
Figure 1 Åke Senning during the press conference held in the auditorium of the former Cantonal Hospital Zurich after the first successful heart transplantation in Switzerland on 14 April 1969 (Source: SRF).
DOI: https://doi.org/10.4414/smw.2020.20192
Based on the leading work of Norman Shumway and colleagues [1–3], the first heart transplantation worldwide was realised by Christiaan Barnard at Groote Schuur Hospital in Cape Town, South Africa, on 3 December 1967. The 53-year-old Louis Washkansky received the heart of 25-year-old Denise Darvall, who suffered from severe brain injury due to a traffic accident [1, 2]. In 2017, on the 50th anniversary of the worldwide first heart transplantation, DKC Cooper, a contemporary surgical colleague of Barnard in South Africa, portrayed the circumstances around this epochal event [1]. At that time, brain death was not yet recognised legally. According to the law in South Africa, a patient could be considered dead when he or she was declared dead by a physician. Barnard took advantage of this statement and interpreted it such that he could use brain death as a criterion for declaring a patient dead. Nevertheless his plan was, according to the ethical rules set by the Groote Schuur authorities, to wait until complete heart arrest before removing the heart for transplant, because he did not want to be caught up in legal affairs. Barnard also obeyed another ethical rule of the Groote Schuur authorities in the politically charged apartheid atmosphere, which required that both donor and recipient be of white race [3].
After the local neurosurgeons declared the accident victim brain dead and her father agreed to donation, Barnard decided to do the transplant [2]. Approximately 6 minutes after ventilation was discontinued, the heart went into ventricular fibrillation, which was considered functional heart arrest. The chest was opened, and a heart-lung machine was connected to cool down the heart with cold oxygenated blood and reduce the duration of warm ischaemic injury. Ischaemic heart arrest was 8 minutes in normothermia and 14 minutes at 22°C [3]. The heart was excised and transferred to the adjacent operating room where the recipient was already prepared. Thus, the heart for the first heart transplantation in the world was explanted from a “non-heart-beating donor” or, as it is called today, a “donation after circulatory-determined death (DCD) donor” [4]. The heart was implanted into the recipient according to the technique described by Shumway and Lower [5]. Only at the third attempt could the patient be weaned successfully from extracorporeal circulation after a reperfusion time of 3 hours and 41 minutes [3]. This might have been a result of insufficient myocardial protection due to the obligatory wait for circulatory standstill of the donor heart and the recipient’s severe pulmonary hypertension at 11.5 Wood units, which would today be considered an absolute contraindication for heart transplantation [3]. Another interesting detail of the operative procedure was that anaesthesiology monitoring for cardiac surgery in Groote Schuur was rudimentary. Blood pressure was measured with a mercury manometer and central venous pressure using a water manometer. Blood gas analysis only allowed measurement of pCO2 and pH, but not pO2 [3]. Surprisingly, nobody photographed or filmed the operation for which the media would later have offered millions [3].
The patient made a rapid recovery during the first postoperative week. In the second week, he developed pulmonary infiltrates on chest x-ray. Since they were misinterpreted as a sign of rejection and not recognised as an incipient infection, Barnard decided to increase the immunosuppressive therapy. The condition of the patient rapidly deteriorated and he died on day 18 after heart transplantation. The antibiotic treatment which was started after pneumonia was diagnosed came too late. The autopsy confirmed pneumonia as cause of death. The heart showed no signs of rejection.
On 6 December 1967, three days after Barnard’s first heart transplant worldwide, Adrian Kantrowitz performed the first heart transplant in the USA at Maimonides Medical Center in Brooklyn, New York [6]. The recipient was an 18-day-old male infant who received the heart of a 2-day-old anencephalic male. The procedure was carried out in hypothermia without the aid of the heart-lung machine. Although the procedure was technically successful, the transplanted heart stopped beating after 7 hours. After Barnard did his second heart transplant on 2 January 1968, which was the third heart transplant worldwide [1], Norman Shumway performed the first successful adult heart transplant in the USA on 6 January 1968, at Stanford. Shumway’s patient died after 15 days from liver and kidney failure and internal bleeding [7]. Barnard’s second patient lived for almost 19 months, but then died from heart failure due to graft atherosclerosis [1, 8].
On 5 February 1968, PK Sen performed the first heart transplantation in Asia at King Edward Memorial Hospital in Bombay [9]. In Europe, Christian Cabrol performed the first heart transplantation on 27 April 1968, at the Hôpital La Pitie Salpêtrière in Paris [10, 11]. Cabrol’s patient died after 3 days from pulmonary embolism. Until the end of the year 1968, 104 heart transplantations were done worldwide [12].
On 14 April 1969, Åke Senning performed the first heart transplantation in Switzerland at the former Cantonal Hospital in Zurich. It was the 126th heart transplantation in the world. A 54-year-old patient received a heart from a 27-year-old donor who was victim of a traffic accident. After the patient was declared dead by a multidisciplinary professorial commission headed by the forensic pathologist and independent of the implant team of cardiac surgery, consent for donation was obtained from the relatives. Since the donor, who had a temperature of 31°C, was haemodynamically unstable, he was connected to the heart-lung machine and cooled down further. In the meantime, the heart was examined and considered suitable for transplantation. Simultaneously, the recipient was taken on the heart-lung machine in the adjacent operating room. When the donor heart developed ventricular fibrillation, it was excised and transported to the recipient’s operating room. After removal of the recipient’s heart, the donor heart was implanted. The implantation took less than 1 hour, the whole surgery lasted 2.5 hours and was technically successful [13].
In the press conference broadcast by Swiss television on the same day, Senning emphasised the importance of the multidisciplinary team approach with participation of almost every clinic of the hospital, which was what made the heart transplantation possible (fig. 1) [13]. Senning stated that he did not do something particularly new. He just did what the other heart transplant surgeons in the world had done before, and what he and his team had practised in the animal laboratory. The surgical technique of heart transplantation was not more difficult than that of a routine heart operation, Senning said. At least five surgeons of his team would have been able to perform the operation. In preparation for the operation, one of his staff members had previously visited Denton Cooley in Houston, where he was able to observe 16–18 heart transplantations.

Figure 1 Åke Senning during the press conference held in the auditorium of the former Cantonal Hospital Zurich after the first successful heart transplantation in Switzerland on 14 April 1969 (Source: SRF).
Senning also noted that the successful operation solved only the smallest problem. The biggest challenge would be the evolving immunological processes between the recipient and the donor heart, which had to be addressed together with the teams of the other clinics. He was optimistic that the immunological experience gained from 50 kidney transplants in Zurich would be of advantage in the management of immunosuppression.
After an initial uneventful course, the patient suffered from pneumonia which caused death 1 month after transplantation. In the summer of 1969, Senning performed the second heart transplantation in Switzerland. The patient recovered well and was discharged home after a few weeks. Three months after the transplant, he suddenly died while working on his farm, most likely owing to acute rejection.
The survival of the first 60 heart transplant patients worldwide was unacceptably low. Only 81% survived more than 1 day after transplantation. One-month survival was just 50%. After 1 year, no more than 18% of patients were alive [12]. Acute rejection and infection were the most frequent causes of death. In the first 30 heart transplant patients in the USA, acute rejection accounted for 40% and infection for 15% of deaths [12]. As a result of the frightening deaths rates, the initial euphoria about heart transplantation declined rapidly. After the enthusiastic 102 heart transplants in 1968, the numbers worldwide decreased to 48 in 1969 and 18 in 1970 [11, 12]. In the light of the disappointing results, the medical establishment, led by the American Heart Association, called for a moratorium on heart transplantation [7]. In February 1973, the Department of Health in Britain issued a moratorium on further heart transplants in the United Kingdom [12]. Almost all major institutions complied, except Norman Shumway in Stanford and Richard Lower at the Medical College of Virginia, who had developed the surgical technique of heart transplantation together with Shumway during his time in Stanford [5, 7, 14]. The Stanford team developed revised protocols for patient selection, since many of the pioneers had realised that the patients who had been selected for transplantation were too sick to survive. Furthermore, the crucial problem of monitoring and treating rejection was approached. Following experimental studies in the dog, Philip Caves at Stanford brought into clinical application the bioptom, which could be inserted through the jugular vein into the right ventricle to obtain pieces of the heart muscle for pathological examination on rejection [15, 16]. This method, which went into clinical routine at Stanford in 1973, made it possible to detect acute rejection earlier than it was previously possible, when the decrease of electrocardiogram voltages was used for diagnosing rejection. Immunosuppression could now be intensified at an earlier stage of acute rejection and, accordingly, be reduced again in time, after the rejection had resolved and before a potentially lethal infection could develop [12]. Margret Billingham at Stanford developed a classification for rejection based on the number of infiltrating leucocytes and the amount of damage to the cardiomyocytes [17–19]. On the basis of her studies, the first grading system of cardiac allograft rejection was later published in 1990 [20].
As a result of these advances, Shumway was able to improve the outcome after heart transplantation considerably. In the period from Shumway’s first heart transplant in 1968 until November 1980, the Stanford team achieved a remarkable 1-year actuarial survival of 68% [21]. The breakthrough in heart transplantation, however, came with the availability of ciclosporin. Its immunosuppressive activity was discovered by Borel and Stähelin in the laboratories of Sandoz in Basel, Switzerland [22, 23]. In 1980, ciclosporin was introduced into the clinical programme at Stanford, which improved outcomes significantly, with 1-year survival approaching 80% [14, 24, 25]. In September 1983, ciclosporin obtained approval by the FDA (Food and Drug Administration of the USA), which paved the way for worldwide clinical application of ciclosporin in transplantation medicine in general and in heart transplantation in particular [26]. Survival following heart transplantation improved significantly under the ciclosporin-based immunosuppressive regimen [21].
In addition to the advances in patient management before and after heart transplantation, as outlined above, the establishment of the brain death concept markedly contributed to the improved outcomes. In 1968, the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death published a report which comprised the first formal definition of brain death [27, 28]. This report was the basis for establishing laws for the legal recognition of brain death in most parts of the world. In the US, the legal basis for declaration of brain death was finalised in 1981 and published in a landmark article in the Journal of the American Medical Association [14, 29].
The key advantage for organ explantation was that the heart could be harvested without being exposed to warm ischaemia. Before brain death was accepted as definition of death, the heart could only be explanted after cessation of the heartbeat, which was considered the precondition to declare a patient dead. This, however, was associated with a period of progressive deterioration and finally stagnation of the circulation, which caused insufficient oxygen and energy supply to the heart in a warm environment. This so-called warm ischaemia is known to injure the heart with a negative impact on outcome after transplantation [30]. In brain dead donors, there is no need to wait for the cessation of the heartbeat. The beating heart can be arrested by cold cardioplegia and subsequently stored on ice. This induces a state of cold ischaemia, which is better tolerated by the myocardium than warm ischaemia [31, 32]. Lower and Shumway demonstrated in the laboratory in a dog model that a heart that was stored in 2–4°C saline for up to 7 hours could successfully be transplanted with normal physiological function [33]. They were the first to prove this concept in the clinic when they transplanted successfully a heart that was exposed to cold storage for transportation from the donor hospital in the periphery of Stanford to the recipient hospital [14]. This method of cold storage allowed the donor pool to expand, since not only hearts from in-house donors could be used, as had been practiced hitherto, but also those from peripheral hospitals [14, 34, 35].
The advances in patient selection and management, monitoring and treatment of rejection, as well as myocardial preservation, resulted in improved outcomes following heart transplantation [21]. This encouraged the global heart transplant community to restart their programmes. The number of heart transplants worldwide exponentially increased from 322 in 1983 to almost 5000 in 1993 [36]. In Zurich, Marko Turina launched the restart of the heart transplant programme in 1985. The number of heart transplants rose from 2 in 1985 to 34 in 1989 (fig. 2). After 1994, the number of heart transplants decreased in Zurich, as well as worldwide, mainly because of the increasing shortage of suitable donors [36] (fig. 2). In recent years, a slight increase in transplant numbers can be observed, which may be in part explained by the success of campaigns for donation and the acceptance for transplantation of donor hearts with higher risk.
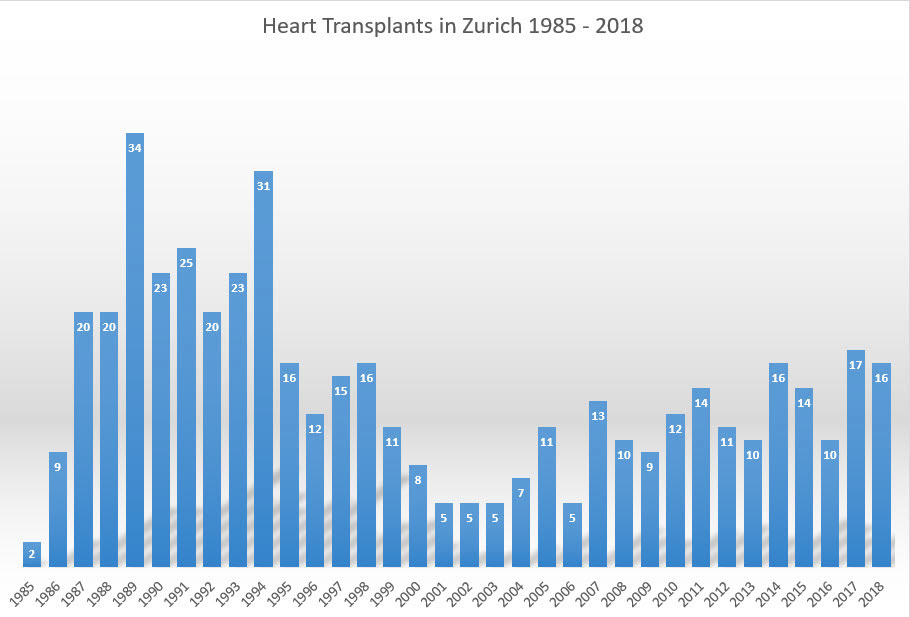
Figure 2 Number of heart transplants in Zurich from restart of the programme in 1985 until 2018.
Under the guidance of Marko Turina, several achievements in heart transplant surgery were accomplished in Zurich with considerable international impact, such as heart transplantation in patients with grown-up congenital heart disease (GUCH), the implementation of the bicaval instead of the standard right atrial anastomosis, and the re-transplantation of an already transplanted heart [37–39]. Although little experience existed worldwide, Zurich commenced heart transplantation in GUCH patients in the late 1980s. Using a modified donor cardiectomy with extensive length of the inflow and outflow tissues, the transplant procedure could be performed successfully without prosthetic material. Complex malformations could be reconstructed by applying the additional tissue taken with the donor heart such that the anatomically normal donor heart fitted in the recipient [37]. The application of the bicaval anastomosis as opposed to the right atrial anastomosis, which was previously the standard technique developed by Lower and Shumway, was associated with two advantages (fig. 3) [38]. Since the sinus node was transplanted with the donor heart, the need for pacemaker implantation decreased significantly, and the transplantation of the complete right atrium preserved its geometry, resulting in a significantly reduced incidence of tricuspid regurgitation. The retransplantation of an already transplanted heart in a second recipient after the first recipient suffered from brain death was recognised as world novelty and constituted a rational solution in an era of organ shortage [39].
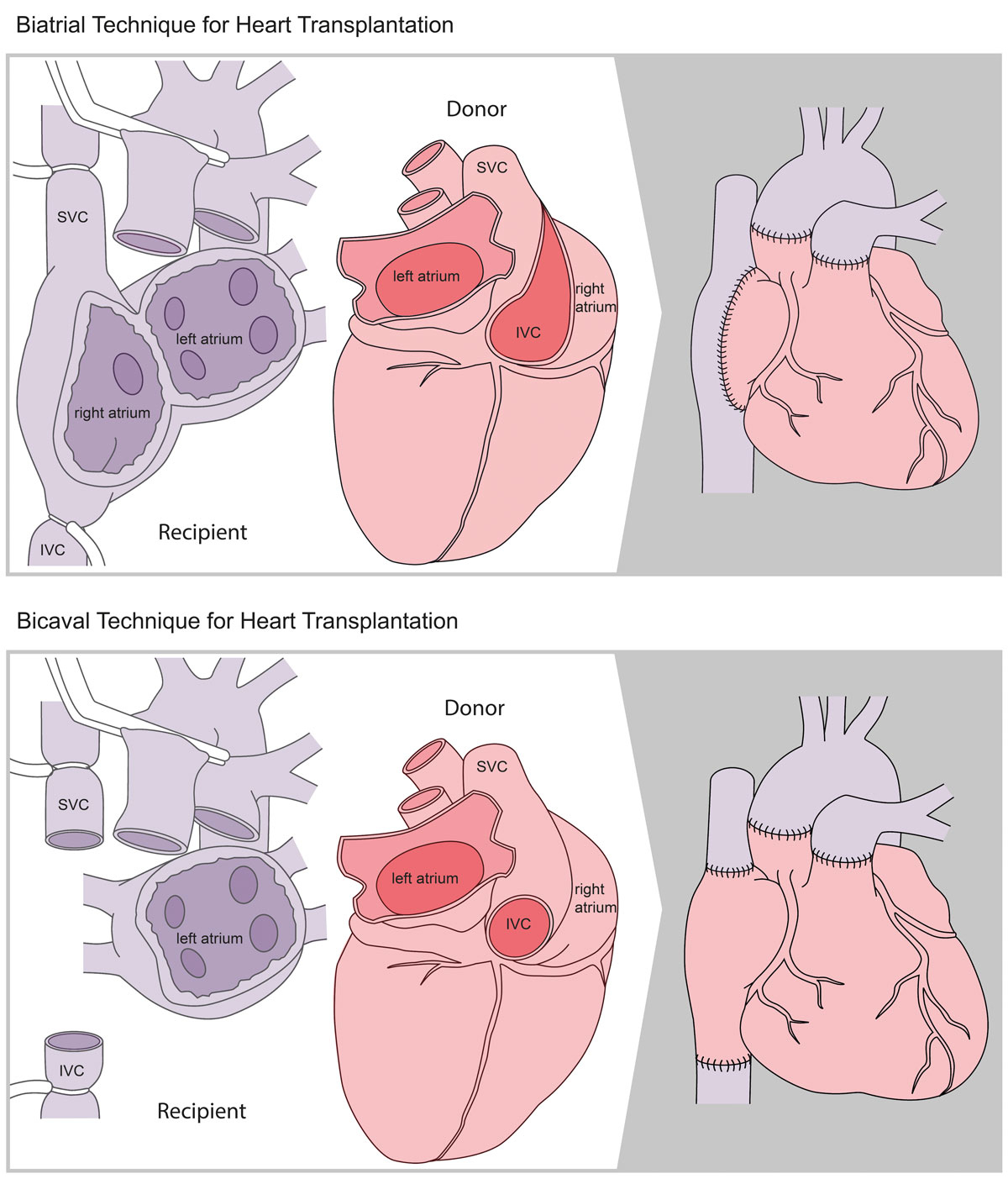
Figure 3 Illustration of the two different surgical techniques for heart transplantation. Upper panel: the classic biatrial technique. Lower panel: the modified bicaval technique.
Outcome following heart transplantation steadily improved, particularly in the early phase after transplantation. This may be attributed to advances in the perioperative management by a multidisciplinary team and a decrease in acute rejection episodes during the first year after transplant [36]. One-year survival improved from 77% in the 1980s to 85% in the 2010s, and 10-year survival increased from 63% to 75% in the same time period [36]. This trend could also be observed in Zurich, with survival rates being superior to the worldwide outcomes reported by the International Society for Heart and Lung Transplantation (ISHLT), particularly over the long term. Previously, we have analysed the long-term outcome of 133 patients transplanted in the first 7 years after restart of the programme in 1985 until 1991. We could show that 20-year actuarial survival was 55.6% (fig. 4), which surpasses the actual worldwide 20-year survival rate of 22.8% reported in the latest analysis of the ISHLT in 2018 [36, 40].
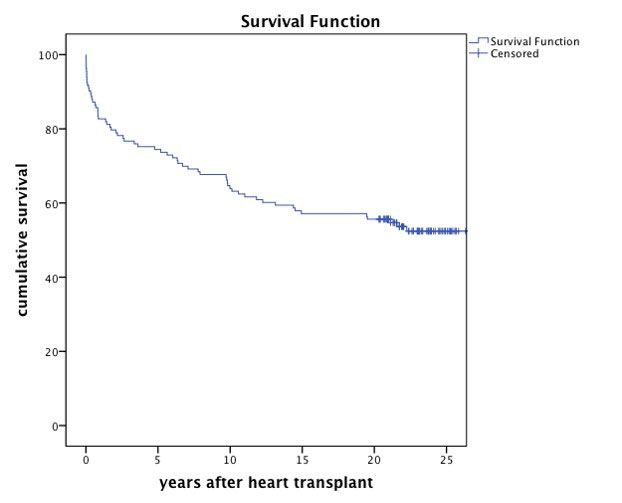
Figure 4 Long-term survival of the first 133 patients transplanted in Zurich from the restart of the programme in 1985 until 1991.
Although heart transplantation evolved into a success story over past decades, it faces some limitations in the current era. Because of the shortage of donors, the number of heart transplants has plateaued over the years in Switzerland and internationally [36, 41, 42]. Various measures have been proposed to enlarge the donor pool, such as the use of marginal donors and, most recently, the modification of the consent for donation and the utilisation of “donation after cardiocirculatory-determined death (DCD)” donors.
Conditions of marginal donors include primarily older age, prolonged ischaemia time, left ventricular hypertrophy and left ventricular dysfunction. Each of these factors has been shown to increase the risk of inferior outcome after transplantation [36, 43, 44]. However, there is notable evidence that transplantation of marginal donor hearts may achieve satisfying outcomes and, thus, is justifiable in order to counteract the donor shortage [45]. The appropriate management of the marginal donor in the period before explantation is the precondition for successful results [46].
The impact of the mode of consent for donation on the donation rate has been discussed controversially. In Switzerland, the “opt-in” or “informed consent” version implies that donation can only be done if the patient has previously agreed to donation and recorded it in a donation card or, if this is not the case, the relatives give their consent. Currently, only 5% of patients carry a donation card. Therefore, in the vast majority of cases, the relatives have to decide about organ donation of a loved one. But more than 50% of the relatives do not know the willingness of the patient to donate. This leads to a more than 50% refusal rate by the relatives of potential organ donors [47]. If the population could be encouraged to document their willingness or unwillingness to donate, the donation rate would probably rise, since, according to a representative survey in 2015, 85% of the Swiss population are in favour for organ donation. To increase the documentation rate, Swisstransplant decided to found a Swiss national organ donor registry which was implemented in October 2018. Within 3 months, 40000 people registered as potential donors [47].
In contrast to the “opt-in” consent, the “presumed consent“ or “opt-out consent” implies that people may become potential organ donors if they have neither explicitly refused organ donation nor documented their unwillingness to donate in a registry during their lifetime. In March 2019, an initiative was filed to the Swiss government that advocates for the change to an extended version of the presumed consent, the so-called “soft opt-out consent” [47]. Adoption of this concept means that the relatives are still asked for consent even though presumed consent could be considered if the deceased person has not rejected organ donation during their lifetime. The Federal Council supports the initiative and suggests a change in the transplant law that was enacted in 2007. The Ministry of Internal Affairs will elaborate a proposal that will be discussed on several political levels before the parliament votes on it.
Swisstransplant anticipates that the change from informed consent to presumed consent increases the donation rate from 18.6 per million people (pmp) in 2018 to more than 20 pmp. Other European countries, such as Spain and Austria, which have adopted the concept of presumed consent, achieved donation rates as high as 46.9 pmp in Spain and 23.5 pmp in Austria in 2017 [48, 49].
Donation after cardiocirculatory-determined death has been shown to achieve successful results in transplantation of organs such as kidneys, livers and lungs [50–53]. In recent years, DCD programs have also been established in heart transplantation. The outcomes can compete with those following transplantation from brain dead donors [4, 54]. As in transplantation of other solid organs, DCD heart transplantation may become a tool to expand the donor pool. Following the successes of the teams in England and Australia, other countries may be encouraged to implement such programmes. The Swisstransplant working group “Heart”, of which the corresponding author of this article is the current president, is currently in the process of evaluating the implementation of DCD heart transplantation in Switzerland.
Other current aspects of heart transplantation include the increasing number of recipients on ventricular assist systems, the growth in numbers of sensitised patients and the morbidities that develop over the long-term following heart transplantation, such as cardiac allograft vasculopathy and malignancy, which have a significant impact on outcome [36]. The discussion of these issues, however, goes beyond the focus of this article.
FR: since January 2018, no personal payments / all payments directly to the University of Zurich. Before 2018, the following conflicts of interest outside the submitted work are reported – grants and personal fees from SJM / Abbott Servier, Novartis and Bayer; personal fees from Zoll, Astra-Zeneca, BMS, Pfizer, Fresenius, Vifor, Roche, Boehringer Ingelheim and Heartware; research grant from Mars.
FM received grants and/or research support from Abbott, Medtronic, Edwards Lifesciences, Biotronic, Boston Scientific, NVT and Terumo; receives consulting fees / honoraria from Abbott, Medtronic, Edwards Lifesciences, SwissVortex, Zerfect, Xeltis, Transseptalsolutions, Cardiovalve and Magenta; has royalty income / international patent rights from Edwards Lifesciences; and is a shareholder of Cardiovalve, CardioGard, Magenta, SwissVortex, Transseptalsolutions, 4Tech and Zerfect.
1 Cooper DKC . Life’s defining moment: Christiaan Barnard and the first human heart transplant. J Heart Lung Transplant. 2017;36(12):1273–5. doi:.https://doi.org/10.1016/j.healun.2017.10.001
2 Barnard CN . The operation. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S Afr Med J. 1967;41(48):1271–4.
3 Turina M . Fifty years of heart transplantation. Cardiovasc Med. 2018;21:209–11.
4 Messer S , Page A , Axell R , Berman M , Hernández-Sánchez J , Colah S , et al. Outcome after heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant. 2017;36(12):1311–8. doi:.https://doi.org/10.1016/j.healun.2017.10.021
5 Lower RR , Shumway NE . Studies on orthotopic homotransplantation of the canine heart. Surg Forum. 1960;11:18–9.
6 Kantrowitz A , Haller JD , Joos H , Cerruti MM , Carstensen HE . Transplantation of the heart in an infant and an adult. Am J Cardiol. 1968;22(6):782–90. doi:.https://doi.org/10.1016/0002-9149(68)90173-2
7White T. Magical moment: The enormity of the first U.S. adult heart transplant. Stanford Medicine, https://stanmed.stanford.edu/2018winter/fifty-years-since-first-us-adult-heart-transplant-stanford.html
8 Thomson JG . Production of severe atheroma in a transplanted human heart. Lancet. 1969;294(7630):1088–92. doi:.https://doi.org/10.1016/S0140-6736(69)90700-4
9 Hosain N . Dr PK Sen - The Bengali Surgeon of the Century. Cardiovasc J. 2011;3(2):254–7. doi:.https://doi.org/10.3329/cardio.v3i2.9200
10 Cabrol C , Associates . Human heart transplantation. Am J Cardiol. 1968;22(6):833–7. doi:.https://doi.org/10.1016/0002-9149(68)90179-3
11 Hosain N , Amin F , Leprince P . Christian Cabrol MD: A Tribute to pioneer cardiac surgeon Christian Cabrol 1 year after his death, on the 50th Anniversary of the First European Human Heart Transplantation that he performed in 1968. Eur Heart J. 2018;39(19):1661–71. doi:.https://doi.org/10.1093/eurheartj/ehy195
12 English ST . The dark early years of heart transplantation: Some lessons learned. J Heart Lung Transplant. 2017;36(12):1279–82. doi:.https://doi.org/10.1016/j.healun.2017.10.005
13Swiss Television. Press Conference on April 14, 1969
14 Hess ML , Hunt S . Conquering the first hurdles in cardiac transplantation: In the footprints of giants. J Heart Lung Transplant. 2017;36(12):1276–8. doi:.https://doi.org/10.1016/j.healun.2017.10.007
15 Caves PK , Billingham ME , Schulz WP , Dong E, Jr , Shumway NE . Transvenous biopsy from canine orthotopic heart allografts. Am Heart J. 1973;85(4):525–30. doi:.https://doi.org/10.1016/0002-8703(73)90498-5
16 Caves PK , Stinson EB , Billingham M , Shumway NE . Percutaneous transvenous endomyocardial biopsy in human heart recipients. Experience with a new technique. Ann Thorac Surg. 1973;16(4):325–36. doi:.https://doi.org/10.1016/S0003-4975(10)65002-3
17 Billingham ME , Caves PK , Dong E, Jr , Shumway NE . The diagnosis of canine orthotopic cardiac allograft rejection by transvenous endomyocardial biopsy. Transplant Proc. 1973;5(1):741–3.
18 Billingham M , Warnke R , Weissman IL . The cellular infiltrate in cardiac allograft rejection in mice. Transplantation. 1977;23(2):171–5. doi:.https://doi.org/10.1097/00007890-197702000-00015
19 Caves PK , Stinson EB , Billingham ME , Rider AK , Shumway NE . Diagnosis of human cardiac allograft rejection by serial cardiac biopsy. J Thorac Cardiovasc Surg. 1973;66(3):461–6. doi:.https://doi.org/10.1016/S0022-5223(19)39805-8
20 Billingham ME , Cary NR , Hammond ME , Kemnitz J , Marboe C , McCallister HA , et al.; The International Society for Heart Transplantation. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. J Heart Transplant. 1990;9(6):587–93.
21 Robbins RC , Barlow CW , Oyer PE , Hunt SA , Miller JL , Reitz BA , et al. Thirty years of cardiac transplantation at Stanford university. J Thorac Cardiovasc Surg. 1999;117(5):939–51. doi:.https://doi.org/10.1016/S0022-5223(99)70375-2
22 Borel JF , Feurer C , Gubler HU , Stähelin H . Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976;6(4):468–75. doi:.https://doi.org/10.1007/BF01973261
23 Heusler K , Pletscher A . The controversial early history of cyclosporin. Swiss Med Wkly. 2001;131(21-22):299–302.
24 Oyer PE , Stinson EB , Jamieson SW , et al. One year experience with cyclosporin A in clinical heart transplantation. J Heart Transplant. 1982;1:285–90.
25 Grattan MT , Moreno-Cabral CE , Starnes VA , Oyer PE , Stinson EB , Shumway NE . Eight-year results of cyclosporine-treated patients with cardiac transplants. J Thorac Cardiovasc Surg. 1990;99(3):500–9. doi:.https://doi.org/10.1016/S0022-5223(19)36981-8
26 Kolata G . FDA speeds approval of cyclosporin. Science. 1983;221(4617):1273. doi:.https://doi.org/10.1126/science.221.4617.1273-a
27 A definition of irreversible coma. Report of the Ad Hoc Committee of the Harvard Medical School to examine the definition of brain death. JAMA. 1968;205(6):337–40. doi:.https://doi.org/10.1001/jama.1968.03140320031009
28 Nikas NT , Bordlee DC , Moreira M . Determination of death and the dead donor rule: A survey of the current law on brain death. J Med Philos. 2016;41(3):237–56. doi:.https://doi.org/10.1093/jmp/jhw002
29 Guidelines for the determination of death. Report of the medical consultants on the diagnosis of death to the President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. JAMA. 1981;246(19):2184–6. doi:.https://doi.org/10.1001/jama.1981.03320190042025
30 Marasco SF , Kras A , Schulberg E , Vale M , Lee GA . Impact of warm ischemia time on survival after heart transplantation. Transplant Proc. 2012;44(5):1385–9. doi:.https://doi.org/10.1016/j.transproceed.2011.12.075
31 Stoica SC , Satchithananda DK , Dunning J , Large SR . Two-decade analysis of cardiac storage for transplantation. Eur J Cardiothorac Surg. 2001;20(4):792–8. doi:.https://doi.org/10.1016/S1010-7940(01)00847-8
32 Ou R , Gavin JB , Esmore DS , Rosenfeldt FL . Increased temperature reduces the protective effect of University of Wisconsin solution in the heart. Ann Thorac Surg. 1999;68(5):1628–34, discussion 1634–5. doi:.https://doi.org/10.1016/S0003-4975(99)00713-4
33 Lower RR , Stofer RC , Hurley EJ , Dong E, Jr , Cohn RB , Shumway NE . Successful homotransplantation of the canine heart after anoxic preservation for seven hours. Am J Surg. 1962;104(2):302–6. doi:.https://doi.org/10.1016/0002-9610(62)90332-X
34 Thomas FT , Szentpetery SS , Mammana RE , Wolfgang TC , Lower RR . Long-distance transportation of human hearts for transplantation. Ann Thorac Surg. 1978;26(4):344–50. doi:.https://doi.org/10.1016/S0003-4975(10)62901-3
35 Watson DC , Reitz BA , Baumgartner WA , Raney AA , Oyer PE , Stinson EB , et al. Distant heart procurement for transplantation. Surgery. 1979;86(1):56–9.
36 Khush KK , Cherikh WS , Chambers DC , Goldfarb S , Hayes D, Jr , Kucheryavaya AY , et al.; International Society for Heart and Lung Transplantation. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplantation Report-2018; Focus Theme: Multiorgan Transplantation. J Heart Lung Transplant. 2018;37(10):1155–68. doi:.https://doi.org/10.1016/j.healun.2018.07.022
37 Carrel T , Neth J , Pasic M , Laske A , Jenni R , Maggiorini M , et al. Should cardiac transplantation for congenital heart disease be delayed until adult age? Eur J Cardiothorac Surg. 1994;8(9):462–8, discussion 469. doi:.https://doi.org/10.1016/1010-7940(94)90015-9
38 Laske A , Carrel T , Niederhäuser U , Pasic M , von Segesser LK , Jenni R , et al. Modified operation technique for orthotopic heart transplantation. Eur J Cardiothorac Surg. 1995;9(3):120–6. doi:.https://doi.org/10.1016/S1010-7940(05)80057-0
39 Pasic M , Gallino A , Carrel T , Maggiorini M , Laske A , von Segesser L , et al. Reuse of a transplanted heart. N Engl J Med. 1993;328(5):319–20. doi:.https://doi.org/10.1056/NEJM199302043280505
40 Rodriguez Cetina Biefer H , Sündermann SH , Emmert MY , Enseleit F , Seifert B , Ruschitzka F , et al. Surviving 20 years after heart transplantation: a success story. Ann Thorac Surg. 2014;97(2):499–504. doi:.https://doi.org/10.1016/j.athoracsur.2013.08.040
41 Schaub S , Immer F , Steiger J . Organ Transplantation in Switzerland. Transplantation. 2019;103(5):853–6. doi:.https://doi.org/10.1097/TP.0000000000002565
42Swisstransplant. Annual Report 2018, p 32. Available at: https://www.swisstransplant.org/fileadmin/user_upload/Swisstransplant/Jahresbericht/Jahresbericht_und_Grafiken_2018/Swisstransplant_Jahresbericht_2018.pdf
43 Wever Pinzon O , Stoddard G , Drakos SG , Gilbert EM , Nativi JN , Budge D , et al. Impact of donor left ventricular hypertrophy on survival after heart transplant. Am J Transplant. 2011;11(12):2755–61. doi:.https://doi.org/10.1111/j.1600-6143.2011.03744.x
44 Costanzo MR , Dipchand A , Starling R , Anderson A , Chan M , Desai S , et al., International Society of Heart and Lung Transplantation Guidelines. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29(8):914–56. doi:.https://doi.org/10.1016/j.healun.2010.05.034
45 Wittwer T , Wahlers T . Marginal donor grafts in heart transplantation: lessons learned from 25 years of experience. Transpl Int. 2008;21(2):113–25. doi:.https://doi.org/10.1111/j.1432-2277.2007.00603.x
46 Wheeldon DR , Potter CD , Oduro A , Wallwork J , Large SR . Transforming the “unacceptable” donor: outcomes from the adoption of a standardized donor management technique. J Heart Lung Transplant. 1995;14(4):734–42.
47Swisstransplant. Annual report 2018, pp 12–14. Available at: https://www.swisstransplant.org/fileadmin/user_upload/Swisstransplant/Jahresbericht/Jahresbericht_und_Grafiken_2018/Swisstransplant_Jahresbericht_2018.pdf
48Nacional de Trasplantes O. Memoria de Actividad 2017: Actividad de donación, p 1. Available at: http://www.ont.es/infesp/Memorias/Forms/AllItems.aspx
49Eurotransplant. Statistical report 2017: Donation, waiting lists and transplants, p 2. Available at: https://www.eurotransplant.org/cms/mediaobject.php?file=803150+020288+Statistical+Report+2017+%28online%291.pdf
50 Pitarch Martínez M , Sánchez Pérez B , León Díaz FJ , Fernández Aguilar JL , Pérez Daga JA , Montiel Casado MC , et al. Donation After Cardiac Death in Liver Transplantation: An Additional Source of Organs With Similar Results to Donation After Brain Death. Transplant Proc. 2019;51(1):4–8. doi:.https://doi.org/10.1016/j.transproceed.2018.02.208
51 Schlegel A , Muller X , Kalisvaart M , Muellhaupt B , Perera MTPR , Isaac JR , et al. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J Hepatol. 2019;70(1):50–7. doi:.https://doi.org/10.1016/j.jhep.2018.10.005
52 Summers DM , Watson CJ , Pettigrew GJ , Johnson RJ , Collett D , Neuberger JM , et al. Kidney donation after circulatory death (DCD): state of the art. Kidney Int. 2015;88(2):241–9. doi:.https://doi.org/10.1038/ki.2015.88
53 Ceulemans LJ , Inci I , Van Raemdonck D . Lung donation after circulatory death. Curr Opin Organ Transplant. 2019;24(3):288–96. doi:.https://doi.org/10.1097/MOT.0000000000000627
54 Chew HC , Iyer A , Connellan M , Scheuer S , Villanueva J , Gao L , et al. Outcomes of Donation After Circulatory Death Heart Transplantation in Australia. J Am Coll Cardiol. 2019;73(12):1447–59. doi:.https://doi.org/10.1016/j.jacc.2018.12.067
FR: since January 2018, no personal payments / all payments directly to the University of Zurich. Before 2018, the following conflicts of interest outside the submitted work are reported – grants and personal fees from SJM / Abbott Servier, Novartis and Bayer; personal fees from Zoll, Astra-Zeneca, BMS, Pfizer, Fresenius, Vifor, Roche, Boehringer Ingelheim and Heartware; research grant from Mars.
FM received grants and/or research support from Abbott, Medtronic, Edwards Lifesciences, Biotronic, Boston Scientific, NVT and Terumo; receives consulting fees / honoraria from Abbott, Medtronic, Edwards Lifesciences, SwissVortex, Zerfect, Xeltis, Transseptalsolutions, Cardiovalve and Magenta; has royalty income / international patent rights from Edwards Lifesciences; and is a shareholder of Cardiovalve, CardioGard, Magenta, SwissVortex, Transseptalsolutions, 4Tech and Zerfect.