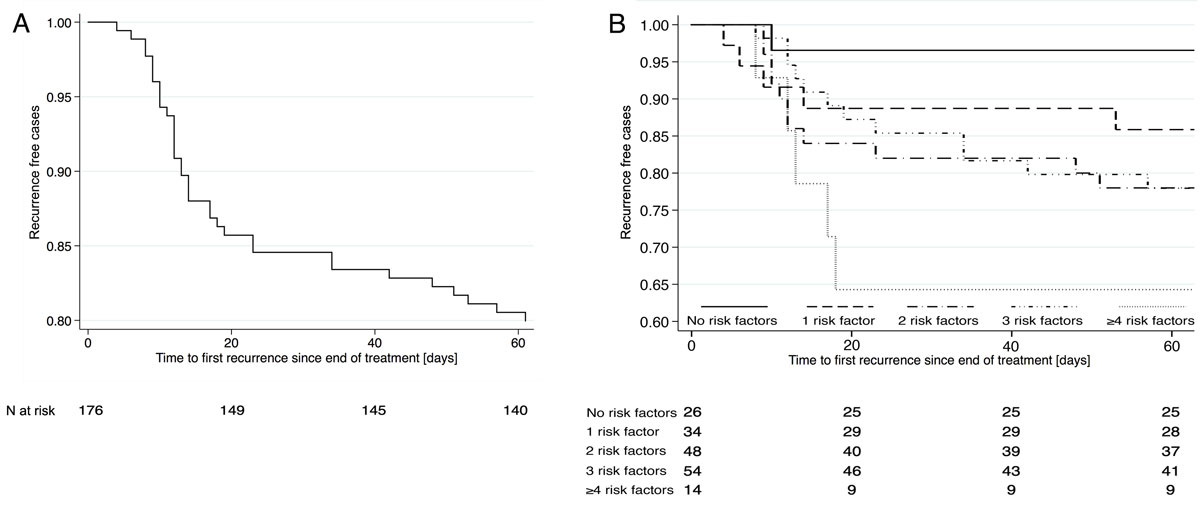
Figure 1 A: Kaplan-Meier curve showing Clostridioides difficile infection recurrence within 60 days after treatment. B: Recurrence by number of risk factors.
DOI: https://doi.org/10.4414/smw.2020.20173
With an average incidence of 4.1 infections/10,000 hospitalisation days, Clostridioides difficile infections (CDIs) have become the leading cause of infectious diarrhoea in patients hospitalised in high-income countries [1–3]. The clinical presentation ranges from mild diarrhoea to life-threatening pseudomembranous colitis and severe sepsis [4], with attributable mortality rates of up to 9.3% [4, 5]. CDI is complicated by high recurrence rates of 15–41% [6–9], which may increase even further with every new episode [4].
For almost three decades metronidazole has been considered the treatment of choice, but recent publications and international guidelines have increasingly favoured other treatment regimens [10, 11]. Vancomycin was shown to outperform metronidazole particularly in severe and complicated CDI, and fidaxomicin showed significantly lower recurrence rates than both metronidazole and vancomycin [10]. In a retrospective analysis, Patel et al. showed an increase in recurrence and mortality rates if CDI was undertreated relative to Infectious Diseases Society of America (IDSA) 2010 guidelines [12], which required the replacement of metronidazole by a more potent drug according to disease severity and risk factors for recurrence [12]. The 2017 IDSA guidelines suggest vancomycin or fidaxomicin, with preference for tapered vancomycin or fidaxomicin for recurrent infections. Metronidazole was mostly abandoned [13]. Despite such evidence metronidazole is still broadly considered the treatment of choice for CDI in Switzerland and in many parts of the world.
We hypothesised that – lacking local epidemiological data and standardised follow-up after hospital discharge – we might underestimate morbidity and mortality of recurrent CDI in our setting, thereby overestimating the cure rate of metronidazole. The aim of our study therefore was to describe CDI epidemiology, recurrence and mortality rates, and risk factors, as well as treatment decisions and cure rates in our hospital. Based on local data and international guidelines, our local CDI management should be revised.
We included all adult in- and outpatients treated for CDI between January 2014 and December 2016 at the Kantonsspital Aarau, a Swiss tertiary care hospital taking care of 27,000 hospitalised patients (140,000 patient days) every year. Data were collected prospectively for quality control in hospital epidemiology; therefore, no formal informed consent was required.
CDI was diagnosed in symptomatic patients with a two-step detection algorithm, as advocated by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) diagnostic guidelines [14]. If specific glutamate dehydrogenase was detected in stool samples a polymerase chain-reaction test for toxigenic C. difficile (GeneXpert C. difficile, Cepheid CA/USA) was conducted in the same sample. This kit also allowed ribotype 027/NAP1/BI to be identified.
Treatment regimen, dosing and duration were at the discretion of the treating physicians. Metronidazole was usually given orally as 500 mg three times daily, vancomycin as 125 mg orally four times daily and fidaxomicin as 200 mg orally twice daily for 10 days each. Patients whose symptoms resolved spontaneously or within one dose of treatment were excluded, as toxin detection in these patients merely indicated colonisation, and the diarrhoea likely had a different cause. Relapse of diarrhoea within 7 days after treatment stop was defined as nonresponse (see table 1 for definitions) and immediate treatment reuptake was considered as one treatment course if the same treatment was resumed. The cumulative length of a treatment consisted of both the initial treatment and a potential reuptake. As a result, the cumulative length of treatment could be longer than a standard 10 days for either oral metronidazole or vancomycin. Recurrence was defined as a new episode of CDI requiring treatment any time after 7 days but within 60 days of treatment stop, the most widely accepted timespan for the epidemiological definition of CDI recurrence as proposed in 2007 by McDonald et al. [15]. Every case was followed up by means of chart review and telephone interviews with patients or relatives and their primary care providers between 60 and 120 days after the last documented treatment had been finished. Details on treatment regimen and length were collected from chart reviews and telephone interviews; adherence was only documented as long as patients were hospitalised (mean length of stay 22.7 days; median 15 (interquartile range [IQR] 8–29).
Table 1 Definitions. Risk factors for recurrence and indicators for severe Clostridioides difficile infection (CDI) included in this analysis are shown in bold.
| Nonresponse | Relapse of diarrhoea within 7 days after treatment stop resulting in re-start. This was not considered a recurrence |
| Recurrence | Relapse of diarrhoea 7–60 days after treatment stop |
| Risk factors for recurrence (adapted from the literature) |
Advanced age (>70 years in our analysis) Severe comorbidity (haematological malignancy in our analysis) Chronic kidney disease stage ≥4 Severe CDI Continued antibiotics other than for CDI Proton pump inhibitor / antacid treatment History of CDI recurrence (not applicable for analysis of first episode) Presence of high risk strains, e.g. 027/NAP1/BI (omitted because of low numbers) |
| Severity | |
| Indicators of severity (adapted from the literature) |
Fever ≥38.5°C
Peritonism (omitted for lack of documentation) White blood cell count >15 × 109/l Serum creatinine increase ≥1.5 times baseline Low serum albumin (<25 g/l in our analysis) |
| Mild/moderate CDI | 0–1 indicators for severe CDI |
| Severe CDI | ≥2 indicators for severe CDI |
| Complicated CDI | Severe CDI plus ileus or severe sepsis or serum lactate >5mmol/l |
| Mortality | Death within 60 days of CDI onset |
The primary endpoints were recurrence of CDI and death. Epidemiological, clinical and laboratory data were assessed for eight risk factors for CDI recurrence described in the literature (table 1) [7, 10, 15, 16]: older age (dichotomised as age above 70 years), severe comorbidities, chronic renal failure (chronic kidney disease stage ≥4, i.e., estimated glomerular filtration rate below 30 ml/min/1.73m2), severe CDI, continued antibiotics other than for CDI, use of proton pump inhibitors (any dosage) or other antacids, history of CDI recurrence and presence of high risk strain types (e.g., 027/NAP1/BI). Severity of the first CDI episode was estimated as the cumulative presence of clinical parameters and biomarkers identified as indicators for a severe course of the disease and mortality by major guidelines [16] and reviews [7]: In addition to the two parameters used in the latest IDSA guidelines [10, 16] – marked leucocytosis >15 × 109/l and acute kidney failure (increase in serum creatinine ≥1.5 times baseline) – we included two parameters suggested by the ESCMID guidelines [10] – fever ≥38.5°C and serum albumin <25 g/l (as a negative acute phase protein and marker for eventual vascular leakage and prolonged malnutrition) (table 1). Reliable data on peritonism and ileus were lacking and therefore were not analysed.
Associations with CDI recurrence were assessed by univariate logistic regression analysis providing odds ratios (ORs) and 95% confidence intervals (CIs). Because of the limited number of endpoints reached, we did not perform multivariate logistic regression analyses in our data, but selected risk factors for recurrence and indicators for severity to be included in a summation score from the literature. Individual risk factors for recurrence were assessed for their predictive potential comparing stratified Kaplan-Meier curves of time to recurrence. As numbers were too small, we did not apply statistical tests for significance for individual risk factors. Finally, the summation score adding one point for each of these risk factors was tested for its predictive value, again using Kaplan-Meier curves and chi-square tests for different threshold values. In the same manner, the indicators for severity of CDI mentioned above were analysed for their individual predictive potential for mortality before selecting an optimal cut off value of the summarised score (1 point for each parameter). Time to death was analysed by Kaplan-Meier statistics, stratified by indicators for severe disease and by cause of death. All calculations were performed using Stata 15.1 (StataCorp, College Station, Texas). All testing was two-sided and p<0.05 was considered to indicate statistical significance.
In the three years between 2014 and 2016 we identified 210 CDI episodes in 191 hospitalised patients, equalling 4.7 infections/10,000 hospital days. Twelve patients in palliative care who were not treated, and three patients with spontaneous clearance of diarrhoea after a single dose of antibiotics who were considered asymptomatic colonisers were excluded from further analysis. Thus, we analysed 176 patients treated for a first CDI episode. The hypervirulent ribotype 027/NAP1/BI accounted for four episodes (2%). Patients were 50% male with a median age of 71 years (IQR 59–79).
The overall recurrence rate after treatment of the first episode (n = 176) was 20% (35 cases), after the first recurrence (n = 30) 13% (4 cases) and after the second recurrence (n = 4) 25% (1 case).
Recurrences mostly occurred during the second week after treatment was stopped, but continued throughout the observation period (fig. 1A). Patient characteristics of the 35 patients with and the 141 without recurrence are shown in table 2. In univariate logistic regression analysis, recurrence was associated with age above 70 years (OR 2.3, 95% CI 1.1–5.0), haematological neoplasia (OR 3.2, 95% CI 1.2–8.5), and proton pump inhibitor use (OR 2.2, 95% CI 1.0–4.6). Given that no other comorbidity or immunodeficiency state showed a significant association with recurrence, we included haematological neoplasia in further analyses.

Figure 1 A: Kaplan-Meier curve showing Clostridioides difficile infection recurrence within 60 days after treatment. B: Recurrence by number of risk factors.
Table 2 Characteristics of patients with and without recurrent Clostridioides difficile infection (CDI). Odds ratios with 95% confidence intervals for recurrence of CDI by univariate logistic regression analysis.
|
Without recurrence
n = 141 (80%) |
With recurrence
n = 35 (20%) |
Odds ratio (95% CI)
(univariate) |
p-value | |
|---|---|---|---|---|
| Age >70 years, n (%) | 64 (45%) | 23 (66%) | 2.31 (1.06 - 4.99) | 0.03 |
| Male sex, n (%) | 70 (50%) | 18 (51%) | 1.07 (0.51–2.25) | 0.85 |
| Antibiotic treatment <30 days before, n (%) | 106 (75%) | 26 (74%) | 0.95 (0.41–2.23) | 0.91 |
| Antibiotics other than for CDI during treatment, n (%) | 68 (48%) | 20 (57%) | 1.43 (0.68–3.02) | 0.35 |
| Inflammatory bowel disease, n (%) | 37 (26%) | 4 (11%) | 0.36 (0.12–1.10) | 0.07 |
| Hospital stay <90 days before, n (%) | 72 (51%) | 24 (69%) | 2.09 (0.95–4.59) | 0.07 |
| ICU <30 days before, n (%) | 34 (24%) | 9 (26%) | 1.09 (0.47–2.55) | 0.84 |
| Chronic infection, n (%) | 36 (26%) | 12 (34%) | 1.52 (0.69–3.37) | 0.30 |
| Solid tumour, n (%) | 31 (22%) | 6 (17%) | 0.73 (0.28–1.93) | 0.53 |
| Haematological malignancy / leukaemia, n (%) | 12 (9%) | 8 (23%) | 3.19 (1.19–8.54) | 0.02 |
| Neutropenia <1000 cells/mm3, n (%) | 7 (5%) | 1 (3%) | 0.56 (0.07–4.73) | 0.60 |
| Solid organ transplantation, n (%) | 13 (9%) | 6 (17%) | 2.04 (0.71–5.81) | 0.18 |
| Prednisone equivalent >20 mg/d, n (%) | 19 (13%) | 4 (11%) | 0.83 (0.26–2.61) | 0.75 |
| Proton pump inhibitors / antacids, n (%) | 54 (38%) | 20 (57%) | 2.15 (1.01–4.55) | 0.05 |
| Diabetes, n (%) | 36 (26%) | 7 (20%) | 0.73 (0.29–1.81) | 0.50 |
| Chronic kidney disease, GFR < 30 ml/min, n (%) | 15 (11%) | 7 (20%) | 2.10 (0.78–5.63) | 0.14 |
| Haemodialysis, n (%) | 3 (2%) | 2 (6%) | 2.79 (0.45–17.36) | 0.27 |
| Serum albumin nadir < 25g/l, n (%)* | 44 (46%) | 11 (50%) | 1.18 (0.47–2.99) | 0.72 |
| Ribotype 027/NAP1/BI, n (%) | 3 (2%) | 1 (3%) | 1.35 (0.14–13.41) | 0.80 |
CI = confidence interval; GFR = glomerular filtration rate; ICU = intensive care unit * Serum albumin available in 118 patients only.
Continuing antibiotic treatment other than for CDI, chronic kidney disease or hypoalbuminaemia did not cause significant differences. In univariate analysis, hospitalisation in the previous 3 months showed a trend for higher recurrence and inflammatory bowel disease for lower recurrence. Recurrence rates correlated with a summation score of 6 established risk factors for recurrence: whereas up to one risk factor was associated with a 10% recurrence rate, two or more risk factors resulted in a 25% recurrence rate (p = 0.03; fig. 1B). This threshold had a sensitivity of 22% (95% CI 15–30), specificity of 89% (79–96), positive predictive value of 81% (64–92) and negative predictive value 36% (28–44) for CDI recurrence. Based on these clinical data we selected ≥2 risk factors as the cut-off to define an increased risk of recurrence. Kaplan-Meier plots for the individual risk factors are shown in figures 2A–F . All risk factors except for severe disease increased recurrences, with the smallest effect for continued antibiotics.
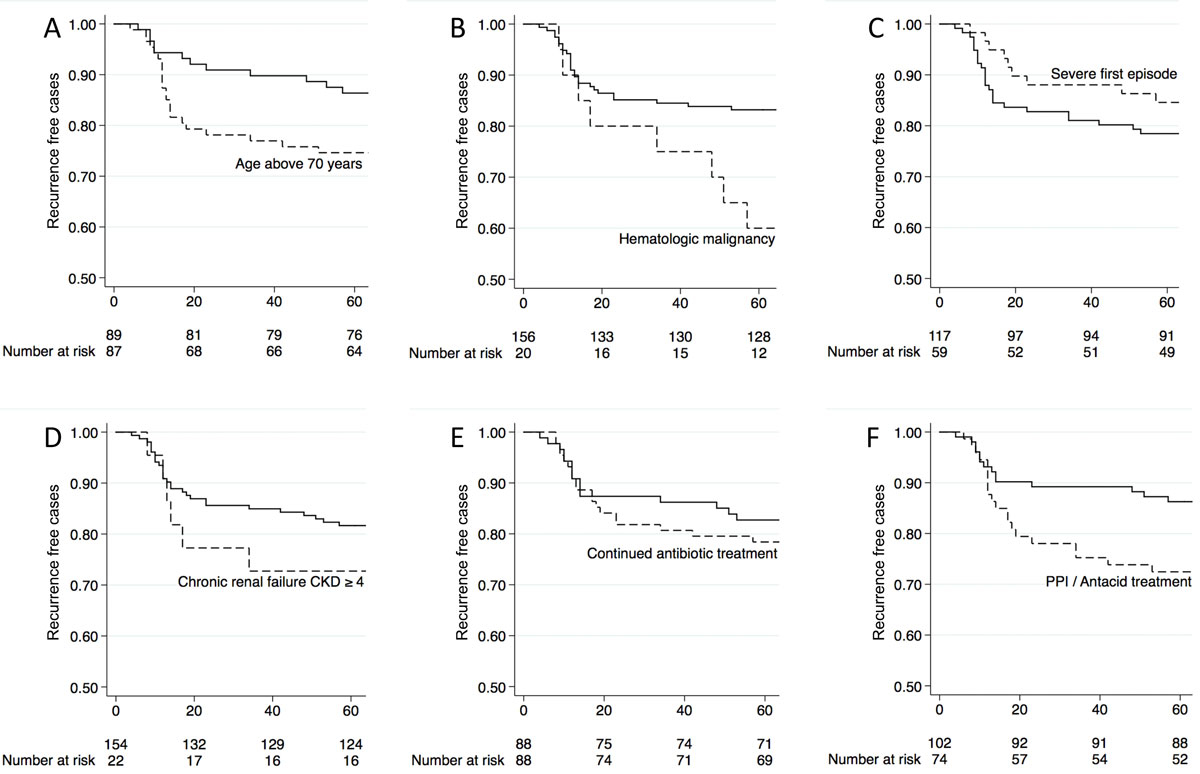
Figure 2 Kaplan-Meier curves showing Clostridioides difficile infection (CDI) recurrence after first episode according to risk factors (shown with the dashed line) of recurrence. A: age >70 years; B: haematological malignancy / leukaemia; C: severe first episode; D: chronic renal failure CKD ≥4; E: continued antibiotic treatment other than for CDI; F: proton pump inhibitor (PPI) or antacid therapy.
The 60-day mortality of all 191 patients was 17% (33 patients, including 12 untreated patients in palliative care), with 4% (8 patients, 24% of total mortality) attributable to CDI (fig. 3A). Among the latter, seven were treated with metronidazole and one with fidaxomicin. In a summation score of four indicators of severe CDI, mortality was 13% with 0–1 indicator, but 24% with 2 or more indicators (p = 0.06; fig. 3B). The latter showed a sensitivity of 24% (95% CI 14–35), specificity of 86% (79–92), positive predictive value 47% (30–65) and negative predictive value 68% (61–75) for 60-day mortality. Based on these clinical data, we defined an episode with at least two indicators of severity as severe.
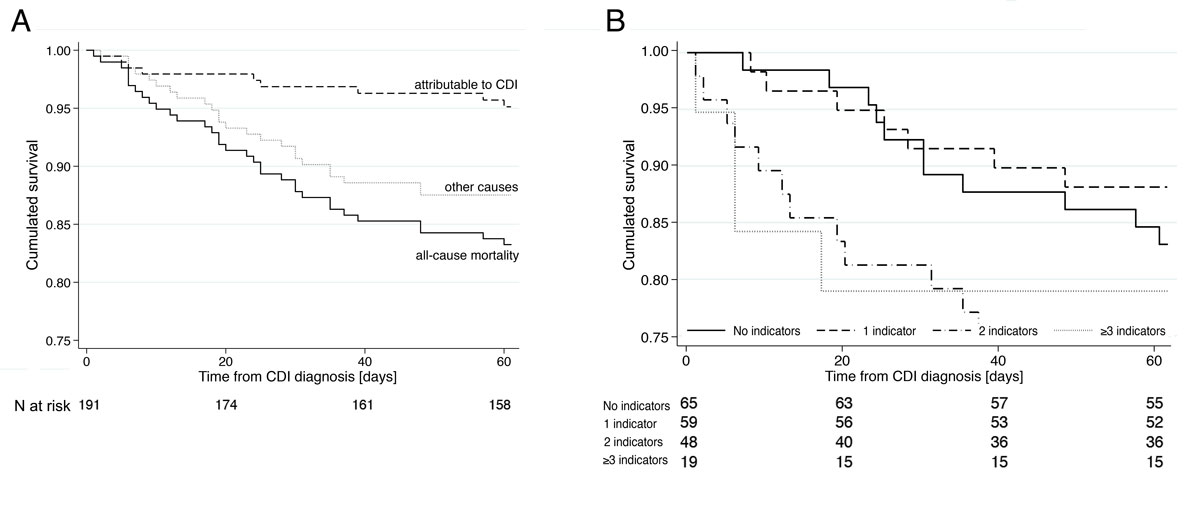
Figure 3 A: Kaplan-Meier curve showing all cause 60-days-mortality (solid), mortality attributable to Clostridioides difficile infections (CDI, dashed) and mortality related to other causes (dotted). B: Overall mortality by number of indicators for severe CDI (temperature ≥38.5°C, leucocytes >15 × 109/l, creatinine increase ≥1.5 times baseline, albumin <25 g/l).
The outcome according to CDI treatment is collected in table 3. We analysed 176 first episodes (34% severe), 30 first recurrences (23% severe) and 4 second recurrences (0% severe). Most patients were treated with metronidazole: it was used for 190/210 treatments overall (90%), including 94% of first episodes, and 73% and 50% of first and second recurrences, respectively. Nonresponse with an immediate re-start of metronidazole was observed in 14/179 patients (8%, all initially treated with metronidazole), including 5/58 patients (9%) with severe and 9/121 (7%) with mild/moderate disease (p = 0.78). Vancomycin was used 9 times (4%), mainly in the first episode, fidaxomicin 11 times (5%), mainly for first and second recurrences. The recurrence rate with metronidazole for a first episode was 20% and significantly higher with at least two risk factors for recurrence (26 vs 9%). Metronidazole treatment of the first and second recurrence failed in 14% (17% for ≥2 risk factors for recurrence vs 0%) and 50% (all exhibiting ≥2 risk factors for recurrence), respectively. None of 9 patients treated with vancomycin showed a recurrence. Recurrence rates after fidaxomicin were 50% (1/2) for the first episode, 14% (1/7) for the first and 0% (0/2) for the second recurrence (numbers too small for statistical comparisons). Severe CDI showed higher mortality but was not associated with higher recurrence rates in any treatment group.
Table 3 Outcome according to Clostridioides difficile infection treatment by episode/recurrence, risk factors for recurrence and severity of disease.
| Treatment | Recurrences / patients treated | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First episode | First recurrence | Second recurrence | ||||||||
| Mild | Severe* | All | Mild | Severe* | All | Mild | Severe* | All | ||
| 26/117 (22%) | 9/59 (15%) | 35/176 (20%) | 4/23 (17%) | 0/7 (0%) | 4/30 (13%) | 1/4 (25%) | – | 1/4 (25%) | ||
| Metronidazole | Total | 25/110 (23%) | 9/56 (16%) | 34/166 (20%) | 3/17 (18%) | 0/5 (20%) | 3/22 (14%) | 1/2 (50%) | – | 1/2 (50%) |
| 0–1 RF | 5/53 (9%) | 0/3 (0%) | 5/56 (9%) | 0/4 (0%) | – | 0/4 (0%) | – | – | – | |
| ≥2 RF | 20/57 (35%) | 9/53 (17%) | 29/110 (26%) | 3/13 (23%) | 0/5 (0%) | 3/18 (17%) | 1/2 (50%) | – | 1/2 (50%) | |
| Vancomycin | Total | 0/5 (0%) | 0/3 (0%) | 0/8 (0%) | 0/1 (0%) | – | 0/1 (0%) | – | – | |
| 0–1 RF | 0/2 (0%) | – | 0/2 (0%) | 0/1 (0%) | – | 0/1 (0%) | – | – | ||
| ≥2 RF | 0/3 (0%) | 0/3 (0%)† | 0/6 (0%) | – | – | – | – | – | – | |
| Fidaxomicin | Total | 1/2 (50%) | – | 1/2 (50%) | 1/5 (2%0) | 0/2 (0%) | 1/7 (14%) | 0/2 (0%) | – | 0/2 (0%) |
| 0–1 RF | 1/2 (50%) | – | 1/2 (50%) | 0/2 (0%) | – | 0/2 (0%) | – | – | ||
| ≥2 RF | – | – | – | 1/3 (33%) | 0/2 (0%) | 1/5 (20%) | 0/2 (0%) | – | 0/2 (0%) | |
RF = risk factor * Severe: ≥2 indicators for severity; † one patient in this group was cured with vancomycin followed by faecal microbiota transplantation. 12 patients in palliative care and 3 patients with resolution of symptoms after the first dose of metronidazole (i.e., spontaneous resolution) were excluded from this analysis.
Our data confirmed CDI to be a serious infection with a recurrence rate of 20% and a 60-day mortality of 17%. CDI seemed to predominantly affect frail and polymorbid patients, as only one quarter of the observed mortality was attributable to this infection. The observation reported in the literature that recurrent CDI increased mortality with an adjusted hazard ratio of 1.33 [5] provides a strong rationale for the critical need of a successful first treatment in this highly vulnerable population. Therefore, newer treatment guidelines have progressively included risk-stratified treatment options, with a choice between metronidazole, vancomycin and fidaxomicin based upon the risk of CDI recurrence and severity.
At 20%, recurrence after the first treatment was frequent in our patients, even though hypervirulent strains were not endemic. A broad range of recurrence rates of between 15% and 41% has been described [6–9], with a reduction in treatment success with every episode [6]. Consistently, the recurrence rate after the second recurrence was 25% in our patients, though numbers were small. The lower recurrence rate after the first recurrence (13 vs 20% after the first episode) might be due to more aggressive treatment, as vancomycin or fidaxomicin were used more often (in 27% as compared with 6% of episodes).
Most guidelines, – including the ones of the Society for Healthcare Epidemiology of America (SHEA)/IDSA [17], the American Society of Gastroenterology (ACG) [18], the Australien Society of Infectious Disesases (ASID) [19] and the ESCMID [10], meta-analyses [7, 20] and systematic reviews [9] have enumerated risk factors for recurrence. In our data, recurrence significantly correlated with age above 70 years, haematological neoplasia and proton pump inhibitor use, whereas chronic kidney disease, prior hospitalisation and solid organ transplantation probably doubled the risk of recurrence without reaching statistical significance in our small sample (table 2). In accordance with our data, haematological neoplasia was identified as a risk factor by Scappaticci et al. [8]. The strong association of prior proton pump inhibitor use with recurrence in our study is remarkable, as previous reports have provided conflicting data on this association [9, 21–24]. In contrast to the literature, we did not show an increase in recurrences when non-CDI antibiotics were continued. Still, there is a good rationale for considering this as a risk factor. In contrast to previous publications, severe CDI was not associated with recurrence in our data. This effect, however, may be underestimated because of censoring by mortality or may have been missed because of the small sample size.
All guidelines provide lists of risk factors for recurrence, but none has correlated their cumulative number with outcome. As some guidelines suggest different treatment regimens for patients with and without a relevant risk of recurrence, discriminatory cut-offs have become mandatory for clinical decision making. Such data currently are lacking with the exception of a recent study by Gerding et al. describing higher recurrence rates in patients with 2 vs 1 risk factors for recurrence [25]. In our patients, the recurrence rate was low (11%) with no more than one risk factor, whereas two or more risk factors more than doubled the recurrence rate (fig. 1B).
In all guidelines, the laboratory definition of severe CDI included marked leucocytosis with a white blood cell count above 15 × 109/l or a more than 50% increase in serum creatinine and severe abdominal symptoms (peritonism or ileus). Many [10, 18, 19, 26], but not all [17], guidelines also included hypoalbuminemia (below 25 or 30 g/l) and elevated serum lactate and fever above 38.5°C. Similarly to the Australian guidelines [19], we defined severe CDI by the presence of at least two indicators of severity including the above-mentioned laboratory parameters except for lactate (because of incomplete data).
A cut-off ≥1 indicator would have classified two thirds of our episodes as severe, whereas the cut-off ≥2 classified one third as severe. This seemed clinically reasonable as up to one indicator correlated with an already relevant mortality of 13%, whereas all-cause mortality almost doubled with ≥2 indicators (fig. 3B). Again, validations of severity cut-offs are lacking in published literature, with the exception of a rarely used severity score proposed by Zar et al. in 2007, without correlating it with mortality [13, 26]. The score included age above 60 years, temperature above 38.3°C, hypoalbuminaemia below 25 mg/l, leucocytosis above 15 × 109/l in the first 48 hours of enrolment, as well as endoscopic evidence of pseudomembranous colitis or treatment in the intensive care unit.
Several guidelines have stratified their treatment suggestions by CDI severity. According to older guidelines issued between 2010 and 2013 [17, 18], such as the IDSA/SHEA or ACG guidelines, mild-moderate first episodes should be treated with metronidazole and severe episodes with oral vancomycin, irrespective of recurrence risk. Patel et al. [12] demonstrated that the 20% of patients under-treated according to these guidelines faced an increased mortality of 44% vs. 11% with an attributable mortality of 22% and 9%, respectively.
In contradiction to these guidelines, we treated only 3/59 (5%) severe and 5/117 (4%) mild/moderate first episodes with vancomycin. Remarkably, CDI severity did not predict recurrence after metronidazole treatment in our patients, whereas presence of at least two risk factors for recurrence strongly did so (25 vs 10%). This contrasts with several meta-analyses, which consistently demonstrated superiority of vancomycin over metronidazole for severe infections [8, 27–30]. Vancomycin superiority in these publications, however, depended on an improved initial response rather than recurrence prevention. In our patients, a possible inferiority of metronidazole as the first treatment might have been overcome by the prolonged metronidazole treatment of the 14/176 (8%) nonresponders, mitigating a potential superiority of vancomycin. The absence of recurrence in any of our 9 patients treated with vancomycin as opposed to 38 recurrences in 190 patients (20%) receiving metronidazole is even more remarkable as only patients with the highest risk for recurrence were treated with vancomycin in our clinic. However, the small number of vancomycin-treated patients and the nonrandomised setting limit this interpretation.
In addition to CDI severity, newer guidelines published between 2014 and 2018 have suggested stratifying treatment according to the risk of CDI recurrence. The ESCMID, WSES and a recent German recommendation statement [10, 31, 32] suggested using vancomycin or fidaxomicin instead of metronidazole for patients with an increased risk of recurrence. ASID suggested vancomycin for the first recurrence and vancomycin tapering or fidaxomicin after multiple recurrences, yet without considering risk factors for recurrence in the first episode [19]. In our cohort, vancomycin was used for the first CDI episode in only 6/118 (5%) patients with ≥2 risk factors for recurrence, and no patient received fidaxomicin. Because of the expected benefit with vancomycin or fidaxomicin reported in the literature, treatment strategies for patients at high risk for recurrence need to be adjusted in our clinic.
The main limitation of our study is the overall low number of patients, particularly on non-metronidazole treatment regimens. The low number of cases and recurrence events limited the degrees of freedom in an extent that we abstained from performing multivariate regression analyses. Also, incomplete clinical (peritonism) and laboratory data (serum albumin, lactate) did not allow a correlation of all established risk factors with outcome.
Despite the relatively small dataset, our results confirmed the findings reported in the literature, except for the association of severe disease with recurrence. Our patients with a severe first CDI episode were as likely to suffer from recurrence as those with mild episodes (16 vs 22%; p = 0.32). Besides the limited power of our dataset, this discrepancy might be related to inconsistent epidemiological definitions of “severe” CDI as well as bias from competing risk by mortality: patients who died during the observation period showed a trend to having a recurrence more often than those who would survive (33 vs 18%; p = 0.097, those patients left untreated for palliative reasons excluded). Because of the high mortality associated with severe episodes, we included severity of CDI as an indication for more efficient treatment in our new treatment algorithm (see below).
Strengths of the study include the comprehensive workup of disease severity and recurrence with a vigorous follow-up, through contact with both patients and treating physicians. We provide the largest clinical dataset for Switzerland so far and were able to confirm the observations of international publications in our setting. In addition, our data is among the first to correlate the number of risk factors for recurrence and indicators of severity with outcome.
The 2017 update of the IDSA/SHEA guidelines preferred vancomycin and fidaxomicin to metronidazole for any CDI [17]. For recurrences after vancomycin treatment, vancomycin tapering or fidaxomicin was suggested. Considering not only these latest guidelines, but also the high cure rate of metronidazole in our patients without risk factors for recurrence, we defined a new treatment algorithm for our hospital (fig. 4): metronidazole is used only to treat the first and second episode of non-severe CDI with 0–1 risk factors for recurrence. Otherwise, vancomycin or fidaxomicin in an extended pulsed regimen, as evaluated by Guery et al. [33], are recommended. Oral vancomycin is proposed without tapering, as recent evidence did not show a difference in recurrence rate [34]. Patients with complicated CDI should receive combined treatment with metronidazole and vancomycin, following the 2017 IDSA update [16]. As severe CDI represents a risk factor for recurrence, and morbidity and mortality might be even higher in a subsequent episode, we propose fidaxomicin in an extended pulsed regimen over 20 days as a follow-up treatment. Clearly, this algorithm needs to be validated prospectively in clinical routine.
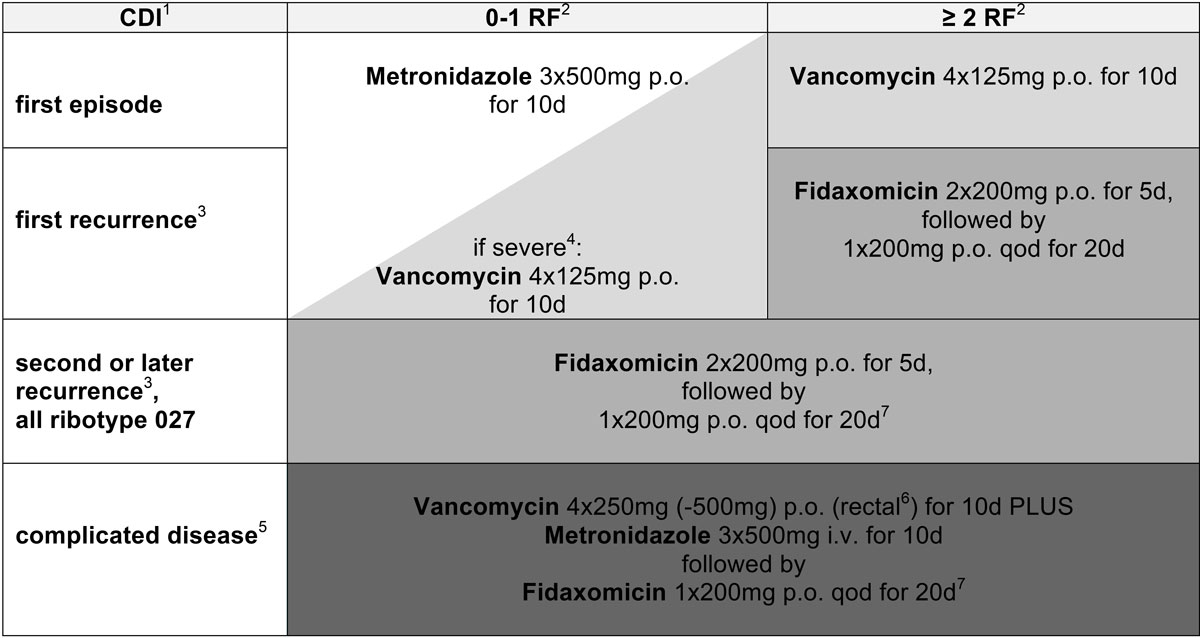
Figure 4 In-house treatment algorithm for Clostridioides difficile infections according to number of episode/recurrence, risk factors for recurrence and severity of disease. 1CDI = Clostridioides difficile infection. 2RF = risk factors for recurrence: age >70 years, severe comorbidity / haematological malignancy, chronic kidney disease stage ≥4, severe CDI, continued antibiotics other than for CDI, proton pump inhibitor use. 3Occurring within a follow-up of 60 days. 4Defined by ≥2 indicators for severe CDI: temperature ≥38.5°C, peritonism, leucocytes >15 × 109/l, creatinine increase ≥1.5 times baseline, albumin <25 g/l. 5Complicated (also fulminant): severe CDI PLUS ileus and/or severe sepsis and/or lactate >5 mmol/l. 6In cases of (sub)ileus. 7For recurrence after tapered fidaxomicin therapy or after complicated disease consider bezlotoxumab or faecal microbiota transplantation in outpatient setting.
If this algorithm were applied to our patients, 64/210 (30%) CDIs would have qualified for metronidazole as primary treatment, 119/210 (57%) for vancomycin and 27/210 (13%) for fidaxomicin, and this would have resulted in a mean increase of treatment costs by CHF 267 (≈USD) per episode (data not shown). This increase has to be weighed against the additional costs caused by each recurrence, estimated to account for USD 13,000–18,000 [35] and likewise a possible reduction in mortality. When these consequences are considered, fidaxomicin has proven cost effective compared with vancomycin and metronidazole in most pharmacoeconomic analyses [36, 37].
A high 60-day mortality suggests CDI to be a strong indicator for frailty; this infection therefore calls for effective treatment strategies. In our cohort, metronidazole was associated with low recurrence at minimal costs in patients with uncomplicated CDI but had relevant shortcomings in risk groups. Readily available clinical and laboratory parameters allow patients with a high risk for recurrence and with severe disease and therefore high mortality to be identified. Because of the superiority of oral vancomycin and fidaxomicin in the literature, which is mirrored in recent guidelines, a risk-guided treatment concept for CDI should be considered.
Parts of these data were presented at the Annual SSI Meeting 2017 and at ECCMID 2018.
No commercial sponsor had any involvement in design and conduct of this study, namely collection, management, analysis and interpretation of the data; and preparation, decision to submit, review, or approval of the manuscript. CAF received honoraria paid to the hospital research funds for advisory boards from Astellas, Gilead, MSD, Pfizer and Viiv. SH received honoraria for advisory boards from Gilead Sciences; all remunerations went to his home institution. All other authors declare no potential conflicts of interest.
1 Magill SS , Edwards JR , Bamberg W , Beldavs ZG , Dumyati G , Kainer MA , et al.; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–208. doi:.https://doi.org/10.1056/NEJMoa1306801
2 Rupnik M , Wilcox MH , Gerding DN . Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7(7):526–36. doi:.https://doi.org/10.1038/nrmicro2164
3 Bauer MP , Notermans DW , van Benthem BH , Brazier JS , Wilcox MH , Rupnik M , et al.; ECDIS Study Group. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377(9759):63–73. doi:.https://doi.org/10.1016/S0140-6736(10)61266-4
4 Pépin J , Routhier S , Gagnon S , Brazeau I . Management and outcomes of a first recurrence of Clostridium difficile-associated disease in Quebec, Canada. Clin Infect Dis. 2006;42(6):758–64. doi:.https://doi.org/10.1086/501126
5 Olsen MA , Yan Y , Reske KA , Zilberberg MD , Dubberke ER . Recurrent Clostridium difficile infection is associated with increased mortality. Clin Microbiol Infect. 2015;21(2):164–70. doi:.https://doi.org/10.1016/j.cmi.2014.08.017
6 Fekety R , McFarland LV , Surawicz CM , Greenberg RN , Elmer GW , Mulligan ME . Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis. 1997;24(3):324–33. doi:.https://doi.org/10.1093/clinids/24.3.324
7 Abou Chakra CN , Pepin J , Sirard S , Valiquette L . Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. PLoS One. 2014;9(6):e98400. doi:. Correction in: PLoS One. 2014;9(8):e107420. doi:https://doi.org/10.1371/journal.pone.0098400
8 Scappaticci GB , Perissinotti AJ , Nagel JL , Bixby DL , Marini BL . Risk factors and impact of Clostridium difficile recurrence on haematology patients. J Antimicrob Chemother. 2017;72(5):1488–95. doi:.https://doi.org/10.1093/jac/dkx005
9 Deshpande A , Pasupuleti V , Thota P , Pant C , Rolston DD , Hernandez AV , et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36(4):452–60. doi:.https://doi.org/10.1017/ice.2014.88
10 Debast SB , Bauer MP , Kuijper EJ ; European Society of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(Suppl 2):1–26. doi:.https://doi.org/10.1111/1469-0691.12418
11 Cohen SH , Gerding DN , Johnson S , Kelly CP , Loo VG , McDonald LC , et al.; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–55. doi:.https://doi.org/10.1086/651706
12 Patel I , Wungjiranirun M , Theethira T , Villafuerte-Galvez J , Castillo N , Akbari M , et al. Lack of adherence to SHEA-IDSA treatment guidelines for Clostridium difficile infection is associated with increased mortality. J Antimicrob Chemother. 2017;72(2):574–81. doi:.https://doi.org/10.1093/jac/dkw423
13 Zar FA , Bakkanagari SR , Moorthi KM , Davis MB . A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302–7. doi:.https://doi.org/10.1086/519265
14 Crobach MJ , Dekkers OM , Wilcox MH , Kuijper EJ . European Society of Clinical Microbiology and Infectious Diseases (ESCMID): data review and recommendations for diagnosing Clostridium difficile-infection (CDI). Clin Microbiol Infect. 2009;15(12):1053–66. doi:.https://doi.org/10.1111/j.1469-0691.2009.03098.x
15 McDonald LC , Coignard B , Dubberke E , Song X , Horan T , Kutty PK ; Ad Hoc Clostridium difficile Surveillance Working Group. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol. 2007;28(2):140–5. doi:.https://doi.org/10.1086/511798
16 Fehér C , Mensa J . A Comparison of Current Guidelines of Five International Societies on Clostridium difficile Infection Management. Infect Dis Ther. 2016;5(3):207–30. doi:.https://doi.org/10.1007/s40121-016-0122-1
17 McDonald LC , Gerding DN , Johnson S , Bakken JS , Carroll KC , Coffin SE , et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987–94. doi:.https://doi.org/10.1093/cid/ciy149
18 Surawicz CM , Brandt LJ , Binion DG , Ananthakrishnan AN , Curry SR , Gilligan PH , et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–98, quiz 499. doi:.https://doi.org/10.1038/ajg.2013.4
19 Trubiano JA , Cheng AC , Korman TM , Roder C , Campbell A , May ML , et al. Australasian Society of Infectious Diseases updated guidelines for the management of Clostridium difficile infection in adults and children in Australia and New Zealand. Intern Med J. 2016;46(4):479–93. doi:.https://doi.org/10.1111/imj.13027
20 Garey KW , Sethi S , Yadav Y , DuPont HL . Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008;70(4):298–304. doi:.https://doi.org/10.1016/j.jhin.2008.08.012
21 McDonald EG , Milligan J , Frenette C , Lee TC . Continuous Proton Pump Inhibitor Therapy and the Associated Risk of Recurrent Clostridium difficile Infection. JAMA Intern Med. 2015;175(5):784–91. doi:.https://doi.org/10.1001/jamainternmed.2015.42
22 Novack L , Kogan S , Gimpelevich L , Howell M , Borer A , Kelly CP , et al. Acid suppression therapy does not predispose to Clostridium difficile infection: the case of the potential bias. PLoS One. 2014;9(10):e110790. doi:.https://doi.org/10.1371/journal.pone.0110790
23 Weiss K , Louie T , Miller MA , Mullane K , Crook DW , Gorbach SL . Effects of proton pump inhibitors and histamine-2 receptor antagonists on response to fidaxomicin or vancomycin in patients with Clostridium difficile-associated diarrhoea. BMJ Open Gastroenterol. 2015;2(1):e000028. doi:.https://doi.org/10.1136/bmjgast-2014-000028
24 Kwok CS , Arthur AK , Anibueze CI , Singh S , Cavallazzi R , Loke YK . Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011–9. doi:.https://doi.org/10.1038/ajg.2012.108
25 Gerding DN , Kelly CP , Rahav G , Lee C , Dubberke ER , Kumar PN , et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection in Patients at Increased Risk for Recurrence. Clin Infect Dis. 2018;67(5):649–56. doi:.https://doi.org/10.1093/cid/ciy171
26 Gomez-Simmonds A , Kubin CJ , Furuya EY . Comparison of 3 severity criteria for Clostridium difficile infection. Infect Control Hosp Epidemiol. 2014;35(2):196–9. doi:.https://doi.org/10.1086/674851
27 Cornely OA , Nathwani D , Ivanescu C , Odufowora-Sita O , Retsa P , Odeyemi IA . Clinical efficacy of fidaxomicin compared with vancomycin and metronidazole in Clostridium difficile infections: a meta-analysis and indirect treatment comparison. J Antimicrob Chemother. 2014;69(11):2892–900. doi:.https://doi.org/10.1093/jac/dku261
28 Li R , Lu L , Lin Y , Wang M , Liu X . Efficacy and Safety of Metronidazole Monotherapy versus Vancomycin Monotherapy or Combination Therapy in Patients with Clostridium difficile Infection: A Systematic Review and Meta-Analysis. PLoS One. 2015;10(10):e0137252. doi:.https://doi.org/10.1371/journal.pone.0137252
29 Di X , Bai N , Zhang X , Liu B , Ni W , Wang J , et al. A meta-analysis of metronidazole and vancomycin for the treatment of Clostridium difficile infection, stratified by disease severity. Braz J Infect Dis. 2015;19(4):339–49. doi:.https://doi.org/10.1016/j.bjid.2015.03.006
30 Nelson RL , Suda KJ , Evans CT . Antibiotic treatment for Clostridium difficile-associated diarrhoea in adults. Cochrane Database Syst Rev. 2017;3:CD004610.
31 Sartelli M , Malangoni MA , Abu-Zidan FM , Griffiths EA , Di Bella S , McFarland LV , et al. WSES guidelines for management of Clostridium difficile infection in surgical patients. World J Emerg Surg. 2015;10(1):38. doi:.https://doi.org/10.1186/s13017-015-0033-6
32 Lübbert C , Lippmann N , von Braun A . Neue Leitlinien und Daten zu Clostridium difficile – Was ändert sich? [New Guidelines and Data to Clostridium difficile - What’s New?]. Dtsch Med Wochenschr. 2018;143(11):787–92. Article in German. doi:. https://doi.org/10.1055/a-0585-9595
33 Guery B , Menichetti F , Anttila VJ , Adomakoh N , Aguado JM , Bisnauthsing K , et al.; EXTEND Clinical Study Group. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients 60 years and older (EXTEND): a randomised, controlled, open-label, phase 3b/4 trial. Lancet Infect Dis. 2018;18(3):296–307. doi:.https://doi.org/10.1016/S1473-3099(17)30751-X
34 Gentry CA , Giancola SE , Thind S , Kurdgelashvili G , Skrepnek GH , Williams RJ, 2nd . A Propensity-Matched Analysis Between Standard Versus Tapered Oral Vancomycin Courses for the Management of Recurrent Clostridium difficile Infection. Open Forum Infect Dis. 2017;4(4):ofx235. doi:.https://doi.org/10.1093/ofid/ofx235
35 Ghantoji SS , Sail K , Lairson DR , DuPont HL , Garey KW . Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect. 2010;74(4):309–18. doi:.https://doi.org/10.1016/j.jhin.2009.10.016
36 Burton HE , Mitchell SA , Watt M . A Systematic Literature Review of Economic Evaluations of Antibiotic Treatments for Clostridium difficile Infection. Pharmacoeconomics. 2017;35(11):1123–40. doi:.https://doi.org/10.1007/s40273-017-0540-2
37 Le P , Nghiem VT , Mullen PD , Deshpande A . Cost-Effectiveness of Competing Treatment Strategies for Clostridium difficile Infection: A Systematic Review. Infect Control Hosp Epidemiol. 2018;39(4):412–24. doi:.https://doi.org/10.1017/ice.2017.303
Equally contributing first authors
SH, NB, EB and CAF planned the study. NB, CS and CAF collected and cleaned epidemiological, clinical and laboratory data. HF provided microbiology data. SH and CAF searched the literature, analysed the data and wrote the first draft of the manuscript. All authors contributed to the manuscript and agreed on its final version.
No commercial sponsor had any involvement in design and conduct of this study, namely collection, management, analysis and interpretation of the data; and preparation, decision to submit, review, or approval of the manuscript. CAF received honoraria paid to the hospital research funds for advisory boards from Astellas, Gilead, MSD, Pfizer and Viiv. SH received honoraria for advisory boards from Gilead Sciences; all remunerations went to his home institution. All other authors declare no potential conflicts of interest.