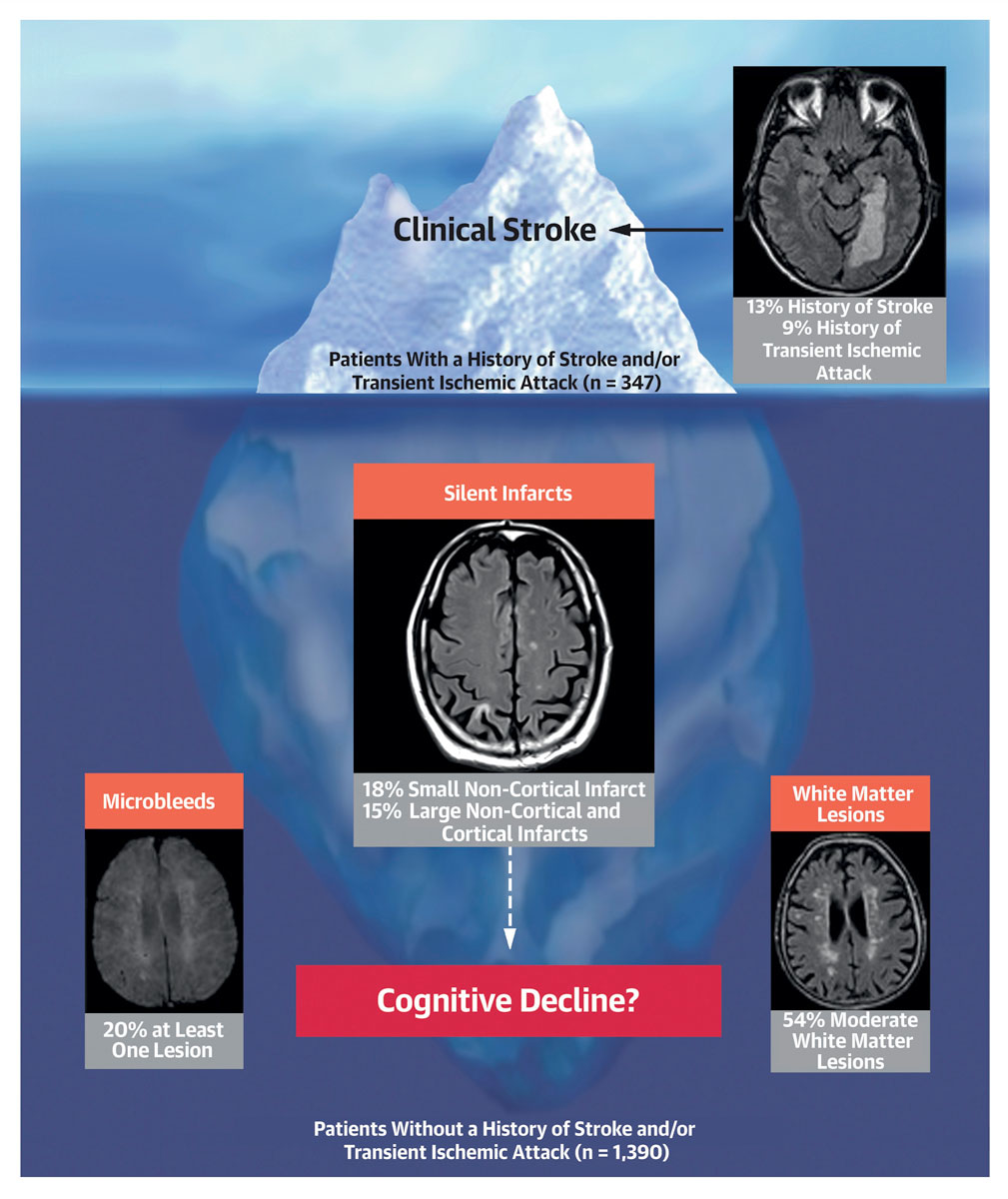
Figure 1 Potential relationships between overt and silent brain lesions and cognitive function in patients with atrial fibrillation (Reproduced with the permission of Elsevier from: Conen et al. J Am Coll Cardiol. 2019;73(9):989–99 [127]).
DOI: https://doi.org/10.4414/smw.2020.20196
atrial fibrillation
brain magnetic resonance imaging
confidence interval
European Society of Cardiology
Montreal Cognitive Assessment
non-vitamin K oral anticoagulant
New York Heart Association
left atrial
large non-cortical and cortical infarcts
relative risk
small non-cortical infarcts
transient ischaemic attack
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in clinical practice [1, 2], and its prevalence is expected to increase in the near future. AF prevalence correlates strongly with age, with estimates ranging from <1% in individuals aged <60 years to >7% among individuals aged 80 years and older [3]. At age 45, the lifetime risk for developing AF is over 20%. In patients with at least one cardiovascular risk factor, such as hypertension, diabetes or smoking, it is as high as 38% [4]. According to longitudinal data from the Rotterdam Study, the lifetime risk of AF at the age of 55 years was 23.8% in men and 22.2% in women [5]. The fact that men seem to have a higher risk of developing AF than women has been observed for many years [6], but the underlying cause remains unclear. Most established cardiovascular risk factors increase the risk of AF development. Among others, the Women’s Health Study showed that hypertension and high body mass index are the strongest potentially modifiable risk factors for incident AF, while the independent contribution of diabetes mellitus seems to be smaller [7–9]. Other known risk factors are a history of coronary heart disease, heart failure and valvular heart disease (predominantly mitral valve stenosis) [10, 11].
The importance of AF as a public health problem is further underscored by its strong and independent association with the risk of stroke, heart failure, death, cognitive dysfunction and a reduced quality of life [11–15]. Stroke and heart failure can be the first manifestations of AF. Patients with established AF have a five-fold increase in their risk of stroke compared to those without arrhythmia [12, 16]. Oral anticoagulation is highly effective at stroke prevention, but residual stroke risk persists in this patient population and current treatment strategies are limited to further reducing this risk. Emerging evidence suggests that patients with AF have a high risk of silent brain lesions, and that these lesions are associated with a decline in cognitive functioning [17].
In this review, we summarise the current evidence on AF risk factors, adverse outcomes in patients with established AF, current treatment strategies, and areas in need of further investigation.
Hypertension is one of the main potentially modifiable risk factors for AF. Epidemiologic studies have reported that the prevalence of hypertension in AF patients ranges from 49 to 90% [18]. Data from the Women’s Health Study suggest that the long-term risk of AF was significantly increased across categories of systolic and diastolic blood pressure, while systolic blood pressure was a better predictor for stroke [7]. Consistent with these observations, data from the Atherosclerosis Risk in Communities (ARIC) Study suggest that elevated blood pressure may be the largest contributor to the overall risk of AF, with an estimated population attributable fraction of 21.6% [19]. The underlying mechanism could include an increase in left ventricular wall thickness or left ventricular stiffness, and the impairment of left ventricular diastolic function associated with hypertension. These processes may cause left atrial (LA) stretch and increase pressure, which leads to remodelling and dilation of the LA, ultimately causing a predisposition to the development of AF [20].
Beyond hypertension, increased body mass index (BMI) and obesity are also key independent risk factors for AF development [8, 21]. Among women free from AF, a total of 18.3% of all new AF cases were attributable to a short-term increase in BMI to >25 kg/m2 [8]. It has been proposed that obesity and elevated body-fat percentages may lead to ventricular diastolic dysfunction, which may cause LA enlargement and increase susceptibility to atrial remodelling [22]. In addition, studies have shown that elevated body-fat percentages may influence myocardial tissue through increased oxidative stress, which could play a role in the initiation of AF [23].
Heavy alcohol consumption has long been known as a risk factor for AF episodes and has been associated with the so-called “holiday heart syndrome” [24]. Prospective cohort studies have investigated the association of moderate to high alcohol consumption and the risk of developing AF. In the Framingham Heart Study, participants who consumed high amounts of alcohol (>36 g/day) had a significantly higher risk of incident AF [25]. Women who consumed ≥2 drinks/day showed a similarly high risk of developing AF in the future [26]. Meta-analysis showed that the risk of AF increases by 8% with every extra 1 drink/day of alcohol consumed [27]. There seems to be no threshold effect, but more studies are needed at the lower end of the drinking spectrum. Nonetheless, the pathophysiological mechanisms responsible for this association could be that alcohol may promote electrical atrial remodelling, producing an arrhythmogenic substrate and causing myocardial fibrosis within the LA [28, 29]. These electrical and mechanical features may act as triggers for AF.
Regular physical activity and exercise training have favourable effects on cardiovascular risk factors, including improvements in hypertension and diabetes through significant weight loss, and through favourable modifications of cardiac structure and function [30–33]. Given that physical activity lowers the risk factors associated with AF, one may assume that the benefit is also evident as a reduction in AF risk. Several population-based studies have shown that moderate amounts of physical activity significantly reduce AF risk [34, 35], while other studies suggest that high-intensity and endurance exercise are associated with a higher risk of developing AF in young athletes and middle-aged men [36–39]. The increased AF risk in endurance athletes is probably mediated by increased vagal tone, higher volume load during exercise, myocardial damage, and enlargement of the LA [40, 41]. Based on the evidence available and the possible U-shaped relationship of physical activity and incident AF, regular moderate physical activity is recommended to prevent AF, while endurance athletes should be counselled that intense sports can promote AF development [42]. The management of athletes with AF is similar to general treatment regimens of AF patients, with a stronger focus on symptom burden reduction.
Taken together, several modifiable cardiovascular risk factors have been identified as associated with AF risk, and hypertension and obesity explain about 50% of the population attributable risk [19]. This underscores the importance of lifestyle and risk factor management in reducing the AF burden in the general population.
Patients with AF have a high risk of stroke, and epidemiologic evidence suggests that 20-30% of all strokes are due to AF [12, 13, 43–45]. Based on this relationship, several clinically applicable stroke risk-stratification schemes have been developed and validated in AF populations [46–48]. The most widely used one is the CHA2DS2-VASc score (congestive heart failure [1 point], hypertension [1 point], age >75 years [2 points], diabetes [1 point], prior stroke, transient ischaemic attack (TIA) or systemic arterial embolism [2 points], vascular disease [1 point], age 65 to 74 years [1 point], female sex [1 point]) [48], which is not only used for stroke risk-stratification, but also aids the decision on whether a patient should receive oral anticoagulation. In general, patients without stroke risk factors do not need antithrombotic therapy, while the great majority of AF patients have stroke risk factors (a CHA2DS2-VASc score of 1 or more for men, and 2 or more for women) and would likely benefit from oral anticoagulation.
Oral anticoagulation effectively reduces stroke risk, by approximately 64% [49, 50]. Vitamin K antagonists (such as phenprocoumon or acenocoumarol) were the oral anticoagulants first used for stroke prevention, and they significantly reduced the risk of stroke in patients with AF [50]. However, the use of vitamin K antagonists is limited by their narrow therapeutic interval, necessitating frequent monitoring and dose adjustments. More recently developed non-vitamin K antagonist oral anticoagulants (NOAC), such as factor Xa inhibitors (apixaban, edoxaban and rivaroxaban) and thrombin inhibitor (dabigatran) have been shown to be at least as effective as vitamin K antagonists for stroke prevention, but safer with regard to adverse events [49]. Clinical trials have showed that NOACs have a lower risk of significant bleeding compared to vitamin K antagonists [49]. This is especially true for intracranial haemorrhage (relative risk [RR] 0.48, 95% confidence interval [CI] 0.39–0.59; p = 0.0001), critical organ bleedings and fatal bleedings. On the other hand, NOAC use is associated with an increase in gastrointestinal bleeding events (RR 1.25, 95% CI 1.01–1.55; p = 0.04) [49]. Additionally, the practical effect of all NOACs is that there is no need for regular anticoagulation monitoring. Based on this recent evidence, the European Society of Cardiology (ESC) guidelines suggest that when oral anticoagulation is initiated in an AF patient who is eligible for oral anticoagulation, an NOAC (apixaban, dabigatran, edoxaban or rivaroxaban) is preferred to a vitamin K antagonist [42]. Data from Switzerland show that over the last few years, a change in the pattern of oral anticoagulation therapy prescription can be observed, with a shift from vitamin K antagonists to NOACs [51].
Evidence on the use of antiplatelet monotherapy for stroke prevention in AF is very limited [52–54]. Data from randomised trials shows that vitamin K antagonists prevent stroke in AF patients better than single or dual antiplatelet therapy with aspirin or clopidogrel [55]. Antiplatelet therapy increases the risk of bleeding, especially dual antiplatelet therapy, for which the risk is comparable with that for oral anticoagulants [56]. Therefore, antiplatelet therapy cannot be recommended for stroke prevention in AF patients.
Despite the evident benefits of oral anticoagulation, there is still an appreciable residual stroke risk, approximately 1.7% per year for vitamin K antagonists and 1.4% per year for NOACs [49]. Unfortunately, there is currently no evidence available which supports the use of one NOAC over the others, or which supports switching from one NOAC to another in patients who have experienced an ischaemic stroke under NOAC therapy. A retrospective study evaluated the prevalence and management of left atrial thrombi in oral anticoagulated patients who had received a transoesophageal echocardiography prior to pulmonary vein isolation [57]. Among 1358 AF patients, only 11 (0.6%) had a thrombus, while 8 were on oral anticoagulation therapy (5 with NOACs and 3 with vitamin K antagonists). There are currently no guidelines on how to treat these patients with an oral anticoagulant. However, thrombus resolution may be achieved in the majority of patients by changing the anticoagulation regimen, such as switching to vitamin K antagonists or to an NOAC, or a different NOAC. In general, depending on the severity and size of the stroke and the presence of an LA thrombus detected by transoesophageal echography, oral anticoagulation should be reinitiated 3 to 14 days after the event onset, and the decision to restart oral anticoagulation must depend on the risk of recurrent stroke outweighing the risk of secondary haemorrhagic transformation and bleeding [42].
The decision on how to treat AF patients with chronic kidney disease who need NOAC therapy may be challenging, and requires an assessment of renal function using a formula for glomerular filtration rate estimation [58]. The CKD-EPI formula is often used in clinical practice because it is easy, since body weight is not included. However, it should be noted that the large NOAC trials used the Cockcroft-Gault formula, which does incorporate body weight into the calculation of renal function. Dose-adjustments should be performed for dabigatran, rivaroxaban and edoxaban in patients who have a creatinine clearance of <50 ml/min. For apixaban, the dose is reduced in the presence of two or more of the following criteria: age >80 years, weight <60 kg, or a serum creatinine level of >133 μmol/l. For patients with a creatinine clearance <30 ml/min or end stage renal disease, no data are available from randomsed trials, since patients with creatinine clearances of <30 ml/min were excluded in all four major NOAC trials. Nonetheless, in Switzerland and Europe the three factor Xa inhibitors (apixaban, edoxaban, rivaroxaban) are approved to a creatinine clearance of 15ml/min, whereas the cut-off for dabigatran is 30 ml/min (due to its high renal excretion). However, NOACs should generally be used with caution in patients with a creatinine clearance of <30 ml/min.
In general, there is limited evidence on whether AF patients with end-stage renal disease on dialysis benefit from oral anticoagulation. Based on a retrospective analysis, apixaban may be associated with better outcomes compared to vitamin K antagonists [59]. Based on scarce evidence, the US Food and Drug Administration approved apixaban for patients with AF on dialysis, and the drug is used off-label in Switzerland and other countries in dialysis patients.
Gastrointestinal bleedings are a frequent complication in patients treated with oral anticoagulation. Although such bleedings are usually not life-threatening, they should not be underestimated. A focused therapy strategy may be required to treat the cause of bleeding (i.e., gastric ulcer, polyps), but oral anticoagulation should be reinitiated to maintain effective stroke prevention. In situations where patients experience recurrent gastrointestinal bleeding events, an alternative NOAC with a potentially different bleeding risk profile may be considered [60]. Proton-pump inhibitors have been proposed as an option for patients who have a history of gastrointestinal bleeding [61–63]. However, the protective effect has only been evaluated in patients receiving antiplatelet therapy or vitamin K antagonists, and data on the preventive effect in NOAC treated patients are limited.
Implantation of an LA appendage occluder as a mechanical alternative to oral anticoagulation may be considered in selected patients with an absolute contraindication for oral anticoagulation (such as patients with cerebral amyloid angiopathy) and in patients with a history of major bleeding, such as intracranial haemorrhage or gastrointestinal bleeding, who are no longer deemed good candidates for oral anticoagulation [42]. However, the overall weight of evidence for LA appendage occlusion is much lower compared to the evidence on NOACs. Therefore, such treatment decisions should be evaluated in the context of the risks and benefits for the individual patient.
Rate and rhythm control interventions, either to improve AF-related symptoms or to restore sinus rhythm, are an integral part of AF management. The decision on which treatment option best fits the individual patient depends on the symptom burden, the patient’s expectations and preferences, the duration of AF, and the patient’s characteristics.
There is little robust evidence available from clinical trials about the best rate control option. Usually, pharmacological rate control can be achieved with beta-blockers, calcium channel blockers (diltiazem, verapamil), and less frequently, digoxin. In the setting of acute new-onset AF, patients should receive rate control interventions using beta-blockers, verapamil or diltiazem [64–66]. Beta-blockers are often the first-line rate-controlling agents [67] because they have better acute heart rate control than calcium channel blockers and digoxin. However, the prognostic benefit of beta-blockers among heart failure patients with reduced ejection fraction is lost in those with AF. An individual, patient-level meta-analysis suggests that beta-blocker therapy leads to a significant reduction in all-cause mortality in heart failure patients with sinus rhythm, but not in patients with AF [68]. Although evidence on the prognostic benefit in heart failure patients with reduced ejection fraction is lacking, the Beta-Blockers in Heart Failure Collaborative Group recommends beta-blockers as a useful first-line rate control agent across all AF patients [69]. This recommendation is mainly based on (1) the good tolerability across patients with sinus rhythm and with AF, (2) the significant functional and symptomatic improvement as a result of beta-blocker administration, and (3) the lack of harm. The use of digoxin may be considered in patients who have concomitant acute systolic heart failure. Verapamil and diltiazem have been shown to improve AF-related symptoms [70]. However, they should not be administered in patients with heart failure because of their negative inotropic effects [66, 71]. Digoxin and digitoxin have been used for rate control for decades, but prescriptions are declining [72]. A randomised trial of heart failure patients showed that digoxin had no mortality benefit compared to placebo, but reduced hospitalisations [73]. Except for critically ill patients, amiodarone should generally not be used for rate-control because of its long-term toxicity. Taken together, the decision on which rate control option best suits a patient should be made on the basis of individual patient characteristics and preferences. Given that all therapies have potential adverse effects, the strategy should be to start at a low dose and then up-titrate until the patient experiences symptom improvements. If pharmacological rate control fails and a patient is not a suitable candidate for rhythm control, a “pace and ablate” strategy (implantation of a permanent pacemaker and ablation of the AV-node) is valuable and has a very high success rate [74–76]. Because it renders patients pacemaker-dependent, it is generally reserved for elderly patients. An additional advantage of this strategy is that patients can stop their rate control medication.
Sinus rhythm restoration and maintenance is one of the cornerstones of AF management. Rhythm control interventions are indicated in AF patients for symptom improvement and in patients who are haemodynamically comprised. Such interventions consist of antiarrhythmic drug therapy, electrical cardioversion or catheter ablation. Although these interventions have been shown to be beneficial in restoring sinus rhythm, some AF patients may still require repeat procedures or a combination therapy. The choice of whether to initiate antiarrhythmic drug therapy must be carefully evaluated and depends on patient preferences, symptom burden, and potential side-effects of the drugs. In general, flecainide, propafenone, sotalol, or less frequently, dronedarone are recommended for treatment of recurrent symptomatic AF episodes in patients without concomitant heart failure [77–79]. Amiodarone is recommended for treatment of recurrent symptomatic AF episodes in patients with a normal structural heart or with concomitant heart failure [80, 81]. Despite its antiarrhythmic effects, this drug also has proarrhythmic effects such as the occurrence of torsades de pointes, which necessitates regular monitoring of the QT interval in patients on therapy [69]. Long-term therapy with amiodarone is associated with high incidence of extracardiac side effects, including pulmonary toxicity, skin discoloration, thyroid dysfunction, corneal deposits and cutaneous reaction [82, 83]. As a consequence, baseline testing and careful monitoring of patients taking amiodarone is crucial. In the setting of acute new-onset AF, electrical cardioversion is the first-line recommendation in patients presenting with haemodynamic instability [84, 85]. However, antiarrhythmic drugs (such as flecainide, amiodarone or vernakalant) can also restore sinus rhythm in an acute setting and reports suggest that in approximately 50% of patients, sinus rhythm can be successfully restored with pharmacological cardioversion [81, 86, 87]. The advantage of electrical cardioversion is that it restores sinus rhythm much quicker than pharmacological cardioversion. Conversely, pharmacological cardioversion has the advantage that is does not require sedation. In some patients, a single bolus of oral flecainide or propafenone can be self-administrated by the patient, the so-called “pill in the pocket” strategy, to restore sinus rhythm [88]. Electrical cardioversion is associated with an increased risk of stroke in AF patients who are not taking oral anticoagulants [89]. This risk can be significantly reduced by administration of oral anticoagulants for at least three weeks prior to the scheduled cardioversion or by performing transoesophageal echography before cardioversion [90]. The current ESC guidelines recommend that in patients who have been in AF for longer than 48 hours, oral anticoagulation should start at least three weeks before the scheduled cardioversion (or a transoesophageal echography should be performed) [42], suggesting that in patients with AF lasting less than 48 hours, this may not be required [91]. However, a large, multicentre, retrospective cohort study showed that although the general stroke risk is low in patients presenting with AF lasting less than 48 hours, the risk becomes unacceptably high in patients with cardiovascular risk factors [89]. Additionally, it is often challenging to specify whether the patient had AF longer than 48 hours or not. We therefore generally recommend three weeks of oral anticoagulation or performing a transoesophageal echography to exclude the presence of LA thrombus prior to cardioversion in all patients. In general, oral anticoagulation should be continued for four weeks after the procedure in all patients, and afterwards a decision on long-term oral anticoagulation should be based on stroke risk assessment.
Catheter ablation is indicated in patients when antiarrhythmic drugs fail to reduce the symptom burden or to restore sinus rhythm. Catheter ablation is more effective at sinus rhythm restoration than antiarrhythmic drug therapy and may therefore also be offered as first-line therapy, especially in younger patients and patients who do not tolerate or do not wish to take anti-arrhythmic drugs long-term [92–95]. Data suggest that the effectiveness of catheter ablation can also be achieved in patients with persistent or long-standing persistent AF [96]. In general, sinus rhythm restoration can be achieved in up to 90% of patients with paroxysmal AF, and in around 50 to 80% with persistent AF [97–101]. Whereas AF ablation improves symptoms, there is currently no evidence that catheter ablation prevents cardiovascular events. Although catheter ablation is a procedure with a high safety profile, complications can occur. In experienced centres, approximately 4% of patients experience complications after catheter ablation, but the majority of complications are usually manageable. The most common are vascular access complications (2%), tamponade (1%) and stroke (<1%) [102, 103]. A significant association between AF ablation operator volume (and hospital volume) and adverse outcomes has been reported, and this underscores the actual clinical relevance of referring patients to experienced operators in large centres [103].
It has been debated whether oral anticoagulation can be withdrawn in patients after successful catheter ablation. A small prospective study found that within 1.3 years after ablation, about two-thirds of patients are able to stay off oral anticoagulation [104]. However, because there are no data available from controlled trials, oral anticoagulation following catheter ablation should generally follow anticoagulation recommendations, regardless of the presumed rhythm outcome.
Given the complexity of the different treatment options for AF, including rate and rhythm control interventions, anticoagulation, and co-existing comorbidities such as heart failure, renal failure, etc., close collaboration between internists and cardiologists is warranted.
The association between AF and heart failure was described many years ago, with several observational studies reporting a prevalence of AF ranging from 13 to 27% in heart failure populations [105–107]. For instance, data from the Framingham Heart Study suggest that out of 1470 participants who developed either heart failure or new AF during follow-up, over one-fourth (26%) developed both heart failure and AF [11]. The severity of heart failure (usually measured with the New York Heart Association [NYHA] classification) is positively correlated with the prevalence of AF [108].
Although evidence of the association between these two diseases is eminent, the causative relationship between the disorders has not been fully determined. Both diseases share common cardiovascular risk factors, including age, hypertension, diabetes, obesity, valvular heart disease and structural heart disease. Consequently, therapies directed towards risk factors associated with heart failure may be protective for the development of AF as well. Retrospective analyses of randomised trials have demonstrated that angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, β-blockers and eplerenone reduce the risk of developing AF in patients with heart failure [109–112]. Previous investigations have shown that persistent AF episodes can cause tachycardia-induced cardiomyopathy and that the elimination of these arrhythmias reverses the haemodynamic and clinical manifestations associated with heart failure [113–115]. Given this, sinus rhythm restoration may not only reduce the burden of AF, but may also improve the haemodynamic properties of the left ventricle and subsequently reduce heart failure hospitalisations. Indeed, there is evidence from randomised trials that catheter ablation may lower heart failure admission rates in AF patients with concomitant heart failure compared to medical therapy [116–119]. In this context, further refinement to how we approach the treatment of patients with AF and concomitant heart failure remains the subject of future research.
Cognitive dysfunction and dementia place an enormous socioeconomic burden on our health care system [120]. Patients with AF have an increased risk of dementia and cognitive decline [14, 17, 121, 122]. A recent meta-analysis of 21 studies demonstrated that the presence of AF was associated with the risk of developing cognitive impairment (RR 1.40, 95% CI 1.19–1.64), and that the risk was even higher in studies that included patients with a history of stroke (RR 2.70, 95% CI 1.82–4.00) [17]. These findings underscore that stroke, which causes structural damage to brain tissue, increases the risk of cognitive decline in patients with AF, but that some of the association seems to be independent of a prior history of overt stroke. An additional factor that might further explain the association between dementia and AF is the higher burden of cardiovascular risk factors in AF patients as compared to individuals without arrhythmia. These cardiovascular risk factors, including hypertension and diabetes, are known to be associated with both AF and dementia [123].
Silent brain lesions can be detected with brain magnetic resonance imaging (bMRI). Up to 20% of individuals from the general population have silent brain infarcts on bMRI [124]. A prospective study performed in individuals free of AF found that the presence of silent brain infarcts on the baseline bMRI was associated with worse performance on neurocognitive tests and a steeper decline in global cognitive function [125]. Also, the presence of silent brain infarcts more than doubled the risk of developing dementia (hazard ratio [HR] 2.26; 95% CI 1.09–4.70). These findings further emphasise that silent infarcts may represent a potential mechanistic correlate of cognitive decline. These relationships have been found in individuals without AF, and there is currently no study that has investigated this association in a high-risk population of AF patients. The Swiss Atrial Fibrillation Cohort (Swiss-AF) Study was designed to investigate the relationship between silent brain lesions and cognitive decline in a large sample of AF patients [126]. Systematic bMRI and cognitive testing using the Montreal Cognitive Assessment (MoCA) were performed in all participants. Cross-sectional analysis revealed that out of 1390 patients without a history of stroke or TIA, 368 (15%) and 387 (18%) had evidence of a previous, silent large non-cortical or cortical infarct (LNCCI) and a small non-cortical infarct (SNCI), respectively (fig. 1) [127]. Importantly, these observations were found in a cohort of patients with a high prevalence of oral anticoagulation (more than 90% on oral anticoagulants) at the time of the bMRI measurements. Patients with silent LNCCI had significantly lower MoCA scores than those with no LNCCI, suggesting a decreased cognitive performance in patients with silent brain lesions. The magnitude of this difference in cognitive performance was similar in patients who had a history of stroke, a finding which suggests that these lesions may explain at least part of the increased risk of cognitive dysfunction in these patients. Whether routine bMRI scanning and cognitive performance testing should be performed in patients with AF is the subject of current research.

Figure 1 Potential relationships between overt and silent brain lesions and cognitive function in patients with atrial fibrillation (Reproduced with the permission of Elsevier from: Conen et al. J Am Coll Cardiol. 2019;73(9):989–99 [127]).
Over the years, significant progress has been made in the field of AF. Hypertension and obesity are the two most important modifiable risk factors for the development of AF, such that lifestyle management and treatment of these risk factors is key for AF prevention. Lowering alcohol consumption and moderate physical activity reduce AF incidence, whereas high endurance sports may increase the risk of incident AF. Stroke risk stratification and oral anticoagulation therapy remain an integral part of AF management, and NOAC therapy is now considered standard of care in patients qualifying for oral anticoagulation. Rate and rhythm control interventions have been shown to reduce the symptoms, and the choice of which treatment best fits the individual patient often depends on the symptom burden, patient characteristics, and patient expectations and preferences. Heart failure remains one of the major comorbidities and complications in AF patients, and their interrelationship needs further evaluation. Patients with AF have an increased risk of cognitive dysfunction, even in the absence of a history of overt stroke or TIA. Further studies are needed to determine the value of routine bMRI screening and testing of cognitive function to improve risk stratification in patients with AF.
DC has a McMaster University Department of Medicine Mid-Career Research Award; his work is supported by the Hamilton Health Sciences RFA Strategic Initiative Program.
DC has received consultant/speaker fees from Servier, Canada. MK has received grants from Bayer, Pfizer-BMS, the Swiss National Science Foundation, and the Swiss Heart Foundation; and lecture or consulting fees from Daiichi-Sankyo, Boehringer Ingelheim, Bayer, Pfizer-BMS, AstraZeneca, Sanofi, Novartis, Merck Sharp & Dohme, Medtronic, Boston Scientific, St. Jude Medical, Biotronik, Sorin, Zoll, Biosense Webster, and Abbott. CSZ has received speaker fees from Vifor Pharma and Novartis. The remaining authors have no relationships with the industry to disclose.
1 Krijthe BP , Kunst A , Benjamin EJ , Lip GY , Franco OH , Hofman A , et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34(35):2746–51. doi:.https://doi.org/10.1093/eurheartj/eht280
2 Miyasaka Y , Barnes ME , Gersh BJ , Cha SS , Bailey KR , Abhayaratna WP , et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–25. doi:.https://doi.org/10.1161/CIRCULATIONAHA.105.595140
3 Go AS , Hylek EM , Phillips KA , Chang Y , Henault LE , Selby JV , et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–5. doi:.https://doi.org/10.1001/jama.285.18.2370
4 Staerk L , Wang B , Preis SR , Larson MG , Lubitz SA , Ellinor PT , et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. BMJ. 2018;361:k1453. doi:.https://doi.org/10.1136/bmj.k1453
5 Heeringa J , van der Kuip DA , Hofman A , Kors JA , van Herpen G , Stricker BH , et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27(8):949–53. doi:.https://doi.org/10.1093/eurheartj/ehi825
6 Magnussen C , Niiranen TJ , Ojeda FM , Gianfagna F , Blankenberg S , Njølstad I , et al.; BiomarCaRE Consortium. Sex Differences and Similarities in Atrial Fibrillation Epidemiology, Risk Factors, and Mortality in Community Cohorts: Results From the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation. 2017;136(17):1588–97. doi:.https://doi.org/10.1161/CIRCULATIONAHA.117.028981
7 Conen D , Tedrow UB , Koplan BA , Glynn RJ , Buring JE , Albert CM . Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation. 2009;119(16):2146–52. doi:.https://doi.org/10.1161/CIRCULATIONAHA.108.830042
8 Tedrow UB , Conen D , Ridker PM , Cook NR , Koplan BA , Manson JE , et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study). J Am Coll Cardiol. 2010;55(21):2319–27. doi:.https://doi.org/10.1016/j.jacc.2010.02.029
9 Schoen T , Pradhan AD , Albert CM , Conen D . Type 2 diabetes mellitus and risk of incident atrial fibrillation in women. J Am Coll Cardiol. 2012;60(15):1421–8. doi:.https://doi.org/10.1016/j.jacc.2012.06.030
10 Benjamin EJ , Levy D , Vaziri SM , D’Agostino RB , Belanger AJ , Wolf PA . Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271(11):840–4. doi:.https://doi.org/10.1001/jama.1994.03510350050036
11 Wang TJ , Larson MG , Levy D , Vasan RS , Leip EP , Wolf PA , et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920–5. doi:.https://doi.org/10.1161/01.CIR.0000072767.89944.6E
12 Conen D , Chae CU , Glynn RJ , Tedrow UB , Everett BM , Buring JE , et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA. 2011;305(20):2080–7. doi:.https://doi.org/10.1001/jama.2011.659
13 Wolf PA , Abbott RD , Kannel WB . Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–8. doi:.https://doi.org/10.1161/01.STR.22.8.983
14 Ott A , Breteler MM , de Bruyne MC , van Harskamp F , Grobbee DE , Hofman A . Atrial fibrillation and dementia in a population-based study. The Rotterdam Study. Stroke. 1997;28(2):316–21. doi:.https://doi.org/10.1161/01.STR.28.2.316
15 Thrall G , Lane D , Carroll D , Lip GY . Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006;119(5):448.e1–19. doi:.https://doi.org/10.1016/j.amjmed.2005.10.057
16 Wolf PA , Dawber TR , Thomas HE, Jr , Kannel WB . Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology. 1978;28(10):973–7. doi:.https://doi.org/10.1212/WNL.28.10.973
17 Kalantarian S , Stern TA , Mansour M , Ruskin JN . Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med. 2013;158(5 Pt 1):338–46. doi:.https://doi.org/10.7326/0003-4819-158-5-201303050-00007
18 Manolis AJ , Rosei EA , Coca A , Cifkova R , Erdine SE , Kjeldsen S , et al. Hypertension and atrial fibrillation: diagnostic approach, prevention and treatment. Position paper of the Working Group ‘Hypertension Arrhythmias and Thrombosis’ of the European Society of Hypertension. J Hypertens. 2012;30(2):239–52. doi:.https://doi.org/10.1097/HJH.0b013e32834f03bf
19 Huxley RR , Lopez FL , Folsom AR , Agarwal SK , Loehr LR , Soliman EZ , et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123(14):1501–8. doi:.https://doi.org/10.1161/CIRCULATIONAHA.110.009035
20 Verdecchia P , Angeli F , Reboldi G . Hypertension and Atrial Fibrillation: Doubts and Certainties From Basic and Clinical Studies. Circ Res. 2018;122(2):352–68. doi:.https://doi.org/10.1161/CIRCRESAHA.117.311402
21 Wang TJ , Parise H , Levy D , D’Agostino RB, Sr , Wolf PA , Vasan RS , et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292(20):2471–7. doi:.https://doi.org/10.1001/jama.292.20.2471
22 Lauer MS , Anderson KM , Kannel WB , Levy D . The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA. 1991;266(2):231–6. doi:.https://doi.org/10.1001/jama.1991.03470020057032
23 Vincent HK , Powers SK , Stewart DJ , Shanely RA , Demirel H , Naito H . Obesity is associated with increased myocardial oxidative stress. Int J Obes Relat Metab Disord. 1999;23(1):67–74. doi:.https://doi.org/10.1038/sj.ijo.0800761
24 Ettinger PO , Wu CF , De La Cruz C, Jr , Weisse AB , Ahmed SS , Regan TJ . Arrhythmias and the “Holiday Heart”: alcohol-associated cardiac rhythm disorders. Am Heart J. 1978;95(5):555–62. doi:.https://doi.org/10.1016/0002-8703(78)90296-X
25 Djoussé L , Levy D , Benjamin EJ , Blease SJ , Russ A , Larson MG , et al. Long-term alcohol consumption and the risk of atrial fibrillation in the Framingham Study. Am J Cardiol. 2004;93(6):710–3. doi:.https://doi.org/10.1016/j.amjcard.2003.12.004
26 Conen D , Tedrow UB , Cook NR , Moorthy MV , Buring JE , Albert CM . Alcohol consumption and risk of incident atrial fibrillation in women. JAMA. 2008;300(21):2489–96. doi:.https://doi.org/10.1001/jama.2008.755
27 Larsson SC , Drca N , Wolk A . Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. 2014;64(3):281–9. doi:.https://doi.org/10.1016/j.jacc.2014.03.048
28 Qiao Y , Shi R , Hou B , Wu L , Zheng L , Ding L , et al. Impact of Alcohol Consumption on Substrate Remodeling and Ablation Outcome of Paroxysmal Atrial Fibrillation. J Am Heart Assoc. 2015;4(11):e002349. doi:.https://doi.org/10.1161/JAHA.115.002349
29 McManus DD , Yin X , Gladstone R , Vittinghoff E , Vasan RS , Larson MG , et al. Alcohol Consumption, Left Atrial Diameter, and Atrial Fibrillation. J Am Heart Assoc. 2016;5(9):e004060. doi:.https://doi.org/10.1161/JAHA.116.004060
30 Cornelissen VA , Fagard RH . Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;46(4):667–75. doi:.https://doi.org/10.1161/01.HYP.0000184225.05629.51
31 Sluik D , Buijsse B , Muckelbauer R , Kaaks R , Teucher B , Johnsen NF , et al. Physical Activity and Mortality in Individuals With Diabetes Mellitus: A Prospective Study and Meta-analysis. Arch Intern Med. 2012;172(17):1285–95. doi:.https://doi.org/10.1001/archinternmed.2012.3130
32 Jakicic JM , Marcus BH , Gallagher KI , Napolitano M , Lang W . Effect of exercise duration and intensity on weight loss in overweight, sedentary women: a randomized trial. JAMA. 2003;290(10):1323–30. doi:.https://doi.org/10.1001/jama.290.10.1323
33 Bhella PS , Hastings JL , Fujimoto N , Shibata S , Carrick-Ranson G , Palmer MD , et al. Impact of lifelong exercise “dose” on left ventricular compliance and distensibility. J Am Coll Cardiol. 2014;64(12):1257–66. doi:.https://doi.org/10.1016/j.jacc.2014.03.062
34 Drca N , Wolk A , Jensen-Urstad M , Larsson SC . Physical activity is associated with a reduced risk of atrial fibrillation in middle-aged and elderly women. Heart. 2015;101(20):1627–30. doi:.https://doi.org/10.1136/heartjnl-2014-307145
35 Mozaffarian D , Furberg CD , Psaty BM , Siscovick D . Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2008;118(8):800–7. doi:.https://doi.org/10.1161/CIRCULATIONAHA.108.785626
36 Aizer A , Gaziano JM , Cook NR , Manson JE , Buring JE , Albert CM . Relation of vigorous exercise to risk of atrial fibrillation. Am J Cardiol. 2009;103(11):1572–7. doi:.https://doi.org/10.1016/j.amjcard.2009.01.374
37 Andersen K , Farahmand B , Ahlbom A , Held C , Ljunghall S , Michaëlsson K , et al. Risk of arrhythmias in 52 755 long-distance cross-country skiers: a cohort study. Eur Heart J. 2013;34(47):3624–31. doi:.https://doi.org/10.1093/eurheartj/eht188
38 Thelle DS , Selmer R , Gjesdal K , Sakshaug S , Jugessur A , Graff-Iversen S , et al. Resting heart rate and physical activity as risk factors for lone atrial fibrillation: a prospective study of 309,540 men and women. Heart. 2013;99(23):1755–60. doi:.https://doi.org/10.1136/heartjnl-2013-303825
39 Abdulla J , Nielsen JR . Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace. 2009;11(9):1156–9. doi:.https://doi.org/10.1093/europace/eup197
40 Wilhelm M , Roten L , Tanner H , Wilhelm I , Schmid JP , Saner H . Atrial remodeling, autonomic tone, and lifetime training hours in nonelite athletes. Am J Cardiol. 2011;108(4):580–5. doi:.https://doi.org/10.1016/j.amjcard.2011.03.086
41 Fortescue EB , Shin AY , Greenes DS , Mannix RC , Agarwal S , Feldman BJ , et al. Cardiac troponin increases among runners in the Boston Marathon. Ann Emerg Med. 2007;49(2):137–43.e1. doi:.https://doi.org/10.1016/j.annemergmed.2006.09.024
42 Kirchhof P , Benussi S , Kotecha D , Ahlsson A , Atar D , Casadei B , et al.; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–962. doi:.https://doi.org/10.1093/eurheartj/ehw210
43 Kishore A , Vail A , Majid A , Dawson J , Lees KR , Tyrrell PJ , et al. Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Stroke. 2014;45(2):520–6. doi:.https://doi.org/10.1161/STROKEAHA.113.003433
44 Sanna T , Diener HC , Passman RS , Di Lazzaro V , Bernstein RA , Morillo CA , et al.; CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–86. doi:.https://doi.org/10.1056/NEJMoa1313600
45 Gladstone DJ , Spring M , Dorian P , Panzov V , Thorpe KE , Hall J , et al.; EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370(26):2467–77. doi:.https://doi.org/10.1056/NEJMoa1311376
46 Gage BF , Waterman AD , Shannon W , Boechler M , Rich MW , Radford MJ . Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–70. doi:.https://doi.org/10.1001/jama.285.22.2864
47 The SPAF III Writing Committee for the Stroke Prevention in Atrial Fibrillation Investigators. Patients with nonvalvular atrial fibrillation at low risk of stroke during treatment with aspirin: Stroke Prevention in Atrial Fibrillation III Study. JAMA. 1998;279(16):1273–7. doi:.https://doi.org/10.1001/jama.279.16.1273
48 Lip GY , Nieuwlaat R , Pisters R , Lane DA , Crijns HJ . Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–72. doi:.https://doi.org/10.1378/chest.09-1584
49 Ruff CT , Giugliano RP , Braunwald E , Hoffman EB , Deenadayalu N , Ezekowitz MD , et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–62. doi:.https://doi.org/10.1016/S0140-6736(13)62343-0
50 Hart RG , Pearce LA , Aguilar MI . Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–67. doi:.https://doi.org/10.7326/0003-4819-146-12-200706190-00007
51 Zimny M , Blum S , Ammann P , Erne P , Moschovitis G , Di Valentino M , et al. Uptake of non-vitamin K antagonist oral anti coagulants in patients with atrial fibrillation - a prospective cohort study. Swiss Med Wkly. 2017;147:w14410.
52 Stroke Prevention in Atrial Fibrillation Study. Final results. Circulation. 1991;84(2):527–39. doi:.https://doi.org/10.1161/01.CIR.84.2.527
53 Olesen JB , Lip GY , Lindhardsen J , Lane DA , Ahlehoff O , Hansen ML , et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: A net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost. 2011;106(4):739–49.
54 Själander S , Själander A , Svensson PJ , Friberg L . Atrial fibrillation patients do not benefit from acetylsalicylic acid. Europace. 2014;16(5):631–8. doi:.https://doi.org/10.1093/europace/eut333
55 Connolly S , Pogue J , Hart R , Pfeffer M , Hohnloser S , Chrolavicius S , et al., ACTIVE Writing Group of the ACTIVE Investigators. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367(9526):1903–12. doi:.https://doi.org/10.1016/S0140-6736(06)68845-4
56 Connolly SJ , Pogue J , Hart RG , Hohnloser SH , Pfeffer M , Chrolavicius S , et al., ACTIVE Investigators. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360(20):2066–78. doi:.https://doi.org/10.1056/NEJMoa0901301
57 Göldi T , Krisai P , Knecht S , Aeschbacher S , Spies F , Zeljkovic I , et al. Prevalence and Management of Atrial Thrombi in Patients With Atrial Fibrillation Before Pulmonary Vein Isolation. JACC Clin Electrophysiol. 2019;5(12):1406–14. doi:.https://doi.org/10.1016/j.jacep.2019.09.003
58 Levey AS , Stevens LA , Schmid CH , Zhang YL , Castro AF, 3rd , Feldman HI , et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi:.https://doi.org/10.7326/0003-4819-150-9-200905050-00006
59 Siontis KC , Zhang X , Eckard A , Bhave N , Schaubel DE , He K , et al. Outcomes Associated With Apixaban Use in Patients With End-Stage Kidney Disease and Atrial Fibrillation in the United States. Circulation. 2018;138(15):1519–29. doi:.https://doi.org/10.1161/CIRCULATIONAHA.118.035418
60 Steffel J , Verhamme P , Potpara TS , Albaladejo P , Antz M , Desteghe L , et al.; ESC Scientific Document Group. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–93. doi:.https://doi.org/10.1093/eurheartj/ehy136
61 Ray WA , Chung CP , Murray KT , Smalley WE , Daugherty JR , Dupont WD , et al. Association of Proton Pump Inhibitors With Reduced Risk of Warfarin-Related Serious Upper Gastrointestinal Bleeding. Gastroenterology. 2016;151(6):1105–12.e10. doi:.https://doi.org/10.1053/j.gastro.2016.08.054
62 Di Minno A , Spadarella G , Spadarella E , Tremoli E , Di Minno G . Gastrointestinal bleeding in patients receiving oral anticoagulation: Current treatment and pharmacological perspectives. Thromb Res. 2015;136(6):1074–81. doi:.https://doi.org/10.1016/j.thromres.2015.10.016
63 Chan EW , Lau WC , Leung WK , Mok MT , He Y , Tong TS , et al. Prevention of Dabigatran-Related Gastrointestinal Bleeding With Gastroprotective Agents: A Population-Based Study. Gastroenterology. 2015;149(3):586–95.e3. doi:.https://doi.org/10.1053/j.gastro.2015.05.002
64 Schreck DM , Rivera AR , Tricarico VJ . Emergency management of atrial fibrillation and flutter: intravenous diltiazem versus intravenous digoxin. Ann Emerg Med. 1997;29(1):135–40. doi:.https://doi.org/10.1016/S0196-0644(97)70319-6
65 Tisdale JE , Padhi ID , Goldberg AD , Silverman NA , Webb CR , Higgins RS , et al. A randomized, double-blind comparison of intravenous diltiazem and digoxin for atrial fibrillation after coronary artery bypass surgery. Am Heart J. 1998;135(5 Pt 1):739–47. doi:.https://doi.org/10.1016/S0002-8703(98)70031-6
66 Scheuermeyer FX , Grafstein E , Stenstrom R , Christenson J , Heslop C , Heilbron B , et al. Safety and efficiency of calcium channel blockers versus beta-blockers for rate control in patients with atrial fibrillation and no acute underlying medical illness. Acad Emerg Med. 2013;20(3):222–30. doi:.https://doi.org/10.1111/acem.12091
67 Kühlkamp V , Bosch R , Mewis C , Seipel L . Use of beta-blockers in atrial fibrillation. Am J Cardiovasc Drugs. 2002;2(1):37–42. doi:.https://doi.org/10.2165/00129784-200202010-00005
68 Kotecha D , Holmes J , Krum H , Altman DG , Manzano L , Cleland JG , et al.; Beta-Blockers in Heart Failure Collaborative Group. Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet. 2014;384(9961):2235–43. doi:.https://doi.org/10.1016/S0140-6736(14)61373-8
69 Kotecha D , Manzano L , Krum H , Rosano G , Holmes J , Altman DG , et al.; Beta-Blockers in Heart Failure Collaborative Group. Effect of age and sex on efficacy and tolerability of β blockers in patients with heart failure with reduced ejection fraction: individual patient data meta-analysis. BMJ. 2016;353:i1855. doi:.https://doi.org/10.1136/bmj.i1855
70 Nikolaidou T , Channer KS . Chronic atrial fibrillation: a systematic review of medical heart rate control management. Postgrad Med J. 2009;85(1004):303–12. doi:.https://doi.org/10.1136/pgmj.2008.068908
71 Goldstein RE , Boccuzzi SJ , Cruess D , Nattel S . Diltiazem increases late-onset congestive heart failure in postinfarction patients with early reduction in ejection fraction. The Adverse Experience Committee; and the Multicenter Diltiazem Postinfarction Research Group. Circulation. 1991;83(1):52–60. doi:.https://doi.org/10.1161/01.CIR.83.1.52
72 Goldberger ZD , Alexander GC . Digitalis use in contemporary clinical practice: refitting the foxglove. JAMA Intern Med. 2014;174(1):151–4. doi:.https://doi.org/10.1001/jamainternmed.2013.10432
73 Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336(8):525–33. doi:.https://doi.org/10.1056/NEJM199702203360801
74 Lim KT , Davis MJ , Powell A , Arnolda L , Moulden K , Bulsara M , et al. Ablate and pace strategy for atrial fibrillation: long-term outcome of AIRCRAFT trial. Europace. 2007;9(7):498–505. doi:.https://doi.org/10.1093/europace/eum091
75 Brignole M , Pokushalov E , Pentimalli F , Palmisano P , Chieffo E , Occhetta E , et al.; APAF-CRT Investigators. A randomized controlled trial of atrioventricular junction ablation and cardiac resynchronization therapy in patients with permanent atrial fibrillation and narrow QRS. Eur Heart J. 2018;39(45):3999–4008. doi:.https://doi.org/10.1093/eurheartj/ehy555
76 Arenja N , Knecht S , Schaer B , Reichlin T , Pavlovic N , Osswald S , et al. Comparison of different approaches to atrioventricular junction ablation and pacemaker implantation in patients with atrial fibrillation. Pacing Clin Electrophysiol. 2014;37(12):1686–93. doi:.https://doi.org/10.1111/pace.12481
77 Roy D , Talajic M , Dorian P , Connolly S , Eisenberg MJ , Green M , et al.; Canadian Trial of Atrial Fibrillation Investigators. Amiodarone to prevent recurrence of atrial fibrillation. N Engl J Med. 2000;342(13):913–20. doi:.https://doi.org/10.1056/NEJM200003303421302
78 Singh BN , Connolly SJ , Crijns HJ , Roy D , Kowey PR , Capucci A , et al.; EURIDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med. 2007;357(10):987–99. doi:.https://doi.org/10.1056/NEJMoa054686
79 Singh BN , Singh SN , Reda DJ , Tang XC , Lopez B , Harris CL , et al.; Sotalol Amiodarone Atrial Fibrillation Efficacy Trial (SAFE-T) Investigators. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005;352(18):1861–72. doi:.https://doi.org/10.1056/NEJMoa041705
80 Letelier LM , Udol K , Ena J , Weaver B , Guyatt GH . Effectiveness of amiodarone for conversion of atrial fibrillation to sinus rhythm: a meta-analysis. Arch Intern Med. 2003;163(7):777–85. doi:.https://doi.org/10.1001/archinte.163.7.777
81 Chevalier P , Durand-Dubief A , Burri H , Cucherat M , Kirkorian G , Touboul P . Amiodarone versus placebo and class Ic drugs for cardioversion of recent-onset atrial fibrillation: a meta-analysis. J Am Coll Cardiol. 2003;41(2):255–62. doi:.https://doi.org/10.1016/S0735-1097(02)02705-5
82 Goldschlager N , Epstein AE , Naccarelli GV , Olshansky B , Singh B , Collard HR , et al.; Practice Guidelines Sub-committee, North American Society of Pacing and Electrophysiology (HRS). A practical guide for clinicians who treat patients with amiodarone: 2007. Heart Rhythm. 2007;4(9):1250–9. doi:.https://doi.org/10.1016/j.hrthm.2007.07.020
83 Vorperian VR , Havighurst TC , Miller S , January CT . Adverse effects of low dose amiodarone: a meta-analysis. J Am Coll Cardiol. 1997;30(3):791–8. doi:.https://doi.org/10.1016/S0735-1097(97)00220-9
84 Crijns HJ , Weijs B , Fairley AM , Lewalter T , Maggioni AP , Martín A , et al. Contemporary real life cardioversion of atrial fibrillation: Results from the multinational RHYTHM-AF study. Int J Cardiol. 2014;172(3):588–94. doi:.https://doi.org/10.1016/j.ijcard.2014.01.099
85 del Arco C , Martín A , Laguna P , Gargantilla P ; Investigators in the Spanish Atrial Fibrillation in Emergency Medicine Study Group (GEFAUR). Analysis of current management of atrial fibrillation in the acute setting: GEFAUR-1 study. Ann Emerg Med. 2005;46(5):424–30. doi:.https://doi.org/10.1016/j.annemergmed.2005.03.002
86 Al-Khatib SM , Allen LaPointe NM , Chatterjee R , Crowley MJ , Dupre ME , Kong DF , et al. Rate- and rhythm-control therapies in patients with atrial fibrillation: a systematic review. Ann Intern Med. 2014;160(11):760–73. doi:.https://doi.org/10.7326/M13-1467
87 Kirchhof P , Andresen D , Bosch R , Borggrefe M , Meinertz T , Parade U , et al. Short-term versus long-term antiarrhythmic drug treatment after cardioversion of atrial fibrillation (Flec-SL): a prospective, randomised, open-label, blinded endpoint assessment trial. Lancet. 2012;380(9838):238–46. doi:.https://doi.org/10.1016/S0140-6736(12)60570-4
88 Alboni P , Botto GL , Baldi N , Luzi M , Russo V , Gianfranchi L , et al. Outpatient treatment of recent-onset atrial fibrillation with the “pill-in-the-pocket” approach. N Engl J Med. 2004;351(23):2384–91. doi:.https://doi.org/10.1056/NEJMoa041233
89 Airaksinen KE , Grönberg T , Nuotio I , Nikkinen M , Ylitalo A , Biancari F , et al. Thromboembolic complications after cardioversion of acute atrial fibrillation: the FinCV (Finnish CardioVersion) study. J Am Coll Cardiol. 2013;62(13):1187–92. doi:.https://doi.org/10.1016/j.jacc.2013.04.089
90 Hansen ML , Jepsen RM , Olesen JB , Ruwald MH , Karasoy D , Gislason GH , et al. Thromboembolic risk in 16 274 atrial fibrillation patients undergoing direct current cardioversion with and without oral anticoagulant therapy. Europace. 2015;17(1):18–23. doi:.https://doi.org/10.1093/europace/euu189
91 Weigner MJ , Caulfield TA , Danias PG , Silverman DI , Manning WJ . Risk for clinical thromboembolism associated with conversion to sinus rhythm in patients with atrial fibrillation lasting less than 48 hours. Ann Intern Med. 1997;126(8):615–20. doi:.https://doi.org/10.7326/0003-4819-126-8-199704150-00005
92 Mont L , Bisbal F , Hernández-Madrid A , Pérez-Castellano N , Viñolas X , Arenal A , et al.; SARA investigators. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study). Eur Heart J. 2014;35(8):501–7. doi:.https://doi.org/10.1093/eurheartj/eht457
93 Wazni OM , Marrouche NF , Martin DO , Verma A , Bhargava M , Saliba W , et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293(21):2634–40. doi:.https://doi.org/10.1001/jama.293.21.2634
94 Oral H , Pappone C , Chugh A , Good E , Bogun F , Pelosi F, Jr , et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354(9):934–41. doi:.https://doi.org/10.1056/NEJMoa050955
95 Stabile G , Bertaglia E , Senatore G , De Simone A , Zoppo F , Donnici G , et al. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation For The Cure Of Atrial Fibrillation Study). Eur Heart J. 2006;27(2):216–21. doi:.https://doi.org/10.1093/eurheartj/ehi583
96 Nyong J , Amit G , Adler AJ , Owolabi OO , Perel P , Prieto-Merino D , et al. Efficacy and safety of ablation for people with non-paroxysmal atrial fibrillation. Cochrane Database Syst Rev. 2016;11:CD012088. doi:.https://doi.org/10.1002/14651858.CD012088.pub2
97 Calkins H , Reynolds MR , Spector P , Sondhi M , Xu Y , Martin A , et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2(4):349–61. doi:.https://doi.org/10.1161/CIRCEP.108.824789
98 Duytschaever M , De Pooter J , Demolder A , El Haddad M , Phlips T , Strisciuglio T , et al. Long-term impact of catheter ablation on arrhythmia burden in low-risk patients with paroxysmal atrial fibrillation: The CLOSE to CURE study. Heart Rhythm. 2019;S1547-5271(19)30997-X. doi:.https://doi.org/10.1016/j.hrthm.2019.11.004
99 Kühne M , Suter Y , Altmann D , Ammann P , Schaer B , Osswald S , et al. Cryoballoon versus radiofrequency catheter ablation of paroxysmal atrial fibrillation: biomarkers of myocardial injury, recurrence rates, and pulmonary vein reconnection patterns. Heart Rhythm. 2010;7(12):1770–6. doi:.https://doi.org/10.1016/j.hrthm.2010.08.028
100 Kühne M , Knecht S , Altmann D , Ammann P , Schaer B , Osswald S , et al. Validation of a novel spiral mapping catheter for real-time recordings from the pulmonary veins during cryoballoon ablation of atrial fibrillation. Heart Rhythm. 2013;10(2):241–6. doi:.https://doi.org/10.1016/j.hrthm.2012.10.009
101 Pavlović N , Sticherling C , Knecht S , Reichlin T , Mühl A , Schaer B , et al. One-year follow-up after irrigated multi-electrode radiofrequency ablation of persistent atrial fibrillation. Europace. 2016;18(1):85–91. doi:.https://doi.org/10.1093/europace/euv020
102 Dagres N , Hindricks G , Kottkamp H , Sommer P , Gaspar T , Bode K , et al. Complications of atrial fibrillation ablation in a high-volume center in 1,000 procedures: still cause for concern? J Cardiovasc Electrophysiol. 2009;20(9):1014–9. doi:.https://doi.org/10.1111/j.1540-8167.2009.01493.x
103 Deshmukh A , Patel NJ , Pant S , Shah N , Chothani A , Mehta K , et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93 801 procedures. Circulation. 2013;128(19):2104–12. doi:.https://doi.org/10.1161/CIRCULATIONAHA.113.003862
104 Zuern CS , Kilias A , Berlitz P , Seizer P , Gramlich M , Müller K , et al. Anticoagulation after catheter ablation of atrial fibrillation guided by implantable cardiac monitors. Pacing Clin Electrophysiol. 2015;38(6):688–93. doi:.https://doi.org/10.1111/pace.12625
105 Middlekauff HR , Stevenson WG , Stevenson LW . Prognostic significance of atrial fibrillation in advanced heart failure. A study of 390 patients. Circulation. 1991;84(1):40–8. doi:.https://doi.org/10.1161/01.CIR.84.1.40
106 Mahoney P , Kimmel S , DeNofrio D , Wahl P , Loh E . Prognostic significance of atrial fibrillation in patients at a tertiary medical center referred for heart transplantation because of severe heart failure. Am J Cardiol. 1999;83(11):1544–7. doi:.https://doi.org/10.1016/S0002-9149(99)00144-7
107 Senni M , Tribouilloy CM , Rodeheffer RJ , Jacobsen SJ , Evans JM , Bailey KR , et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98(21):2282–9. doi:.https://doi.org/10.1161/01.CIR.98.21.2282
108 Maisel WH , Stevenson LW . Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91(6):2–8. doi:.https://doi.org/10.1016/S0002-9149(02)03373-8
109 Vermes E , Tardif JC , Bourassa MG , Racine N , Levesque S , White M , et al. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the Studies Of Left Ventricular Dysfunction (SOLVD) trials. Circulation. 2003;107(23):2926–31. doi:.https://doi.org/10.1161/01.CIR.0000072793.81076.D4
110 Ducharme A , Swedberg K , Pfeffer MA , Cohen-Solal A , Granger CB , Maggioni AP , et al.; CHARM Investigators. Prevention of atrial fibrillation in patients with symptomatic chronic heart failure by candesartan in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Am Heart J. 2006;152(1):86–92. doi:.https://doi.org/10.1016/j.ahj.2005.06.036
111 Nasr IA , Bouzamondo A , Hulot JS , Dubourg O , Le Heuzey JY , Lechat P . Prevention of atrial fibrillation onset by beta-blocker treatment in heart failure: a meta-analysis. Eur Heart J. 2007;28(4):457–62. doi:.https://doi.org/10.1093/eurheartj/ehl484
112 Swedberg K , Zannad F , McMurray JJ , Krum H , van Veldhuisen DJ , Shi H , et al.; EMPHASIS-HF Study Investigators. Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J Am Coll Cardiol. 2012;59(18):1598–603. doi:.https://doi.org/10.1016/j.jacc.2011.11.063
113 Van Gelder IC , Crijns HJ , Blanksma PK , Landsman ML , Posma JL , Van Den Berg MP , et al. Time course of hemodynamic changes and improvement of exercise tolerance after cardioversion of chronic atrial fibrillation unassociated with cardiac valve disease. Am J Cardiol. 1993;72(7):560–6. doi:.https://doi.org/10.1016/0002-9149(93)90352-D
114 Zipes DP . Atrial fibrillation. A tachycardia-induced atrial cardiomyopathy. Circulation. 1997;95(3):562–4. doi:.https://doi.org/10.1161/01.CIR.95.3.562
115 Wilson JR , Douglas P , Hickey WF , Lanoce V , Ferraro N , Muhammad A , et al. Experimental congestive heart failure produced by rapid ventricular pacing in the dog: cardiac effects. Circulation. 1987;75(4):857–67. doi:.https://doi.org/10.1161/01.CIR.75.4.857
116 Marrouche NF , Brachmann J , Andresen D , Siebels J , Boersma L , Jordaens L , et al.; CASTLE-AF Investigators. Catheter Ablation for Atrial Fibrillation with Heart Failure. N Engl J Med. 2018;378(5):417–27. doi:.https://doi.org/10.1056/NEJMoa1707855
117 Jones DG , Haldar SK , Hussain W , Sharma R , Francis DP , Rahman-Haley SL , et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. 2013;61(18):1894–903. doi:.https://doi.org/10.1016/j.jacc.2013.01.069
118 Anselmino M , Matta M , D’Ascenzo F , Bunch TJ , Schilling RJ , Hunter RJ , et al. Catheter ablation of atrial fibrillation in patients with left ventricular systolic dysfunction: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2014;7(6):1011–8. doi:.https://doi.org/10.1161/CIRCEP.114.001938
119 Khan MN , Jaïs P , Cummings J , Di Biase L , Sanders P , Martin DO , et al.; PABA-CHF Investigators. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359(17):1778–85. doi:.https://doi.org/10.1056/NEJMoa0708234
120 Kraft E , Marti M , Werner S , Sommer H . Cost of dementia in Switzerland. Swiss Med Wkly. 2010;140:w13093.
121 Kwok CS , Loke YK , Hale R , Potter JF , Myint PK . Atrial fibrillation and incidence of dementia: a systematic review and meta-analysis. Neurology. 2011;76(10):914–22. doi:.https://doi.org/10.1212/WNL.0b013e31820f2e38
122 Thacker EL , McKnight B , Psaty BM , Longstreth WT, Jr , Sitlani CM , Dublin S , et al. Atrial fibrillation and cognitive decline: a longitudinal cohort study. Neurology. 2013;81(2):119–25. doi:.https://doi.org/10.1212/WNL.0b013e31829a33d1
123 Gottesman RF , Albert MS , Alonso A , Coker LH , Coresh J , Davis SM , et al. Associations Between Midlife Vascular Risk Factors and 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol. 2017;74(10):1246–54. doi:.https://doi.org/10.1001/jamaneurol.2017.1658
124 Vermeer SE , Koudstaal PJ , Oudkerk M , Hofman A , Breteler MM . Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2002;33(1):21–5. doi:.https://doi.org/10.1161/hs0102.101629
125 Vermeer SE , Prins ND , den Heijer T , Hofman A , Koudstaal PJ , Breteler MM . Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215–22. doi:.https://doi.org/10.1056/NEJMoa022066
126 Conen D , Rodondi N , Mueller A , Beer J , Auricchio A , Ammann P , et al. Design of the Swiss Atrial Fibrillation Cohort Study (Swiss-AF): structural brain damage and cognitive decline among patients with atrial fibrillation. Swiss Med Wkly. 2017;147:w14467. doi:.https://doi.org/10.4414/smw.2017.14467
127 Conen D , Rodondi N , Müller A , Beer JH , Ammann P , Moschovitis G , et al.; Swiss-AF Study Investigators. Relationships of Overt and Silent Brain Lesions With Cognitive Function in Patients With Atrial Fibrillation. J Am Coll Cardiol. 2019;73(9):989–99. doi:.https://doi.org/10.1016/j.jacc.2018.12.039
DC has a McMaster University Department of Medicine Mid-Career Research Award; his work is supported by the Hamilton Health Sciences RFA Strategic Initiative Program.
DC has received consultant/speaker fees from Servier, Canada. MK has received grants from Bayer, Pfizer-BMS, the Swiss National Science Foundation, and the Swiss Heart Foundation; and lecture or consulting fees from Daiichi-Sankyo, Boehringer Ingelheim, Bayer, Pfizer-BMS, AstraZeneca, Sanofi, Novartis, Merck Sharp & Dohme, Medtronic, Boston Scientific, St. Jude Medical, Biotronik, Sorin, Zoll, Biosense Webster, and Abbott. CSZ has received speaker fees from Vifor Pharma and Novartis. The remaining authors have no relationships with the industry to disclose.