Implementation of a multiprofessional, multicomponent delirium management guideline in two intensive care units, and its effect on patient outcomes and nurse workload: a pre-post design retrospective cohort study
DOI: https://doi.org/10.4414/smw.2020.20185
Maria
Schubertab, Dominique
Bettexc, Peter
Steigerd, Roger
Schürchef, Alois
Hallerg, Jasmina
Bogdanovich, David Garcia
Nuñezij, Urs
Schwarzk, Martin
Siegemundl
aSchool of Health Professions, ZHAW Zurich University of Applied Science, Winterthur, Switzerland
bCentre of Clinical Nursing Science, University Hospital Zurich, Switzerland
cInstitute of Anaesthesiology, University of Zurich and University Hospital Zurich, Switzerland
dDivision of Surgical Intensive Care, University Hospital Zurich, Switzerland
eDepartment of Entomology, Virginia Tech, Blacksburg, USA
fClinical Trial Unit, Institute of Social and Preventive Medicine, University of Bern, Switzerland
gCentre for Intensive Care, Cantonal Hospital Winterthur, Switzerland
hFaculty of Medicine, Department Public Health, Institute of Nursing Science, University of Basel, Switzerland
iDepartment of Psychiatry and Psychotherapy, University Hospital Zurich, Switzerland
jCentre for Gender Variance, University Hospital Basel, Switzerland
k Division of Neurology, University of Zurich and University Hospital Zurich, Switzerland
lIntensive Care Unit, Department of Intensive Care Medicine, University Hospital Basel, Switzerland
Summary
AIM OF THE STUDY
Delirium is a frequent intensive care unit (ICU) complication, affecting 26% to 80% of ICU patients, often with serious consequences. This study aimed to evaluate the effectiveness, costs and benefits of following a standardised multiprofessional, multicomponent delirium guideline on eight outcomes: delirium prevalence and duration, lengths of stay in ICU and hospital, in-hospital mortality, duration of mechanical ventilation, and cost and nursing hours per case. It also aimed to explore the associations of delirium with length of ICU stay, length of hospital stay and duration of mechanical ventilation.
METHODS
This retrospective cohort study used a pre-post design. ICU patients in an historical control group (n = 1608) who received standard ICU care were compared with a postintervention group (n = 1684) who received standardised delirium management – delirium risk identification, preventive measures, screening and treatment – with regard to eight outcomes. The delirium management guideline was developed and implemented in 2012 by a group of experts from the study hospital. As appropriate, descriptive statistics and multivariate, multilevel models were used to compare the two groups and to explore the association between delirium occurrence and the selected outcomes.
RESULTS
Twelve percent of the 1608 historical controls and 20% of the 1684 postintervention patients were diagnosed with delirium according to the ICD-10 delirium diagnosis codes. Patients being treated for heart disease, and those with septic shock, ARDS, renal insufficiency (acute or chronic), older age and higher numbers of comorbidities were significantly more likely to develop delirium during their stay. Multivariate models comparing the historical controls with the post intervention group indicated significant differences in delirium period prevalence (odds ratio 1.68, 95% confidence interval [CI] 1.38–2.06; p <0.001), length of stay in the ICU (time ratio [TR] 0.94, CI 0.89–1.00; p = 0.048), cost per case (median difference 3.83, CI 0.54–7.11; p = 0.023) and duration of mechanical ventilation (TR 0.84, CI 0.77–0.92; p <0.001). The observed differences in the other four outcomes – in-hospital mortality, delirium duration, length of stay in the hospital, and nursing hours per case – were not significant. Delirium was a significant predictor for prolonged duration of mechanical ventilation and for both ICU and hospital stay.
CONCLUSION
Standardised delirium management, specifically delirium screening, supports timely detection of delirium in ICU patients. Increased awareness of delirium after the implementation of standardised multiprofessional, multicomponent management leads to increased therapeutic attention, a prolongation of ICU stay and increased costs, but with no influence on mortality.
Abbreviations:
- ARDS
-
acute respiratory distress syndrome
- CHOP
-
CH (Swiss) Operation Classification
- FSO
-
Federal Statistical Office
- LEP
-
Leistungserfassung in der Pflege, (care performance and process documentation)
- LOS
-
length of stay
- ICD-10
-
International Statistical Classification of Diseases and Related Health Problems 10th Revision
- ICDSC
-
Intensive Care Delirium Screening Checklist
- ICU
-
intensive care unit
- MDSi
-
minimal data set ICU
- OR
-
odds ratio
- RASS
-
Richmond Agitation Sedation Scale
- SAPS II
-
Simplified Acute Physiology Score II
- TR
-
time ratio
Introduction
Delirium – a sudden, acute deterioration of mental status – is a frequent complication in intensive care unit (ICU) patients. Its reported prevalence and incidence rates in ICU patients vary between 26% and 80% [1–3]. Delirium is linked with negative patient and economic outcomes. Compared with non-delirious ICU patients, the delirious are six times more likely to develop further complications, especially acute respiratory distress syndrome (ARDS), pneumonia, pulmonary oedema or cardiac arrhythmias [4, 5] and to need skilled care, i.e., rehabilitation, after discharge [4, 5]. Further, they are two to three times as likely to die [4–8], stay on average seven to eight days longer in the ICU [4, 5, 8], and require an average of seven more days of mechanical ventilation [4, 5]. The reported additional cost attributable to delirium ranges from EUR ~1,200 (USD ~1300) in a German study [8] to USD 9014 in a USA study [9]. Assuming that 721,600 to 2,263,200 ICU patients develop delirium each year in the US, this corresponds to additional annual healthcare costs ranging from USD 6.6 billion to 20.4 billion [9]. A recent Swiss study calculated that the cost in cases involving delirium was twice as high as in those that did not (respectively CHF 40,000 vs 16,662) [10].
Delirium can result from a multitude of factors. Predisposing and precipitating factors such as age (≥65 years), previous delirium, male gender, dementia, comorbidities such as heart failure, visual and/or auditory impairment, psychoactive medication, and regular consumption of tobacco or alcohol. In ICU patients, these factors commonly converge with intraoperative events and serious conditions such as polytrauma, sepsis, shock, infection, stroke, requirement for mechanical support or transcutaneous pacing, antiarrhythmic agents or use of therapeutic hypothermia, for example after a cardiac arrest [1–3, 11, 12]. The resulting accumulation of predisposing risk factors and precipitators greatly increases the risk of delirium.
For appropriate delirium management, early identification of relevant risk factors is crucial. With this in mind, standardised delirium management routines can increase recognition of at-risk patients [13]. In recent years, several single and multicomponent delirium management guidelines have been developed. Of these, multiprofessional, multicomponent delirium practice guidelines focusing on the prevention, screening, diagnosis and treatment of delirium offer the most effective strategy to minimise delirium rates, related symptoms, associated prolonged length of stay (LOS) and overall in-patient costs [13–16]. However, evidence supporting the benefits and efficiency of such multicomponent guidelines is rare. To date, research has focused predominantly on techniques to improve delirium screening – to help identify delirium onset or delirium-risk patient, and / or to measure the benefits of pharmacological treatments [13] [17]. Some researchers have simultaneously applied multiple guidelines dealing, for example, with pain, agitation and delirium, and studied the effects on selected outcomes [14, 18].
Noting the high frequency of delirium in ICU patients in the study hospital (a Swiss tertiary teaching hospital), a multiprofessional quality improvement and practice development project was launched in 2011 to improve the prevention, early recognition and appropriate treatment of delirium in hospitalised patients. In the context of this project, an interprofessional project team developed, implemented and evaluated an evidence-based multiprofessional, multicomponent delirium management guideline [10, 19–21].
This study is part of a larger delirium health service programme Delir-Path. The aim of this study was to evaluate the effectiveness, costs and benefits of the implemented standardised delirium management guidelines by comparing ICU patients in an historical control (preintervention) group with those in a postintervention group, with regard to eight outcomes: LOS in the ICU and hospital, in-hospital mortality, delirium prevalence and duration, duration of mechanical ventilation, cost and nursing hours per case. We hypothesised that (1) the postintervention group’s LOS in the ICU and (2) in hospital would be shorter, (3) in-hospital mortality and (4) delirium prevalence rates would be lower, (5) delirium duration in those who developed a delirium would be shorter, (6) the duration of mechanical ventilation would be shorter and (7) the cost and (8) nursing hours per case would be lower; further, we hypothesised that delirium in surgical ICU patients would be significantly associated with (9) longer ICU LOS, (10) hospital LOS and (11) mechanical ventilation duration.
Materials and methods
Study design, setting and sampling
In this single centre cohort study, which used a pre-post design to allow evaluation of the effects efficiency and costs of the implemented standardised delirium management guideline, an historical group of 1608 patients treated in two surgical ICUs (before implementation of this guideline) were compared with a postintervention group of 1684 ICU patients treated in the same ICUs after the introduction of the guideline, with reference to the selected outcomes. All eligible patients consecutively admitted to a cardio-surgical ICU (ICU 1) and a thoracic-visceral-surgical ICU (ICU 2) from 1 January 1 to 31 December 2013 were assigned to the postintervention group. Those admitted to the same ICUs between January and December 2011 were assigned to the historical control group. To be included, patients had to fulfil the following criteria: (1) adult (age ≥18 years) ICU patient and (2) no history of abuse of medication or other substances, except possibly alcohol.
Intervention and standard care
In addition to standard ICU care, patients in the postintervention group received standardised multiprofessional delirium management following the chosen guideline. This guideline includes four main components:
-
-
-
- For patients with delirium,
The professional and interprofessional responsibilities for each component are colour-coded for quick access.
As a first step, the delirium management guideline was implemented in nine units (seven non-ICUs, two ICUs) using practice development and quality improvement strategies including specific interprofessional clinic/unit teams. In addition to implementing the guideline, this team supported the nurses and physicians, answered related questions, and delivered a delirium education programme of professional and interprofessional lectures and eLearning components. Using techniques including feedback loops, they also monitored delirium prevalence rates, numbers of patients screened for delirium and numbers gauged at risk for a delirium. The benefits and efficiency of the guideline were evaluated after 6 months. Because of the indicated benefits, the standardised delirium management system was implemented in all in-patient units using the same implementation strategies [10, 19–21].
Patients in the control group (hospitalised in the study hospital before the implementation of the standardised delirium management) received standard care. This included all necessary standard intensive care therapy (e.g., ventilation, pharmacological treatment to stabilise cardiac and/or circulatory disturbances and, if necessary, sedation management), with any non-standardised delirium prevention, diagnosis and treatment in use at that time.
Primary and secondary outcomes
The three primary outcomes were: (1) LOS (in days) in the ICU: the number of days the patient was hospitalised in the ICU; (2) LOS in hospital: the number of days the patient was hospitalised in the hospital, including the ICU stay; (3) in-hospital mortality: the percentage of patients who died at any time during hospitalisation, either in the ICU or elsewhere.
The five secondary outcomes were: (1) ICU period prevalence of delirium: the percentage of patients who, at any time during their ICU stay, were delirious, based on an ICD-10 delirium diagnosis (F050, F051, F058, F059, F104) and additionally (in the postintervention group) on one or more ICDSC assessment scores (ICDSC ≥4; (2) delirium duration: the number of days a postintervention group patient, who was screened with the ICDSC, spent in a delirium (ICDSC Score ≥4); (3) duration of mechanical ventilation: the number of days the patient spent on a ventilator; (4) costs: the sum of all individual health care costs and overheads per case; and (5) nursing workload: the number of hours spent per case on direct patient care.
Primary and secondary outcomes were adjusted for the following confounders (delirium risk factors, delirium causes): gender (male/female), age, septic shock, ARDS, acute renal insufficiency, chronic renal insufficiency, hepatic insufficiency (based on ICD-10 classification), sedation (based on RASS data [23]), Simplified Acute Physiology Score II (SAPS II) [24] and Charlson comorbidity index [25]. Further variables used were medical diagnosis (ICD-10), treatment/interventions: heart intervention such as cardiovascular surgery, heart valves, heart septum surgeries, neurological intervention such as computed tomography, fluoroscopy or sonography, injection of anaesthetic and analgesic into the spinal canal for pain therapy) codes of Swiss Operation (CHOP) classification [26], alcohol dependency (ICD-10; yes/no) and type of admission. The relevant data (on primary and secondary outcomes) were extracted from the following databases: Minimal Data Set ICU (MDSi) [27], Swiss Federal Statistical Office (FSO) data set [28], ICDSC Assessment data [22], Care Performance and Process Documentation (Leistungserfassung in der Pflege; LEP) [29].
Data analysis
Descriptive statistics (mean and ± standard deviation, median with 25% and 75% quartiles, or counts and percentage rates) were used to describe characteristics of the patients in the postintervention and control groups based on ten baseline values. ICD-10 diagnoses [30] and interventions were categorised into five groups (see table 1 below). Missing values were replaced with the normal value where appropriate (e.g., a patient with no alcohol abuse diagnosis was assumed not to abuse alcohol). We conducted a multivariate, multilevel analysis to explore the effect, efficiency and costs of the implemented standardised delirium management guideline with respect to the selected primary and secondary outcomes. In the crude and multivariate analysis, patients who stayed longer than 7 days in the ICU or 60 days in the hospital, who died in the ICU or were mechanically ventilated for longer than 48 hours were censored. Censoring was chosen for mechanical ventilation because the majority of patients were ventilated for less than 48 hours. The restricted mean LOS (95% CI) presented was also restricted with respect to 7- and 60-day boundaries. To compare the LOS duration of delirium and mechanical ventilation between the postintervention group and the historical control group, we used parametric survival models with accelerated failure time metrics. In-hospital mortality was compared using binomial generalised linear models, with delirium as a predictor. Delirium ICU and hospitalisation period prevalences were compared with logistic regression based on ICD-10 diagnoses [30] and ICDSC scores [19]. In the case of ICDSC scores the analysis was restricted to ICU 1, as only this unit had scores for both 2011 and 2013. We used quantile regression methods to analyse the highly skewed data regarding distributed costs and nursing hours per case, and binomial generalised linear models (logistic regressions) to analyse the association between delirium and in-hospital mortality. For these we present both parametrically modelled and Kaplan-Meier time-to-event curves. ICU LOS and mechanical ventilation duration were modelled using a gamma distribution. Hospital LOS was modelled using log-logistic distribution, and delirium duration was modelled using a log-normal distribution. Distributions for these time-to-event models were selected based on the Akaike Information Criterion [31]. Statistical analyses were performed in Stata 13.0 and R 3.1.1. Figures were created in R 3.1.1 [32]. All tests presented use a two-tailed α = 0.05.
Table 1 Baseline characteristics of the historical control group and postintervention group.
| |
Total
(2011, 2013)
|
Historical control
(2011)
|
After intervention
(2013)
|
p-value
|
| N/n |
3292 |
1608 |
1684 |
|
| Gender female, n (%) |
1025 (31%) |
499 (31%) |
526 (31%) |
0.91 |
| Age at admission (years), mean ± SD |
61.8 ± 14.5 |
61.8 ± 14.4 |
61.8 ± 14.6 |
0.905 |
| Admission planned, n (%) |
2011 (61%) |
976 (61%) |
1035 (61%) |
0.668 |
| Alcohol abuse (ICD-10 codes), n (%) |
94 (3%) |
49 (3%) |
45 (3%) |
0.532 |
| RASS at baseline, mean ± SD |
−2.6 ± 1.9 |
−2.6 ± 1.9 |
−2.5 ± 1.9 |
0.440 |
| SAPS II, mean ± SD |
33.0 ± 17.0 |
31.1 ± 16.3 |
34.8 ± 17.4 |
<0.001 |
| Charlson comorbidity index score, mean ± SD |
2.5 ± 1.5 |
2.5 ± 1.5 |
2.4 ± 1.5 |
0.281 |
| Interventions (MDSi), n (%) |
Heart interventions |
2038 (62%) |
1004 (62%) |
1034 (61%) |
0.542 |
| Neurological interventions |
12 (0%) |
0 (0%) |
12 (1%) |
<0.001 |
| Septic shock, n (%) |
245 (7%) |
109 (7%) |
136 (8%) |
0.163 |
| ARDS, n (%) |
87 (3%) |
41 (3%) |
46 (3%) |
0.828 |
| Acute renal insufficiency, n (%) |
557 (17%) |
261 (16%) |
296 (18%) |
0.307 |
| Chronic renal insufficiency, n (%) |
364 (11%) |
179 (11%) |
185 (11%) |
0.912 |
| Hepatic insufficiency, n (%) |
64 (2%) |
27 (2%) |
37 (2%) |
0.313 |
| ICD-10 chapter N/n |
3226 |
1601 |
1625 |
0.093 |
| |
Diseases of circulatory system, n (%) |
1922 (60%) |
916 (57%) |
1006 (62%) |
| Neoplasms, n (%) |
440 (14%) |
233 (15%) |
207 (13%) |
| Diseases of the digestive system, n (%) |
200 (6%) |
109 (7%) |
91 (6%) |
| Diseases of respiratory system, n (%) |
157 (5%) |
80 (5%) |
77(5%) |
| Other, n (%) |
507 (16%) |
263 (16%) |
244 (15%) |
| CHOP Main intervention N/n |
3219 |
1600 |
1619 |
<0.001 |
| |
Heart (non-aorta), n (%) |
1801 (56%) |
892 (56%) |
909 (56%) |
| Gastrointestinal tract, n (%) |
407 (13%) |
226 (14%) |
181 (11%) |
| Respiratory system, n (%) |
233 (7%) |
130 (8%) |
103 (6%) |
| Transplantation, n (%) |
202 (6%) |
112 (7%) |
90 (6%) |
| Other, n (%) |
576 (18%) |
240 (15%) |
336 (21%) |
| CHOP Secondary intervention N/n |
3205 |
1582 |
1623 |
<0.001 |
| |
Heart (non-aorta), n (%) |
1391 (43%) |
788 (50%) |
603 (37%) |
| Gastrointestinal tract, n (%) |
277 (9%) |
175 (11%) |
102 (6%) |
| Respiratory system, n (%) |
175 (5%) |
108 (7%) |
67 (4%) |
| No intervention, n (%) |
143 (4%) |
4 (0%) |
139 (9%) |
| Other, n (%) |
1219 (38%) |
507 (32%) |
712 (44%) |
Ethical considerations
This study (PB_2016-01264) was approved by the responsible ethics board of the Kantonale Ethikkommission des Kanton Zurich and carried out in accordance with the Declaration of Helsinki, taking into consideration local regulations and standards.
Results
Description of the postintervention and historical control groups
A total of 3496 patients (1620 in 2011, 1876 in 2013) were hospitalised following cardiac or thoracic visceral surgery in the two selected ICUs during the study periods. Based on the inclusion criteria listed above, 204 patients were excluded. Of the 3292 patients included, 1608 hospitalised in 2011 were assigned to the (historical) control group and 1684 hospitalised in 2013 were assigned to the postintervention group. Besides considerable differences in the SAPS II there were no notable differences between the two groups in terms of baseline characteristics (table 1).
Effectiveness, costs, benefits of the delirium management guideline
Delirium prevalence
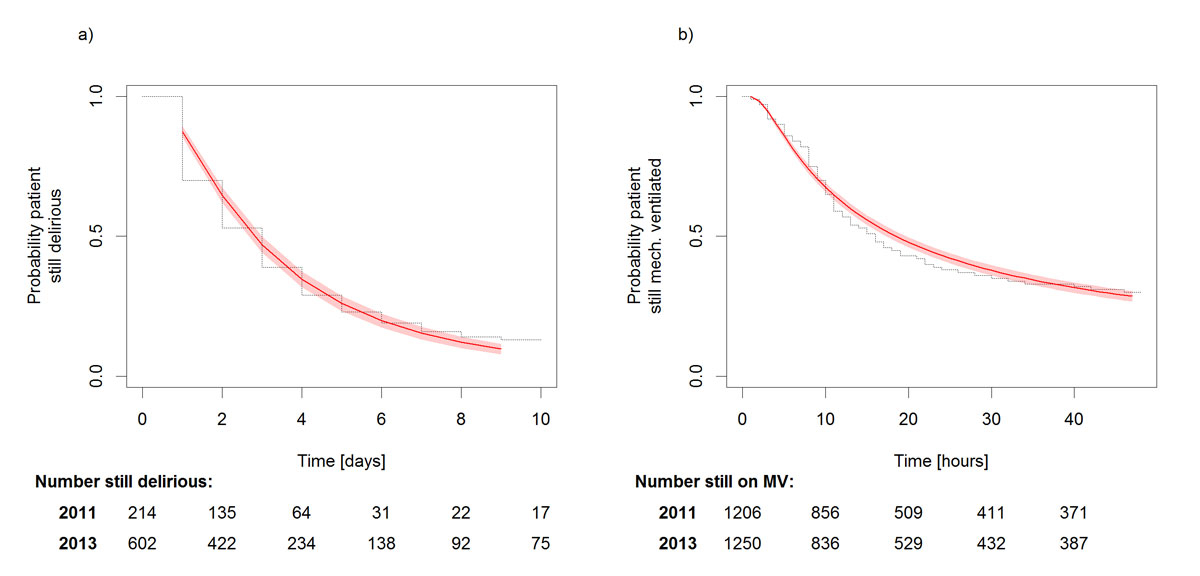
Of the 3292 included patients, 534, 200 (12%) in the control group and 334 (21%) in the postintervention group received a delirium diagnosis based on their ICD-10 code. Based on the ICDSC scores ≥4, 816 patients, 214 in the control group and 602 in the postintervention group patients, were diagnosed as delirious. When interpreting these results, it should be borne in mind that in 2011 the ICDSC assessment was implemented in only one of the ICUs, in the context of a nursing thesis. However, in both the crude and the adjusted model, the changes in the delirium rate over time were significant (table 2).
Table 2 Crude odds ratios for delirium incidence based on ICD-10 or ICDSC, and delirium duration.
|
Variable
|
Delirium incidence ICD-10
|
Delirium incidence ICDSC
|
Delirium duration ICDSC
|
|
n/N
|
OR (95% CI)
|
p-value
|
n/N
|
OR (95% CI)
|
p-value
|
n
|
Mean (95% CI)
|
TR (95% CI)
|
p-value
|
| Overall |
534/3292 |
|
|
816/3292 |
|
|
816 |
3.7 (3.5–4.0) |
|
|
| Year 2011 |
200/1608 |
|
|
214/1608 |
|
|
214 |
3.2 (2.9–3.6) |
|
|
| Year 2013 |
334/1684 |
1.8 (1.4–2.1) |
<0.001 |
602/1684 |
3.6 (3.0–4.3) |
<0.001 |
602 |
3.9 (3.7–4.2) |
1.2 (1.1–1.4) |
0.006 |
| Gender |
Male |
375/2267 |
|
|
538/2267 |
|
|
538 |
3.9 (3.6–4.1) |
|
|
| Female |
159/1025 |
0.9 (0.8–1.1) |
0.463 |
278/1025 |
1.2 (1.0–1.4) |
0.037 |
278 |
3.5 (3.2–3.9) |
0.9 (0.8–1.1) |
0.324 |
| Heart intervention |
No |
173/1254 |
|
|
258/1254 |
|
|
258 |
4.5 (4.0–4.9) |
|
|
| Yes |
361/2038 |
1.3 (1.1–1.6) |
0.004 |
558/2038 |
1.5 (1.2–1.7) |
<0.001 |
558 |
3.43 (3.20–3.7) |
0.8 (0.7–0.9) |
0.003 |
| Neurological intervention |
No |
532/3280 |
|
|
809/3280 |
|
|
809 |
3.8 (3.5–4.0) |
|
|
| Yes |
2/12 |
1.0 (0.2–4.7) |
0.968 |
7/12 |
4.3 (1.4–13.5) |
0.013 |
7 |
3.9 (1.0–6.8) |
0.8 (0.4–1.7) |
0.618 |
| Septic shock |
No |
448/3047 |
|
|
700/3047 |
|
|
700 |
3.24 (3.04–3.44) |
|
|
| Yes |
86/245 |
3.2 (2.4–4.2) |
< 0.001 |
116/245 |
3.0 (2.3–3.9) |
<0.001 |
116 |
7.02 (6.37–7.66) |
2.9 (2.4–3.5) |
<0.001 |
| ARDS |
No |
506/3205 |
|
|
775/3205 |
|
|
775 |
3.59 (3.38–3.80) |
|
|
| Yes |
28/87 |
2.5 (1.6–4.0) |
<0.001 |
41/87 |
2.8 (1.8–4.2) |
<0.001 |
41 |
6.8 (5.7–7.8) |
2.6 (1.9–3.5) |
<0.001 |
| AKI |
No |
367/2735 |
|
|
555/2735 |
|
|
775 |
3.6 (3.4–3.8) |
|
|
| Yes |
167/557 |
2.8 (2.2, 3.4) |
<0.001 |
261/557 |
3.5 (2.9–4.2) |
<0.001 |
41 |
6.8 (5.7–7.8) |
2.6 (1.9–3.5) |
<0.001 |
| CKD |
No |
440/2928 |
|
|
674/2928 |
|
|
674 |
3.51 (3.28–3.73) |
|
|
| Yes |
94/364 |
2.0 (1.5–2.6) |
<0.001 |
142/364 |
2.1 (1.7–2.7) |
<0.001 |
142 |
4.85 (4.30–5.40) |
1.5 (1.3–1.8) |
<0.001 |
| Hepatic failure |
No |
522/3228 |
|
|
788/3228 |
|
|
788 |
3.62(3.41–3.83) |
|
|
| Yes |
2/64 |
1.1 (0.6–2.3) |
0.584 |
28/64 |
2.4 (1.5–4.0) |
<0.001 |
28 |
7.86 (6.61–9.11) |
3.8 (2.5–5.8) |
<0.001 |
In the crude model using ICD-10-based delirium diagnoses, compared with the overall sample, patients treated for heart disease, and those with septic shock, ARDS, acute kidney injury, chronic kidney disease, older age and higher numbers of comorbidities were significantly more likely to develop delirium during their stay. Neither female gender, neurological disease nor hepatic failure increased the probability of developing delirium. However, the crude model using ICDSC-diagnosed delirium indicated that all the tested factors increased the probability of developing a delirious state (table 2). In both crude models, baseline RASS scores were significantly linked with delirium (table 2).
Delirium duration
Five hundred and seventy-two ICU patients identified as delirious (ICDSC ≥4) spent a mean of 3 days (3.56 in 2013, 3.29 in 2011) delirious. Changes in the time ratio for delirium duration before versus after introduction of the delirium management guideline were not significant in either the adjusted or the crude models (table 2). Compared with the other patients, those with septic shock, ARDS, acute kidney injury, chronic kidney disease or hepatic failure remained significantly longer in delirium, whereas those with heart disease had a significantly shorter mean duration. Gender, age, neurological or other interventions, baseline RASS level and number of comorbidities had no significant effect on delirium duration (table 2).
LOS in ICU and hospital
Patients typically stayed roughly 2.5 days in the ICU (2011: 2.48, 2013: 2.56) with overall stays of 17 days (in 2011) and 18 days (in 2013) in the hospital (table 3). The crude model indicated that postintervention patients stayed on average significantly longer in the ICU, but not significantly longer in the hospital than the historical controls (table 3). Adjusting for potential confounders confirmed these findings and their significance (fig. 1).
Table 3 Effectiveness, costs and benefits of the delirium management guideline.
|
Endpoints/outcomes
|
2011
|
2013
|
Crude models
(2013/ 2011)
|
Adjusted models
(2013/2011)
|
|
Logistic regression models
|
n/N
|
OR (95% CI)
|
p-value
|
OR (95% CI)
|
p-value
|
| Delirium prevalence |
ICD-10 |
200/1608 |
334/1684 |
1.8 (1.4–2.1) |
<0.001 |
1.7 (1.4–2.1) |
<0.001 |
| ICD-10 ICU 1 |
121/971 |
244/1102 |
|
|
|
|
| ICD-10 ICU 2 |
79/636 |
90/578 |
|
|
|
|
| ICDSC* ICU 1 |
201/971 |
371/1102 |
1.94 (1.6–2.4) |
<0.001 |
1.8 (1.5–2.3) |
<0.001 |
| ICSC ICU 2 |
No data |
|
|
|
|
|
|
Parametric survival models
|
Crude restricted mean (95% CI)
|
TR (95% CI)
|
p-value
|
TR (95% CI)
|
p-value
|
| ICU LOS (days) |
2.5 (2.4–2.6) |
2.6 (2.5–2.7) |
1.1 (1.0–1.1) |
0.132 |
0.9 (0.9–1.0) |
0.048 |
| In-hospital LOS (days) |
17.1 (16.4–17.8) |
18.1 (17.4–18.8) |
1.1 (1.0–1.1) |
0.015 |
1.0 (1.0–1.1) |
0.214 |
| Delirium duration (days)*
|
3.3 (2.9–3.7) |
3.6 (3.6–3.4) |
1.1 (0.98–1.3) |
0.102 |
1.1 (1.0–1.3) |
0.059 |
| Duration mechanical ventilation (days) |
23.8 (22.8–24.8) |
23.6 (22.6–24.6) |
0.96 (0.9–1.1) |
0.429 |
0.8 (0.8–0.9) |
<0.001 |
|
Binomial general linear models
|
n/N
|
n/N
|
OR (95% CI)
|
p-value
|
OR (95% CI)
|
p-value
|
| In-hospital mortality |
84/1608 |
96/1684 |
1.1 (0.8–1.5) |
0.548 |
0.68 (0.5–1.0) |
0.063 |
|
Quantitative regression methods/models
|
Median difference (95% CI)
|
Median difference (95% CI)
|
Median difference (95% CI)
|
p-value
|
Median difference (95% CI)
|
p-value
|
| Cost per case (thousands of CHF) |
49.4 (47.4–51.5) |
55.9 (54.0–57.9) |
6.5 (3.7–9.3) |
<0.001 |
3.8 (0.5–7.1) |
0.023 |
| Nursing hours/case |
26.0 (24.3–27.7) |
28.5 (26.9–30.5) |
2.6 (0.2–5.0) |
0.037 |
−0.09 (−4.0 – −3.9) |
0.500 |
Duration of mechanical ventilation
In both years, patients spent on average just under one day (2011: 23.75 hours, 2013: 23.55 hours) on mechanical ventilation. The crude model indicated that the difference was non-significant, whereas the confounder-adjusted model indicated that the mean duration of mechanical ventilation was significantly shorter in the postintervention group (table 3).
In-hospital mortality
In 2011, 96 and in 2013, 84 patients died during their hospital stay. Neither the crude nor the adjusted model indicated any significant mortality rate difference between the historical-control and postintervention group (table 3).
Cost
The total cost per case increased from CHF 49,440 in 2011 to CHF 55,940 in 2013. Both the crude and the adjusted model indicated that the median cost increase was significant (table 3).
Nursing hours per case
In 2011, nurses spent on average roughly 26 hours and in 2013, 29 hours per case on direct patient care. Although this difference appeared significant in the unadjusted model, adjustment for confounders indicated that it was non-significant (table 3).
Associations between delirious state and ICU LOS, hospital LOS and mechanical ventilation duration
For both the 2011 and 2013 groups, the adjusted model indicated that delirium was significantly associated with LOS – both in the ICU and in hospital – and duration of mechanical ventilation. Delirium increased predicted ICU LOS by a factor of 2.66 and increased hospital LOS by a factor of 1.62. Predicted duration of mechanical ventilation increased by a factor of 2.33 in delirious patients (table 4).
Table 4 Associations between delirium and ICU length of stay, hospital length of stay and mechanical ventilation.
|
Variables
|
Delirious patients
|
Non-delirious patients
|
TR (95% CI)
|
p-value
|
| ICU length of stay (days) |
4.57 (4.37–4.78) |
2.11 (2.03–2.19) |
2.66 (2.43–2.92) |
<0.001 |
| Hospital length of stay (days) |
25.07 (23.63–26.51) |
16.07 (15.58–16.56) |
1.62 (1.52–1.74) |
<0.001 |
| Duration mechanical ventilation (hours) |
34.20 (32.66–35.74) |
20.98 (20.22–21.74) |
2.33 (2.04–2.65) |
<0.001 |
Discussion
The purpose of this study was to evaluate the effectiveness, costs and benefits of a standardised delirium management guideline implemented in two ICUs of a Swiss tertiary care hospital with reference to eight selected patient and healthcare system outcomes. After implementation of the guideline, the duration of mechanical ventilation decreased significantly as hypothesised. However, contrary to our hypothesis, ICU LOS, delirium prevalence and cost per case increased significantly. No significant changes occurred in hospital LOS, delirium duration, in-hospital mortality or nursing hours per case. These results are in line with those of previous studies that indicated delirium is a significant predictor for ICU LOS [4, 8, 33] and duration of mechanical ventilation [4, 33], and showed that the implementation of a multicomponent delirium programme increased the detection of delirium. These results do not support a connection between intensive care delirium and hospital LOS.
These mixed results call for a differentiated interpretation. On the one hand, they show that the implemented guideline led to a reduction in mechanical ventilation duration independent of multiple disease-associated factors. Since prolonged mechanical ventilation has been linked to several risk factors such as ventilator-associated pneumonia, increased costs and higher 1-year mortality [34, 35], this is a positive finding.
On the other hand, combined with the unexpected results demonstrated by significant changes contrary to what was hypothesised in three of the selected outcomes, the absence of significant changes in five more outcomes suggests extrinsic and intrinsic factors should be considered. For example, the (statistically significant, but clinically irrelevant) increase in the ICU LOS can be partly explained by changes in the hospital infrastructure (opening of a large new intermediate care facility [IMC]) and the more frequent use of certain treatments (e.g., extracorporeal membrane oxygenation). Due to the opening of the new IMC, to which all stable patients are transferred, the proportion of unstable critically ill patients requiring catecholamines, mechanical ventilation, etc., had risen in both of the ICUs observed. The significantly higher SAPS II scores [24] in the postimplementation group support this assumption. In the current study, it was not possible to control for such changes. To increase external validity, it would be important for future studies to consider these and similar factors in their design.
As delirium is underdiagnosed in the absence of standardised screening (those with hypoactive symptomatology in particular are not recognised and are therefore underreported [36]), the establishment of standard diagnostics in everyday clinical practice results initially in a higher sensitivity and therefore in increased delirium detection and documentation, which explains the observed increases in delirium diagnoses. However, this effect is time dependent: the selected analytical timeframe (the time interval between the start of implementation and the evaluation of its effects) plays a decisive role. As such, this study’s analytical timeframe might have been too short to show any effect on the selected outcomes, particularly with respect to changes in ICU infrastructure during the observational period.
Other aspects also need to be discussed and considered when interpreting the delirium prevalence rate. For example, the poor agreement between delirium prevalence rates based on delirium ICD-10 codes [30] and those based on ICDSC scores [22] may indicate either an underreporting bias when using the ICD-10 code or insufficient specificity of ICDSC scores. Although both the implemented delirium management guideline and the algorithm clearly specify that at the end of the diagnostic process any results indicating delirium need to be documented in the electronic patient record as either a medical or a nursing diagnosis, this step is sometimes omitted, i.e., implementation is not 100% successful. Reasons include the principle that, as successful implementation requires cultural change, its completeness differs between units: gaps and disruptions arise in interprofessional processes, especially communication, leading to differing consideration among the involved professionals regarding, for example, the idea that failures to correctly document delirium diagnoses can have negative consequences for a patient. With the involvement of interprofessional teams at the unit or clinic, we aimed to support this cultural change via adaptation, where appropriate, of the relevant processes. With regard to the overall success of an implementation, the importance of involving all relevant stakeholders, including senior leaders and frontline providers, is supported by the results of other studies and quality improvement projects that use Lean Sigma methodology [37]. Since a cultural change requires time, our chosen time-frame might simply have been too short to show an effect in the expected direction.
In addition, the manifold interdependencies between risk factors precipitating or predisposing to delirium and/or causes make recognising and managing those factors and causes very challenging. Furthermore, in the critical care setting, given the complexity and instability of the patient’s other conditions, delirium risk factors may not receive top priority, or the underlying cause, such as sepsis or ARDS, may not respond immediately to treatment. Therefore, it is impossible to prevent all delirium in ICU patients.
Although our results did not match our hypotheses, they fit well into the overall picture of previous literature on the implementation of delirium guidelines. Reports on the implementation of multifaceted care approaches for the prevention and mitigation of a delirium in ICU patients or the ABCDE (Awakening, Breathing, Coordination, Delirium Monitoring/ Management, and Early Exercise/Mobility) bundles also show contradictory results. In fact, the evidence remains inconclusive: several studies have found significant decreases in ICU and/or hospital LOS, in-hospital and short-term mortality, delirium prevalence and duration, duration of mechanical ventilation, and total cost of ICU hospitalisation, whereas others have found no significant changes in these outcomes [9, 14, 16, 18, 38–45].
This study has several notable strengths and limitations. Its strengths include the large sample size and a fully representative sample of patients. Relevant limitations include the chosen design, which allows neither causal inferences nor control of confounders or changes over time such as legislative/policy shifts or staff turnover over the 2 years between datasets. To calculate delirium prevalence rates and the other primary and secondary outcomes, we combined objective clinical data extracted from well-established databases with delirium assessment data generated via validated instruments such as the ICDSC [22] once per shift. This and our extensive process of data validation, which we conducted to ensure both the quality of the data and the internal validity of the study, are also strengths. However, as bases for delirium prevalence calculations, the ICD-10 codes [30] and ICDSC assessment scores [22] varied considerably. As we know from feedback from physicians working in the study hospital, delirium diagnosis was not always documented at the end of the diagnostic process as defined in the implemented delirium algorithm. This might have contributed to the differences between results.
To evaluate the effectiveness of the implemented standardise delirium management, we compared the entire control group with the entire postintervention group. This lack of selectivity may have led to significant unexpected changes in effect direction. In future studies of outcomes including ICU and hospital LOS, length of mechanical ventilation, mortality, cost and nursing hours per case, it would be advisable to include only patients diagnosed with delirium.
Conclusions
Standardised delirium management supports the detection of delirium in ICU patients and may contribute to improved outcomes in critical care cases, such as shorter length of stay in the ICU. However, the increased awareness of delirium after the implementation of a standardised multiprofessional, multicomponent management process leads to increased therapeutic effort, resulting in prolongation of ICU stay and higher costs but with no significant effect on mortality.
To overcome the influence of an initial learning effect on the delirium rates and to clarify this study’s conflicting results, further research will be necessary in our study context. In order to better understand delirium’s causal relationships with ICU and hospital LOS, costs and mortality, we strongly advise research teams to employ study designs that allow causal inferences.
Acknowledgements
We thank the Gottfried und Julia Bangerter-Rhyner Foundation, the Swiss Academy of Medical Sciences, and the involved hospital for co-financing this study.
Prof. Maria Schubert, PhD, RN, ZHAW Zurcher University of Applied Science, School of Health Professions, Institute of Nursing, Co-Head Research Unit for Nursing Science and MSc in Nursing, Technikumstr. 81, PO Box, CH-8401 Winterthur, maria.schubert[at]zhaw.ch
References
1
Morandi
A
,
Jackson
JC
. Delirium in the intensive care unit: a review. Neurol Clin. 2011;29(4):749–63. doi:.https://doi.org/10.1016/j.ncl.2011.08.004
2
Rudiger
A
,
Begdeda
H
,
Babic
D
,
Krüger
B
,
Seifert
B
,
Schubert
M
, et al.
Intra-operative events during cardiac surgery are risk factors for the development of delirium in the ICU. Crit Care. 2016;20(1):264. doi:.https://doi.org/10.1186/s13054-016-1445-8
3
Pandharipande
P
,
Cotton
BA
,
Shintani
A
,
Thompson
J
,
Pun
BT
,
Morris
JA, Jr
, et al.
Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34–41. doi:.https://doi.org/10.1097/TA.0b013e31814b2c4d
4
van den Boogaard
M
,
Schoonhoven
L
,
van der Hoeven
JG
,
van Achterberg
T
,
Pickkers
P
. Incidence and short-term consequences of delirium in critically ill patients: A prospective observational cohort study. Int J Nurs Stud. 2012;49(7):775–83. doi:.https://doi.org/10.1016/j.ijnurstu.2011.11.016
5
Zhang
Z
,
Pan
L
,
Ni
H
. Impact of delirium on clinical outcome in critically ill patients: a meta-analysis. Gen Hosp Psychiatry. 2013;35(2):105–11. doi:.https://doi.org/10.1016/j.genhosppsych.2012.11.003
6
Ely
EW
,
Shintani
A
,
Truman
B
,
Speroff
T
,
Gordon
SM
,
Harrell
FE, Jr
, et al.
Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–62. doi:.https://doi.org/10.1001/jama.291.14.1753
7
Weinrebe
W
,
Johannsdottir
E
,
Karaman
M
,
Füsgen
I
. What does delirium cost? An economic evaluation of hyperactive delirium. Z Gerontol Geriatr. 2016;49(1):52–8. doi:.https://doi.org/10.1007/s00391-015-0871-6
8
Pauley
E
,
Lishmanov
A
,
Schumann
S
,
Gala
GJ
,
van Diepen
S
,
Katz
JN
. Delirium is a robust predictor of morbidity and mortality among critically ill patients treated in the cardiac intensive care unit. Am Heart J. 2015;170(1):79–86, 86.e1. doi:.https://doi.org/10.1016/j.ahj.2015.04.013
9
Milbrandt
EB
,
Deppen
S
,
Harrison
PL
,
Shintani
AK
,
Speroff
T
,
Stiles
RA
, et al.
Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32(4):955–62. doi:.https://doi.org/10.1097/01.CCM.0000119429.16055.92
10
Schubert
M
,
Schürch
R
,
Boettger
S
,
Garcia Nuñez
D
,
Schwarz
U
,
Bettex
D
, et al.
A hospital-wide evaluation of delirium prevalence and outcomes in acute care patients - a cohort study. BMC Health Serv Res. 2018;18(1):550. doi:.https://doi.org/10.1186/s12913-018-3345-x
11
McPherson
JA
,
Wagner
CE
,
Boehm
LM
,
Hall
JD
,
Johnson
DC
,
Miller
LR
, et al.
Delirium in the cardiovascular ICU: exploring modifiable risk factors. Crit Care Med. 2013;41(2):405–13. doi:.https://doi.org/10.1097/CCM.0b013e31826ab49b
12
Ibrahim
K
,
McCarthy
CP
,
McCarthy
KJ
,
Brown
CH
,
Needham
DM
,
Januzzi
JL, Jr
, et al.
Delirium in the Cardiac Intensive Care Unit. J Am Heart Assoc. 2018;7(4):e008568. doi:.https://doi.org/10.1161/JAHA.118.008568
13
Arumugam
S
,
El-Menyar
A
,
Al-Hassani
A
,
Strandvik
G
,
Asim
M
,
Mekkodithal
A
, et al.
Delirium in the Intensive Care Unit. J Emerg Trauma Shock. 2017;10(1):37–46. doi:.https://doi.org/10.4103/0974-2700.199520
14
Balas
MC
,
Vasilevskis
EE
,
Olsen
KM
,
Schmid
KK
,
Shostrom
V
,
Cohen
MZ
, et al.
Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit Care Med. 2014;42(5):1024–36. doi:.https://doi.org/10.1097/CCM.0000000000000129
15
Baron
R
,
Binder
A
,
Biniek
R
,
Braune
S
,
Buerkle
H
,
Dall
P
, et al.; DAS-Taskforce 2015. Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS-Guideline 2015) - short version. Ger Med Sci. 2015;13:Doc19. doi:.https://doi.org/10.3205/000223
16
Collinsworth
AW
,
Priest
EL
,
Campbell
CR
,
Vasilevskis
EE
,
Masica
AL
. A Review of Multifaceted Care Approaches for the Prevention and Mitigation of Delirium in Intensive Care Units. J Intensive Care Med. 2016;31(2):127–41. doi:.https://doi.org/10.1177/0885066614553925
17
Miller
RR, 3rd
,
Wesley Ely
E
. Delirium and cognitive dysfunction in the intensive care unit. Semin Respir Crit Care Med. 2006;27(3):210–20. doi:.https://doi.org/10.1055/s-2006-945532
18
Awissi
DK
,
Bégin
C
,
Moisan
J
,
Lachaine
J
,
Skrobik
Y
. I-SAVE study: impact of sedation, analgesia, and delirium protocols evaluated in the intensive care unit: an economic evaluation. Ann Pharmacother. 2012;46(1):21–8. doi:.https://doi.org/10.1345/aph.1Q284
19
Boettger
S
,
Knöpfel
S
,
Schubert
M
,
Garcia Nuñez
D
,
Plichta
MM
,
Klaghofer
R
, et al.
Pipamperone and delirium: a preliminary evaluation of its effectiveness in the management of delirium and its subtypes. Swiss Med Wkly. 2017;147:w14471. doi:.https://doi.org/10.4414/smw.2017.14471
20
Boettger
S
,
Meyer
R
,
Richter
A
,
Fernandez
SF
,
Rudiger
A
,
Schubert
M
, et al.
Screening for delirium with the Intensive Care Delirium Screening Checklist (ICDSC): Symptom profile and utility of individual items in the identification of delirium dependent on the level of sedation. Palliat Support Care. . 2019;17(1):74–81. doi:.https://doi.org/10.1017/s1478951518000202
21
Boettger
S
,
Nuñez
DG
,
Meyer
R
,
Richter
A
,
Fernandez
SF
,
Rudiger
A
, et al.
Delirium in the intensive care setting: A reevaluation of the validity of the CAM-ICU and ICDSC versus the DSM-IV-TR in determining a diagnosis of delirium as part of the daily clinical routine. Palliat Support Care. 2017;15(6):675–83. doi:.https://doi.org/10.1017/S1478951516001176
22
Bergeron
N
,
Dubois
MJ
,
Dumont
M
,
Dial
S
,
Skrobik
Y
. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27(5):859–64. doi:.https://doi.org/10.1007/s001340100909
23
Sessler
CN
,
Gosnell
MS
,
Grap
MJ
,
Brophy
GM
,
O’Neal
PV
,
Keane
KA
, et al.
The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–44. doi:.https://doi.org/10.1164/rccm.2107138
24
Le Gall
JR
,
Lemeshow
S
,
Saulnier
F
. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–63. doi:.https://doi.org/10.1001/jama.1993.03510240069035
25
Sundararajan
V
,
Henderson
T
,
Perry
C
,
Muggivan
A
,
Quan
H
,
Ghali
WA
. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57(12):1288–94. doi:.https://doi.org/10.1016/j.jclinepi.2004.03.012
26Bundesamt für Statistik. Schweizerische Operationsklassifikation (CHOP) ICD-9-CM, volume 3, version 10.0, 2008. Neuchatel: Bundesamt für Statistik BFS; 2008. https://www.bfs.admin.ch/bfs/de/home/statistiken/kataloge-datenbanken/publikationen.assetdetail.344563.html
27SGI-SSMI. Minimaler Datensatz der SGI (MDSI), vol. 24d_2013. http://docplayer.org/12224800-Minimaler-datensatz-der-sgi-mdsi.html.
28Bundesamt für Statistik. Statistik der stationären Betriebe des Gesundheitswesens, Medizinische Statistik der Krankenhäuser. Neuchatel: Bundesamt für Statistik, Abteilung Bevölkerung und Beschäftigung; 1997. pp 1–43. https://www.bfs.admin.ch/bfsstatic/dam/assets/230430/master
29Baumberger D, Kuehne G, Hieber S. LEP Making nurses’ work visible. LEP Nursing 3.3. In: vol. 2, March 2007 edn. St. Gallen, Switzerland: LEP-AG; 2007. pp 1–10.
30DIMDI. International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10), [Internationale statistische Klassifikation der Krankheiten und verwandter Gesundheitsprobleme 10. Revision], Version 2013. In., vol. CD-10-GM Version 2013, 21.09.2012 edn. Koeln, Germany: Deutschen Institut für Medizinische Dokumentation und Information (DIMDI) 2013. https://www.dimdi.de/static/de/klassifikationen/icd/icd-10-gm/kode-suche/htmlgm2013/
31
Akaike
H
. A New Look at the Statistical Model Identification. IEEE Trans Automat Contr. 1974;19(6):716–23. doi:.https://doi.org/10.1109/TAC.1974.1100705
32Team RDC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008.
33
Salluh
JI
,
Wang
H
,
Schneider
EB
,
Nagaraja
N
,
Yenokyan
G
,
Damluji
A
, et al.
Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. 2015;350:h2538. doi:.https://doi.org/10.1136/bmj.h2538
34
Loss
SH
,
de Oliveira
RP
,
Maccari
JG
,
Savi
A
,
Boniatti
MM
,
Hetzel
MP
, et al.
The reality of patients requiring prolonged mechanical ventilation: a multicenter study. Rev Bras Ter Intensiva. 2015;27(1):26–35. doi:.https://doi.org/10.5935/0103-507x.20150006
35
Cox
CE
,
Carson
SS
,
Lindquist
JH
,
Olsen
MK
,
Govert
JA
,
Chelluri
L
; Quality of Life After Mechanical Ventilation in the Aged (QOL-mechanical ventilation) Investigators. Differences in one-year health outcomes and resource utilization by definition of prolonged mechanical ventilation: a prospective cohort study. Crit Care. 2007;11(1):R9. doi:.https://doi.org/10.1186/cc5667
36
Mossello
E
,
Tesi
F
,
Di Santo
SG
,
Mazzone
A
,
Torrini
M
,
Cherubini
A
, et al.; Italian Study Group on Delirium. Recognition of Delirium Features in Clinical Practice: Data from the “Delirium Day 2015” National Survey. J Am Geriatr Soc. 2018;66(2):302–8. doi:.https://doi.org/10.1111/jgs.15211
37
Aparanji
K
,
Kulkarni
S
,
Metzke
M
,
Schmudde
Y
,
White
P
,
Jaeger
C
. Quality improvement of delirium status communication and documentation for intensive care unit patients during daily multidisciplinary rounds. BMJ Open Qual. 2018;7(2):e000239. doi:.https://doi.org/10.1136/bmjoq-2017-000239
38
Skrobik
Y
,
Ahern
S
,
Leblanc
M
,
Marquis
F
,
Awissi
DK
,
Kavanagh
BP
. Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg. 2010;111(2):451–63. doi:.https://doi.org/10.1213/ANE.0b013e3181d7e1b8
39
Kram
SL
,
DiBartolo
MC
,
Hinderer
K
,
Jones
RA
. Implementation of the ABCDE Bundle to Improve Patient Outcomes in the Intensive Care Unit in a Rural Community Hospital. Dimens Crit Care Nurs. 2015;34(5):250–8. doi:.https://doi.org/10.1097/DCC.0000000000000129
40
Dale
CR
,
Kannas
DA
,
Fan
VS
,
Daniel
SL
,
Deem
S
,
Yanez
ND, 3rd
, et al.
Improved analgesia, sedation, and delirium protocol associated with decreased duration of delirium and mechanical ventilation. Ann Am Thorac Soc. 2014;11(3):367–74. doi:.https://doi.org/10.1513/AnnalsATS.201306-210OC
41
Mehta
S
,
Burry
L
,
Cook
D
,
Fergusson
D
,
Steinberg
M
,
Granton
J
, et al.; SLEAP Investigators; Canadian Critical Care Trials Group. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: a randomized controlled trial. JAMA. 2012;308(19):1985–92. doi:.https://doi.org/10.1001/jama.2012.13872
42
Bryczkowski
SB
,
Lopreiato
MC
,
Yonclas
PP
,
Sacca
JJ
,
Mosenthal
AC
. Delirium prevention program in the surgical intensive care unit improved the outcomes of older adults. J Surg Res. 2014;190(1):280–8. doi:.https://doi.org/10.1016/j.jss.2014.02.044
43
Girard
TD
,
Kress
JP
,
Fuchs
BD
,
Thomason
JW
,
Schweickert
WD
,
Pun
BT
, et al.
Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–34. doi:.https://doi.org/10.1016/S0140-6736(08)60105-1
44
Schweickert
WD
,
Pohlman
MC
,
Pohlman
AS
,
Nigos
C
,
Pawlik
AJ
,
Esbrook
CL
, et al.
Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–82. doi:.https://doi.org/10.1016/S0140-6736(09)60658-9
45
Hshieh
TT
,
Yue
J
,
Oh
E
,
Puelle
M
,
Dowal
S
,
Travison
T
, et al.
Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512–20. doi:.https://doi.org/10.1001/jamainternmed.2014.7779