to pres. | Pneumothorax | Air repartition |
Drainage
(side) |
Therapy | Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Age (y) | Side | Tension | Cause | |||||||||||
| [7] | Al-Ashaal | 2011 | W | 25 | CBDS | UnC | Immediate | R | T | I | Prp, Pp, Pm | R | OP | 14 | |
| [8] | Bartosz | 2014 | U | U | U | U | PCP | U | U | U | U | U | U | U | |
| [47] | Brueck | 2010 | W | 39 | CBDS | Div | ES, StEx, stent | Immediate | B | IV | Prp, Pp, Pm, SE | OL | Cons | 7 | |
| [9] | Colemont | 1988 | W | 62 | Stricture | ES, stent | Immediate | R | IV | Prp, Pm, SE | Ø | Cons | S | ||
| [10] | Doerr | 1996 | W | 81 | CBDS | Div | Immediate | R | II | Pp, Pm, SE | R | Cons | S | ||
| [11] | Ferrara | 2009 | M | 82 | CBDS | ES, StEx | Immediate | L | T | IV | Prp, Pp, Pm, SE | L | Cons | 9 | |
| [12] | Fujii | 2010 | W | 73 | Stricture | Biliodig | Stent | Immediate | B | III | Prp, Pp, Pm, SE | B | Cons | 7 | |
| [13] | García-Cano | 2016 | W | 79 | CBDS | ES, StEx | Early | R | III | Prp, Pm, SE | Ø | Cons | 7 | ||
| [14] | Garmon | 2013 | W | 23 | CBDS | Immediate | B | T | I | Prp, Pp, Pm, SE | O1 | OP | 5 | ||
| [15] | Goddard | 2016 | M | 68 | Stricture | Tumour | UnC | Immediate | B | II | Prp, Pp, Pm, SE | OR | Cons | 6 | |
| [16] | Greilich | 2016 | W | U | CBDS | ES, stent | Immediate | B | Tracheal tear | Pm, SE | U | OP | 21 | ||
| [17] | Gya | 1989 | W | 63 | CBDS | PCP | Late | R | II | Prp, Pm, SE | R | OP | S | ||
| [18] | Han | 2008 | M | 63 | CBDS | Ulc | UnC | Immediate | R | T | I | Pp | R | OP | S |
| [19] | Hui | 2001 | W | 89 | CBDS | BII+RY | LARCP, UnC | Immediate | R | T | III | R | Cons | S | |
| [20] | Iyilikci | 2009 | W | 24 | CBDS | ES, StEx | Immediate | B | T | II | SE | B | OP | 13 | |
| [21] | Keber | 2015 | W | 63 | CBDS | Endoscopic alt. | StEx | Immediate | B | II | Prp, Pp, SE | B | OP | S | |
| [22] | Keskin | 2014 | W | 56 | CBDS | ES, StEx | Immediate | B | II | Prp, Pp, Pm, SE | B | OP | 7 | ||
| [23] | Kocaman | 2009 | M | 24 | Stricture | Stent | Immediate | B | III | Prp, Pp, Pm, SE | B | OP | 8 | ||
| [24] | Kogure | 2014 | U | U | U | Surgical alt. | DBE | U | U | T | U | U | U | U | U |
| [25] | Lagoudianakis | 2006 | M | 55 | CBDS | Ect | PCP, UnC | Late | R | II | Prp, SE | Ø | Cons | 16 | |
| [26] | Linssen | 2013 | W | 89 | CBDS | Div | UnC | Immediate | L | II | Pp, Pm, SE | L | Cons | S | |
| [27] | Lopes | 2009 | U | U | U | RY | Immediate | U | T | III | 1 | Cons | U | ||
| [28] | Lutchmansingh | 2013 | W | 57 | Stricture | ES, stent | Immediate | B | U | Prp, Pm, SE | OL | Cons | U | ||
| [29] | Makni | 2012 | W | 40 | CBDS | ES, StEx | Late | R | T | I | Prp, Pp, Pm, SE | R | OP | S | |
| [30] | Markogiannakis | 2007 | W | 56 | CBDS | ES, StEx | Immediate | B | T | IV | Prp, Pp, Pm, SE | B | Cons | 10 | |
| [48] | Menéndez | 2012 | W | 79 | CBDS | Early | B | I | Prp, Pp, Pm, SE | U | OP | S | |||
| [31] | Morley | 1997 | W | 80 | Stricture | Ulc | PCP | Immediate | R | T | I | Pp, SE | R | OP | S |
| [32] | Neofytou | 2013 | W | 45 | CBDS | ES, StEx | Immediate | R | II | Prp, Pp, Pm, SE | R | Cons | 5 | ||
| W | 94 | CBDS | Immediate | R | T | Valsalva | R | Cons | 8 | ||||||
| [33] | Ozgonul | 2010 | W | 62 | Stricture | Stent | Late | B | T | II | Prp, Pp, Pm, SE | B | OP | S | |
| [34] | Plönes | 2012 | W | 67 | Stricture | Stent | Immediate | B | I | Pm, SE | O1 | Cons | 9 | ||
| [35] | Rappaport | 2017 | M | 65 | Stricture | Stent | Late | B | I | Prp, Pm | B | OP | S | ||
| [36] | Samies | 2015 | W | 81 | Stricture | Div | ES, stent | Immediate | L | IV | Prp, Pm, SE | Ø | Cons | S | |
| [37] | Sampaziotis | 2010 | W | 68 | CBDS | ES, StEx | Immediate | B | Valsalva | Prp, Pp, Pm, SE | B | Cons | 4 | ||
| [38] | Savides | 1993 | W | 79 | Stricture | Tumour | ES, stent | Immediate | B | I | Prp, Pm, SE | B | OP | 12 | |
| [39] | Scarlett | 1994 | W | 59 | Stricture | PCP | Immediate | R | II | Prp, Pp | R | Cons | 5 | ||
| [40] | Schepers | 2012 | W | 76 | CBDS | Tumour | ES, StEx, stent | Immediate | B | T | II | Prp, Pp, Pm, SE | B | Cons | 10 |
| M | 77 | Stricture | PCP, stent | Immediate | B | II | Prp, Pm, SE | Ø | Cons | 7 | |||||
| W | 88 | CBDS | Div | ES | Early | R | T | II | Prp, Pm, SE | R | Cons | S | |||
| W | 58 | Jaundice | PCP | Immediate | R | II | Prp, Pm | Ø | Cons | S | |||||
| [41] | Schiavon | 2010 | W | 79 | CBDS | Div | ES, StEx | Immediate | R | II | Pm, SE | Ø | Cons | 10 | |
| [42] | Schilling | 2014 | M | 41 | Stricture | Biliodig | Stent | Immediate | R | T | Stent migration | U | R | OP | S |
| [43] | Seymann | 2010 | W | 78 | CBDS | Div | StEx | Immediate | B | II | Prp, Pm, SE | B | Cons | S | |
| [44] | Shen | 2015 | W | 82 | Stricture | ES, stent | Immediate | B | T | II | Prp, Pm, SE | O1 | Cons | S | |
| [45] | Shimatani | 2009 | U | U | U | RY | DBE | U | U | U | SE | 1 | Cons | S | |
| [46] | Song | 2009 | W | 78 | CBDS | ES, StEx | Late | R | T | II | Prp, Pp, Pm, SE | R | Cons | † | |
| [4] | Stapfer |
2000 | W | 74 | U | Immediate | U | I | SE | U | OP | † | |||
| M | 43 | U | Immediate | U | T | I | SE | U | OP | 16 | |||||
| [49] | Valkovský | 2014 | W | 68 | CBDS | Div | StEx | Immediate | B | II | Prp, Pm, SE | B | Cons | 20 | |
Ø = no drainage; † = deceased; I, II, III, IV = type of perforation according to Stapfer; alt. = alteration; B = bilateral; BII = Billroth II operation; Biliodig = biliodigestive anastomosis; CBDS = common bile duct stone(s); Cons = conservative treatment; DBE = Double balloon enteroscopy; Div = peri-ampullary diverticulum; Ect = ectopic papilla; ES = standard endoscopic sphincterotomy; F = female; L = left; LARCP = laparoscopic assisted ERCP; M = male; O1 = only one side; OP = surgical treatment; OR = only right; OL = only left; Outcome = hospital stay (in days), survival in deceased patients or in unreported stay duration; Patho. = underlying pathology; PCP = precut papillotomy; Pm = pneumomediastinum; Pp = pneumoperitoneum; pres. = presentation; Prp = pneumoretroperitoneum; R = right; RY = roux-en-Y operation; S = survived; SE = subcutaneous emphysema; StEx = stone extraction; T = tension; U = unreported; Ulc = ulceration; UnC = unsuccessful cannulation
The results of the quality assessment analysis are displayed in figure 2. For the assesment of the two included reviews, we only considered the quality of their original cases. By design, all case reports and retrospective studies had a selection bias. Exposure (ERCP) was always ascertained, in retrospective studies the outcome (pneumothoraces) was often insufficiently documented. The causes of pneumothoraces were better documented in case reports; however, other causes were not always actively excluded in Stapfer IV injuries. Reporting quality was mostly sufficient to allow an adequate extraction of data, with the exception of retrospective studies.
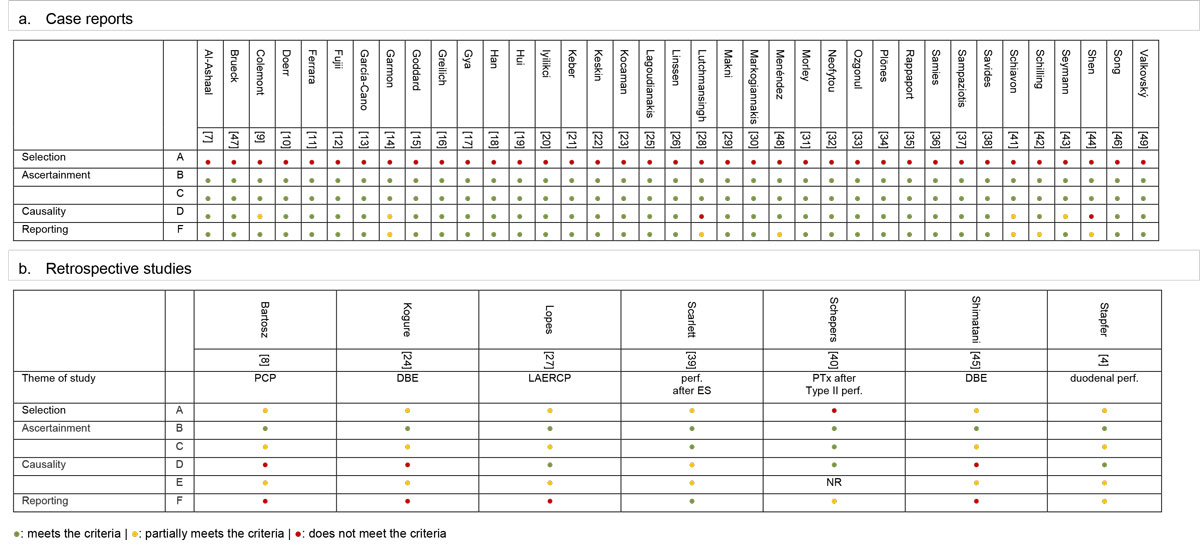
Figure 2 Summary of quality assessment adapted from Murad [5].
A: Does the patient(s) represent(s) the whole experience? Might patients with similar presentation not have been reported?
B: Was the ERCP adequately ascertained?
C: Was the pneumothorax adequately ascertained?
D: Was a plausible etiology proposed, thoroughly described, or other causes ruled out?
E: Was follow-up long enough for outcomes to occur? (NR for case reports)
F: Is/are the case(s) described with sufficient details to allow research replication or inferences to the medical practice?
DBE = double balloon endoscopy; ES = endoscopic sphincterotomy; NR = not relevant; PCP = precut papillotomy; perf. = perforation; PTx = pneumothorax
Table 3 summarises the characteristics of the included cases. There were 36 female and 9 male patients (the gender of 4 patients was not reported), with a median age of 67 years (range 23–94). Atypical local anatomy was present in 21 cases (43%). Peri-ampullary diverticula, a peri-ampullary tumour, a duodenal ulceration or a pancreatic neoplasia that deformed the duodenum were noted on endoscopy in 16, 4, 4 and 2% of cases, respectively. One additional case presented with an ectopic papilla [25]. Six patients had relevant prior surgery, namely one unspecified surgical modification necessitating a double-balloon enteroscopy [24]; three roux-en-Y gastrojejunal reconstructions [19, 27, 45]; two biliodigestive anastomoses, one performed during a liver transplantation [42] and one performed during the repair of a congenital biliary obstruction [12]. One patient had a modified papilla due to previous ES [21]. The main interventional procedures in the entire patient collective were an ES (39%) and a PCP (14%), as well as stenting (31%) or stone extraction (31%). In 6 patients (12%), the intended common bile duct cannulation was not achieved.
Table 3 Summary of findings.
|
n
(N = 49) |
% or range | |||
|---|---|---|---|---|
| Patient demographics | Gender | Female | 36 | 74% |
| Male | 9 | 18% | ||
| Unreported | 4 | 8% | ||
| Median age, y | 67 | 23–94 | ||
| Anatomy | No anomaly reported | 28 | 58% | |
| Atypical anatomy | 21 | 42% | ||
| Peri-ampullary diverticula | 8 | 16% | ||
| Surgical alterations | 6 | 12% | ||
| Peri-ampullary or duodenal tumours | 3 | 6% | ||
| Ulcerations | 2 | 4% | ||
| Ectopic papilla | 1 | 2% | ||
| Endoscopic alteration | 1 | 2% | ||
| Procedures | Standard endoscopic sphincterotomy | 19 | 39% | |
| Precut papillotomy | 7 | 14% | ||
| Stent | 15 | 31% | ||
| Stone extraction | 15 | 31% | ||
| Unsuccessful cannulation | 6 | 12% | ||
| Pneumothorax | Bilateral | 22 | 45% | |
| Right | 18 | 37% | ||
| Left | 3 | 6% | ||
| Unreported | 6 | 12% | ||
| Tension pneumothorax | 19 | 39% | ||
| Cause of pneumothorax | Type I perforation | 11 | 23% | |
| Type II perforation | 20 | 41% | ||
| Type III perforation | 5 | 10% | ||
| Type IV perforation | 5 | 10% | ||
| Other | 4 | 8% | ||
| Unreported | 4 | 8% | ||
| Time to pneumothorax | Immediate (<1 h) | 36 | 74% | |
| Early (1–6 h) | 3 | 6% | ||
| Late (>6 h or after discharge from hospital) | 7 | 14% | ||
| Unreported | 3 | 6% | ||
| Pleural drainage | All pneumothoraces drained | 30 | 62% | |
| Unilateral drainage in bilateral pneumothoraces | 6 | 12% | ||
| Not drained | 7 | 14% | ||
| Unreported | 6 | 12% | ||
| Therapy for the perforation | Surgical repair | 18 | 37% | |
| Conservative treatment | 29 | 59% | ||
| Unreported | 2 | 4% | ||
| Outcome | Mean hospital stay, d | 9.8 | 4–21 | |
| Median stay, d | 8.5 | |||
| Death | 2 | 4% | ||
In most cases, the cause of the pneumothorax was a duodenal perforation near (type II = 41%) or distant from the papilla (type I = 23%). The next most frequent cause was the appearance of air in a retroperitoneal location, but without a proven perforation (type IV = 10%). Five type III perforations were found: two were due to guide-wire injuries in gastrointestinal strictures [12, 23]; one was caused by the trapping of a CBDS between the wall and a balloon during dilatation [13]; and two were due to insufflation, with air tracking through a previously inserted PTCD catheter [19, 27]. Of the latter two, one resulted from overpressure in the afferent loop of a roux-en-Y reconstruction, with air consecutively tracking through the drained bile ducts, the liver parenchyma, and, eventually, the pleural space; and the other one was the result of an iatrogenic injury due to a transdiaphragmatic catheter insertion. In one rather peculiar case, a very late onset pneumothorax occurred, owing to a biliary stent dislocation and perforation in an intrathoracic herniated colon, which caused a colothoracic fistula [42]. Finally, three publications reported extra-intestinal causes, including a tracheal perforation [16] and a forced Valsalva manoeuvre [32, 37].
Most reported pneumothoraces (74%) were diagnosed peri-interventionally, as a result of the immediate onset of clinical symptoms; only 6% were diagnosed early after the intervention. In 7 cases (14%) with no or few specific symptoms, the pneumothorax was diagnosed as late as 3 days after the procedure [46]. Moreover, the stent migration was diagnosed at an unclear, but even later time point [42].
Variable diagnostic tools were used to identify the site of perforation, including peri-interventional cholangiography (or fluoroscopy), X-rays, and CT scans. Pneumothoraces were described as bilateral, right-sided or left-sided, in 22, 18, and 3 cases, respectively. In 6 cases, the exact localisation of the pneumothorax was not reported. Nineteen patients (39%) had clinical or radiological signs of tension pneumothoraces. Subcutaneous chest emphysema was present in 78% of cases, and one had extended to the orbital region [9]. Pneumomediastinum, pneumoretroperitoneum, and pneumoperitoneum were present in 69, 65, and 45% of cases, respectively.
Most gastrointestinal injuries (59%) could be managed conservatively, with antibiotics and bowel rest, with or without a nasogastric suction tube. However, patients with type I perforations nearly always required surgery (10/11). The one exception was a patient with an oesophageal tear, which was detected as contrast extravasation on imaging, but it could not be identified during an oesophagoscopy [34]. This patient recovered with antibiotics and nasogastric suction alone.
Surgical repair consisted of a direct suture alone [38], combined with an omental patch [31] or combined with a cholecystectomy and T-tube insertion [7, 18, 48]. In others, pyloric exclusion and gastrojejunostomy [4, 29], tube duodenostomy [4, 20, 33], colostomy and mucous fistula [42] were performed. In another two cases the perforation could not be identified at laparotomy [17, 23]. In one case with a tracheal tear treatment consisted of stent placement and interposition of an intercostal muscle flap [16]. Finally, four publications did not report any details on the surgical intervention [14, 21, 22, 35].
Conservative management without a chest tube was successful in 7 cases of uncomplicated pneumothoraces. All 19 tension pneumothoraces were systematically drained. Only 2 of these 19 patients underwent immediate decompression [14, 44].
The mean hospital stay was 9.8 days (range 4–21). Two patients with perforations died. One refused surgery [46] and the other developed septic shock after a 2-cm lateral duodenal wall perforation was treated with a pyloric exclusion and gastrojejunostomy [4].
We found that the current published evidence on post-ERCP pneumothoraces was limited. The present review included more than double the number of pneumothorax cases after ERCP, compared with the previous reviews.
Article screening and data gathering were conducted by two authors. However, this study had some potential limitations, besides the already addressed quality issues of the included articles. First, there might have been a reporting bias due to a reluctance of authors to expose disastrous complications, as suggested previously by Schepers [40]. In addition, there might have been a publication bias, because we found studies only on dramatic cases; recently published cases were almost exclusively about bilateral or tension pneumothoraces. Other biases might have arisen because of the heterogeneous case report descriptions and collected data; this heterogeneity might be partly explained by the different professions of the authors (e.g., gastroenterologists, anaesthesiologists, emergency physicians and even cardiologists). However, despite these limitations, we provided a summary of the current evidence, and we highlighted the practices and some correlations and properties that were not previously discussed.
Intervention-related pneumothoraces are always due to leakage of intraluminal air. Therefore, it seems appropriate to compare our findings with studies that reported ERCP-linked perforations. Women and older patients were predominantly affected by this complication. Savides [38] suggested that age-related thinning of the duodenal wall might be a determining factor. However, age and female gender are also the most predominant factors in patients suffering from gallstone disease. Therefore, intervention-related pneumothoraces are much more related to ERCP performed to treat CBDS and much less directly to these two factors [50]. Although studies have shown that women were more prone to developing post-ERCP pancreatitis, no link has been demonstrated between patient sex and a susceptibility to perforations [51].
Consistently with a previous retrospective case control study [52], we identified several local and interventional risk factors for the occurrence of ERCP-related pneumothorax, including an ES, PCP, dilated common bile duct, a dilatation manoeuvre and atypical anatomy. Other recognised contributing factors include the duration of the procedure and the insufflation time after the injury [53]. Because the insufflation time probably determines the amount of air that will leak through the injury, it might contribute to the development of significant pneumothoraces. As a result of the above-mentioned lack of data in the reviewed cases, corroboration was not possible.
Strategies to avoid ERCP-linked complications were not discussed in the reviewed articles. However, such strategies are nicely described in the literature: for example, a magnetic resonance cholangiopancreatography (MRCP)-first approach might minimise unnecessary diagnostic ERCPs; however, it remains a matter of debate whether this approach might diminish the overall complication rate [54]. Anatomical information gained by an MRCP could, in combination with demographic and clinical factors, provide a partial basis for the anticipation of risky constellations. Intra-interventional bail-out strategies could additionally limit injuries in complicated cannulations. Conducting another ERCP after a few days is often successful and should be considered, particularly in the setting of bleeding or increasing papillary oedema. Alternative strategies might also be considered, such as a PTCD or even a surgical revision. These strategies could at least minimise the intervention time and the need for PCPs [55].
The pathophysiological mechanisms of pneumothorax formation during ERCP have been described in the literature. In summary, there are four possible routes to the mediastinal and pleural spaces: (1) in a retroperitoneal perforation, air can track throughout the continuous fascial planes, up to the thoracic cavity [56]; (2) in a free peritoneal perforation, air can communicate between the abdominal and thoracic cavities through diaphragmatic orifices (peritoneal stomata) in porous diaphragm syndromes [23]; (3) in the absence of a true perforation, air can enter the mucosa at a weak spot (e.g., anastomosis, diverticulum) and track along the perineural and perivascular sheaths [12]; (4) finally, air from a ruptured alveolus (through hyperventilation, the Valsalva manoeuvre, or excess positive pressure during ventilation) can track into the perivascular sheath, which could lead to pulmonary interstitial emphysema, and eventually, cause endoscopy-independent pneumothoraces, also known as the Macklin effect [10].
To the best of our knowledge, the right-sided predominance of pneumothoraces remains unexplained. We postulated that air might follow the path of least resistance. Indeed, the development of left pneumothoraces might be discouraged by the presence of adhesions between the retroperitoneum and the anterior aortic sheath, which might form an anatomic barrier.
The consistent presence of a pneumomediastinum in bilateral pneumothoraces suggested bilateral mediastinal tears caused by a tension pneumomediastinum with air leaking into both pleural cavities. Alternatively, some patients might have a congenital or surgically acquired communication between the two pleural spaces; this is known as “buffalo chest syndrome” [57]. In this condition, bilateral pneumothoraces could occur without a pneumomediastinum [20].
The high prevalence of tension (39%) and bilateral (45%) pneumothoraces among the reviewed cases might be due to the above-mentioned publication bias, because only severe cases were published. Alternatively, high pressure insufflation might be another contributing factor; this pressure could either cause decompression to the second hemithorax or lead to the development of tension.
The clinical signs and diagnosis of an ERCP-related perforation have been described extensively [4, 12, 39, 58]. A thoraco-abdominal CT scan is the diagnostic gold standard for evaluating the problem (fig. 3) [59]. The classical clinical signs of small pneumothoraces, such as diminished breath sounds and tympanic percussion, are frequently overlooked, particularly in patients who are otherwise stable post-ERCP. Subcutaneous emphysema, which was often present in the reported cases (78%), is a highly suspicious sign of an air-containing organ perforation, even without pneumothorax formation. A tension pneumothorax should be suspected when patients exhibit neck vein distention, tracheal deviation, tachycardia, and/or desaturation. Moreover, decompensation signs, such as respiratory distress or circulatory instability, should rouse suspicion of a tension pneumothorax, and after clinical confirmation, it should lead to immediate decompression, without further delay, even for imaging. However, in stable patients suspected of having a perforation with pneumothorax, radiographic evaluation should be performed before drainage, as suggested by the authors of a recent review [60].
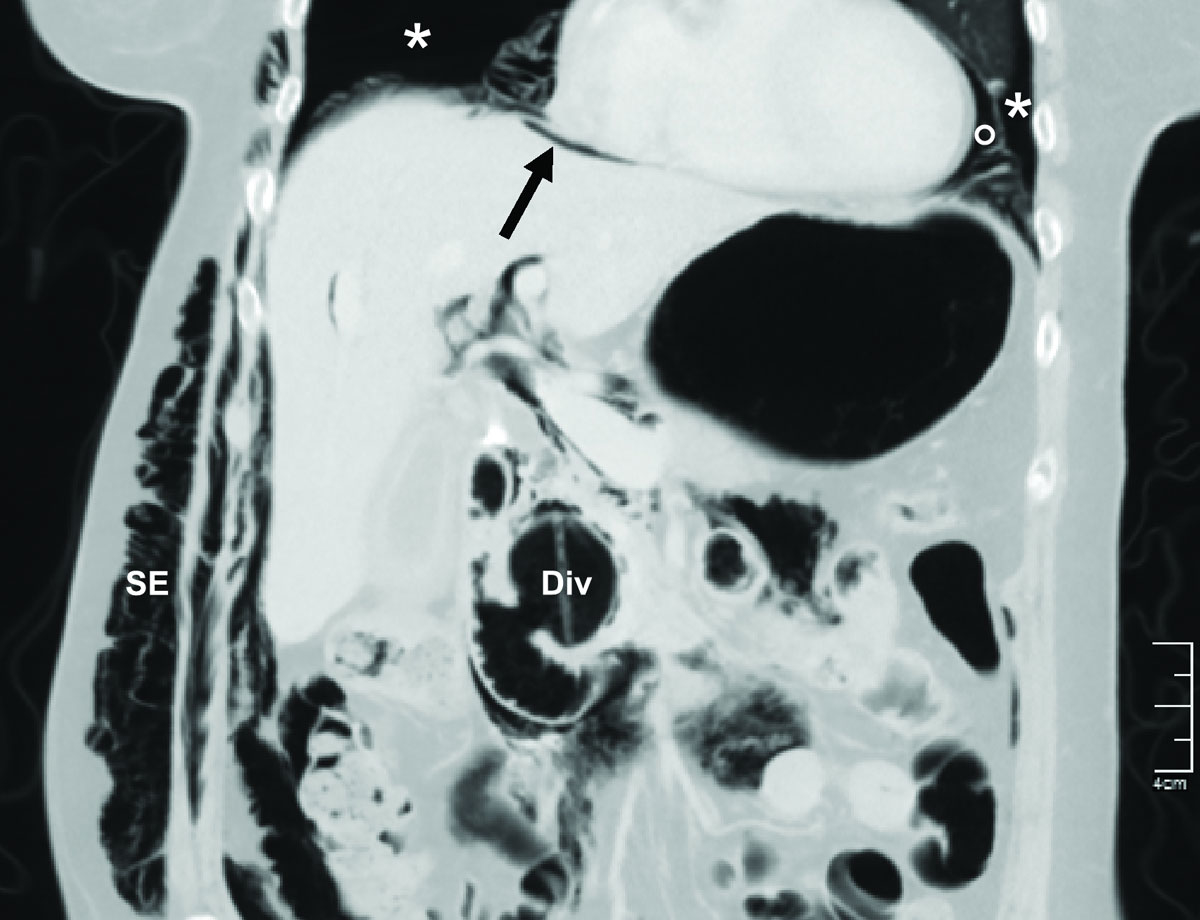
Figure 3 Post-ERCP abdominal computed tomography scan of a 75-year old patient at our institution demonstrating bilateral pneumothoraces (*), pneumomediastinum (°), pneumoperitoneum (arrow), and subcutaneous emphysema (SE). A peri-ampullary diverticulum (Div) is also present. A smaller but significant pneumothorax could have been missed, since the thorax was incompletely scanned.
A simple chest X-ray or sonography [53, 61] may exclude relevant pneumothorax formations. However, in accordance with the newest recommendations from the European Society of Gastrointestinal Endoscopy on iatrogenic endoscopic perforations [59], we strongly recommend an imaging evaluation with a thoraco-abdominal CT scan in such patients. The presence of a duodenal diverticulum, the performance of a PCP or ES and long procedure times should be considered reasons to lower the threshold for additional diagnostic studies after the intervention. In the specific case of intra-interventional suspicion of perforation and the beginning of a tension pneumothorax, the use of fluoroscopy, which is typically available in the ERCP-room, might enable an immediate diagnosis [18, 22, 31, 40]. However, minimum pneumothorax formations might be overlooked, due to the supine positioning. Finally, a loss of image resolution might be an indirect sign of extraluminal air formation. This finding should suggest the need for additional imaging studies, such as CT.
It would be appropriate to tailor therapies for treating perforations and pneumothoraces. A step-by-step approach has been advocated, based on the degree of perforation and the evolution of the patient’s injury [4, 12, 39, 58]. In our review, we found that type I perforations were commonly managed operatively, except for small oesophageal tears that could be detected with CT but not by endoscopy [34]. Types II and III perforations were mainly managed conservatively, which is consistent with literature recommendations. However, the time and specifications of antibiotic therapy and bowel rest have not been standardised.
Early treatments for pneumothoraces include supplemental oxygen, close monitoring and iterated evaluations of drainage procedures. Drainage should be evaluated according to the amount of collapsed pulmonary tissue and patient compensation, which also depends on previous lung function. One-sided drainage may be sufficient, even in bilateral pneumothoraces. Therefore, in symmetrical pneumothoraces without instability, decompression and drainage should first be performed on the right side, and then, according to the evolution, a tailored contralateral drainage might be warranted. In any case, pleural drainage might accelerate recovery. In the literature, hospital stays were only reported for four of seven patients who did not receive drainage. As mentioned above, a tension pneumothorax requires prompt management.
It is of pivotal importance to monitor the patient after ERCP. A review [2] suggested that patients in good general condition, without additional risk factors, might be eligible for outpatient ERCPs. The authors mentioned that only 10% of all post-ERCP complications that required treatment presented symptoms after 24 hours. The present review demonstrated that late symptom onset occurred in 14% of patients. Therefore, close monitoring for at least 6 hours after the procedure is mandatory, and at our institutions an overnight stay is standard.
None of the patients included in this review died as a direct result of a pneumothorax; in the patients who died, the perforation and related sepsis were ultimately the cause of death. However, a missed pneumothorax could be lethal when left untreated [60], particularly in patients who require intubation for a surgical revision. Therefore, we advocate that pneumothorax formation should be actively excluded in cases of ERCP-linked perforation, before any further action is taken.
In conclusion, pneumothorax is a rare, but significant complication of ERCP, and it might not become evident until days after the procedure. In particular, after unsuccessful attempts to cannulate the common bile duct, it is mandatory to observe the patient closely. Because of the rarity of this complication, it is essential to report new cases frequently to ensure that physicians remain alert to this phenomenon. Additionally, reviews of reported cases might further refine our knowledge of diagnostic and treatment modalities.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors; the authors remain independent of any funding influence and declare no support from any organisation for the submitted work. All authors declare no financial relationships with any organisations that might have an interest in the submitted work in the previous three years and no other relationships or activities that could appear to have influenced the submitted work.
1 Loperfido S Angelini G Benedetti G Chilovi F Costan F De Berardinis F Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998;48(1):1–10 .https://doi.org/10.1016/S0016-5107(98)70121-X
2 Jeurnink SM Poley JW Steyerberg EW Kuipers EJ Siersema PD . ERCP as an outpatient treatment: a review. Gastrointest Endosc. 2008;68(1):118–23 .https://doi.org/10.1016/j.gie.2007.11.035
3 Moher D Liberati A Tetzlaff J Altman DG PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41 .https://doi.org/10.1016/j.ijsu.2010.02.007
4 Stapfer M Selby RR Stain SC Katkhouda N Parekh D Jabbour N Management of duodenal perforation after endoscopic retrograde cholangiopancreatography and sphincterotomy. Ann Surg. 2000;232(2):191–8 .https://doi.org/10.1097/00000658-200008000-00007
5 Murad MH Sultan S Haffar S Bazerbachi F . Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–3 .https://doi.org/10.1136/bmjebm-2017-110853
6Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane. 2019. Available from www.training.cochrane.org/handbook.
7 Al-Ashaal YI Hefny AF Safi F Abu-Zidan FM . Tension pneumothorax complicating endoscopic retrograde cholangiopancreatography: case report and systematic literature review. Asian J Surg. 2011;34(1):46–9. Published online April 26, 2011 .https://doi.org/10.1016/S1015-9584(11)60018-3
8 Bartosz C Kovach A Forssell K Winter M Kothari S Ullah A Needle Knife Sphincterotomy for Biliary Access at ERCP: Experience At Two United States Academic Tertiary Referral Centers: 288. Am J Gastroenterol. 2014;109:S88. doi:.https://doi.org/10.14309/00000434-201410002-00288
9 Colemont LJ Pelckmans PA Moorkens GH Van Maercke YM . Unilateral periorbital emphysema: an unusual complication of endoscopic papillotomy. Gastrointest Endosc. 1988;34(6):473–5 .https://doi.org/10.1016/S0016-5107(88)71440-6
10 Doerr RJ Kulaylat MN Booth FV Corasanti J . Barotrauma complicating duodenal perforation during ERCP. Surg Endosc. 1996;10(3):349–51. Published online March 01, 1996 .https://doi.org/10.1007/BF00187390
11 Ferrara F Luigiano C Billi P Jovine E Cinquantini F D’Imperio N . Pneumothorax, pneumomediastinum, pneumoperitoneum, pneumoretroperitoneum, and subcutaneous emphysema after ERCP. Gastrointest Endosc. 2009;69(7):1398–401 .https://doi.org/10.1016/j.gie.2008.08.004
12 Fujii L Lau A Fleischer DE Harrison ME . Successful nonsurgical treatment of pneumomediastinum, pneumothorax, pneumoperitoneum, pneumoretroperitoneum, and dubcutaneous emphysema following ERCP. Gastroenterol Res Pract. 2010;2010:289135. Published online June 14, 2010 .https://doi.org/10.1155/2010/289135
13 García-Cano J Ferri-Bataller R Gómez-Ruiz CJ . Common bile duct perforation sealed with a metal fully-covered stent. Rev Esp Enferm Dig. 2016;108(8):495–6. Published online August 25, 2016.
14 Garmon EH Contreras E Conley J . Tension pneumothorax and widespread pneumatosis after endoscopic retrograde cholangiopancreatography. Anesthesiology. 2013;119(3):699 .https://doi.org/10.1097/ALN.0b013e31827bcdba
15 Goddard AG McConomy B Bathla G Furqan M Silverman WB . Air leakage in multiple compartments after endoscopy. Cleve Clin J Med. 2016;83(10):705–7. Published online October 12, 2016 .https://doi.org/10.3949/ccjm.83a.15168
16 Greilich NB Gasanova I Farrell B Joshi GP . The diagnosis and management of patient with delayed symptoms from a tracheal tear. A A Case Rep. 2016;6(8):230–3 .https://doi.org/10.1213/XAA.0000000000000289
17 Gya D Sali A Angus D . Subcutaneous emphysema and pneumothorax following endoscopic sphincterotomy. Aust N Z J Surg. 1989;59(11):900–2 .https://doi.org/10.1111/j.1445-2197.1989.tb07038.x
18 Han M-L Wu Y-M Liu K-L Su W-C Wang H-P . Tension pneumothorax after an ERCP in a patient with hepatic hydrothorax and sealed-off perforated duodenal ulcer. Gastrointest Endosc. 2008;68(4):771–2, discussion 772 .https://doi.org/10.1016/j.gie.2008.03.007
19 Hui C-K Lai K-C Yuen M-F Lam S-K . Tension pneumothorax complicating ERCP in a patient with a Billroth II gastrectomy. Gastrointest Endosc. 2001;54(2):254–6 .https://doi.org/10.1067/mge.2001.114962
20 Iyilikci L Akarsu M Duran E Sarikaya HB Biyikli B . Duodenal perforation and bilateral tension pneumothorax following endoscopic sphincterotomy. J Anesth. 2009;23(1):164–5 .https://doi.org/10.1007/s00540-008-0710-7
21 Keber T Mojškerc K Makuc J . Bilateral Pneumothorax and Subcutaneous Emphysema following Endoscopic Retrograde Cholangiopancreatography with Sphincterectomy. Signa Vitae. 2015;10(2):192–201. doi:.https://doi.org/10.22514/SV102.122015.13
22 Keskin K Başkurt M Aktürk F Conkbayır C . A rare cause of chest pain mimicking myocardial infarction. Turk Kardiyol Dern Ars. 2014;42(5):472–4 .https://doi.org/10.5543/tkda.2014.27163
23 Kocaman O Sipahi M Cubukçu A Baykara ZN Hülagü S . Porous diaphragm syndrome after ERCP in a patient with bile duct stricture. Turk J Gastroenterol. 2009;20(2):157–8. Published online June 17, 2009.
24 Kogure H Watabe H Yamada A Isayama H Tsujino T Nagano R Su1456 Therapeutic ERCP Using Short Double-Balloon Enteroscopy in Patients With Surgically Altered Anatomy. Gastrointest Endosc. 2011;73(4):AB271. doi:.https://doi.org/10.1016/j.gie.2011.03.509
25 Lagoudianakis EE Tsekouras D Papadima A Genetzakis M Pattas M Giannopoulos P Pneumothorax complicating endoscopic sphincterotomy successfully treated conservatively. Acta Gastroenterol Belg. 2006;69(3):342–4. Published online December 16, 2006.
26 Linssen VD Tan ACITL Schouten JA . Massive subcutaneous emphysema, unilateral pneumothorax, pneumomediastinum and pneumoperitoneum after endoscopic retrograde cholangiopancreatography. Netherlands Journal of Critical Care. 2013;17(4):16–8.
27 Lopes TL Clements RH Wilcox CM . Laparoscopy-assisted ERCP: experience of a high-volume bariatric surgery center (with video). Gastrointest Endosc. 2009;70(6):1254–9 .https://doi.org/10.1016/j.gie.2009.07.035
28 Lutchmansingh D Ryu C Amzuta I . The Path Less Traveled: Bilateral Pneumothoraces as a Complication of Endoscopic Retrograde Cholangiopancreatography. Chest. 2013;144(4):494A. doi:.https://doi.org/10.1378/chest.1671537
29 Makni A Chebbi F Ben Safta Z . Pneumoretroperitoneum, bilateral pneumothorax and emphysema following endoscopic biliary sphincterotomy. Acta Chir Belg. 2012;112(4):307–9 .https://doi.org/10.1080/00015458.2012.11680844
30 Markogiannakis H Toutouzas KG Pararas NV Romanos A Theodorou D Bramis I . Bilateral pneumothorax following endoscopic retrograde cholangiopancreatography: a case report. Endoscopy. 2007;39(Suppl 1):E195. doi:.https://doi.org/10.1080/00015458.2012.11680844
31 Morley AP Lau JYW Young RJ . Tension pneumothorax complicating a perforation of a duodenal ulcer during ERCP with endoscopic sphincterotomy. Endoscopy. 1997;29(4):332 .https://doi.org/10.1055/s-2007-1004205
32 Neofytou K Petrou A Savva C Petrides C Andreou C Felekouras E Pneumothorax following ERCP: Report of two cases with different pathophysiology. Case Rep Med. 2013;2013:206564 .https://doi.org/10.1155/2013/206564
33 Ozgonul A Cece H Sogut O Demir D Kurkcuoglus IC . Pneumoperitoneum, pneumoretroperitoneum and bilateral pneumothorax caused by ERCP. J Pak Med Assoc. 2010;60(1):60–1. Published online January 09, 2010.
34 Plönes T Reuland A-K Passlick B . Bilateral pneumothoraces, pneumomediastinum and subcutaneous emphysema as a rare complication of endoscopic cholangiopancreatography. BMJ Case Rep. 2012;2012(dec03 1):bcr2012007412 .https://doi.org/10.1136/bcr-2012-007412
35 Rappaport DE Solano JJ Edlow JA . Bilateral pneumothoraces as a complication of endoscopic retrograde cholangiopancreatography. J Emerg Med. 2017;52(4):573–5 .https://doi.org/10.1016/j.jemermed.2016.11.023
36 Samies N 3rd Reidman D Franga D . Diffuse subcutaneous emphysema after endoscopic retrograde cholangiopancreatography with subsequent pneumothorax and pneumomediastinum. Am Surg. 2015;81(2):E67–9.
37 Sampaziotis F Wiles A Shaukat S Dickinson RJ . Bilateral pneumothorax and subcutaneous emphysema following endoscopic retrograde cholangiopancreatography: a rare complication. Diagn Ther Endosc. 2010;2010:894045. Published online August 11, 2010 .https://doi.org/10.1155/2010/894045
38 Savides T Sherman S Kadell B Cryer H Derezin M . Bilateral pneumothoraces and subcutaneous emphysema after endoscopic sphincterotomy. Gastrointest Endosc. 1993;39(6):814–7 .https://doi.org/10.1016/S0016-5107(93)70273-4
39 Scarlett PY Falk GL . The management of perforation of the duodenum following endoscopic sphincterotomy: a proposal for selective therapy. Aust N Z J Surg. 1994;64(12):843–6 .https://doi.org/10.1111/j.1445-2197.1994.tb04561.x
40 Schepers NJ van Buuren HR . Pneumothorax following ERCP: report of four cases and review of the literature. Dig Dis Sci. 2012;57(8):1990–5. Published online March 31, 2012 .https://doi.org/10.1007/s10620-012-2150-3
41 Schiavon LL Rodrigues RA Nakao FS Di Sena VO Ferrari AP Libera ED Jr . Subcutaneous emphysema, pneumothorax and pneumomediastinum following endoscopic sphincterotomy. Gastroenterol Res. 2010;3(5):216–8. Published online September 20, 2010 .https://doi.org/10.4021/gr232w
42 Schilling M Sailer AS Heinz G Kutilek M . Intrathorakale Perforation von Gallenwegsstents bei St. p. Lebertransplantation. RoFo Fortschr Geb Rontgenstr Nuklearmed. 2014;186(9):887–8 .https://doi.org/10.1055/s-0033-1356313
43 Seymann GB Savides T Richman KM . Massive subcutaneous emphysema after endoscopic retrograde cholangiopancreatography. Am J Med. 2010;123(9):e15–6 .https://doi.org/10.1016/j.amjmed.2009.12.040
44 Shen T-C Chen C-P Chen C-H . An elderly woman with air leakage. Intern Med. 2015;54(5):533–4 .https://doi.org/10.2169/internalmedicine.54.3652
45 Shimatani M Matsushita M Takaoka M Koyabu M Ikeura T Kato K Effective “short” double-balloon enteroscope for diagnostic and therapeutic ERCP in patients with altered gastrointestinal anatomy: a large case series. Endoscopy. 2009;41(10):849–54 .https://doi.org/10.1055/s-0029-1215108
46 Song SY Lee KS Na KJ Ahn BH . Tension pneumothorax after endoscopic retrograde pancreatocholangiogram. J Korean Med Sci. 2009;24(1):173–5. Published online March 10, 2009 .https://doi.org/10.3346/jkms.2009.24.1.173
47 Brueck M Bandorski D Rauber K Lotterer E . [Pneumoretroperitoneum and bilateral pneumothorax after endoscopic biliary sphincterotomy]. Dtsch Med Wochenschr. 2010;135(17):853–6 .https://doi.org/10.1055/s-0030-1253667
48 Menéndez P Padilla D Villarejo P García A . Neumoperitoneo, neumoretroperitoneo, neumotórax bilateral, neumomediastino y enfisema subcutáneo secundario a CPRE. Rev Gastroenterol Peru. 2012;32(1):94–7. [Spanish].
49 Valkovský J Vrána J Šmíd J Kraus J Kovala P Kremer M Neobvyklá komplikace ERCP. Gastroenterologie a Hepatologie. 2014;68:209–11. Czech. doi:http://redakce.ambitmedia.cz/index.php/gh/article/view/410
50 Ko CW Lee SP . Epidemiology and natural history of common bile duct stones and prediction of disease. Gastrointest Endosc. 2002;56(6, Suppl):S165–9 .https://doi.org/10.1016/S0016-5107(02)70005-9
51 Williams EJ Taylor S Fairclough P Hamlyn A Logan RF Martin D Risk factors for complication following ERCP; results of a large-scale, prospective multicenter study. Endoscopy. 2007;39(9):793–801 .https://doi.org/10.1055/s-2007-966723
52 Enns R Eloubeidi MA Mergener K Jowell PS Branch MS Pappas TM ERCP-related perforations: risk factors and management. Endoscopy. 2002;34(4):293–8 .https://doi.org/10.1055/s-2002-23650
53 Cotton PB Lehman G Vennes J Geenen JE Russell RCG Meyers WC Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37(3):383–93 .https://doi.org/10.1016/S0016-5107(91)70740-2
54 Bhat M Romagnuolo J da Silveira E Reinhold C Valois E Martel M Randomised clinical trial: MRCP-first vs. ERCP-first approach in patients with suspected biliary obstruction due to bile duct stones. Aliment Pharmacol Ther. 2013;38(9):1045–53 .https://doi.org/10.1111/apt.12481
55 Chen Q Jin P Ji X Du H Lu J . Management of difficult or failed biliary access in initial ERCP: A review of current literature. Clin Res Hepatol Gastroenterol. 2019;43(4):365–72 .https://doi.org/10.1016/j.clinre.2018.09.004
56 Maunder RJ Pierson DJ Hudson LD . Subcutaneous and mediastinal emphysema. Pathophysiology, diagnosis, and management. Arch Intern Med. 1984;144(7):1447–53 .https://doi.org/10.1001/archinte.1984.00350190143024
57 Hartin DJ Kendall R Boyle AA Atkinson PRT . Case of the month: Buffalo chest: a case of bilateral pneumothoraces due to pleuropleural communication. Emerg Med J. 2006;23(6):483–6 .https://doi.org/10.1136/emj.2005.030981
58 Howard TJ Tan T Lehman GA Sherman S Madura JA Fogel E Classification and management of perforations complicating endoscopic sphincterotomy. Surgery. 1999;126(4):658–63, discussion 664–5 .https://doi.org/10.1016/S0039-6060(99)70119-4
59 Paspatis GA Dumonceau J-M Barthet M Meisner S Repici A Saunders BP Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2014;46(8):693–711 .https://doi.org/10.1055/s-0034-1377531
60 Leigh-Smith S Harris T . Tension pneumothorax--time for a re-think? Emerg Med J. 2005;22(1):8–16 .https://doi.org/10.1136/emj.2003.010421
61 Machado NO . Management of duodenal perforation post-endoscopic retrograde cholangiopancreatography. When and whom to operate and what factors determine the outcome? A review article. JOP. 2012;13(1):18–25.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors; the authors remain independent of any funding influence and declare no support from any organisation for the submitted work. All authors declare no financial relationships with any organisations that might have an interest in the submitted work in the previous three years and no other relationships or activities that could appear to have influenced the submitted work.