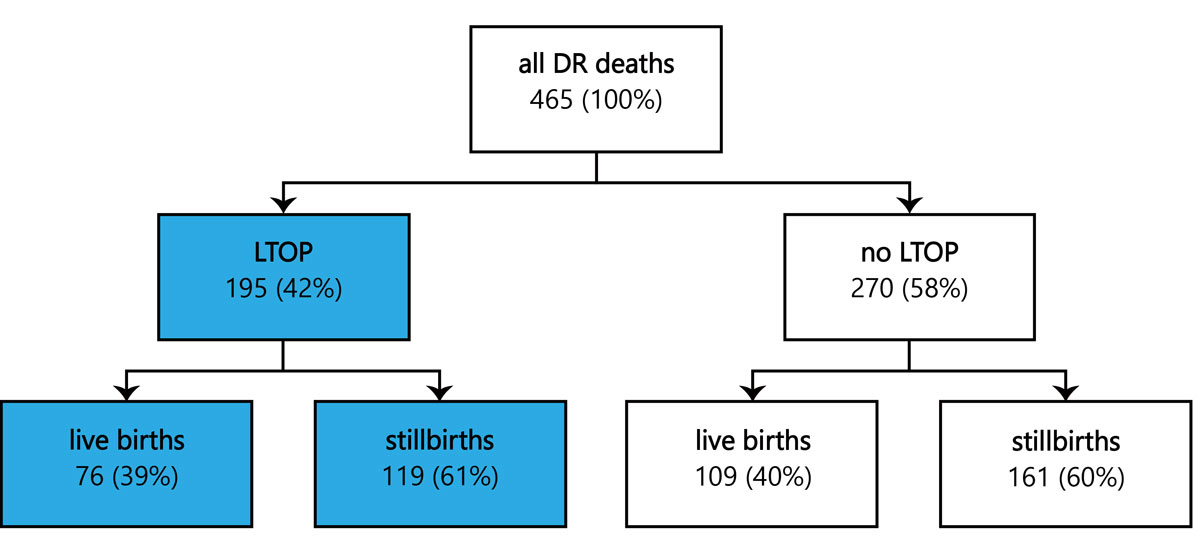
Figure 1 Overview of all delivery room (DR) deaths recorded by the SNSF project 67 over a 3-year period from 1 July 2012 to 30 June 2015; in 195 cases labour was induced for late termination of pregnancy (LTOP).
DOI: https://doi.org/10.4414/smw.2020.20186
birth weight
congenital heart disease
central nervous system
extremely low gestational age newborn
hypertension, elevated liver enzymes, low platelets
late termination of pregnancy
neonatal intensive care unit
In Switzerland, induced abortion is legal up to the 12th week of pregnancy at the written request of a pregnant woman who claims that she is in a state of distress (so-called “Fristenregelung” or “régime du délai”). Beyond the first trimester, termination of a pregnancy is exempt from penalty if it is deemed necessary by a physician to prevent the pregnant woman from sustaining serious physical injury or serious psychological distress (so-called “Indikationsregelung” or “régime des indications”). The Swiss Criminal Code stipulates that the risk to the mother must be greater the more advanced the pregnancy is [1], implying that the value of a fetus increases with advancing gestational age.
Worldwide, annual induced abortion rates have decreased from 40 induced abortions per 1000 women aged 15–44 years in 1990–1994 to 35 in 2010–2014 [2]. According to the most recent “Abortion Worldwide 2017” report from the Guttmacher Institute [3], Switzerland has one of the lowest induced abortion rates worldwide (5 induced abortions per 1000 women aged 15–49 years in 2014). The Swiss Federal Statistical Office estimates that approximately 95% of these induced abortions occur prior to the 12th week of pregnancy and only 5% at a later stage [4].
There is no generally accepted definition of what constitutes late termination of pregnancy (LTOP), except that it describes the termination of an advanced pregnancy – the threshold ranging from 12 to 20 or even >24 weeks of gestation [5, 6].
In a retrospective cohort study of all deaths among extremely low gestational age newborns (ELGANs) born between 22 and 27 completed weeks of gestation in Switzerland over a 3-year period [7], researchers unexpectedly found that 42% of all delivery room deaths in this population followed induction of labour for LTOP. Subsequently, the Swiss National Ethics Committee explored legal, medical and ethical aspects of LTOP. The Committee summarised its findings in a recently published position statement [8]. Presumably, the vast majority of LTOPs in our cohort were performed by early labour induction. Surgical terminations are only rarely performed at this stage of pregnancy in Switzerland [9]. Details of the methods used for LTOP were not collected.
The aim of the present study was to analyse in depth indications for LTOP, rates of live and stillbirths after LTOP, as well as circumstances of delivery room deaths of live born infants after LTOP in Switzerland over a 3-year period.
This retrospective multicentre observational study was undertaken at nine Swiss Level III perinatal centres. From data of the Swiss National Science Foundation (SNSF) Project 67 “End-of-life decision-making in extremely low birth weight infants in Switzerland”, all delivery room deaths following induction of labour for LTOP among ELGANs (gestational age between 22 0/7 and 27 6/7 weeks) over a 3-year period (1 July 2012 to 30 June 2015) were analysed. Both live and stillborn infants were included. Because of the initial study design, LTOPs at higher gestational ages were not recorded.
Gestational age was defined as the postmenstrual age in weeks and days, and was the best obstetric estimate based on ultrasound and/or date of the beginning of the last menstrual period. The period between 24 0/7 weeks and 24 6/7 weeks, for example, is termed 24 completed weeks of gestation: the fetus has completed 24 weeks and is in the 25th week of gestation.
Patients were identified using clinical records, electronic databases and birth log books. De-identified data were entered into REDCap (Research Electronic Data Capture), a secure online database, by trained research assistants on-site. The system featured easy-to-use online screen forms and electronic data validation checks to minimise data entering errors. Confidentiality was assured by password protection.
The indications for LTOP were classified as (a) maternal emergencies, such as haemorrhage, sepsis secondary to chorioamnionitis, preeclampsia or HELLP syndrome (hypertension, elevated liver enzymes, low platelets) or acute psychiatric disorders; and (b) fetal anomalies severe enough to cause significant maternal psychological distress. The latter were further subdivided into the following categories according to the organ system mostly affected by the malformation: central nervous system (CNS) malformations, congenital heart diseases (CHD), pulmonary malformations (including pleural effusions), renal malformations, and skeletal disorders. The term multiple malformations was used when multiple organ systems were involved. The term chromosomal anomalies was used when known genetic disorders were diagnosed regardless of the organ systems affected. Disorders that could not be classified into one of the above categories were listed individually.
For each patient, date and time of birth, as well as the perinatal centre where the infant was born, were registered. Additional demographic data included gestational age, birth weight, sex and whether the infant was a singleton or child from a multiple birth. For stillborn infants, we attempted to determine whether fetal death occurred spontaneously or resulted from cardiac injection of digoxin or potassium chloride to stop the fetal heart. For live born infants, the type of delivery, any postnatal interventions (e.g., the administration of sedatives and/or analgesics) and time until death were recorded.
Data collection was limited to the level III perinatal centres at the University Hospitals of Basel, Bern, Geneva, Lausanne and Zurich, and the level III perinatal centres at the Cantonal Hospitals of Aarau, Chur, Lucerne and St Gallen.
Continuous variables are presented as median and range, dichotomous variables are presented as absolute numbers and percentages.
Data collection and evaluation for this study were approved by the Swiss Federal Commission for Privacy Protection in medical research and the Swiss ethical review boards (KEK-ZH-Nr2014-0551 and KEK-ZH-Nr2014-0552).
Over the 3-year study period, a total of 465 delivery room deaths among ELGANs (22–27 completed weeks of gestation) were identified. Of these, 195 (42%) occurred in the context of LTOP, and resulted in live births in 76 (39%) and stillbirths in 119 (61%) of cases (fig. 1).

Figure 1 Overview of all delivery room (DR) deaths recorded by the SNSF project 67 over a 3-year period from 1 July 2012 to 30 June 2015; in 195 cases labour was induced for late termination of pregnancy (LTOP).
A total of 126 (65%) LTOPs occurred at 22–23 weeks of gestation, 36 (18%) at 24 weeks of gestation, and 33 (17%) at 25–27 weeks of gestation. The rate of live births did not correlate with gestational age and varied between 11% and 50% (fig. 2).
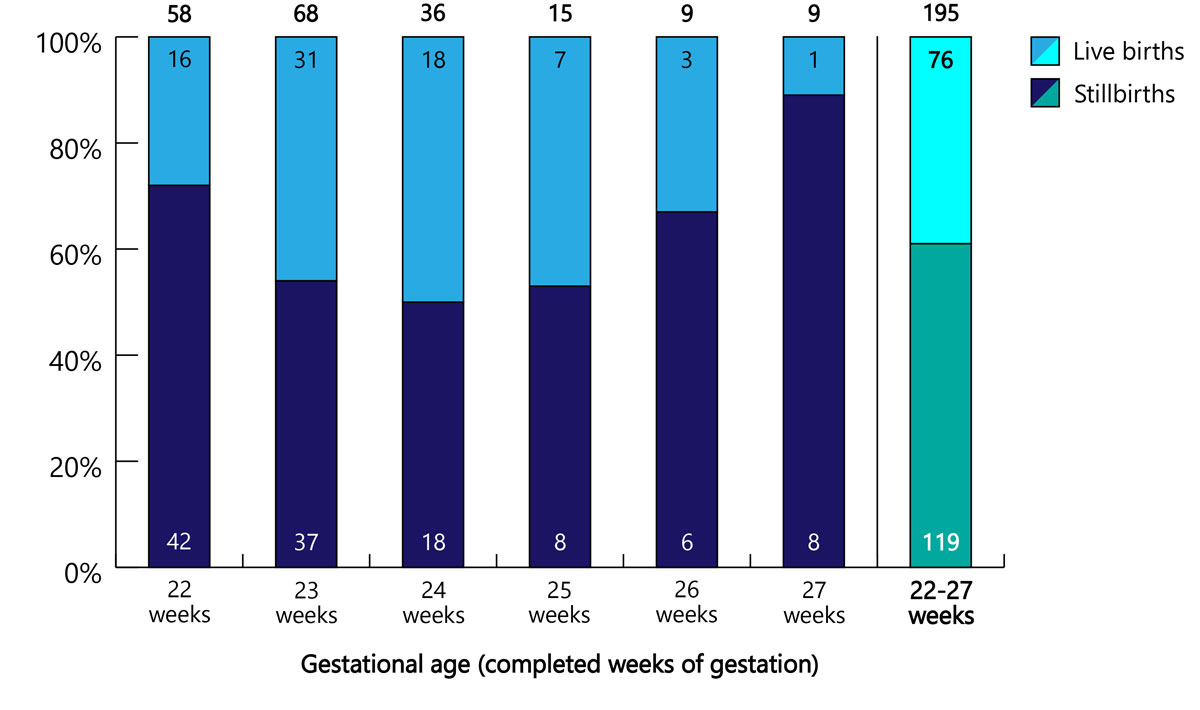
Figure 2 Numbers of later-terminations of pregnancy by gestational age, with rates of live and still births (n and %).
Demographic data were comparable between live and stillborn infants with a median gestational age and birthweight of 23 3/7 weeks and 550 g versus 24 0/7 weeks and 530 g, respectively (table 1). Fetal death caused by injection of a cardioplegic drug was documented in three cases (for details see table S1 in appendix 1).
Table 1 Patient demographics.
| Characteristics |
Total
(n = 195) |
Live births
(n = 76) |
Stillbirths
(n = 119) |
|---|---|---|---|
| Gestational age in weeks, median (range) | 23 3/7 (22 0/7 to 27 6/7) |
23 3/7 (22 1/7 to 27 6/7) |
24 0/7 (22 0/7 to 27 6/7) |
| Birthweight in g, median (range) |
540 (110–1300) | 550 (200–1300) | 530 (110–1250) |
| Male gender, n (%) | 104 (53%) | 42 (55%) | 62 (52%) |
| Multiples, n (%) | 16 (8%) | 8 (11%) | 8 (7%) |
Overall, CNS malformations, including myelomeningoceles (n = 46), chromosomal anomalies (n = 29), severe CHD (n = 22), multiple malformations (n = 18) and maternal emergencies (n = 22), accounted for 70% of all LTOPs (table 2). The most frequent diagnoses for each of the above categories are listed in table 3.
Table 2 Indications for late termination of pregnancy with gestational age, birthweight and rate of live births.
| Indication for LTOP | n | % of all LTOP |
Median gestational age in weeks
(range) |
Median birthweight in g
(range) |
Live births
n (%) |
|---|---|---|---|---|---|
| CNS malformations | 46 | 23.6 | 23 3/7 (22 1/7 to 27 6/7) |
568 (240–1300) |
22/46 (48%) |
| Chromosomal anomalies | 28 | 14.3 | 23 2/7 (22 0/7 to 27 2/7) |
530 (111–780) |
6/28 (21%) |
| CHD | 23 | 11.8 | 23 4/7 (22 0/7 to 27 6/7) |
600 (410–880) |
10/23 (43%) |
| Multiple malformations | 17 | 8.7 | 23 3/7 (22 3/7 to 26 6/7) |
570 (370–1005) |
5/17 (29%) |
| Miscellaneous | 60 | 30.8 | 23 6/7 (22 0/7 to 27 6/7) |
550 (140–1160) |
20/60 (33%) |
| Maternal emergencies | 21 | 10.8 | 23 0/7 (22 1/7 to 27 2/7) |
490 (330–790) |
13/21 (62%) |
| Total | 195 | 100 | 23 3/7 (22 0/7 to 27 6/7) |
540 (110–1300) |
76/195 (39%) |
CNS = central nervous system; CHD = congenital heart disease; LTOP = late termination of pregnancy. An expanded version of this table is available in table S2, appendix 1.
Table 3 Most frequent diagnoses listed for organ system categories and maternal emergencies.
| Indication for LTOP | Most frequent specific diagnoses |
|---|---|
| CNS malformations (n = 46) | Myelomeningocele (n = 11) |
| Massive hydrocephalus (n = 10) | |
| Agenesis of corpus callosum (n = 5) | |
| Chromosomal anomalies (n = 28) | Trisomy 21 (n = 10) |
| Trisomy 18 (n = 5) | |
| Triploidy (n = 4) | |
| CHD (n = 23) | Hypoplastic left heart syndrome (n = 7) |
| Unspecified complex CHD (n = 6) | |
| Pulmonary malformations (n = 5) | Congenital diaphragmatic hernia (n = 2) |
| Renal malformations (n = 8) | Bilateral renal agenesis (n = 3) |
| Bilateral multicystic dysplastic kidneys (n = 2) | |
| Skeletal disorders (n = 11) | (Suspected) Osteogenesis imperfecta (n = 5) |
| Thanatophoric dysplasia type 1 (n = 2) | |
| Multiple malformations (n = 17) | Unspecified multiple malformations (n = 5) |
| Fetal akinesia syndrome (n = 3) | |
| Maternal emergencies (n = 21) | Chorioamnionitis (n = 8) |
| (Pre)eclampsia, HELLP syndrome (n = 8) | |
| Psychiatric disorder (n = 3) |
CNS = central nervous system; CHD = congenital heart disease; HELLP = hypertension, elevated liver enzymes, low platelets; LTOP = late termination of pregnancy
When data were analysed separately for LTOPs below the current limit of viability in Switzerland (22 0/7 to 23 6/7), at the grey zone of the current limit of viability in Switzerland (24 0/7 to 24 6/7) and above the current limit of viability in Switzerland (25 0/7 to 27 6/7), some variations between the five most common indications were noted. At less than 24 completed weeks of gestation, CNS malformations were the leading cause for LTOP (accounting for 29% in this age group), whereas at the grey zone of the limit of viability, CNS malformations, chromosomal anomalies, and CHD were listed with similar frequencies (accounting for 8%, 11% and 14% in this age group, respectively). Above the limit of viability, CNS malformations and chromosomal anomalies were predominant (accounting for 18% and 21%, respectively) (fig. 3).
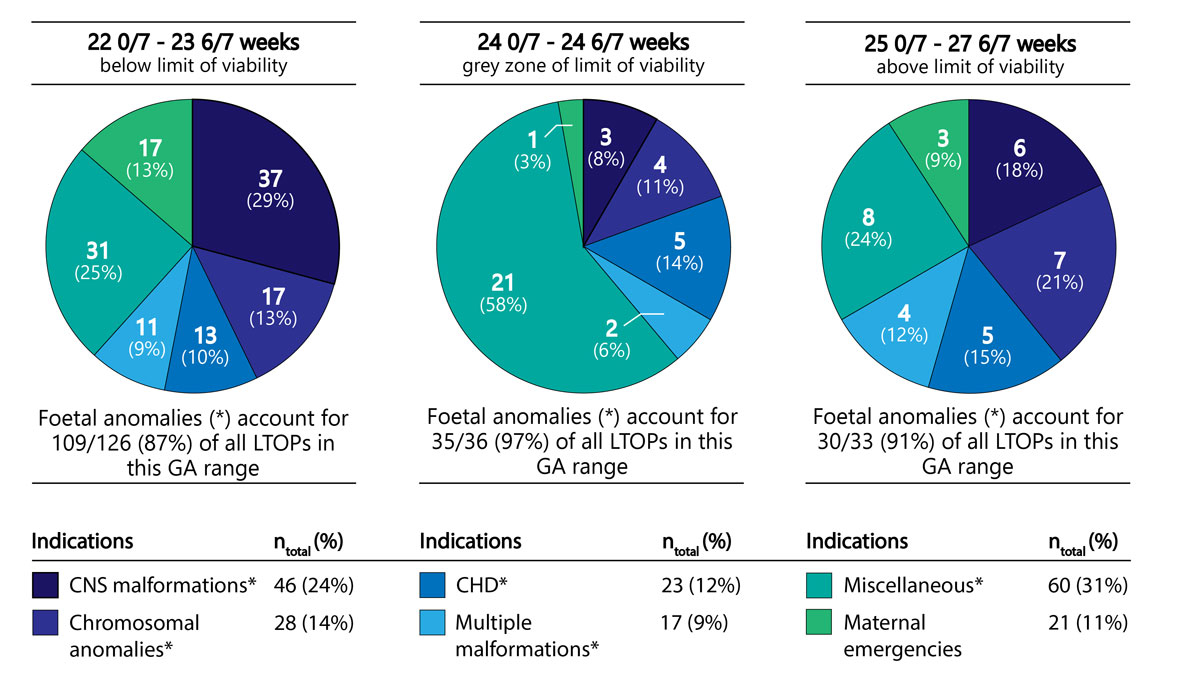
Figure 3 Comparison of the indications for LTOP at <24 completed weeks (below current limit of viability in Switzerland), 24 weeks (grey zone of current limit of viability in Switzerland) and ≥24 completed weeks (above current limit of viability in Switzerland) (CNS = central nervous system; CHD = congenital heart disease; LTOP = late termination of pregnancy; GA = gestational age).
Most of the LTOPs for maternal emergencies occurred at less than 24 completed weeks of gestation (82%) and resulted in 11 live births and 7 stillbirths. Only four patients were delivered with a gestational age ≥24 0/7 weeks (i.e., beyond the limit of viability suggested in the current Swiss guidelines [10]), three of whom were born alive. Of these, two infants weighed less than 500 g and one infant was delivered owing to chorioamnionitis; none of them received any life-sustaining therapies (fig. 4).
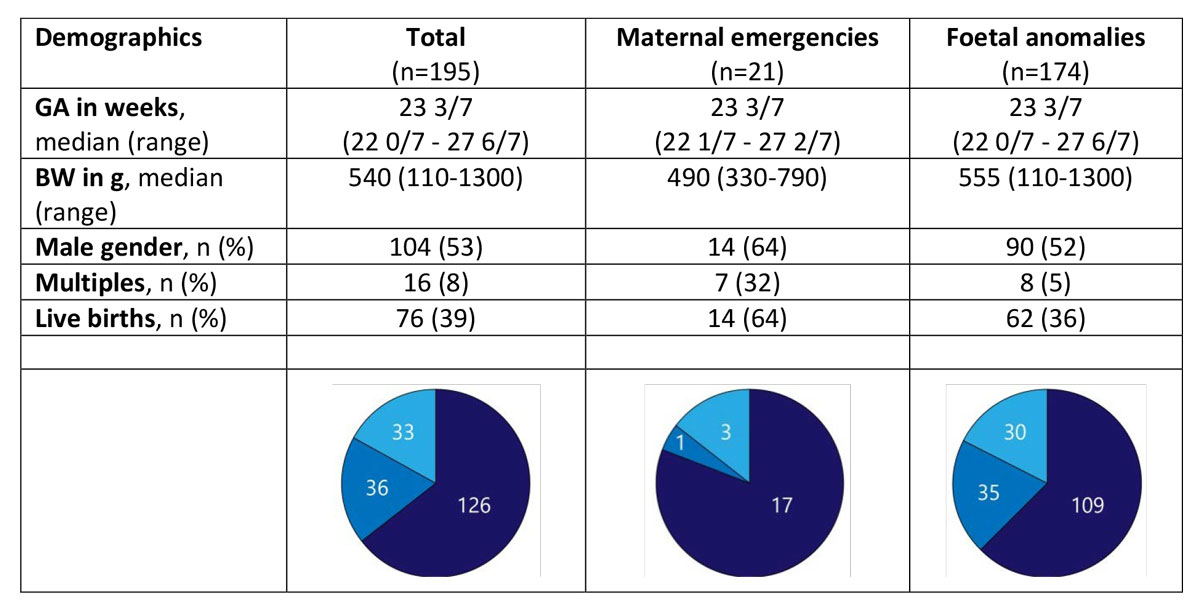
Figure 4 Demographics and gestational age categories (22 0/7 to 23 6/7: below current limit of viability in Switzerland, 24 0/7 to 24 6/7: grey zone of current limit of viability in Switzerland; 25 0/7 to 27 6/7: above current limit of viability in Switzerland) of late terminations of pregnancy for maternal emergencies and fetal anomalies (GA = gestational age; BW = birth weight).
Live births occurred frequently after LTOP, and were observed both after Caesarean section (n = 16, 21%) and vaginal delivery (n = 60, 79%). For the most common indications, live births were observed in 64% (maternal emergencies), 48% (CNS malformations), 41% (CHD), 33% (multiple malformations) and 21% (chromosomal anomalies) of cases (table 2 and table S2 in appendix 1).
All infants born alive after LTOP (n = 76) died in the delivery room without resuscitation attempts. Time until death among live born infants was documented for only 10 (13%) patients and ranged from 2 minutes to 4 hours. The use of analgo-sedative drugs for palliative care of the neonate was reported for only one patient; it was therefore either extremely rare or, alternatively, incompletely documented.
A large proportion (42%) of delivery room deaths among ELGANs in Swiss Level III perinatal centres occur after LTOP (fig. 1). This unexpected finding can in part be explained by the fact that life-sustaining therapies are never initiated after LTOP. The infant’s death is the anticipated outcome. In contrast, in infants born at or beyond the limit of viability (defined as ≥24 0/7 weeks of gestation in Switzerland) after spontaneous labour or induction of labour for reasons other than LTOP, resuscitation is usually attempted and rarely fails (in less than 10% of cases) [7]. Such infants are then admitted to a neonatal intensive care unit (NICU) for provisional intensive care, where the clinical evolution will help to clarify whether life-sustaining therapies should be continued or withdrawn. The Swiss Recommendations for Perinatal Care at the Limit of Viability suggest that life support should be continued as long as there is reasonable hope for survival and the infant’s burden of intensive care is acceptable. If, on the other hand, the health care team and the parents recognise that, in the light of a very poor prognosis, the burden of the currently used therapies has become disproportionate, redirection of care is appropriate [10].
In our cohort, the majority of LTOPs (89%) followed the detection of fetal anomalies. Swiss law does not allow LTOP for fetopathic indications per se, but only if the fetal anomalies are considered to be severe enough to cause significant maternal psychological distress. This contrasts with induced abortion laws in many other countries. In his global review of second trimester induced abortion laws, Boland found that 69 out of 191 countries specifically authorise induced abortions for fetal impairments [11]. The definitions of what sort of fetal impairment is sufficiently serious to justify an induced abortion vary considerably. Specific time limits on induced abortion for fetal impairments are mentioned in only some of these laws (16 weeks in 5 countries, 24 weeks in 10 countries, 32 weeks in 1 country, “viability” in 2 countries) [11]. Interestingly, Swiss law does not define any time limits.
It has been reported that significant differences concerning practice of LTOP exist between Swiss hospitals, both with regard to accepted indications and method of termination. This may lead to LTOP tourism – both within Switzerland and abroad [9].
The finding that CNS malformations, chromosomal anomalies, severe CHD and multiple malformations were the most commonly encountered diagnostic categories of fetal anomalies (accounting for 59% of all LTOP cases in the present study) has been reported from other European countries [6, 12] (tables 2 and 3 ). For example, in a French population-based cohort study, Monier et al. found that the same diagnostic categories were responsible for 70% of all LTOPs performed between 22 and 31 completed weeks of pregnancy [13]. The EUROCAT working group analysed routinely collected data on congenital anomalies from 19 registries in 12 European countries in which LTOP for fetal anomalies is legal. Of all LTOPs performed at a gestational age of 24 weeks or more, 81% were due to CNS malformations, chromosomal anomalies, severe CHD or multiple malformations [6].
In our study, at less than 24 weeks of gestation, CNS malformations was the leading diagnostic category for LTOP, accounting for 29% of the LTOPs in this gestational age group. In contrast, at later stages of gestation, CNS malformations accounted for only 13% of all LTOPs, and miscellaneous disorders accounted for the majority (41%) (fig. 3, table S2). In an analysis of 53,000 pregnancies from a single centre in the USA, Schechtman et al. observed that severe CNS anomalies appear to provide a much greater incentive for induced abortion than non-CNS anomalies of the same severity. In fact, the probability of termination for grade 3 CNS anomalies (defined as an anomaly with the potential for serious impact on quality of life) was higher than for grade 4 non-CNS anomalies (defined as anomalies incompatible with life) [12].
Congenital malformations are increasingly diagnosed in utero as a result of advances in prenatal screening [14]; however, prognostic uncertainty can be considerable and should be acknowledged when parents are counselled. There are malformations, such agenesis of the corpus callosum (five patients in our cohort), for which the neurodevelopmental outcome cannot be reliably predicted, particularly if this is an isolated finding (one patient in our cohort) (table S3 in appendix 2). In fact, it has been reported to be normal in the majority of cases. Nevertheless, fine and gross motor control, coordination, language and cognitive status can be impaired in some of these children. In addition, neurodevelopmental and overall outcome depend on associated anomalies that are not always detected prenatally [15].
The most severe malformations are often termed “lethal”. However, Wilkinson et al. warned that this term is flawed as there is no agreed definition, and its use hinders clear communication [16]. Even for some of the most frequently cited lethal malformations (renal agenesis, anencephaly, thanatophoric dwarfism, trisomy 13 or 18, holoprosencephaly, triploidy and hydranencephaly) occasional long-term survivors have been reported. Wilkinson et al. argued that the term should be avoided altogether and be replaced by a set of five key questions [16]: (1) What is the diagnosis and how certain is it? (2) What is the likelihood of survival beyond the newborn period if life-sustaining treatment is provided? (3) What is the likely duration of survival if life-sustaining treatment is provided? (4) What is the range of possible physical or cognitive impairments if the newborn survives? (5) What is the burden of treatment required to keep the baby alive?. Information given to the prospective parents should be precise, comprehensive and unbiased. Healthcare professionals ought to be aware that the way messages are relayed (so-called message framing) significantly influences the parents’ decisions [17].
The most frequent medical conditions that led to LTOP because of maternal emergencies were premature prolonged rupture of membranes, preeclampsia and psychiatric disorders (table 3). In the present study, maternal emergencies contributed only 11% of all LTOPs, and the majority of them (n = 18; 11 live births, 7 stillbirths) occurred at less than 24 weeks of gestation. The fact that none of the live born infants received any life-sustaining therapies is not surprising since the current Swiss guidelines for perinatal care at the limit of viability recommend that comfort care is appropriate for most infants born at less than 24 completed weeks of gestation. Provisional intensive care should be initiated only in selected cases with favourable prenatal indicators for improved survival and neurodevelopmental outcome [10].
Only four infants were born after LTOP for maternal emergencies at more than 24 completed weeks of gestation. For the three live born infants, a birthweight of less than 500 g in two infants and chorioamnionitis in one infant were probably considered to be significant negative prognostic factors leading to a decision not to attempt resuscitation of these infants in the delivery room.
The timing of LTOP is likely to be influenced by the current Swiss guidelines on perinatal care, which suggest the grey zone of the limit of viability to be around 24 weeks of gestation [10]. The fact that LTOPs are judged to be more acceptable at earlier gestational ages, when extrauterine survival is not possible, potentially exerts significant time pressure on the decision-making process.
On the other hand, the present study also demonstrated that the lower border of the limit of viability can be crossed when LTOPs are performed in Switzerland. Although 65% (n = 126) of LTOPs occurred below the current limit of viability (<24 0/7 weeks), 18% (n = 36) fell into what is considered to be the grey zone of the limit of viability (24 0/7 to 24 6/7 weeks) and another 17% (n = 33) were performed at an even later stage (>24 6/7 weeks) (figs 2 and 3 ).
According to a recently published study from France, a country with a liberal attitude towards induced abortions, LTOPs are responsible for almost a quarter of all very preterm births between 22 0/7 and 31 6/7 weeks of gestation [13]. Compared with Switzerland, higher rates of LTOPs at more advanced gestational ages were observed in the French study: only 36% were performed at less than 24 weeks of gestation, 25% at 24 weeks of gestation, and 39% at 25 0/7 to 27 6/7 weeks of gestation. At least in part, this can be explained by the fact that second trimester screening ultrasound examinations are scheduled at 22–24 weeks of gestation in France, as opposed to 19–22 weeks of gestation in many other European countries [13]. A potential advantage of second trimester screening at a later stage in pregnancy is that it might reduce prognostic uncertainty.
The fact that 39% of infants born after LTOP showed signs of life after birth is not surprising since the induction of fetal death by injection of a cardioplegic drug is rarely practised in Switzerland. The rate of live births did not correlate with gestational age: it peaked at 24 weeks of gestation and then decreased again (fig. 2). This can perhaps be explained by the more severe fetal impairments required by Swiss law for LTOPs at more advanced gestational ages. Alternatively, there may be some unreported cases of fetal deaths caused by injection of a cardioplegic drug.
In their population-based cohort study from France, Monier et al. found that fetal death caused by injection of a cardioplegic drug was common, with an overall rate of 73%, increasing from 48% and 59% at 22 and 23 weeks of gestation, respectively, to 80–92% at later stages of pregnancy [13]. This contrasts very strongly with a recorded overall rate of only 1.5% in the present study from Switzerland. In their guidelines concerning LTOP for fetal abnormality, the Royal College of Obstetricians and Gynaecologists in the UK recommends that fetal death caused by injection of a cardioplegic drug be routinely offered after 21 6/7 weeks of gestation because live birth becomes increasingly common after 22 weeks of gestation: “Where the fetal abnormality is not lethal and termination of pregnancy is being undertaken after 21 6/7 weeks of gestation, failure to perform feticide could result in live birth and survival, an outcome that contradicts the intention of the abortion” [13].
The use of analgo-sedative drugs for palliative care of the neonate was reported for only 1 of 76 live born patients. It was therefore either extremely rare or, alternatively, incompletely documented. This is obviously of concern and contrasts with the approach taken to infants dying following redirection of care after admission to a NICU, where more than 50% of patients receive opiates and/or benzodiazepines [7]. Available evidence from the literature suggests that this is a common phenomenon in the delivery room setting and not restricted to preterm infants [18–20].
Data collection was restricted to infants born between 22 0/7 to 27 6/7 weeks of gestation in Swiss level III perinatal centres. Therefore, LTOPs outside of this gestational age range (<22 0/7 weeks or >27 6/7 weeks) were not recorded. It would have been particularly interesting to have had data on LTOPs after 27 6/7 weeks of gestation, which were not collected as a result of the initial study design. Furthermore, some ELGANs (including those born after LTOP) who died in institutions not certified as perinatal centres might have been missed. However, comparison with data provided by the Swiss Federal Statistical Office shows that the group of infants born after LTOP in other hospitals is likely to be small (personal communication). Finally, medical records are hospital specific and non-uniform; data quality is likely to vary. There may be hospital-specific data sources to which the investigators did not have access. Therefore, the possibility that some cases were misclassified or missed altogether cannot be excluded.
To the best of our knowledge, this is the first comprehensive study of LTOPs between 22 0/7 and 27 6/7 weeks of gestation in Switzerland. LTOPs contribute significantly to mortality rates among ELGANs. LTOPs have an important impact on extremely preterm birth and perinatal mortality rates, and should be included in statistical analyses. Detailed documentation of both live and stillbirths after LTOP should be collected in a dedicated specialised registry [13]. Currently, documentation of LTOPs is often incomplete. Uniform reporting should be established and include information on indication(s), the decision-making process, the method of termination used and details of palliative care provided after live births.
Live births are common among infants delivered in the context of LTOP. Such infants are entitled to benefit from comprehensive palliative care; they should not be treated differently from infants born after spontaneous labour or induction of labour for maternal reasons who die in the delivery room or in the NICU after redirection of care.
As suggested by a recent statement of the Swiss National Ethics Committee [8], development of national guidelines for LTOPs would be highly desirable to harmonise practice and guarantee equal standards of care, regardless of where the induced abortion takes place. Clearly defined roles and tasks of gynaecologists, midwives and neonatologists would ensure good interprofessional collaboration and ease the emotional burden for parents and professionals confronted with such difficult situations. In addition, adequate follow-up with psychological support of parents having experienced LTOP should be guaranteed. The roles of fetal death induced by injection of a cardioplegic drug and palliative births (continuation of pregnancy after detection of severe fetal anomalies followed by spontaneous birth and comfort care) should be explored.
Table S1 Details of cases with fetal death caused by injection of a cardioplegic drug.
| Case | Indication for LTOP | Gestational age (weeks) | Birthweight (g) |
|---|---|---|---|
| 1 | Cystic fibrosis | 24 6/7 | 620 |
| 2 | Mosaicism with partial trisomy 17q and partial monosomy 12p, hypoplastic aortic arch | 25 4/7 | 730 |
| 3 | Trisomy 9 | 27 2/7 | 730 |
Table S2 Indications for late termination of pregnancy with gestational age, birthweight and rate of live births.
| Indication for LTOP | n | % of all LTOP |
Median gestational age in weeks
(range) |
Median birthweight in g
(range) |
Live births
n (%) |
|---|---|---|---|---|---|
| CNS malformations | 46 | 23.6 | 23 3/7 (22 1/7 to 27 6/7) |
568 (240–1300) |
22/46 (48%) |
| Chromosomal anomalies | 28 | 14.3 | 23 2/7 (22 0/7 to 27 2/7) |
530 (111–780) |
6/28 (21%) |
| CHD | 23 | 11.8 | 23 4/7 (22 0/7 to 27 6/7) |
600 (410–880) |
10/23 (43%) |
| Maternal emergencies | 21 | 10.8 | 23 0/7 (22 1/7 to 27 2/7) |
490 (330–790) |
13/21 (62%) |
| Multiple malformations | 17 | 8.7 | 23 3/7 (22 3/7 to 26 6/7) |
570 (370–1005) |
5/17 (29%) |
| Skeletal disorders | 11 | 5.6 | 23 3/7 (22 2/7 to 26 5/7) |
580 (440–1005) |
5/11 (45%) |
| Oligo-/anhydramnion | 11 | 5.6 | 23 0/7 (22 1/7 to 24 5/7) |
540 (400–710) |
5/11 (45%) |
| Renal malformations | 8 | 4.1 | 23 3/7 (22 3/7 to 25 4/7) |
615 (370–1060) |
1/8 (13%) |
| Severe IUGR | 7 | 3.6 | 24 3/7 (22 3/7 to 27 0/7) |
330 (220–500) |
3/7 (43%) |
| Fetal infections | 6 | 3.1 | 23 5/7 (22 0/7 to 24 5/7) |
460 (370–635) |
2/6 (33%) |
| Pulmonary malformation | 5 | 2.6 | 23 1/7 (22 1/7 to 25 0/7) |
680 (600–990) |
1/5 (20%) |
| TTTS | 4 | 2.1 | 24 4/7 (24 2/7 to 24 5/7) |
450 (140–640) |
2/4 (50%) |
| Cystic fibrosis | 3 | 1.5 | 24 6/7 (22 3/7 to 27 6/7) |
620 (430–1010) |
0/3 (0%) |
| Fetal hydrops | 2 | 1.0 | 24 3/7 (22 2/7 to 26 3/7) |
920 (680–1160) |
0/2 (0%) |
| Sacrococcygeal teratoma | 1 | 0.5 | 24 3/7 NA |
820 NA |
1/1 (100%) |
| Peutz-Jeghers syndrome | 1 | 0.5 | 24 2/7 NA |
660 NA |
0/1 (0%) |
| Unknown | 1 | 0.5 | 27 0/7 NA |
730 NA |
0/1 (0%) |
| Total | 195 | 100 | 23 3/7 (22 0/7 to 27 6/7) |
540 (110–1300) |
76/195 (39%) |
CNS = central nervous system; CHD = congenital heart disease; IUGR = intrauterine growth restriction; TTTS = twin-twin transfusion syndrome; LTOP = late termination of pregnancy
Appendix 2 is available as a separate file at https://smw.ch/article/doi/smw.2020.20186.
This work was supported by grant No. 406740_139303 of the Swiss National Science Foundation (SNSF NRP 67 Project “End-of-Life Decisions”). The funder had no role in trial design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the trial and had final responsibility for the decision to submit for publication.
The authors declare no conflict of interest.
1Schweizer Strafgesetzbuch, Artikel 119. 2011, Mar 23.
2 Sedgh G , Bearak J , Singh S , Bankole A , Popinchalk A , Ganatra B , et al. Abortion incidence between 1990 and 2014: global, regional, and subregional levels and trends. Lancet. 2016;388(10041):258–67. doi:.https://doi.org/10.1016/S0140-6736(16)30380-4
3Singh S, Remez L, Sedgh G, Kwok L, Onda T. Abortion Worldwide 2017: Uneven Progress and Unequal Access. https://www.guttmacher.org/report/abortion-worldwide-2017, accessed 01 April 2019.
4Bundesamt für Statistik. Schwangerschaftsabbrüche. https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/gesundheitszustand/reproduktive/schwangerschaftsabbrueche.html, accessed 21 April 2019.
5Schwarzenegger C. Schwangerschaftsabbruch in der Spätphase - Kriminologische und rechtsdogmatische Perspektiven. Nomos. 2011. http://www.zora.uzh.ch/49959, accessed 01April 2019.
6 Garne E , Khoshnood B , Loane M , Boyd P , Dolk H ; EUROCAT Working Group. Termination of pregnancy for fetal anomaly after 23 weeks of gestation: a European register-based study. BJOG. 2010;117(6):660–6. doi:.https://doi.org/10.1111/j.1471-0528.2010.02531.x
7 Berger TM , Steurer MA , Bucher HU , Fauchère JC , Adams M , Pfister RE , et al.; Swiss Neonatal End-of-Life Study Group. Retrospective cohort study of all deaths among infants born between 22 and 27 completed weeks of gestation in Switzerland over a 3-year period. BMJ Open. 2017;7(6):e015179. doi:.https://doi.org/10.1136/bmjopen-2016-015179
8Nationale Ethikkommission im Bereich der Humanmedizin. Zur Praxis des Abbruchs im späteren Verlauf der Schwangerschaft - Ethische Erwägungen und Empfehlungen. 2018. Available at: https://www.nek-cne.admin.ch/inhalte/Themen/Stellungnahmen/NEK_Stellungnahme_Abbruch_im_spaeteren_Verlauf_der_Schwangerschaft_D.pdf
9 Rey A , Seidenberg A . Schwangerschaftsabbruch: die Praxis der Spitäler und Kliniken in der Schweiz. Schweiz Arzteztg. 2010;91:(13):551–4. doi:https://doi.org/10.4414/saez.2010.15075
10 Berger TM , Bernet V , El Alama S , Fauchère JC , Hösli I , Irion O , et al. Perinatal care at the limit of viability between 22 and 26 completed weeks of gestation in Switzerland. 2011 revision of the Swiss recommendations. Swiss Med Wkly. 2011;141:w13280. doi:.https://doi.org/10.4414//smw.2011.13280
11 Boland R . Second trimester abortion laws globally: actuality, trends and recommendations. Reprod Health Matters. 2010;18(36):67–89. doi:.https://doi.org/10.1016/S0968-8080(10)36521-9
12 Schechtman KB , Gray DL , Baty JD , Rothman SM . Decision-making for termination of pregnancies with fetal anomalies: analysis of 53,000 pregnancies. Obstet Gynecol. 2002;99(2):216–22. doi:.https://doi.org/10.1097/00006250-200202000-00010
13 Monier I , Lelong N , Ancel P-Y , Benachi A , Khoshnood B , Zeitlin J , et al. Indications leading to termination of pregnancy between 22+0 and 31+6 weeks of gestational age in France: A population-based cohort study. Eur J Obstet Gynecol Reprod Biol. 2019;233(233):12–8. doi:.https://doi.org/10.1016/j.ejogrb.2018.11.021
14 Courtwright AM , Laughon MM , Doron MW . Length of life and treatment intensity in infants diagnosed prenatally or postnatally with congenital anomalies considered to be lethal. J Perinatol. 2011;31(6):387–91. doi:.https://doi.org/10.1038/jp.2010.124
15 D’Antonio F , Pagani G , Familiari A , Khalil A , Sagies T-L , Malinger G , et al. Outcomes Associated With Isolated Agenesis of the Corpus Callosum: A Meta-analysis. Pediatrics. 2016;138(3):e20160445. doi:.https://doi.org/10.1542/peds.2016-0445
16 Wilkinson DJ , Thiele P , Watkins A , De Crespigny L . Fatally flawed? A review and ethical analysis of lethal congenital malformations. BJOG. 2012;119(11):1302–8. doi:.https://doi.org/10.1111/j.1471-0528.2012.03450.x
17 Haward MF , Murphy RO , Lorenz JM . Message framing and perinatal decisions. Pediatrics. 2008;122(1):109–18. doi:.https://doi.org/10.1542/peds.2007-0620
18 Koper JF , Bos AF , Janvier A , Verhagen AAE . Dutch neonatologists have adopted a more interventionist approach to neonatal care. Acta Paediatr. 2015;104(9):888–93. doi:.https://doi.org/10.1111/apa.13050
19 Garten L , Glöckner S , Siedentopf J-P , Bührer C . Primary palliative care in the delivery room: patients’ and medical personnel’s perspectives. J Perinatol. 2015;35(12):1000–5. doi:.https://doi.org/10.1038/jp.2015.127
20 Janvier A , Meadow W , Leuthner SR , Andrews B , Lagatta J , Bos A , et al. Whom are we comforting? An analysis of comfort medications delivered to dying neonates. J Pediatr. 2011;159(2):206–10. doi:.https://doi.org/10.1016/j.jpeds.2011.01.022
This work was supported by grant No. 406740_139303 of the Swiss National Science Foundation (SNSF NRP 67 Project “End-of-Life Decisions”). The funder had no role in trial design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the trial and had final responsibility for the decision to submit for publication.
The authors declare no conflict of interest.