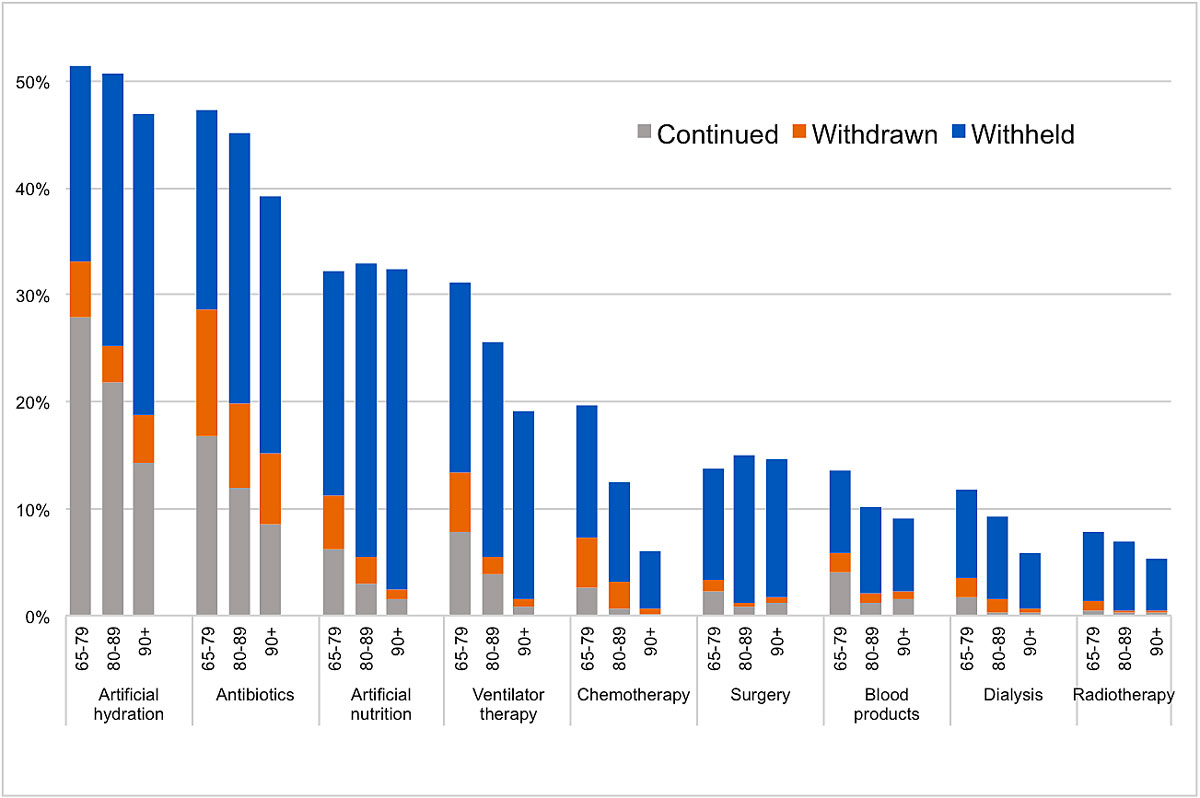
Figure 1 Weighted percentages of cases where treatment was continued, withdrawn, or withheld by age group: 65–79 (n = 835), 80–89 (n = 1242) and 90+ (n = 765) years old.
DOI: https://doi.org/10.4414/smw.2020.20177
As a result of demographic aging in developed countries, the proportion of deaths of patients aged 80 years or older has risen sharply in recent decades. In Switzerland, this proportion has now reached over one half of all deaths in men and more than two thirds of all deaths in women [1]. The aging of the population as well as medical technological developments occur alongside changes in medical care and decision making at the end of life [2]. There is little information available on the circumstances and quality of care in the final phase of older patients’ lives [3–5]. Particularly for patients aged 90 years or older, there are no specific investigations into the incidence and characteristics of medical end-of-life decisions (MELDs). The oldest old have been reported to have less access to specialist or palliative care, to receive adequate pain and symptom treatment less often, and to be excluded from decision making more often [6]. For this reason, it is highly relevant to gain more insight into what medical decisions are made towards the end of life in these patients and whether age is a determining factor.
MELDS include the administration of drugs to alleviate pain and other symptoms even when such treatment may hasten the patient’s death (APS), as well as decisions to forgo potentially life-sustaining treatment, namely withholding and/or withdrawing a certain medical therapy. These decisions account for the vast majority of MELDs [2, 7, 8]. Withholding treatment means the decision not to initiate a treatment that is aimed at prolonging life, whereas withdrawing treatment means to discontinue an ongoing treatment [9–12]. Both types of decision can hasten death and thus may create a tension between prolonging life and providing the best possible quality of life. For this reason, despite their frequency in end-of-life care, such decisions are often difficult and stressful for medical health professionals [9, 13, 14].
Several studies have shown that APS was used less often in older patients [3, 15, 16], whereas other studies did not find any age differences [8, 17, 18]. A systematic review by Rietjens et al. [2] showed that in most of the studies reviewed, APS was used less often in the 80+-year-olds, but the overall effect was not significant. According to several studies, forgoing potentially life-sustaining treatment occurred more often in older patients (80+) than in younger ones [2, 8, 9, 16–20]. In older patients, withholding treatment was generally more frequent than withdrawing treatment [21]. Other studies found that age is unrelated to the frequency of end-of-life decisions in general [15, 22, 23] and that the incidence of forgoing treatment in particular did not significantly differ with age [3, 15]. But many of these studies did not differentiate between withholding and withdrawing treatment, and none of them considered the treatments that were continued until death. However, it is essential to analyse the frequency of treatments withdrawn in relation to the total number of treatments given, that is, cases in which a treatment was withdrawn plus cases in which that treatment was continued until death. We therefore chose a new approach and compared withholding treatment not only with withdrawing but also with continuing it [24]. For this purpose, we restricted our analyses to non-sudden, expected deaths since only these cases were eligible for an MELD.
The first aim of our study was to describe the incidences of intensified APS and of treatments withheld/withdrawn among the oldest old patients in Switzerland prior to death and to analyse whether they differ with age. The second aim was to study whether age impacts the frequency of withholding, withdrawing and continuing specific types of treatment.
Between 1 August 2013 and 31 January 2014, the Swiss Federal Statistical Office weekly drew a random sample of death certificates, encompassing 21.3% of deaths of people aged 1 year or older in the German-speaking part of Switzerland, 41.1% in the French-speaking part and 62.9% in the Italian-speaking part. Certifying physicians were sent a total of 8963 questionnaires, of which 3173 (63.5%), 1538 (51.9%) and 617 (61.7%) were returned from the German-, French-, and Italian-speaking regions, respectively, up to 11 June 2014. To ensure anonymity of both patients and physicians, the questionnaires were sent to the Swiss Academy of Medical Science where they were anonymised before being made available for research.
The standardised survey collected information about MELDs regarding the patient, as well as physician demographic information. Physicians were asked to indicate when they had first encountered the patient (including possibly not until after death) and whether death had been sudden and completely unexpected. Further questions inquired after treatment decisions made at the end of life and the decision-making process. Multiple-choice questions asked about specific treatments that were continued until death, or withheld or withdrawn before death. Demographic information about the patient was collected from the death certificate. More details are given elsewhere [23].
We included all people aged 65 years or older at the time of death who did not die suddenly and completely unexpectedly as assessed by the responding physician (n = 3678). We excluded those who died from a physician-assisted death or with whom the responding physician did not have contact before death. Cases without APS where only “other treatment” or “other medications” were continued, withdrawn or withheld were also excluded (n = 134). This led to a final sample of n = 2842.
Data were weighted to adjust for region-, age-, and sex-specific differences in response rates and to ensure the sample was representative of all deaths in the sample period. The regions (German-, French-, and Italian-speaking) were weighted according to their proportion of deaths in the total Swiss population.
Descriptive statistics (weighted %) were used to assess the characteristics of the study population and the frequency of the different MELDs. Multivariable logistic regression (odds ratios [ORs], 95% confidence intervals [CIs]) was used to test the association between patient and care characteristics and odds of specific MELDs. All analyses were adjusted for sex, marital status, cause of death, and place of death. Deceased persons were classified into three age groups: 65–79 (reference group), 80–89, and 90+ years old. Study methods have been described in more detail elsewhere [25].
The study was issued a waiver as being unproblematic by the Zurich Cantonal Ethics Board (KEK-StV-Nr. 23/13).
Mean age of the study population was 83.8 years (95% CI 83.5–84.2; table 1). There were slightly more women than men (56.4%) with the percentage rising with age: 43.7% in the youngest age group, 56.6% in the intermediate group and 69.1% in the oldest one. Overall, 37.5% of all patients were married, with the proportion decreasing with age (57.8%, 38.7%, and 14.5%, respectively).
Table 1 Characteristics of the study population (n = 2842).
|
All (65–90+)
n = 2842 |
65–79
n = 835 |
80–89
n = 1242 |
90+
n = 765 |
||
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Age (years), mean (95% CI) | 83.8 (83.5–84.2) | ||||
| Sex | Male | 1271 (43.6%) | 467 (56.3%) | 565 (43.4%) | 239 (30.9%) |
| Female | 1571 (56.4%) | 368 (43.7%) | 677 (56.6%) | 526 (69.1%) | |
| Marital status | Married | 1081 (37.5%) | 484 (57.8%) | 485 (38.7%) | 112 (14.5%) |
| Unmarried* | 1761 (62.5%) | 351 (42.2%) | 757 (61.3%) | 653 (85.5%) | |
| Cause of death | Neoplasm | 819 (27.5%) | 404 (47.7%) | 311 (23.6%) | 104 (12.7%) |
| Cardiovascular disease | 707 (25.4%) | 139 (16.2%) | 318 (26.4%) | 250 (33.4%) | |
| Respiratory disease | 333 (11.2%) | 91 (11.0%) | 147 (11.3%) | 95 (11.3%) | |
| Disease of the nervous system | 553 (19.9%) | 108 (13.6%) | 268 (21.6%) | 177 (23.7%) | |
| Other or unknown | 430 (16.0%) | 93 (11.5%) | 198 (17.0%) | 139 (19.0%) | |
| Place of death | Hospital | 1102 (37.7%) | 467 (54.1%) | 468 (37.0%) | 167 (22.0%) |
| Palliative care unit | 117 (4.3%) | 71 (9.2%) | 37 (3.1%) | 9 (1.2%) | |
| Nursing home | 955 (35.2%) | 153 (19.7%) | 430 (35.8%) | 372 (50.5%) | |
| Elderly care residence | 382 (12.9%) | 47 (5.4%) | 182 (14.3%) | 153 (18.5%) | |
| Home | 279 (9.6%) | 95 (11.4%) | 121 (9.5%) | 63 (7.7%) | |
| Other | 7 (0.3%) | 2 (0.3%) | 4 (0.3%) | 1 (0.2%) | |
| MELDs | Any MELD | 2350 (83.8%) | 678 (81.7%) | 1031 (84.6%) | 641 (84.8%) |
| Intensified APS | 1941 (68.8%) | 560 (67.2%) | 850 (68.9%) | 531 (70.3%) | |
| Any potentially life-sustaining treatment withheld/withdrawn | 1959 (70.7%) | 577 (69.7%) | 866 (72.3%) | 516 (69.1%) | |
| Any potentially life-sustaining treatment continued/withdrawn | 1195 (39.4%) | 449 (52.2%) | 498 (37.7%) | 248 (28.9%) | |
| Any potentially life-sustaining treatment continued until death | 897 (29.2%) | 334 (38.8%) | 380 (28.4%) | 183 (20.4%) | |
APS = alleviation of pain and other symptoms; CI = confidence interval; MELD = medical end-of-life decision Unweighted n, weighted % * Includes single, divorced, widowed, and “other”.
In the youngest age group, the most common cause of death was neoplasm (47.7%). This proportion decreased with age (23.6% in the 80–89-year-olds, 12.7% in the 90+-year-olds). On the other hand, there was an increase in cardiovascular diseases with age (16.2% in the 65–79-year-olds vs 26.4% in the 80–89-year-olds and 33.4% in the 90+-year-olds) and a smaller increase of diseases of the nervous system (13.6 vs 21.6 and 23.7%, respectively). Respiratory disease was rarer (11.2% of all cases) and did not substantially vary across age groups. The same was true for “other or unknown” diagnoses (16.0% of all cases).
The most common places of death were hospital (37.7%) and nursing home (35.2%), followed by elderly care residence (12.9%), home (9.6%), palliative care unit (4.3%) and “other” (0.3%). The youngest patients were most likely to die in a hospital (54.1%), whereas patients aged 90 years or older were most likely to die in a nursing home (50.5%).
In 83.8% of all individuals in our study population at least one MELD was made, and the proportion slightly increased with age (81.7 vs 84.6 vs 84.8%; table 1). However, this increase was not significant in logistic regression analysis controlling for place of death and sociodemographic characteristics (OR 1.24, 95% CI 0.96–1.60 for the 80–89-year-olds and OR 1.28, 95% CI 0.94–1.74 for the 90+-year-olds compared with the 65–79-year-olds; table 2). There were no significant differences in the frequency of at least one MELD between different causes of death, places of death or regarding sex and marital status.
Table 2 Age and other determinants of medical end-of-life decisions*: multi-variable logistic regression (n = 2842).
|
Any MELD†
n = 2350 |
Treatments withheld/withdrawn†
n = 1959 |
APS†
n = 1941 |
|||||
|---|---|---|---|---|---|---|---|
| n (%)‡ | OR (95% CI) | n (%)‡ | OR (95% CI) | n (%)‡ | OR (95% CI) | ||
| Age (years) | 65–79 | 678 (81.7%) | Ref. | 577 (69.7%) | Ref. | 560 (67.2%) | Ref. |
| 80–89 | 1031 (84.6%) | 1.24 (0.96–1.60) | 866 (72.3%) | 1.12 (0.91–1.38) | 850 (68.9%) | 1.16 (0.95–1.43) | |
| 90+ | 641 (84.7%) | 1.28 (0.94–1.74) | 516 (69.1%) | 0.97 (0.75–1.24) | 531 (70.3%) | 1.29 (1.01–1.66) | |
| Sex | Male | 1032 (82.6%) | Ref. | 874 (70.7%) | Ref. | 858 (68.3%) | Ref. |
| Female | 1318 (84.7%) | 1.21 (0.96–1.52) | 1085 (70.7%) | 1.01 (0.84–1.21) | 1083 (69.2%) | 1.06 (0.88–1.27) | |
| Marital status | Unmarried§ | 1452 (83.4%) | Ref. | 1206 (70.1%) | Ref. | 1201 (68.7%) | Ref. |
| Married | 898 (84.4%) | 1.27 (0.99–1.63) | 753 (71.6%) | 1.08 (0.88–1.32) | 740 (68.9%) | 1.07 (0.88–1.30) | |
| Cause of death | Neoplasm | 666 (82.3%) | Ref. | 538 (68.1%) | Ref. | 586 (71.4%) | Ref. |
| Cardiovascular disease | 576 (83.0%) | 0.99 (0.75–1.32) | 472 (68.1%) | 1.03 (0.82–1.30) | 474 (68.4%) | 0.82 (0.65–1.04) | |
| Respiratory disease | 274 (83.6%) | 1.05 (0.74–1.49) | 221 (67.4%) | 0.97 (0.73–1.30) | 222 (68.0%) | 0.82 (0.61–1.09) | |
| Disease of the nervous system | 468 (85.0%) | 1.10 (0.80–1.51) | 413 (76.0%) | 1.50 (1.15–1.95) | 370 (67.2%) | 0.77 (0.60–0.99) | |
| Other or unknown | 366 (86.2%) | 1.22 (0.87–1.72) | 315 (74.9%) | 1.37 (1.04–1.81) | 289 (67.4%) | 0.77 (0.59–1.01) | |
| Place of death¶ | Hospital | 1009 (83.8%) | Ref. | 859 (71.8%) | Ref. | 839 (69.6%) | Ref. |
| Home | 531 (80.8%) | 0.78 (0.60–1.02) | 424 (65.9%) | 0.75 (0.60–0.94) | 442 (66.2%) | 0.83 (0.66–1.03) | |
| Nursing home | 810 (85.8%) | 1.10 (0.85–1.43) | 676 (72.4%) | 0.98 (0.79–1.21) | 660 (69.6%) | 0.99 (0.80–1.22) | |
APS = alleviation of pain and other symptoms; CI = confidence interval; MELD = medical end-of-life decisions; OR = odds ratio * Mutually adjusted for all other listed variables † Patients with treatments withheld/withdrawn and APS combined are included in all three regression models ‡ Unweighted n, weighted % (percentage of MELD) § Includes single, divorced, widowed and “other” ¶ Home includes elderly care residence and “other”. Hospital includes palliative care unit
The use of intensified APS slightly increased with age (67.2% in the youngest vs 68.9% in the second and 70.3% in the oldest group; table 1). Logistic regression (table 2) showed that the increase was significant for the oldest group compared with the youngest one (OR 1.29, 95% CI 1.01–1.66), but not for the second age group (OR 1.16, 95% CI 0.95–1.43).
Patients who died from non-cancer causes were less likely to receive APS than those who died from cancer, although the difference was only significant for diseases of the nervous system (OR 0.77, 95% CI 0.60–0.99). The frequency of APS was similar in all settings, ranging from 66.2 to 69.6% (compared with hospital deaths: OR 0.83, 95% CI 0.66–1.03 for home deaths and OR 0.99, 95% CI 0.80–1.22 for nursing home deaths).
Withholding or withdrawing a potentially life-sustaining treatment occurred most often in the 80–89-year-olds (72.3%), followed by the 65–79-year-olds (69.7%), and the 90+-year-olds (69.1%). However, logistic regression showed no significant age-related differences (OR 1.12, 95% CI 0.91–1.38 for the 80–89-year-olds and OR 0.97, 95% CI 0.75–1.24 for the 90+-year-olds). Regarding the different causes of death, 76.0% of all patients with diseases of the nervous system and 74.9% of all patients with “other or unknown” diagnoses had a decision to withhold or withdraw treatment, which was significantly more common than in cancer patients (OR 1.50, 95% CI 1.15–1.95 and OR 1.37, 95% CI 1.04–1.81, respectively). No significant differences were found for the other causes of death (OR 1.03, 95% CI 0.82–1.30 for cardiovascular diseases and OR 0.97, 95% CI 0.73–1.30 for respiratory diseases). When the different settings were compared, the frequency of withholding or withdrawing treatment was significantly less likely at home (65.9%) than in hospitals (71.8%; OR 0.75, 95% CI 0.60–0.94), and as likely in nursing homes (72.4%) as in hospitals (OR 0.98, 95% CI 0.79–1.21).
The decision to apply a potentially life-sustaining treatment that was either withdrawn later or continued until death was made in 39.4% of all patients, with rates decreasing with increasing age (52.2% in the 65–79-year-olds, 37.7% in the 80–89-year-olds, and 28.9% in the 90+-year-olds). In 29.2% of all patients such a treatment was continued until death – a proportion that also decreased with age (38.8% in the 65-79-year-olds, 28.4% in the 80-89-year-olds, and 20.4% in the 90+-year-olds).
When analysing the frequency of withholding/withdrawing treatment with and without the explicit intention of hastening death separately, we found no significant age pattern for either group (not shown in table).
Figure 1 shows the weighted percentages of withholding, withdrawing and continuing all nine specific types of treatment for the three age groups separately. All treatments were attempted most often in the youngest age group. There was a clear and consistent decrease with age of treatment attempts in artificial hydration and nutrition, antibiotics, ventilator therapy, chemotherapy and dialysis. For surgery, blood products and radiotherapy the proportion increased slightly in the oldest age group compared with the middle group. But for all these treatments, case numbers in older patients were low. The proportions of withdrawing an already attempted treatment were approximately the same for all age groups across all types of treatment. Withholding a treatment was clearly more frequent than withdrawing the same treatment for all types of treatment in all age categories. Furthermore, withholding a therapy was more common than attempting (withdrawing plus continuing) the same therapy for all types of treatment in all age groups except for artificial hydration and antibiotics, which were given more frequently than withheld in the youngest age group, and artificial hydration, which was withheld about as often as given in the second age group. The proportions of patients for whom a specific treatment was taken into consideration (sum of continued, withdrawn and withheld) decreased with age for all types of treatment except for artificial nutrition and surgery. The decrease with age was most pronounced in chemotherapy and ventilator therapy.

Figure 1 Weighted percentages of cases where treatment was continued, withdrawn, or withheld by age group: 65–79 (n = 835), 80–89 (n = 1242) and 90+ (n = 765) years old.
The four most common types of treatment were artificial hydration, antibiotics, artificial nutrition and ventilator therapy. These four treatments were analysed in more detail by comparing the frequency of withholding the different therapies to withdrawing or continuing them (table 3). The greatest increase with age was found for ventilator therapy. The 80-89-year-olds were nearly three times as likely to have ventilator therapy withheld rather than withdrawn or continued than the 65–79-year-olds (OR 2.83, 95% CI 1.82–4.41). The 90+-year-olds were six times as likely (OR 6.17, 95% CI 2.89–13.17). A clear increase with age was also found for artificial nutrition (OR 2.33, 95% CI 1.46–3.71 and OR 4.44, 95% CI 2.28–8.65, respectively), as well as for antibiotics (OR 1.53, 95% CI 1.11–2.09 and OR 1.57, 95% CI 1.05–2.35, respectively). Age had no independent influence on artificial hydration (OR 1.05, 95% CI 0.71–1.54 for the 80–89-year-olds and OR 0.95, 95% CI 0.61–1.47 for the 90+-year-olds).
Table 3 Withholding the four most common types of treatment compared to those for whom treatment was continued or withdrawn* : multi-variable logistic regression.
|
Artificial hydration
n = 1360 |
Antibiotics
n = 1184 |
Artificial nutrition
n = 853 |
Ventilator therapy
n = 675 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%)† | OR (95% CI) | n (%)† | OR (95% CI) | n (%)† | OR (95% CI) | n (%)† | OR (95% CI) | |||||
| Age (years) | 65–79 | 141 (35.7%) | Ref. | 141 (39.4%) | Ref. | 163 (65.0%) | Ref. | 135 (56.8%) | Ref. | |||
| 80–89 | 273 (50.2%) | 1.05 (0.71–1.54) | 278 (56.2%) | 1.53 (1.11–2.09) | 203 (83.5%) | 2.33 (1.46–3.71) | 220 (78.9%) | 2.83 (1.82–4.41) | ||||
| 90+ | 189 (60.2%) | 0.95 (0.61–1.47) | 170 (61.1%) | 1.57 (1.05–2.35) | 204 (92.3%) | 4.44 (2.28–8.65) | 121 (91.8%) | 6.17 (2.89–13.17) | ||||
| Sex | Male | 225 (40.0%) | Ref. | 251 (45.4%) | Ref. | 279 (73.7%) | Ref. | 210 (66.0%) | Ref. | |||
| Female | 378 (55.2%) | 1.19 (0.86–1.64) | 338 (58.6%) | 1.32 (0.99–1.77) | 391 (86.3%) | 1.40 (0.93–2.13) | 266 (81.0%) | 1.54 (1.01–2.36) | ||||
| Marital status | Unmarried‡ | 400 (53.9%) | Ref. | 380 (54.9%) | Ref. | 426 (85.8%) | Ref. | 289 (80.2%) | Ref. | |||
| Married | 293 (40.3%) | 1.00 (0.70–1.42) | 209 (48.0%) | 1.09 (0.79–1.49) | 244 (72.9%) | 1.00 (0.64–1.56) | 187 (65.7%) | 0.94 (0.60–1.47) | ||||
| Cause of death | Neoplasm | 152 (45.4%) | Ref. | 146 (52.8%) | Ref. | 201 (82.7%) | Ref. | 140 (85.6%) | Ref. | |||
| Cardiovascular disease | 119 (43.8%) | 0.62 (0.41–0.95) | 102 (46.9%) | 0.51 (0.34–0.76) | 125 (78.4%) | 0.38 (0.21–0.68) | 117 (68.0%) | 0.25 (0.14–0.46) | ||||
| Respiratory disease | 61 (43.5%) | 0.52 (0.33–0.85) | 87 (39.3%) | 0.33 (0.22–0.50) | 59 (79.2%) | 0.39 (0.19–0.79) | 69 (68.7%) | 0.29 (0.14–0.58) | ||||
| Disease of the nervous system | 185 (66.2%) | 0.89 (0.55–1.45) | 164 (73.2%) | 1.15 (0.73–1.81) | 200 (85.9%) | 0.45 (0.24–0.84) | 93 (77.8%) | 0.27 (0.14–0.54) | ||||
| Other or unknown | 86 (39.7%) | 0.93 (0.57–1.54) | 90 (48.2%) | 0.65 (0.42–1.00) | 85 (70.7%) | 0.34 (0.18–0.64) | 57 (64.6%) | 0.24 (0.12–0.49) | ||||
| Place of death§ | Hospital | 92 (13.5%) | Ref. | 160 (30.4%) | Ref. | 219 (62.3%) | Ref. | 217 (59.3%) | Ref. | |||
| Home | 184 (81.4%) | 29.26 (20.09–42.60) | 148 (70.6%) | 5.24 (3.57–7.67) | 165 (91.1%) | 4.82 (2.73–8.51) | 102 (93.0%) | 6.36 (3.08–13.15) | ||||
| Nursing home | 327 (83.2%) | 32.64 (22.25–47.89) | 281 (72.5%) | 5.51 (3.99–7.60) | 286 (94.8%) | 8.98 (4.87–16.58) | 157 (92.2%) | 5.32 (2.88–9.84) | ||||
CI = confidence interval; OR = odds ratio * Mutually adjusted for all other listed variables † Unweighted n, weighted % (percentage of MELD) ‡ Includes single, divorced, widowed and “other” § Home includes elderly care residence and ‘other’. Hospital includes palliative care unit.
Place of death had a very strong impact on all four types of treatment and all of them were more often withheld at home or in nursing homes than in the hospital. This was especially true for artificial hydration, which was withheld about 30 times more often at home (OR 29.26, 95% CI 20.09–42.60) or in nursing homes (OR 32.64, 95% CI 22.25–47.89) than in the hospital.
With respect to cause of death, there was a significant association between the underlying disease and any of the four types of treatment. A decision to withhold rather than attempt ventilator therapy and artificial nutrition was significantly less common in patients who died from non-cancer causes than those who died from cancer. For antibiotics and artificial hydration, the proportion was significantly lower only for cardiovascular and respiratory diseases compared with cancer.
Withholding ventilator therapy instead of attempting it was significantly more common among women (OR 1.54, 95% CI 1.01–2.36), whereas no significant sex differences could be found for the three other types of treatment studied in more detail. Marital status had no significant impact on withholding any of the four treatments.
To our knowledge, this is the first nationwide study of MELDs that specifically includes and focuses on the oldest old patients while differentiating between withholding, withdrawing, and continuing treatment until death. Our results show a number of substantial age differences in the most common MELDs and confirm that the oldest old patients are not a homogeneous group, but that there are relevant differences between the ages 80-89 and 90+ years.
The frequency of APS increased across the three age groups and varied from 67.2% to 70.3%. The increase was significant for the oldest old (90+) compared to the youngest age group (65–79). To the best of our knowledge, this gradient has never been described. Previous studies showed a decreasing use of APS with age [3, 15, 16] or no significant age differences at all [2, 8, 17, 18]. A reason for this discrepancy may be that we compared the 90+ and 80–89-year-old groups with the 65–79-year-old patients, whereas all other studies compared 80+-year-olds to <80-year-olds. In addition, almost all previous studies on APS, with the exception of one [15], assigned to each death only one MELD, namely the decision with the most explicit intention of shortening life, whereas we allowed more than one MELD per case. This may of course contribute to the difference in results. An explanation as to why elderly people received more intensified APS may be that they more often prefer treatment options that aim to provide the best possible quality of life rather than prolonging life at all costs [26]. Our study showed that APS was most frequent in cancer patients, which is in line with findings of other studies [16, 18].
In more than four out of five non-sudden deaths in patients aged 65 years or older, at least one MELD had been made prior to death. The incidence of MELDs in total did not significantly differ with age, which is consistent with previous research [3, 15, 17, 22]. The frequency of withholding/withdrawing treatment did not significantly differ with age, either. Only two previous reports came to the same conclusion [3, 15]. They chose the same approach as we did and included only non-sudden, expected deaths in the denominator. This has been shown to be more valid since only these deaths were eligible for an MELD [3]. Most previous studies found an increasing incidence of withholding/withdrawing treatment with age and most of them included all deaths in the denominator [8, 9, 16, 18]. This approach might skew the data because younger patients may be more likely to die from sudden, unexpected deaths. In addition, the prioritisation of MELDs in nearly all previous studies could be a relevant reason why our study had different results. All studies that found a significant age difference used this method, whereas the only study that permitted more than one MELD per case [15] found no significant age difference in withholding/withdrawing treatment, which is consistent with the approach and result of our study.
In the analysis of decisions with the explicit intention of hastening death, no significant age differences were found. Seale et al. [22] reported the same finding and Chambaere et al. [15] even showed a decrease in withholding or withdrawing treatment with the intention to hasten death. This indicates that the elderly, who are often considered a vulnerable patient group [2], are not more frequently exposed to withholding or withdrawing treatment with intentional life-shortening. Other studies support this finding, showing that decisions to withhold or withdraw life-prolonging treatment were most often made because there was no chance of improvement and the patient was recognised as being near death, which led to promoting patient comfort as the main goal of treatment [9, 22, 27].
A reason why there were no significant age differences in withholding/withdrawing treatment in general may be that it is a too broad and nonspecific category. The various types of treatment can have very different characteristics regarding the circumstances in which they are used, the potential life-prolonging effect or the burden of treatment [28]. Therefore, we analysed them separately.
Although the frequency of withholding/withdrawing treatment did not depend on age, significant age differences were found when different types of therapies were analysed separately and when withholding a particular treatment was compared with applying (withdrawing plus continuing) the same treatment. Withholding treatment was generally more common than withdrawing and continuing it for nearly all types of treatment, especially in the oldest patients (90+). This is consistent with findings from other studies [9, 20, 21]. The proportion of patients for whom a particular treatment was attempted clearly decreased with age for nearly all treatments. Increased comorbidity and mortality risk in older age can lead to treatments being considered less promising from the start and therefore being withheld rather than attempted [2, 21]. In addition, older patients often prefer comfort care rather than prolonging life at all costs [9]. Moreover, it has been shown that even after adjustment for patients’ care preferences and prognosis, severely ill older patients are treated less aggressively than younger ones [29]. For withholding artificial hydration compared with attempting it, no significant age differences could be found. However, place of death had a strong impact on artificial hydration, which was about 30 times more likely to be withheld at home or in nursing homes than in hospital. The benefits and burdens of the use of artificial hydration in end-of-life care are still controversial [30–32]. There are studies that found no effect of artificial hydration on symptom control, whereas others argue that it is still considered the minimum standard of end-of-life care [24, 33, 34]. Our results suggest that the latter is particularly the case in hospitals.
High-technology treatments with a high potential of being burdensome, such as surgery, oncotherapy, dialysis or radiotherapy, were rarely considered (sum of withholding, withdrawing and continuing) and much more often withheld than withdrawn or continued. This was particularly true for the oldest old patients and corresponds to the current ethical view that therapies with little benefit compared with burden should be avoided [35].
The decision to continue a potentially life-sustaining treatment until death was made less often in older patients, whereas the proportion of treatments withdrawn compared with applied was about the same over all three age groups for all types of treatments. Potentially life-sustaining treatments were less often applied in older patients, which explains why they were less often continued until death: a treatment that has never been used cannot be continued. However, the frequency of withdrawing an already applied treatment did not decrease with age. The decision to withdraw an already applied treatment therefore appears to be not primarily dependent on age, but could also be related to factors such as the current clinical status and the burden of symptoms.
The mortality follow-back study method used was rated as highly reliable [3, 8, 16–18, 36] and even if the optimal phrasing of the questionnaire is controversial [37, 38], this kind of study is still considered the gold standard for investigating end-of-life decision making on a population level [23]. A strength of the questionnaire is that only descriptive questions were asked and no sensitive terms such as “passive euthanasia” were used [28], since it is known that there are still uncertainties among physicians as to what these terms mean [39–42]. Other methodological strengths were the high response rates (63.5%, 51.9%, and 61.7% in the German-, French-, and Italian-speaking region, respectively) and the large and representative region-wide sample sizes for all deaths in Switzerland in 2013. This allowed us to analyse all age groups up to the oldest old in different settings, to disentangle withdrawing, withholding and continuing treatment, and to examine the individual types of treatment separately. Compared with previous studies, our study therefore provides a highly accurate picture of the end-of-life decisions in the oldest old, providing greater insight into clinical practice.
Nevertheless, some limitations need to be considered. The case numbers for rarer types of treatment, especially among the oldest old patients, were too low for reliable quantitative analyses. Larger studies to assess age differences in the oldest old in all types of treatments are needed. Since the data were collected retrospectively, there is always a risk of memory bias. To minimise this problem, time between death and completion of the questionnaire was kept as short as possible and physicians were able to use the patient’s medical records as a memory aid. Although the response rates were relatively high, there is still the possibility of nonresponse bias, especially since MELDs with a life-shortening effect are a sensitive topic. However, studies that investigated nonresponders showed that nonresponse did not cause sociodemographic distortion and was most often due to a lack of time and not because of more relevant reasons such as the physician’s personal attitude towards end-of-life decisions [22, 23, 43, 44]. Our study is based on self-reports of physicians and therefore depends on their assessment of the situation; however, this approach is the most appropriate to examine their medical end-of-life practices [23]. In addition, we had no information as to whether a decision to withhold, withdraw or continue a therapy was appropriate or whether the decision was in line with patients’ preferences.
And finally, we did not take into account possible regional variations. A previous analysis revealed that MELDs in total and decisions to withhold or withdraw treatment in particular were more common in the German-speaking part of Switzerland than in the French- and Italian-speaking regions. However, no significant regional differences were found for different types of treatment, except for artificial hydration being more likely to be withheld in the German- than in the French-speaking part of Switzerland [24, 25].
In conclusion, we can state that MELDs were common among patients above the age of 65 years, and that the frequency of any MELD versus none did not significantly vary by age. However, the use of APS significantly increased in patients over 90 years old, which is in line with previous research. The incidence of withholding/withdrawing treatment did not significantly differ with age. However, if one considers that potentially life-sustaining treatments were generally attempted less often in oldest old (80+ years) than in younger old patients (65–79 years), the relative frequency of withholding such treatments increased for most of the treatments studied. Since demographic aging occurs in all developed countries, many of our results may be generalised to other countries.
Nonetheless, it is important to consider that MELDs in themselves do not say anything about the quality of end-of-life care [9]. Our study could not investigate whether certain MELDs contributed to the best possible quality of treatment and dying. Further studies are needed to determine whether MELDs are in line with best care practices, patient’s wishes and best possible quality of life. In addition, insight into the physicians’ understanding of the benefits and burdens of different life-prolonging treatments would be helpful to assess the need for better education in end-of-life care and promote quality of care. A recent analysis of the Swiss 2013 MELD study revealed that the physician’s training in end-of-life care (e.g., country of education or year of graduation) had a significant impact on the frequency of MELDs and the propensity to discuss MELDs with patients and relatives [45]. And lastly, clearer guidelines would be desirable to help physicians understand how to deal with the often challenging decision to withhold, withdraw or continue different treatments in complex situations [21].
We thank the Swiss Federal Statistical Office for having sampled deaths for our study and the Swiss Academy of Medical Sciences (SAMS) for ensuring anonymity of questionnaires. We are indebted to the many physicians who completed the questionnaires and provided the data for this study.
This study was supported by the Swiss National Science Foundation (grant 406740-139309, National Research Program 67 “End-of-Life”), and the Palliative Care Research funding program of the Swiss Academy of Medical Sciences, the Gottfried and Julia Bangerter-Rhyner Foundation and the Stanley Thomas Johnson Foundation (grant PC 03/16).
The authors report no conflicts of interest.
1Bundesamt für Statistik. Todesfälle. 2018 [last accessed 2019 Aug 2]. Available from: https://www.bfs.admin.ch/bfs/de/home/statistiken/bevoelkerung/geburten-todesfaelle/todesfaelle.html.
2 Rietjens JA , Deschepper R , Pasman R , Deliens L . Medical end-of-life decisions: does its use differ in vulnerable patient groups? A systematic review and meta-analysis. Soc Sci Med. 2012;74(8):1282–7. doi:.https://doi.org/10.1016/j.socscimed.2011.12.046
3 De Gendt C , Bilsen J , Mortier F , Vander Stichele R , Deliens L . End-of-life decision-making and terminal sedation among very old patients. Gerontology. 2009;55(1):99–105. doi:.https://doi.org/10.1159/000163445
4 De Gendt C , Bilsen J , Van Den Noortgate N , Lambert M , Stichele RV , Deliens L . Prevalence of patients with do-not-resuscitate status on acute geriatric wards in Flanders, Belgium. J Gerontol A Biol Sci Med Sci. 2007;62(4):395–400. doi:.https://doi.org/10.1093/gerona/62.4.395
5 Lloyd-Williams M , Kennedy V , Sixsmith A , Sixsmith J . The end of life: a qualitative study of the perceptions of people over the age of 80 on issues surrounding death and dying. J Pain Symptom Manage. 2007;34(1):60–6. doi:.https://doi.org/10.1016/j.jpainsymman.2006.09.028
6 Lloyd A , Kendall M , Carduff E , Cavers D , Kimbell B , Murray SA . Why do older people get less palliative care than younger people? Eur J Palliat Care. 2016;23(3):132–7.
7 Bosshard G , Zellweger U , Bopp M , Schmid M , Hurst SA , Puhan MA , et al. Medical end-of-life practices in Switzerland: A comparison of 2001 and 2013. JAMA Intern Med. 2016;176(4):555–6. doi:.https://doi.org/10.1001/jamainternmed.2015.7676
8 van der Heide A , Deliens L , Faisst K , Nilstun T , Norup M , Paci E , et al.; EURELD consortium. End-of-life decision-making in six European countries: descriptive study. Lancet. 2003;362(9381):345–50. doi:.https://doi.org/10.1016/S0140-6736(03)14019-6
9 Martins Pereira S , Pasman HR , van der Heide A , van Delden JJ , Onwuteaka-Philipsen BD . Old age and forgoing treatment: a nationwide mortality follow-back study in the Netherlands. J Med Ethics. 2015;41(9):766–70. doi:.https://doi.org/10.1136/medethics-2014-102367
10 Sprung CL , Cohen SL , Sjokvist P , Baras M , Bulow HH , Hovilehto S , et al.; Ethicus Study Group. End-of-life practices in European intensive care units: the Ethicus Study. JAMA. 2003;290(6):790–7. doi:.https://doi.org/10.1001/jama.290.6.790
11 Levin PD , Sprung CL . Withdrawing and withholding life-sustaining therapies are not the same. Crit Care. 2005;9(3):230–2. doi:.https://doi.org/10.1186/cc3487
12 Howard DS , Pawlik TM . Withdrawing medically futile treatment. J Oncol Pract. 2009;5(4):193–5. doi:.https://doi.org/10.1200/JOP.0948501
13 Teixeira C , Ribeiro O , Fonseca AM , Carvalho AS . Ethical decision making in intensive care units: a burnout risk factor? Results from a multicentre study conducted with physicians and nurses. J Med Ethics. 2014;40(2):97–103. doi:.https://doi.org/10.1136/medethics-2012-100619
14 Hurst SA , Perrier A , Pegoraro R , Reiter-Theil S , Forde R , Slowther AM , et al. Ethical difficulties in clinical practice: experiences of European doctors. J Med Ethics. 2007;33(1):51–7. doi:.https://doi.org/10.1136/jme.2005.014266
15 Chambaere K , Rietjens JA , Smets T , Bilsen J , Deschepper R , Pasman HR , et al. Age-based disparities in end-of-life decisions in Belgium: a population-based death certificate survey. BMC Public Health. 2012;12(1):447. doi:.https://doi.org/10.1186/1471-2458-12-447
16 van der Maas PJ , Van Delden JJ , Pijnenborg L , Looman CW . Euthanasia and other medical decisions concerning the end of life. Lancet. 1991;338(8768):669–74. doi:.https://doi.org/10.1016/0140-6736(91)91241-L
17 Deliens L , Mortier F , Bilsen J , Cosyns M , Vander Stichele R , Vanoverloop J , et al. End-of-life decisions in medical practice in Flanders, Belgium: a nationwide survey. Lancet. 2000;356(9244):1806–11. doi:.https://doi.org/10.1016/S0140-6736(00)03233-5
18 van der Maas PJ , van der Wal G , Haverkate I , de Graaff CL , Kester JG , Onwuteaka-Philipsen BD , et al. Euthanasia, physician-assisted suicide, and other medical practices involving the end of life in the Netherlands, 1990-1995. N Engl J Med. 1996;335(22):1699–705. doi:.https://doi.org/10.1056/NEJM199611283352227
19 Groenewoud JH , van der Heide A , Kester JG , de Graaff CL , van der Wal G , van der Maas PJ . A nationwide study of decisions to forego life-prolonging treatment in Dutch medical practice. Arch Intern Med. 2000;160(3):357–63. doi:.https://doi.org/10.1001/archinte.160.3.357
20 Pijnenborg L , van der Maas PJ , Kardaun JW , Glerum JJ , van Delden JJ , Looman CW . Withdrawal or withholding of treatment at the end of life. Results of a nationwide study. Arch Intern Med. 1995;155(3):286–92. doi:.https://doi.org/10.1001/archinte.1995.00430030080009
21 Bosshard G , Nilstun T , Bilsen J , Norup M , Miccinesi G , van Delden JJ , et al.; European End-of-Life Consortium. Forgoing treatment at the end of life in 6 European countries. Arch Intern Med. 2005;165(4):401–7. doi:.https://doi.org/10.1001/archinte.165.4.401
22 Seale C . Hastening death in end-of-life care: a survey of doctors. Soc Sci Med. 2009;69(11):1659–66. doi:.https://doi.org/10.1016/j.socscimed.2009.09.025
23 Schmid M , Zellweger U , Bosshard G , Bopp M , Swiss M ; Swiss Medical End-Of-Life Decisions Study Group. Medical end-of-life decisions in Switzerland 2001 and 2013: Who is involved and how does the decision-making capacity of the patient impact? Swiss Med Wkly. 2016;146:w14307. doi:.https://doi.org/10.4414/smw.2016.14307
24 Penders YWH , Bopp M , Zellweger U , Bosshard G ; Swiss Medical End-of-Life Decisions Study Group. Continuing, Withdrawing, and Withholding Medical Treatment at the End of Life and Associated Characteristics: a Mortality Follow-back Study. J Gen Intern Med. 2019. doi:.https://doi.org/10.1007/s11606-019-05344-5
25 Hurst SA , Zellweger U , Bosshard G , Bopp M ; Swiss Medical End-of-Life Decisions Study Group. Medical end-of-life practices in Swiss cultural regions: a death certificate study. BMC Med. 2018;16(1):54. doi:.https://doi.org/10.1186/s12916-018-1043-5
26 Biola H , Sloane PD , Williams CS , Daaleman TP , Zimmerman S . Preferences versus practice: life-sustaining treatments in last months of life in long-term care. J Am Med Dir Assoc. 2010;11(1):42–51. doi:.https://doi.org/10.1016/j.jamda.2009.07.005
27 Buiting HM , Rietjens JA , Onwuteaka-Philipsen BD , van der Maas PJ , van Delden JJ , van der Heide A . A comparison of physicians’ end-of-life decision making for non-western migrants and Dutch natives in the Netherlands. Eur J Public Health. 2008;18(6):681–7. doi:.https://doi.org/10.1093/eurpub/ckn084
28 Bosshard G , Faisst K , Fischer S , Minder R , Zellweger U , Tschopp A , et al. Begrenzung lebenserhaltender Massnahmen bei Patienten am Lebensende in der deutschsprachigen Schweiz -- Resultate einer Todesfallstudie [Forgoing life-sustaining measures in patients at the end of life in the German-speaking part of Switzerland: results of a death certificate study]. Dtsch Med Wochenschr. 2005;130(50):2887–92. doi:.https://doi.org/10.1055/s-2005-923321
29 Hamel MB , Lynn J , Teno JM , Covinsky KE , Wu AW , Galanos A , et al. Age-related differences in care preferences, treatment decisions, and clinical outcomes of seriously ill hospitalized adults: lessons from SUPPORT. J Am Geriatr Soc. 2000;48(S1, Suppl):S176–82. doi:.https://doi.org/10.1111/j.1532-5415.2000.tb03129.x
30 Onwuteaka-Philipsen BD , Pasman HR , Kruit A , van der Heide A , Ribbe MW , van der Wal G . Withholding or withdrawing artificial administration of food and fluids in nursing-home patients. Age Ageing. 2001;30(6):459–65. doi:.https://doi.org/10.1093/ageing/30.6.459
31 Raijmakers NJ , van Zuylen L , Costantini M , Caraceni A , Clark J , Lundquist G , et al.; OPCARE9. Artificial nutrition and hydration in the last week of life in cancer patients. A systematic literature review of practices and effects. Ann Oncol. 2011;22(7):1478–86. doi:.https://doi.org/10.1093/annonc/mdq620
32 Buiting HM , van Delden JJ , Rietjens JA , Onwuteaka-Philipsen BD , Bilsen J , Fischer S , et al.; EURELD-Consortium. Forgoing artificial nutrition or hydration in patients nearing death in six European countries. J Pain Symptom Manage. 2007;34(3):305–14. doi:.https://doi.org/10.1016/j.jpainsymman.2006.12.006
33 Bükki J , Unterpaul T , Nübling G , Jox RJ , Lorenzl S . Decision making at the end of life--cancer patients’ and their caregivers’ views on artificial nutrition and hydration. Support Care Cancer. 2014;22(12):3287–99. doi:.https://doi.org/10.1007/s00520-014-2337-6
34 Morita T , Shima Y , Adachi I ; Japan Palliative Oncology Study Group. Attitudes of Japanese physicians toward terminal dehydration: a nationwide survey. J Clin Oncol. 2002;20(24):4699–704. doi:.https://doi.org/10.1200/JCO.2003.10.155
35 Welie JV , Ten Have HA . The ethics of forgoing life-sustaining treatment: theoretical considerations and clinical decision making. Multidiscip Respir Med. 2014;9(1):14. doi:.https://doi.org/10.1186/2049-6958-9-14
36 van der Heide A , Onwuteaka-Philipsen BD , Rurup ML , Buiting HM , van Delden JJ , Hanssen-de Wolf JE , et al. End-of-life practices in the Netherlands under the Euthanasia Act. N Engl J Med. 2007;356(19):1957–65. doi:.https://doi.org/10.1056/NEJMsa071143
37 Seale C . End-of-life decisions in the UK: a response to van der Heide and colleagues. Palliat Med. 2009;23(6):567–8. doi:.https://doi.org/10.1177/0269216309106500
38 van der Heide A , Onwuteaka-Philipsen B , Deliens L , van Delden JJ , van der Maas PJ . End-of-life decisions in the United Kingdom. Palliat Med. 2009;23(6):565–6, author reply 567–8. doi:.https://doi.org/10.1177/0269216309106457
39 Höfling W . Integritätsschutz und Patientenautonomie am Lebensende [Integrity and autonomy at the end of life]. Dtsch Med Wochenschr. 2005;130(14):898–900. doi:.https://doi.org/10.1055/s-2005-865105
40 Spittler JF . Flüssigkeitsverzicht. Ethische Massstabsfindung in der gesellschaftlichen Kontroverse [Dehydration. Ethical standards finding in the social controversy]. Dtsch Med Wochenschr. 2005;130(4):171–4. doi:.https://doi.org/10.1055/s-2005-837391
41 van Oorschot B , Lipp V , Tietze A , Nickel N , Simon A . Einstellungen zur Sterbehilfe und zu Patientenverfügungen -- Ergebnisse einer Befragung von 727 Arzten [Attitudes on euthanasia and medical advance directives]. Dtsch Med Wochenschr. 2005;130(6):261–5. doi:.https://doi.org/10.1055/s-2005-837410
42 Weber M , Schildmann J , Schüz J , Herrmann E , Vollmann J , Rittner C . Ethische Entscheidungen am Lebensende--Kenntnisstand und Einstellungen Medizinstudierender [Ethical decision-making at the end of life - knowledge and attitudes of medical students]. Dtsch Med Wochenschr. 2004;129(28-29):1556–60. doi:.https://doi.org/10.1055/s-2004-828989
43 Onwuteaka-Philipsen BD , Muller MT , van der Wal G , van Eijk JTM , Ribbe MW . Active voluntary euthanasia or physician-assisted suicide? J Am Geriatr Soc. 1997;45(10):1208–13. doi:.https://doi.org/10.1111/j.1532-5415.1997.tb03771.x
44 Fischer S , Miccinesi G , Hornung R , Bosshard G , Deliens L , van der Heide A , et al., EURELD consortium. Responders and non-responders in a study on medical end-of-life decisions in Denmark, the Netherlands, Sweden and Switzerland. Soz Praventivmed. 2006;51(1):24–33. doi:.https://doi.org/10.1007/s00038-005-0004-x
45 Bopp M , Penders YWH , Hurst SA , Bosshard G , Puhan MA ; Swiss End-of-Life Decisions Study Group. Physician-related determinants of medical end-of-life decisions - A mortality follow-back study in Switzerland. PLoS One. 2018;13(9):e0203960. doi:.https://doi.org/10.1371/journal.pone.0203960
This study was supported by the Swiss National Science Foundation (grant 406740-139309, National Research Program 67 “End-of-Life”), and the Palliative Care Research funding program of the Swiss Academy of Medical Sciences, the Gottfried and Julia Bangerter-Rhyner Foundation and the Stanley Thomas Johnson Foundation (grant PC 03/16).
The authors report no conflicts of interest.