Roadmap for the treatment of heart failure patients after hospital discharge: an interdisciplinary consensus paper
DOI: https://doi.org/10.4414/smw.2020.20159
Christian
Muellera, Klaus
Ballyb, Marc
Buserc, Andreas J.
Flammerd, Jean-Michel
Gaspoze, François
Machf, Giorgio
Moschovitisg, Matthias
Paulh, Andreas
Zellerb, Ellen
Heitlingeri, Bianca
Fayj, Thomas
Rosemannk
aUniversitäres Herzzentrum und Cardiovascular Research Institute Basel (CRIB), Universitätsspital Basel, Switzerland
bUniversitäres Zentrum für Hausarztmedizin beider Basel, Universität Basel, Switzerland
cKantonsspital St. Gallen, Switzerland
dUniversitäres Herzzentrum, UniversitätsSpital Zürich, Switzerland
eClinique des Grangettes, Chêne-Bougeries, Switzerland
fHôpitaux Universitaires de Genève, Switzerland
gEnte Ospedaliero Cantonale, Ospedale Regionale di Lugano, Switzerland
hKantonsspital Luzern, Switzerland
iH+O Communications Ltd., Küssnacht am Rigi, Switzerland
jNovartis Pharma Schweiz AG, Rotkreuz, Switzerland
kInstitut für Hausarztmedizin, Universität Zürich, Switzerland
Summary
The transition period from the hospital to the outpatient setting is a critical phase when managing heart failure. A well-structured transition is paramount and helps to ensure a tight follow-up schedule for the heart failure patient, thereby improving treatment outcomes. This article aims to provide guidance for the first three follow-up visits after hospital discharge, with a focus on monitoring heart failure patients and up-titrating their medication in primary care.
Introduction
Heart failure (HF) is a serious chronic condition associated with periodic exacerbations leading to frequent hospitalisation [1]. Indeed, it is the leading cause of hospitalisation in patients over 65 years of age [1–3]. HF patients are discharged from the hospital in a vulnerable phase characterised by high mortality and morbidity. In a Swiss trial, approximately 20% of HF patients were readmitted to the hospital within the first 30 days of discharge [4]. Similarly, data gathered from two other studies conducted in Switzerland showed that at 3 months mortality was 18% and the rate of rehospitalisation was 26% [3, 5].
Several strategies have been developed to reduce mortality and morbidity during the vulnerable post-discharge period, including coordinated discharge planning and the development of a well-structured follow-up treatment plan [1]. This seems to be an area where cardiology could learn a lot from oncology with its predefined treatment schedules aiming to best balance treatment efficacy and tolerability. An integrative and collaborative patient care approach following hospital discharge has been shown to reduce the mortality risk by approximately 50% (from 15.5% to 7.2%) [6]. In particular, prescheduled follow-up visits, adherence to therapy and up-titration of HF medication are important aspects of reducing the mortality risk during the transition phase after hospital discharge [1]. Consequently, according to European Society of Cardiology (ESC) Guidelines, patients with chronic HF should be followed up in a multidisciplinary environment (evidence level IA) in which general practitioners (GPs) and cardiologists play a key role [1, 7, 8].
The aim of this consensus paper is to set clear treatment targets for the first three follow-up visits, and to provide GPs and cardiologists with recommendations for an optimal patient follow-up during the transition phase after discharge of HF patients hospitalised for acute decompensation in Switzerland. We address the aspect of feasibility, highlighting current hurdles for implementation, and also concepts on how to overcome them. In particular, these recommendations also provide guidance on the optimal monitoring of HF patients when up-titrating HF medication, which may be challenging owing to its tolerability, concomitant comorbidities and polypharmacy. The recommendations for monitoring apply universally to all HF patients, whereas the recommendations for up-titration of disease-modifying drugs are specific for HF with reduced ejection fraction (HFrEF)
The benefit of a well-structured transition phase
There is a growing body of evidence suggesting that HF patients are particularly vulnerable to disruptions in care during the transition phase [7, 9, 10]. For example, it has been shown that readmission rates can be reduced if the first outpatient follow-up visit is scheduled prior to hospital discharge [1]. In line with this study, the 2016 ESC guidelines for acute and chronic HF propose a care plan ensuring a well-structured transition phase to improve treatment outcomes [1].
The criteria for a well-structured transition phase are detailed in figure 1. The discharge letter ideally provides information on the clinical course including body weight, blood pressure, potassium and renal function prior to discharge, the therapy initiated in hospital and – importantly – recommendations on the follow-up schedule and up-titration strategy [1, 11]. The HF diary developed by the Schweizerische Herzstiftung should be given to the patient at discharge or at the latest at the first outpatient visit, as it contributes to a well-structured transition from hospital to primary care [12]. In the HF patient’s daily diary, “My heart diary”, provided by the Swiss Heart Failure foundation, the patient can (or should) record body weight, blood pressure, heart rate, and other symptom-related observations [13]. It is suggested that patients bring their HF diary to each follow-up visit with their GP or cardiologist. Another cornerstone of the HF diary is a detailed medication plan. This plan should list the prescribed drugs, including their active substances and brand names, as well as provide instructions on how and when the drugs should be taken. The GP and the cardiologist should be notified whether the patient has been informed about the prognosis of the disease while still at hospital or if the patient has expressed wishes regarding life-prolonging measures and palliative care [14]. In the case of patients with severe HF, the patient, the cardiologist and the GP should be involved in the decision to transition to palliative care. However, it is challenging to select the time-point for this transition as symptoms and quality of life keep changing for HF patients. Thus, it is recommended to regularly assess the patients and analyse their palliative scores, which provide an objective assessment of the patient’s condition [1].
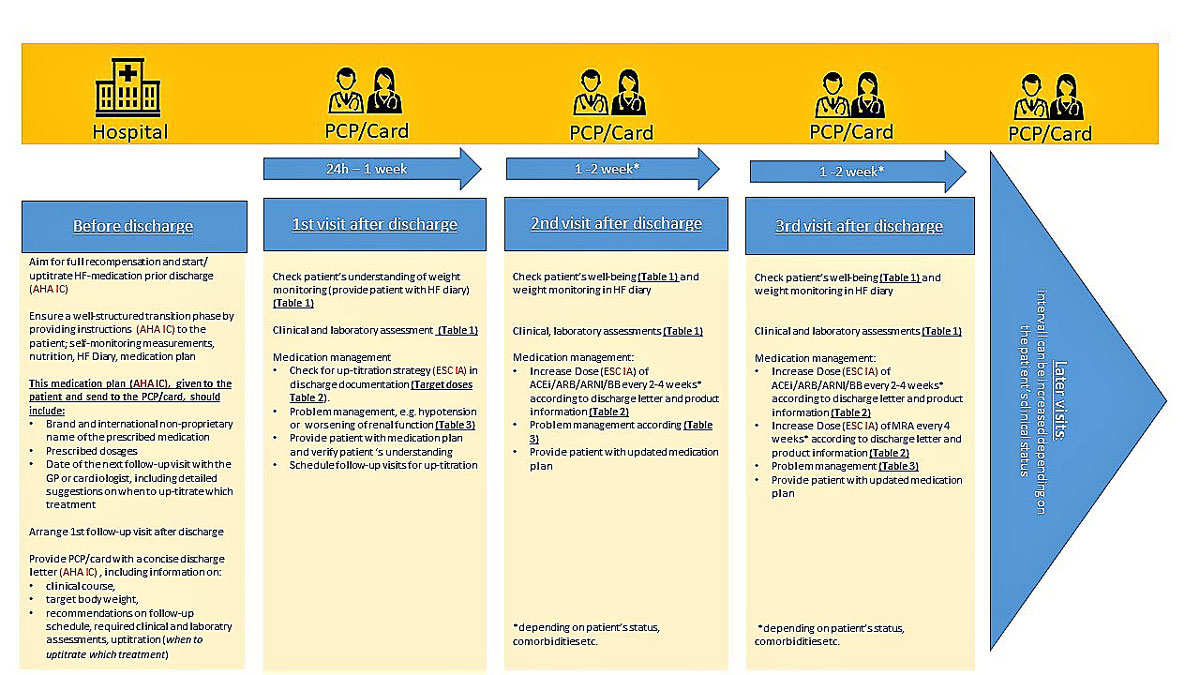
Figure 1
Up-titration roadmap for patients with HFrEF. ACEI = angiotensin-converting-enzyme inhibitor; AE = adverse event; AHA = American Heart Association; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor-neprilysin inhibitor; BB = beta blockers; ESC = European Society of Cardiology; GP = General Practitioner; HFrEF = heart failure (HF) with reduced ejection fraction; MRA = mineralocorticoid receptor antagonist; PCP = primary care physician. Based on the scientific content of the publications from Hickey 2016 [45] with data from Ponikowski et al. 2016 [1], Yancy 2013/2017 [47, 48] and the Canadian Cardiovascular Society 2017 [30].
To ensure a timely follow-up schedule, a collaborative care network with HF specialists, cardiologists and GPs should be established. Consistently with the 2016 ESC guidelines on HF, the present author group recommends that the first follow-up visit take place within the first 7–10 days after hospital discharge [1]. Patients with severe HF, however, should see a GP or cardiologist within 1–3 days [15–17]. Recommendations for monitoring HF patients beyond the first follow-up visit are currently limited and depend on the individual patient’s needs. This author group further suggests that the second visit take place 7–10 days after the first visit. The interval between follow-up visits can be increased for the third and later follow-up visits depending on the patient’s clinical status. A referral to a cardiologist, on the other hand, is recommended every 3 months for patients with New York Heart Association (NYHA) stage III–IV HF [18, 19].
Together these measures ensure a close collaboration between GPs and cardiologists, thus enabling HF patients to receive the right support and care after hospital discharge. Furthermore, these measures are a prerequisite for optimal follow-up and up-titration strategies and, therefore, contribute to reducing the risk of death or rehospitalisation [1].
Feasibility
Several hurdles for implementation exist. First, most GPs/cardiologists have busy schedules, making it challenging to add on additional patients at rather short notice. Second, GPs/cardiologists may not have all relevant medical information of the recent hospitalisation available early on after discharge owing to delays in the finalisation of the discharge letter or lack of details in the discharge letter (e.g., missing details on body weight, blood pressure, potassium and renal function). Third, some patients may not have a GP/cardiologist at the time of hospitalisation. Fourth, frailty may limit the ability of the patient to visit the office of the GP/cardiologist.
For successful implementation of the transition programme, each of these hurdles must be addressed and ultimately overcome. To improve practical feasibility, the implementation of cardiology and/or HF networks that share patient information and use common electronic health records, joint online booking systems for GP/cardiology slots (similar to those used extensively to book hotel rooms worldwide), such as docbox, nurse-coordinated HF management programmes [20, 21], and tele-monitoring programmes, such as the Swiss Care4Cardio, will all have important roles [22, 23].
General assessments during follow-up visits
Recommended assessments to be performed during the first three follow-up visits with the GP or the cardiologist are summarised in table 1 [1]. These include the evaluation of risk factors that increase the risk of rehospitalisation due to HF. Monitoring of physical symptoms (rales, oedema, body weight changes), laboratory parameters (B-type natriuretic peptide [BNP] or N-terminal pro b-type natriuretic peptide [NT-pro-BNP], electrolytes, haemoglobin, renal function), and drug-related adverse events (hypotension, hyperkalaemia, worsening renal function) are likewise of particular relevance [1, 26].
Table 1 Assessments in primary care for monitoring HF patient status.
| Assessments to monitor patient status [16, 24] |
Patient well-being |
Physical and social activities, independent care (e.g., ability to climb stairs, carry groceries, jog or run [as if to catch a bus], do housework or gardening, walk a certain distance on level ground, have sex, dress, shower/bathe) |
| Symptoms such as shortness of breath, swelling in your feet, ankles, legs, fatigue |
| Patient education / self-care |
The patient should be aware of the importance of self-monitoring and documenting their body weight on a daily basis. |
| Life-style: physical activity |
Knowledge about “red flags” and what to do if any of the following warning signs occur:
‒ increase in body weight
‒ dyspnoea
‒ fainting
‒ worsening symptoms |
| Palliative care: Patients with late-stage HF should be informed about palliative care options |
| Therapy adherence |
Verify medication plan and adherence to prescribed therapy |
| Verify whether the patient would benefit from additional measures, such as a dosette boxe or home-care support |
| Assessments to monitor HF status [16, 19, 25] |
Cardiac function |
Heart rate and heart rhythm |
| Laboratory assessments |
Kidney function (eGFR and SCr) |
| Serum electrolytes (potassium) |
| Clinical assessments |
Body weight |
| Jugular venous pressure, crackling in the lungs (these provide valuable insights for adjusting diuretic therapy) |
| Peripheral oedema |
| Blood pressure (to assess potential orthostatic hypotension) |
| Palpitations |
| Volume status |
Patient education
The follow-up visits should also be used to educate the HF patients on the importance of self-monitoring body weight and other signs /symptoms of deterioration. Besides the “heart failure diary”, patients may use apps, such “life with heart failure” or the “electronic heart failure diary”, for self-monitoring [27]. This authors group considers patient education and empowerment an import pillar of HF management. In addition, patients need to learn how to react to imminent decompensation (calling their GP, self-adjusting the diuretic dose).
Medication management after hospital discharge
The importance of up-titration after hospital discharge
Treatment of HF with angiotensin converting-enzyme inhibitors (ACEIs), or angiotensin II receptor blockers (ARBs) if the ACEI is not tolerated, angiotensin receptor-neprilysin inhibitor (ARNI), beta-blockers and mineralocorticoid receptor antagonists (MRAs) form the basis of the management of patients with HFrEF (fig. 2). Adjustment and up-titration of these therapeutic agents are critical for successful HF management. Based on pivotal trials, the 2016 ESC guidelines on HF recommend tight follow-up and up-titration schedules until the highest tolerated dose is achieved [1]. However, only a minority of patients enrolled in the ESC Heart Failure Long-Term Registry received the target dose of an ACEI (29% of patients) or beta-blocker (18% of patients) despite their positive impact [28]. Conversely, a careful reduction of loop diuretics may be feasible in the majority of stable chronic HF patients without signs of volume overload [29].
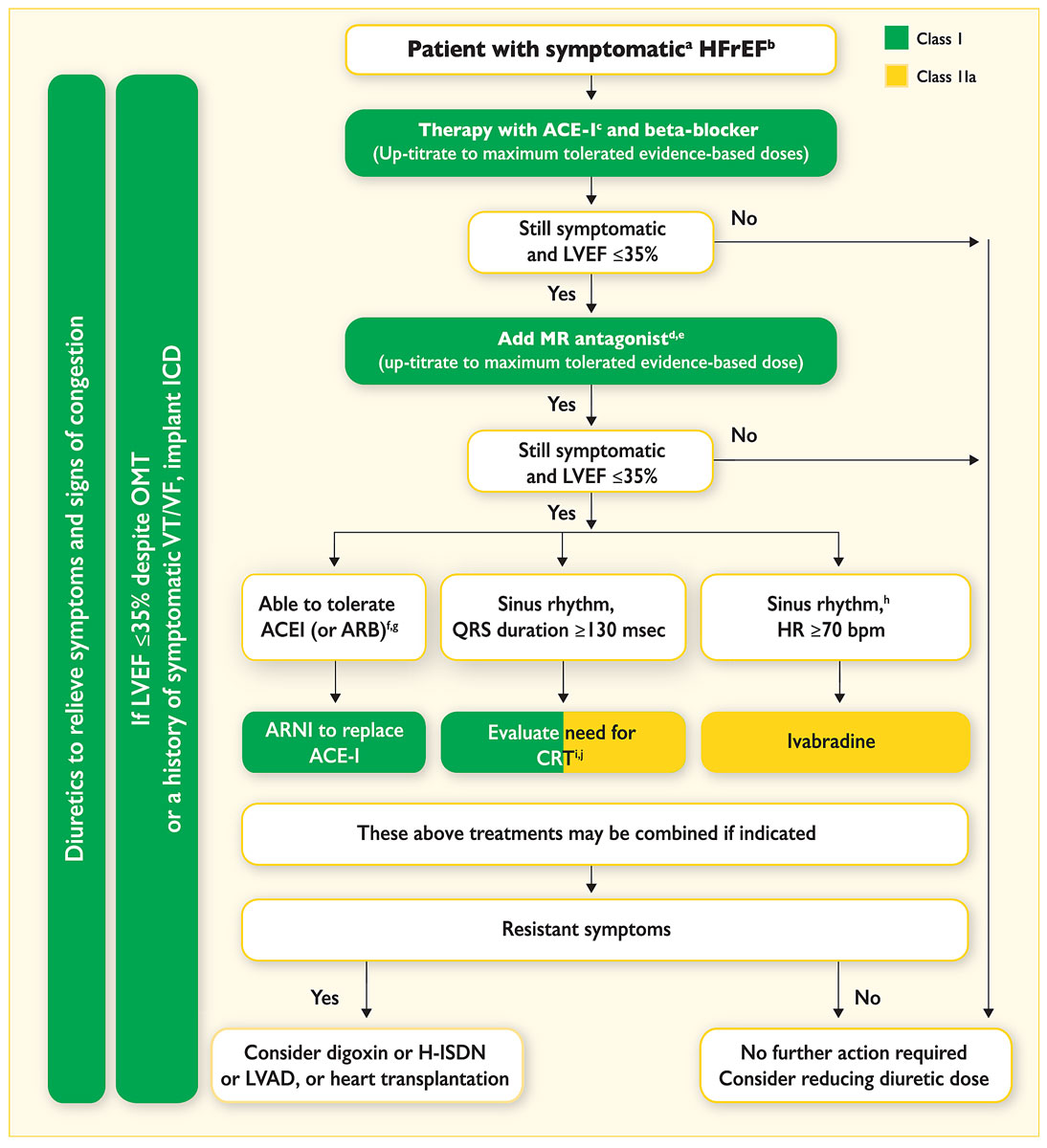
Figure 2
Treatment algorithms of symptomatic HFrEF patients [
1
].
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; ARNI = angiotensin receptor neprilysin inhibitor; CRT = cardiac resynchronisation therapy; HFrEF = heart failure with reduced ejection fraction; H-ISDN = hydralazine and isosorbide dinitrate; HR = heart rate; ICD = implantable cardioverter defibrillator; LVAD = left ventricular assist device; LVEF = left ventricular ejection fraction; MR = mineralocorticoid receptor; OMT = optimal medical therapy; VF = ventricular fibrillation; VT = ventricular tachycardia.
a Symptomatic = NYHA Class II-IV. b HFrEF = LVEF <40%. c If ACEI not tolerated / contraindicated, use ARB. d If MR antagonist not tolerated / contraindicated, use ARB. e With a hospital admission for HF within the last 6 months or with elevated natriuretic peptides (BNP >250 pg/ml or NTproBNP >500 pg/ml in men and 750 pg/ml in women). f With an elevated plasma natriuretic peptide level (BNP ≥150 pg/ml or plasma NT-proBNP ≥600 pg/ml, or if HF hospitalisation within recent 12 months plasma BNP ≥100 pg/ml or plasma NT-proBNP ≥400 pg/ml). g In doses equivalent to enalapril 10 mg b.i.d. h With a hospital admission for HF within the previous year. I CRT is recommended if QRS ≥130 msec and LBBB (in sinus rhythm). j CRT should/may be considered if QRS ≥130 msec with non-LBBB (in a sinus rhythm) or for patients in AF provided a strategy to ensure bi-ventricular capture in place (individualised decision). Adapted from Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al.; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(27):2129–200 [1].
Guidance on up-titrating heart failure medication
Table 2 provides both GPs and cardiologists with a checklist for initiating and up-titrating HF medication. An overview is depicted in figure 1. This up-titration strategy should be followed in all HFrEF patients as long as the therapy is tolerated. The up-titration strategy should be adjusted in the case of adverse events such as hypotension, hyperkalaemia or worsening renal function [1, 30]. Recommendations for adjusting HF medication in HFrEF patients experiencing such adverse events are summarised in table 3.
Table 2 Target doses and contraindications of common therapies for HFrEF patients, according to the Swiss Summary of Product Characteristics.
|
Class
|
Class contraindications
|
Drug
|
Target Dose
|
Up-titration interval [30] |
Specific contraindications
|
| ACEI |
Hypersensitivity (hereditary angio-oedema, or angio-oedema in the past during therapy with ACEI/ARB), combination with aliskirene in DM II/renal insufficiency, pregnancy/breastfeeding |
Enalapril [31] |
10–20 mg b.i.d. |
2–4 weeks |
– |
| Lisinopril [32] |
5–20 mg o.d. |
| Perindopril[29] |
2.5–5.0 mg o.d. |
Dialysis and haemofiltration |
| Ramipril [33] |
10 mg o.d. |
Dialysis and hemofiltration, uni- or bilateral renal stenosis, creatinine clearance <20 ml/min |
| ARB |
Candesartan [34] |
32 mg o.d. |
Severe hepatic impairment and/or cholestasis |
| Losartan [35] |
150 mg o.d. |
| Valsartan [36] |
160 mg b.i.d. |
Creatinine clearance <10 ml/min |
| ARNI |
Sacubitril/Valsartan [37] |
200 mg b.i.d. |
eGFR <10ml/min |
| BB |
Hypersensitivity, AV-block 2nd/3rd degree; sick sinus syndrome, sinoatrial block, symptomatic bradycardia (<60 beats/min) before initiation; symptomatic hypotension (<100 mm Hg) before initiation, severe pAVK / Raynaud’s syndrome, severe bronchial asthma, untreated phaeochromocytoma, metabolic acidosis |
Bisoprolol† [38] |
10 mg o.d. |
Hepatic insufficiency/impairment, acute decompensated heart failure, cardiogenic shock, pregnancy/breastfeeding, bradycardia (<50 beats/minutes)
Stable dose of diuretics, digoxin, ACEi, ARB for at least 2 weeks before initiating of nebivolol |
| Carvedilol† [39] |
25–100 mg‡ b.i.d. |
| Metoprolol succinate† [40] |
200 mg o.d. |
| Nebivolol† [41] |
10 mg o.d. |
| MRA*
|
Hypersensitivity, hyperkalaemia |
Spironolactone [42] |
25–50 mg o.d. |
4 weeks |
Acute renal failure (creatinine clearance <30ml/ml), anuria, Addison’s disease, hyponatraemia, combination with eplerenone |
| Eplerenone [43] |
50 mg o.d. |
Potassium >5.0 mmol/l before initiation, eGFR <30 ml/min/1.73m2), hepatic insufficiency (Child-Pugh C), combination with potassium-sparing diuretics, Cyp3A4-inhibitors, potassium supplements, dual RAAS blockade |
Table 3 Adjustment of HF medication in the case of adverse events. Adapted from Ponikowski et al. 2016 [1, 44].
|
Adverse event
|
ACEI/ARB/ARNI
|
BB
|
MRA
|
Diuretic
|
| Worsening signs or symptoms (increasing dyspnoea, fatigue, oedema, weight gain) |
|
In patients with signs of increasing congestion, the diuretic dose should be increased.
In the event of severe deterioration, the BB dose should be reduced to 50%. In the case of marked fatigue, the BB dose should be reduced to 50% and it should be taken in the night. The patient should be reassessed within 1–2 weeks. |
|
|
| Asymptomatic hypotension |
No change in therapy required. |
Dose may be reduced if the patient shows no signs or symptoms of congestion. |
| Symptomatic hypotension |
Dizziness and light-headedness are common adverse events and improve over time.
Reduce the dose if the patient experiences dizziness or light-headedness without showing signs or symptoms of congestion.
Dose adaption of diuretics, antihypertensive medication [44]; treatment with nitrates, calcium-channel blockers and other vasodilators should be stopped or the dose reduced.
If the patient shows no signs or symptoms of congestion, a reduction of the diuretic dose can be considered.
Alternative causes for hypotension, e.g. hypovolaemia, should be treated [44]. |
| Cough |
ACEI-induced cough does not usually require a change in therapy. However, substitution of an ARB is recommended in case of troublesome, ACEI-induced cough |
|
| Worsening renal function |
Concomitant nephrotoxic drugs, including NSAIDs should be stopped if urea or creatinine levels rise excessively.
ACEI (or ARB) dose should be reduced to half and blood chemistry re-checked within 1–2 weeks if the rise in creatinine levels persists at 50% above baseline, 266 μmol/l (3 mg/dl) or eGFR <25 ml/min/1.73 m2.
ACEI (or ARB) therapy should be stopped if creatinine levels increase by >100%, to >310 μmol/l (3.5 mg/d-) or eGFR <20 ml/min/1.73 m2.
ARNI therapy (Swiss Summary of Product Characteristics):
In the case of renal dysfunction, concomitant medication should be evaluated and a temporary dose reduction or withdrawal of ARNI is recommended [44].
ARNI therapy is contra-indicated in patients with eGFR <10ml/min/1.73m [1, 44]. |
No change in therapy required. |
Due to an increased risk of renal dysfunction, the triple combination of an ACEi, ARB and MRA is not recommended.
Nephrotoxic drugs such as NSAIDs should be avoided.
Male HF patients treated with spironolactone should be switched to eplerenone if they develop breast discomfort or gynaecomastia. |
Thiazide diuretic therapy should be stopped if used with concomitant loop diuretics.
A reduction of the diuretic dose should be considered if urea or creatinine creatinine levels rise excessively.
Thiazide diuretic therapy should be stopped if used with concomitant loop diuretics. |
| Hyperkalaemia |
Potassium supplements or retaining agents should be stopped. A reduction of the diuretic dose should be considered if potassium levels rise excessively, if there are no signs of congestion.
Close monitoring of potassium levels is recommended especially for patients with risk factors: severe renal insufficiency, diabetes mellitus, hyperaldosteronism [44].
If the increase in potassium levels persists above ≥5.5 mmol/l, the ACEI (or ARB) dose should be reduced to half and blood chemistry re-checked within 1–2 weeks.
ACEI (or ARB) therapy should be stopped temporarily if potassium levels rise to >6.0 mmol/l.
In the event of hyperkalaemia, concomitant medication should be evaluated and a temporary dose reduction or withdrawal of ARNI is recommended [44] |
No change in therapy required. |
The triple combination of an ACEI, ARB and MRA is not recommended.
Other potassium-sparing or -retaining agents should be avoided.
Some low-salt supplements can lead to increased serum potassium levels.
Normal-to-high potassium levels are described in HF patients if they are receiving digoxin. |
| Hypokalaemia or hypomagnaesaemia |
|
The ACEI or ARB dose should be increased.
MRA should be added.
Potassium/magnesium supplements |
| Hyponatraemia |
|
If the patient is volume depleted, either:
‒ stop treatment with thiazide
‒ switch to a loop diuretic
‒ reduce the dose of loop diuretics
‒ stop treatment with loop diuretics
If the patient is volume overloaded:
‒ increase the loop diuretic dose
‒ consider ultrafiltration
‒ consider i.v. inotropic support
‒ consider treatment with an AVP antagonist
Advise the patient to restrict fluids. |
| Hypovolaemia or dehydration |
|
The diuretic dose may be reduced depending on the volume status. |
| Insufficient diuretic response or diuretic resistance |
|
Consider adding an MRA or increasing the MRA dose.
The diuretic (loop, thiazides, non-thiazide-sulphonamide) dose should be increased.
The loop diuretic should be administered more frequently or on empty stomach
Loop diuretics may be combined with thiazide or metolazone.
Patients on furosemide may be switched to bumetanide or torasemide.
Ultrafiltration or short term i.v. infusion of loop diuretic can be considered. |
Management of adverse events is part of the up-titration strategy. Patients with asymptomatic hypotension (systolic blood pressure [SBP] 90–100 mm Hg) usually do not require any changes to therapy [45]. In patients with symptomatic hypotension (characterised by dizziness, light-headedness and confusion; SBP ≤90 mm Hg), however, treatment with nitrates, calcium-channel blockers and other vasodilators should be either stopped entirely or administered at reduced doses [45].
The probability for symptomatic hypotension is increased in patients with volume and sodium depletion [31–33, 35–37, 44]. Therefore, a reduction of the diuretic dose should be considered in compensated patients not showing signs or symptoms of congestion [45]. If these measures do not improve the patient’s clinical status, the dose of ACEi, ARB, ARNI or beta-blocker may be temporarily reduced in a step-by-step manner [38–41] and the patient has to be reassessed within one week [45]. Renal insufficiency may also increase the risk of hypotension [32, 35]. Furthermore, postural hypotension in HF patients with autonomic dysfunction or stroke can limit the optimal dosing of HF medication [1]. In these patients, a reduction of the diuretic dose may reduce the severity of the interaction [1]. Treatment of other pharmacological conditions such as depression (tricyclic antidepressants) or prostatic obstruction (alpha-adrenoceptor blockers) may cause hypotensive interactions with HF medication [1].
As hyperkalaemia may occur in HF patients, regular monitoring of serum potassium levels is necessary [1]. Particularly in dehydrated or septic patients, serum potassium, creatinine and urea levels should be closely followed [45]. Additional risk factors for hyperkalaemia include age, renal failure and diabetes mellitus [43]. If serum potassium levels increase to ≥5.5 mmol/l, the doses of potassium-retaining agents and renin-angiotensin-aldosterone system (RAAS) inhibitors should be halved and levels rechecked after few days. Short term cessation is required if potassium rises to >6.0 mmol [1]. However, RAAS inhibitors should be reintroduced as soon as possible with continued close monitoring of potassium levels [1].
Furthermore, worsening renal function or chronic kidney disease (CKD) are also commonly seen among HF patients [1]. Both CKD and HF share many risk factors, including diabetes, hypertension and hyperlipidaemia [1]. Heart failure patients are more vulnerable to acute renal failure following a destabilising event, such as a dehydrating illness, over-diuresis or the addition of nephrotoxic medication (e.g., antibiotics such as trimethoprim or gentamicin, or non-steroidal anti-inflammatory drugs, which are even contraindicated in HFrEF) [1]. Worsening renal function is common during initiation and up-titration of RAAS inhibitors [1]. Usually, the reduction in renal function is minor and should not lead to treatment discontinuation [1]. However, patients showing a substantial increase in serum creatinine levels (50% above baseline, 266 μmol/l / 3 mg/dl or estimated glomerular filtration rate <25 ml/min/1.73 m2) should be evaluated thoroughly and assessed for possible renal artery stenosis, excessive hyper- or hypovolaemia and concomitant medication [1].
Limitations
Many recommendations provided in this review are supported by the latest European Society of Cardiology and American Heart Association / American College of Cardiology guidelines for the management of patients with HF (see also fig. 1). However, as we tried to provide as concrete and specific suggestions as possible to facilitate implementation into routine clinical care, we also wish to highlight that some recommendations provided by this expert group are expert opinion only and not yet supported by data from prospective outcome studies.
Conclusions
Managing HF successfully after hospital discharge requires a seamless interaction between the patient, the GP and the cardiologist [15]. In particular, the transition phase after hospital discharge is a critical and vulnerable phase in the management of HF [45]. Here, both GPs and cardiologists have an important role in monitoring and treating HF patients. In this article, we provide a summary of recommendations on how to up-titrate HF medication, which clinical and biomedical parameters to assess during the follow-up visits and how the patients can contribute to optimising their treatment outcomes. A well-structured transition phase is critical in HF management and the management of chronic diseases in general, especially considering that the proportion of patients with chronic diseases is continuously increasing [46].
Clinical relevance
Heart failure is a serious chronic disease associated with frequent hospitalisation [1–4]. The transition from hospital to primary care is a critical and vulnerable phase requiring close collaboration between patient, GP and cardiologist [1]. A cornerstone of follow-up care is the up-titration of HF medication. However, only a minority of HF patients achieve the target dose for ACEi and beta-blockers [28]. This article provides both GPs and cardiologists with suggested up-titration strategies and the optimal management of adverse events in an attempt to further improve HF management in Switzerland.
References
1
Ponikowski
P
,
Voors
AA
,
Anker
SD
,
Bueno
H
,
Cleland
JGF
,
Coats
AJS
, et al.; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(27):2129–200. doi:.https://doi.org/10.1093/eurheartj/ehw128
2
Stewart
S
,
Ekman
I
,
Ekman
T
,
Odén
A
,
Rosengren
A
. Population impact of heart failure and the most common forms of cancer. Circ Cardiovasc Qual Outcomes. 2010;3(6):573–80. doi:.https://doi.org/10.1161/CIRCOUTCOMES.110.957571
3
Muzzarelli
S
,
Leibundgut
G
,
Maeder
MT
,
Rickli
H
,
Handschin
R
,
Gutmann
M
, et al.; TIME-CHF Investigators. Predictors of early readmission or death in elderly patients with heart failure. Am Heart J. 2010;160(2):308–14. doi:.https://doi.org/10.1016/j.ahj.2010.05.007
4
Garnier
A
,
Rouiller
N
,
Gachoud
D
,
Nachar
C
,
Voirol
P
,
Griesser
AC
, et al.
Effectiveness of a transition plan at discharge of patients hospitalized with heart failure: a before-and-after study. ESC Heart Fail. 2018;5(4):657–67. doi:.https://doi.org/10.1002/ehf2.12295
5
Ritter
M
,
Laule-Kilian
K
,
Klima
T
,
Christ
A
,
Christ
M
,
Perruchoud
A
, et al.
Gender differences in acute congestive heart failure. Swiss Med Wkly. 2006;136(19-20):311–7.
6
Metra
M
,
Gheorghiade
M
,
Bonow
RO
,
Dei Cas
L
. Postdischarge assessment after a heart failure hospitalization: the next step forward. Circulation. 2010;122(18):1782–5. doi:.https://doi.org/10.1161/CIRCULATIONAHA.110.982207
7
McDonagh
TA
,
Blue
L
,
Clark
AL
,
Dahlström
U
,
Ekman
I
,
Lainscak
M
, et al.; European Society of Cardiology Heart Failure Association Committee on Patient Care. European Society of Cardiology Heart Failure Association Standards for delivering heart failure care. Eur J Heart Fail. 2011;13(3):235–41. doi:.https://doi.org/10.1093/eurjhf/hfq221
8
Lee
DS
,
Stukel
TA
,
Austin
PC
,
Alter
DA
,
Schull
MJ
,
You
JJ
, et al.
Improved outcomes with early collaborative care of ambulatory heart failure patients discharged from the emergency department. Circulation. 2010;122(18):1806–14. doi:.https://doi.org/10.1161/CIRCULATIONAHA.110.940262
9
Coleman
EA
,
Williams
MV
. Executing high-quality care transitions: a call to do it right. J Hosp Med. 2007;2(5):287–90. doi:.https://doi.org/10.1002/jhm.276
10
Kripalani
S
,
Jackson
AT
,
Schnipper
JL
,
Coleman
EA
. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–23. doi:.https://doi.org/10.1002/jhm.228
11Workshop zum Spitalaustrittsmanagement des herzinsuffizienten Patienten (Organisation: Novartis). Luzern; 5 December 2018.
12Herzstiftung S. Internetplattform Schweizerische Herzstiftung 2019; https://www.swissheart.ch/de/shop/produkt/produktdetail/detail/fuer-patienten/herzinsuffizienz-herztagebuch.html. Accessed 27 September 2019.
13Schweizerische Herzstiftung. Herztagebuch. Available at: https://www.swissheart.ch/de/shop/produkt.html?tx_nezzoshop_detail[action]=detail&tx_nezzoshop_detail[categoryId]=62&tx_nezzoshop_detail[controller]=Product&tx_nezzoshop_detail[productId]=93&cHash=2cf3ca7e874b9d18e55758122bf971e6. Accessed February 2019.
14
Stocker
R
,
Close
H
,
Hancock
H
,
Hungin
APS
. Should heart failure be regarded as a terminal illness requiring palliative care? A study of heart failure patients’, carers’ and clinicians’ understanding of heart failure prognosis and its management. BMJ Support Palliat Care. 2017;7(4):464–9. doi:.https://doi.org/10.1136/bmjspcare-2016-001286
15Heart Failure Policy Network. The handbook of multidisciplinary and integrated heart failure care. 2018; http://www.hfpolicynetwork.eu/wp-content/uploads/2018/09/HFPN_handbookD_DIGITAL.pdf. Accessed 18 December 2018.
16Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin (DEGAM). DEGAM Leitlinie Nr. 9. Herzinsuffizienz - Teil 1, no. 9. 2006; http://www.herzschwaeche-info.de/fileadmin/user_upload/Dokumente/Wissenschaftliche_Quellen/Leitlinie_Dtsch_Ges_Allgemeinmedizin.pdf. Accessed 18 December 2018.
17Coleman E. The Post-Hospital Follow-Up Visit: A Physician Checklist to Reduce Readmissions. California Health Care Foundation. 2010. Available at: https://www.chcf.org/wp-content/uploads/2017/12/PDF-PostHospitalFollowUpVisit.pdf. Accessed 18 December 2018.
18Santitätsbetrieb S. Klinischer Betreuungspfad für den Patienten mit Herzinsuffizienz. Available at: http://sakam.it/sakam/images/pdf/Sonderausbildung_2014-2017/21.05.2015_Chronische_Herzinsuffizienz_PCA_scompenso_cardiaco_DE.pdf. Accessed 18 December 2018.
19Nationale VersorgungsLeitlinie. Chronische Herzinsuffizienz Kurzfassung. 2013; https://www.dgthg.de/upload/pdf/herzinsuffizienz-2aufl-vers1-kurz.pdf. Accessed 18 December 2018.
20
Angermann
CE
,
Störk
S
,
Gelbrich
G
,
Faller
H
,
Jahns
R
,
Frantz
S
, et al.; Competence Network Heart Failure. Mode of action and effects of standardized collaborative disease management on mortality and morbidity in patients with systolic heart failure: the Interdisciplinary Network for Heart Failure (INH) study. Circ Heart Fail. 2012;5(1):25–35. doi:.https://doi.org/10.1161/CIRCHEARTFAILURE.111.962969
21
Güder
G
,
Störk
S
,
Gelbrich
G
,
Brenner
S
,
Deubner
N
,
Morbach
C
, et al.
Nurse-coordinated collaborative disease management improves the quality of guideline-recommended heart failure therapy, patient-reported outcomes, and left ventricular remodelling. Eur J Heart Fail. 2015;17(4):442–52. doi:.https://doi.org/10.1002/ejhf.252
22BAG CK. Referenzrahmen Selbstmanagement-Förderung bei chronischen Krankheiten und Sucht. Konzeptionelle Klärung, Umsetzungsbeispiel und strategische Emfpfehlungen 2018, Available at: https://www.npg-rsp.ch/fileadmin/npg-rsp/Themen/BAG_2018_Referenzrahmen_Selbstmanagement.pdf. Accessed 23 July 2019.
23
Inglis
SC
,
Clark
RA
,
McAlister
FA
,
Ball
J
,
Lewinter
C
,
Cullington
D
, et al.
Structured telephone support or telemonitoring programmes for patients with chronic heart failure. Cochrane Database Syst Rev. 2010;8:CD007228. doi:.https://doi.org/10.1002/14651858.CD007228.pub2
24Rosemann A. Guidelines Herzinsuffizienz. Available at: https://www.medix.ch/media/herzinsuffizienz.pdf. Accessed 18 December 2018. 2014.
25
Nicholls
MG
,
Richards
AM
; Christchurch Cardioendocrine Research Group. Disease monitoring of patients with chronic heart failure. Heart. 2007;93(4):519–23. doi:.https://doi.org/10.1136/hrt.2005.078519
26
Dunlay
SM
,
Gheorghiade
M
,
Reid
KJ
,
Allen
LA
,
Chan
PS
,
Hauptman
PJ
, et al.
Critical elements of clinical follow-up after hospital discharge for heart failure: insights from the EVEREST trial. Eur J Heart Fail. 2010;12(4):367–74. doi:.https://doi.org/10.1093/eurjhf/hfq019
27
Arulnathan
A
,
Vaaheesan
S
,
Denecke
K
. A mobile application for self-monitoring for patients with heart failure. Healthcare of the Future. 2019:113–7.
28
Maggioni
AP
,
Anker
SD
,
Dahlström
U
,
Filippatos
G
,
Ponikowski
P
,
Zannad
F
, et al.; Heart Failure Association of the ESC. Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2013;15(10):1173–84. doi:.https://doi.org/10.1093/eurjhf/hft134
29
Martens
P
,
Verbrugge
FH
,
Boonen
L
,
Nijst
P
,
Dupont
M
,
Mullens
W
. Value of routine investigations to predict loop diuretic down-titration success in stable heart failure. Int J Cardiol. 2018;250:171–5. doi:.https://doi.org/10.1016/j.ijcard.2017.10.018
30Canadian Cardiovascular Society. Heart Failure Medication Initiation and Titration. Available at: https://www.ccs.ca/images/Guidelines/Tools_and_Calculators_En/HF_Med_Algorithms_Aug_2017.pdf. Accessed 18 December 2018.
31Enalapril Helvepharm® Swiss Prescribing Information (Status April 2016). Available at: https://www.swissmedicinfo.ch Acessed 23 January 2019.
32Lisinopril Axapharm® Swiss Prescribing Information (Status June2018). Available at: https://www.swissmedicinfo.ch Acessed 18 December 2018.
33Ramipril® Swiss Prescribing Information (Status September 2016). Available at: https://www.swissmedicinfo.ch Acessed 23 January 2019.
34Candesartan Sandoz® Swiss Prescribing Information (Status December 2015) Available at: https://www.swissmedicinfo.ch. Acessed 23 January 2019.
35Losartan Sandoz® Swiss Prescribing Information (Status June 2015). Available at: https://www.swissmedicinfo.ch Acessed 23 January 2019.
36Diovan® Swiss Prescribing Information (Status September 2015). Available at: https://www.swissmedicinfo.ch Acessed 23 January 2019.
37Entresto® Swiss Prescribing Information (Status April 2018). Available at: https://www.swissmedicinfo.ch Acessed 23 January 2019.
38Concor® Swiss Prescribing Information (Status August 2018). Available at: https://www.swissmedicinfo.ch Acessed 23 January 2019.
39Dilatrend® Swiss Prescribing Information (Status March 2015). Available at: https://www.swissmedicinfo.ch Acessed 23 January 2019.
40Beloc Zok® Swiss Prescribing Information (Status March 2015). Available at: https://www.swissmedicinfo.ch Acessed 23 January 2019.
41Nebilet® Swiss Prescribing Information (Status March 2015). Available at: https://www.swissmedicinfo.ch Acessed 23 January 2019.
42Aldactone® Swiss Prescribing Information (Status December 2018). Available at: https://www.swissmedicinfo.ch. Acessed 23 January 2019.
43Inspra® Swiss Prescribing Information (Status October 2016). Available at: https://www.swissmedicinfo.ch Acessed 23 January 2019.
44Coversum® Swiss Prescribing Information (Status March 2018). Available at: https://www.swissmedicinfo.ch Acessed 24 January 2019.
45
Hickey
A
,
Suna
J
,
Marquart
L
,
Denaro
C
,
Javorsky
G
,
Munns
A
, et al.
Improving medication titration in heart failure by embedding a structured medication titration plan. Int J Cardiol. 2016;224:99–106. doi:.https://doi.org/10.1016/j.ijcard.2016.09.001
46
Wagner
EH
,
Groves
T
. Care for chronic diseases. BMJ. 2002;325(7370):913–4. doi:.https://doi.org/10.1136/bmj.325.7370.913
47
Yancy
CW
,
Jessup
M
,
Bozkurt
B
,
Butler
J
,
Casey
DE, Jr
,
Colvin
MM
, et al.
2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776–803. doi:.https://doi.org/10.1016/j.jacc.2017.04.025
48
Yancy
CW
,
Jessup
M
,
Bozkurt
B
,
Butler
J
,
Casey
DE, Jr
,
Drazner
MH
, et al.; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. doi:.https://doi.org/10.1016/j.jacc.2013.05.019

