
Figure 1 Blood transfusion services in Switzerland (data from the Swiss Red Cross). The white spots symbolise the inter-regional Blood Transfusion Services between three Swiss districts.
DOI: https://doi.org/10.4414/smw.2020.20178
Anaemia is a common problem in neonatal intensive care [1]. Low erythropoietin levels and the necessity to take frequent blood samples make premature infants particularly vulnerable [1–3]. As a consequence, the vast majority of extremely low birth weight infants (birth weight less than 1000 g) require numerous red blood cell transfusions (RBCTs) during their neonatal period [1, 2, 4–8].
Despite the frequent use of neonatal RBCT, there are few guidelines in Switzerland addressing this issue. In addition, there is a remarkable paucity of literature and, accordingly, little evidence regarding the indication for RBCT in newborns. Many neonatologists use a particular haematocrit or haemoglobin level to indicate RBCT. This level varies and depends on the degree of prematurity, the postnatal age and the clinical state of the baby, including oxygen requirement and need for ventilatory support [8]. Apart from the beneficial effects of RBCT for infants in neonatal intensive care units, there is growing concern about adverse short-term consequences of RBCT, particularly in very preterm infants born before 32 weeks gestational age [4]. Moreover, recent studies suggest an association between transfusion practices and later neurocognitive outcomes in these tiniest infants [3, 5, 9, 10].
In addition, uncertainty remains with regard to the preparation and storage of blood products, transfusion volume and rate, cardiorespiratory monitoring of patients and documentation of the intervention. Since sound evidence for these issues is lacking, guidelines have been developed on a national basis, for example in France [11], Germany [12], the United Kingdom [13], Canada [5], Australia and New Zealand [14].
In Switzerland, the Swiss Blood Transfusion Service (BTS) of the Swiss Red Cross published mandatory guidelines for all 11 regional blood transfusion services (RBTSs) to ensure standardisation of preparation, logistics and quality control of blood products (fig. 1) [15–17]. The RBTS is the sole provider of blood components to hospitals supervised by Swissmedic [18]. Some hospitals have an in-house RBTS. In such cases, the RBTS is responsible for the entire supply chain. In hospitals without an internal RBTS, local haematology laboratories take responsibility for certain processes such as storage and irradiation of blood products. In contrast to the national standardised processes adopted by the RBTSs, there are no guidelines on irradiation, indication and administration of red blood cells at the local level of hospital laboratories and neonatal units. Therefore, we hypothesised that there is consistency among BTSs, but no consensus among local laboratories and Swiss neonatologists regarding blood transfusion practices in newborns.

Figure 1 Blood transfusion services in Switzerland (data from the Swiss Red Cross). The white spots symbolise the inter-regional Blood Transfusion Services between three Swiss districts.
This was a cross-sectional study based on two questionnaires developed with a publicly available online tool for surveys (findmind.ch). Full texts of both questionnaires are provided in the appendices. The main focus was placed on the neonatal survey.
PubMed was searched for neonatal literature and international guidelines on red blood cell transfusion practices and was restricted to publications in English. The search was performed in April 2019 and updated in July 2019. RAM and CR screened the literature and identified relevant publications without prespecified in- and exclusion criteria.
The study did not collect information on biological material or medical personal data and does not fall within the scope of human research law. Ethical approval was not required.
In October 2016, the first questionnaire was sent to the 9 BTSs and 16 hospital laboratories in Switzerland responsible for processing neonatal red blood cells, according to the Swiss Red Cross guidelines (fig. 1) [17]. The questionnaire was developed with the help of the blood transfusion unit of the University Hospital Zurich to reflect the guidelines of the Swiss BTS [15, 16] and included 30 questions (appendix 1). For data conformity, all RBTSs were asked to provide a list of neonatal units they prepare and provide red blood cells for. All data were recorded and stored anonymously. The BTSs and hospital laboratories answered the questionnaire between October and December 2016.
The second questionnaire was sent in April 2016 to all level II and level III neonatal units accredited by the Committee for the Accreditation of Neonatal Units (CANU) of the Swiss Neonatal Society, endorsed by the FMH [19, 20]. According to CANU, level III units include a neonatal intensive care unit. Level II units provide intermediate care and use noninvasive respiratory support with (level IIB) or without (level IIA) continuous positive airway pressure support. In addition, two paediatric intensive care units and five neonatal units not yet accredited by CANU were contacted. Thus, a total of 34 units were invited to participate – 10 level III perinatal centres, 17 level II neonatal units, 2 paediatric intensive care units and 5 with pending CANU classification. The questionnaire, developed by neonatologists (RAM, CR) was sent to the medical head of each unit and consisted of twelve questions related to the unit’s blood transfusion practices in the treatment of newborns (appendix 2). Moreover, participants were asked to provide a copy of their local blood transfusion guidelines, if they had any. No centre was excluded from the survey. The neonatal units answered the questionnaire between April and December 2016.
One investigator (GL) sent the surveys, collected all data and performed descriptive analysis of the results under the supervision, control and responsibility of RAM and CR.
Eighteen (72%) of the 25 RBTSs and laboratories answered the survey questions. All but three (88%) followed the guidelines of the BTS and Swiss Red Cross. The majority of laboratories (14/17) prepare full pouches of about 350–400 ml and nearly the same number (13/17) also deliver split pouches of 60–80 ml each, depending on availability. Irradiation procedures of blood products differed from one centre to the other: 2/15 (13%) do not irradiate at all, 4/15 (27%) irradiate blood for all newborn infants and 9/15 (60%) irradiate blood for premature infants only, although the gestational age limits range from 24 to 37 weeks. In 15/17 (88%) responding RBTS and laboratories, blood bags are shared locally. Storage times range from 5 to 49 days (median 28 days; fig. 2). Determination of the newborn’s blood group before RBCT is mandatory and is performed on a blood sample from the baby (16/16, 100%), but also on umbilical blood (6/16, 38%). Nine out of 16 (56%) laboratories deliver blood bags with O negative red blood cells, whereas the remaining laboratories (7/16, 44%) provide red blood cells compatible with the blood group of the mother, the baby or both. In that case, compatibility is tested for the first RBCT only.
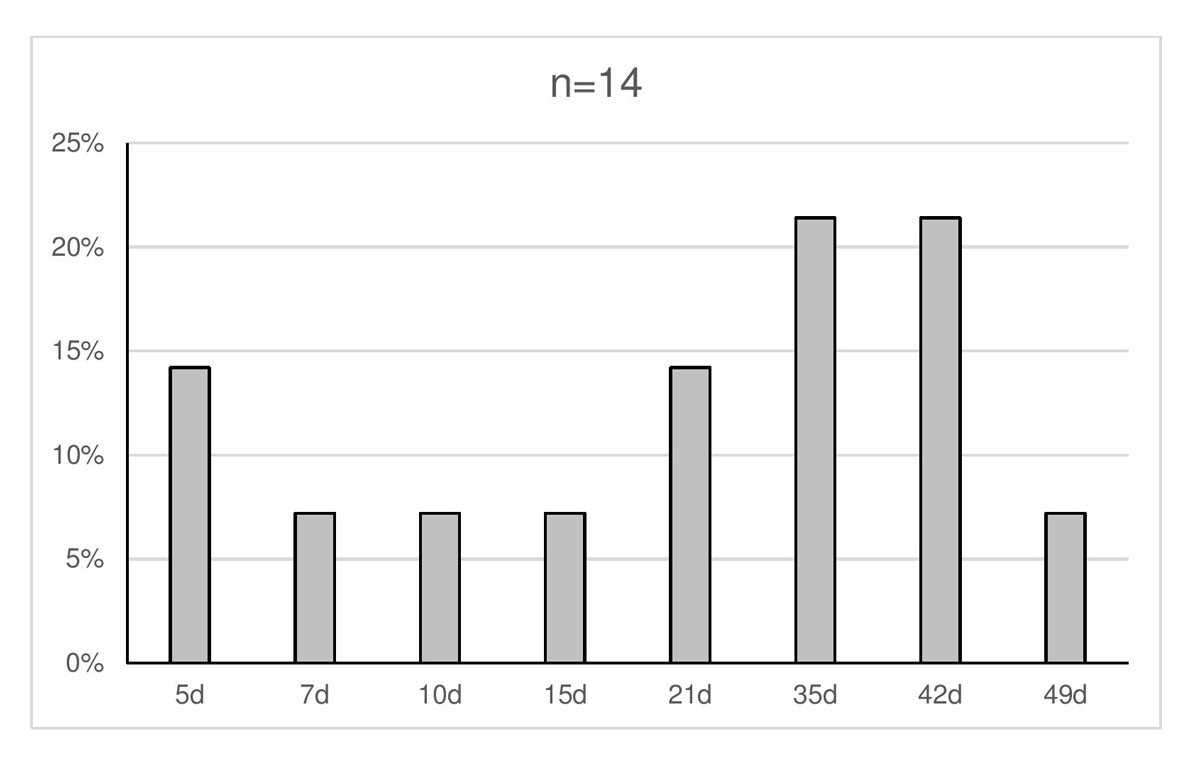
Figure 2 Maximum shelf life of red blood cells: X axis is days (d), Y axis is the percentage of participants providing this response.
Five out of 34 neonatal units (15%) do not transfuse red blood cells. These five units are small and rarely encounter situations where transfusions are required; this makes referral to a larger centre reasonable if a transfusion is indicated. Twenty-six (90%) of the remaining 29 units participated in the survey.
The cantons of Zurich and Vaud are most strongly represented, with five (19%) neonatal units each. Sixteen (62%) neonatal units purchase blood products from local haematology laboratories, ten (38%) units order from RBTS. All neonatal units document the patient’s vital parameters before and during RBCT, but there are differences in approach, ranging from pulse oximetry alone to combined monitoring of pulse oximetry, heart rate and blood pressure. Twenty-four (92%) units noted that they would check the haematocrit or haemoglobin level between 30 minutes to several hours after the end of the transfusion.
Units use various transfusion volumes; six (23%) neonatal units transfuse 10 ml/kg, another six (23%) transfuse 15 ml/kg and five (19%) transfuse 20 ml/kg. The remaining nine (35%) neonatal units reported various blood volumes for RBCT, usually ranging from 10 to 20 ml/kg. Participants reported the main determinants for transfusion volume to be haematocrit or haemoglobin levels prior to transfusion, differentiation between acute and chronic anaemia, clinical state of the baby, concomitant pathologies such as bronchopulmonary dysplasia or congenital heart disease, and importantly, the neonatologist in charge.
Transfusion rates for red blood cells vary; 11 (44%) units transfuse at a rate of 5 ml/kg/h, 7 (28%) units use 2–4 ml/kg/h and 5 (20%) units 10 ml/kg/h. Two (8%) neonatal units use individual transfusion rates based on the clinical indication. Some participants reported a transfusion break of one hour in the middle of the RBCT.
A number of vascular access sites are used for RBCT; red blood cells are given through a peripheral venous catheter in all 26 (100%) units, through an umbilical venous catheter in 20 (77%) units, a central venous catheter in 9 (35%) units, and an umbilical arterial catheter in 5 (19%) units.
Twelve of the 26 (46%) participating neonatal units, including all level III perinatal centres, apply local guidelines for RBCT. The units without internal guidelines either follow those of their reference level III centres or consider the clinical aspect of the baby to indicate RBCT.
The present study shows little agreement in RBCT practices among local laboratories and neonatal units. In contrast to our hypothesis, we also found some variability in the preparation and logistics of blood products among RBTSs, despite the implementation of national guidelines. These findings may be explained by a lack of evidence for both the supply chain of red blood cells at the regional level and transfusion practices at the local hospitals.
In the following section, we discuss and compare our findings with national guidelines of other countries in Europe, North America and Australia/New Zealand and the available literature for the five main topics (practices for red blood cell preparation and storage, transfusion threshold, transfusion volume, transfusion rate, and vascular access) [5, 11–14].
In Switzerland, volumes of split products are dependent on the initial standard product volume. Therefore a four-pouch single unit has a volume of 60–80 ml and a two-pouch single unit a volume of 120–160 ml. One of the present study’s aims was to document the practices in the RBTSs and local laboratories for preparation and storage of blood products for newborns, in particular with regard to irradiation procedures and storage of red blood cells. Since RBTSs claim to follow the guidelines of the Swiss Red Cross [15, 16], we were surprised to find differences among laboratories.
The Swiss Red Cross recommends irradiating blood products that will be administered to premature infants. Given that preterm infants are immature and have some degree of immunological incompetency, irradiated blood could protect this fragile population from blood-related immunological complications. This recommendation is followed inconstantly by the RBTSs, as demonstrated by the various indications reported by participants for blood product irradiation in Switzerland. Indeed, there is no evidence for a clear benefit of irradiating blood. The British and French haematology societies recommend irradiating blood transfused to newborns who were exposed to intrauterine transfusions, who need exchange or massive transfusions, or who have an inherited cellular immunodeficiency [11, 21]. It may be argued that extremely premature infants are immunocompromised, which may be the reason why the Australian and New Zealand guidelines suggest a gestational age below 28 weeks and a birth weight below 900 g as indications for blood irradiation [14]. However, red blood cell bags transfused to newborns are leucocyte depleted, mainly to avoid transmission of cytomegalovirus. The almost complete absence of viable lymphocytes in transfused blood units makes the probability of transfusion-associated graft versus host disease negligible for most patients, except for those suffering from hereditary or acquired immunodeficiencies. Further, irradiation leads to a reduced shelf life of the red blood cells. There was only partial consensus on this aspect and no clear recommendations among members of the European Committee on Blood Transfusion in their “Guide to the preparation, use and quality assurance of blood components” published in 2015 [22].
How long may red blood cells be stored before transfusion? In the present study, storage times ranged from 5 to 49 days (median 28 days; see fig. 2). Again, very little evidence means there is a lack of consensus. In the French guidelines, storage time varies, depending on several parameters such as gestational age, birth weight, clinical state and transfusion volume. The Swiss BTS and the British guidelines recommend a maximum storage time of 35 days. The Canadian, USA, Australian and New Zealand guidelines extend the shelf life to 42 days [11, 16, 21, 23, 24]. In a randomised controlled trial including 377 premature infants with a birth weight of less than 1250 g, Fergusson et al. did not demonstrate a negative effect of a long (up to 42 days) versus a short (less than 7 days) storage time on mortality or acute organ failure [10]. No evidence exists to justify a red blood cell storage time of more than 42 days.
The present study shows that a minority of Swiss neonatal units have guidelines on transfusion thresholds, but variability from one centre to another is high. Thus, comparison with the international literature is of great interest and, not surprisingly, there is little consensus across the world.
Recent literature emphasises that RBCTs are not purely beneficial for the newborn infant. They also carry the risk of adverse effects, including an increase in mortality and morbidities such as necrotising enterocolitis, retinopathy of prematurity or long-term neurodevelopmental impairment [3, 8–10, 25–27]. Thus, a cautious restrictive strategy seems appropriate.
The British Committee for Standards in Haematology published guidelines in 2016 with a restrictive RBCT policy [13]. The thresholds depend on the postnatal age and the need for respiratory support. Guidelines from Australia and New Zealand consider – apart from postnatal age and the need for oxygen or respiratory support – individual clinical factors, such as severity of illness, anticipated blood loss, quality of nutrition and site of sampling [14]. Germany also takes a restrictive stance [12]. France adopted transfusion policies based on haematocrit or haemoglobin levels and a number of clinical and laboratory parameters [11]. Howarth et al. summarised the major international practice guidelines on RBCT in newborns [8].
In 2011, Whyte and Kirpalani published a Cochrane Review on low versus high haemoglobin thresholds for blood transfusion for preventing morbidity and mortality in very low birth weight infants [28]. Four trials could be included; they did not show an impact of the transfusion threshold on mortality and serious morbidity. A single trial of this systematic review reported no difference in the neurodevelopmental outcome at 18 to 21 months between the low and high RBCT threshold groups [26, 27]. The authors concluded that further trials would be required to define the impact of transfusion practices on long-term outcome. The results of two large randomised controlled trials evaluating the effects of transfusion thresholds (liberal versus restrictive) on neurodevelopment of very preterm infants are eagerly awaited (ETTNO and TOP Trial [4, 29]).
To some extent, the variability in transfusion thresholds seen in national guidelines and the literature may be explained by the number of clinical variables considered before transfusing a newborn infant, such as postnatal age or need for respiratory support [2, 8].
The blood volume transfused to a newborn infant is of great importance, particularly in the extremely premature infant. A low volume can expose the baby to more transfusions, whereas a high volume can lead to cardiopulmonary deterioration due to volume overload. In addition, high transfusion volumes will slow down erythropoiesis. In the present survey, commonly transfused red blood cell volumes ranged from 10 to 20 ml/kg. Some units also take further criteria into consideration, such as the haemoglobin level or the weight of the baby. In the literature, transfusion volumes between 10 and 20 ml/kg are most frequently found. The French guidelines recommend 15 ml/kg in premature infants born before 32 weeks of gestation or with a birth weight below 1500 g, and 20 ml/kg for older and heavier babies [11]. Canada, France and the UK recommend 10 to 20 ml/kg [5, 11, 21]. In a randomised pilot study in infants with a birth weight of less than 1500 g, Paul et al. compared a transfusion volume of 10 ml/kg with 20 ml/kg and found that the transfusion of 20 ml/kg red blood cells produced a significantly greater increase in haemoglobin and haematocrit levels than a transfusion of 10ml/kg, without detrimental effects on pulmonary compliance and resistance [6]. Von Lindern et al. analysed the long-term outcome of extremely premature infants receiving either 15 or 20 ml/kg red blood cells. Again, no significant difference in neurodevelopment was observed [9]. However, in the case of severe anaemia or hypovolaemic shock, higher RBCT volumes may be required. Close monitoring of these patients is essential in order to avoid transfusion-associated circulatory overload or heart failure [21].
In summary, current evidence does not provide a definite volume recommendation; however, the majority of authors recommend an upper limit of 15 ml/kg in order to avoid volume overload.
Apart from the transfusion volume, the transfusion rate is of interest. Again, a baby with a compromised cardiopulmonary situation may benefit from a low rate. However, a higher transfusion rate may allow the use of a single blood bag within the maximum time allowed. In our survey, we found RBCT rates between 2 and 10 ml/kg per hour. The rate should be higher in acute situations such as hypovolaemic shock [5]. Some units do not have guidelines and, again, neonatologists seem to determine the transfusion rates based on the age of the baby, the haematocrit or haemoglobin levels and the clinical condition. Venkatesh et al. found no differences between transfusion times of 3 versus 12 hours with regard to intraventricular haemorrhage, necrotising enterocolitis and nosocomial infections [25]. However, both transfusion rates used in this study may be too low for the chosen outcomes.
The consensus in international guidelines is to transfuse at a rate of 5 ml/kg per hour [11, 14, 21]. However, this recommendation is empirical and not evidence based.
All neonatal units in our survey use intravenous peripheral lines as vascular access for RBCT. However, RBCTs are also given through venous and arterial umbilical catheters, and central venous catheters. A separate peripheral line is the ideal intravenous access, allowing slow transfusion rates and preventing infection [13]. However, in the extremely premature newborns or those in a poor medical condition, inserting a peripheral intravenous cannula may be difficult. There is a complete lack of literature on the preferred vascular access for RBCT. If central venous or even arterial access is used for RBCT, inherent risks including infections, thrombosis or clotting of the catheter must be considered. For compatibility and osmolality reasons, no parallel solution should be infused during the RBCT [13]. Every vascular access for RBCT must be documented. In addition, adverse reactions to transfusions have to be reported to the national “haemovigilance system” as advised by Stainsby on behalf of the Serious Hazards of Transfusion (SHOT) steering group [30].
The strength of the present study is the high participation rate, particularly among neonatologists. Ninety percent of the neonatal units – reflecting 98% of newborns in Switzerland – responded to the questionnaire, which makes our results representative for the whole neonatal population. The main limitations include the fact that some questions were only partially answered and that answers to an online questionnaire usually reflect the opinion of the participant and are not necessarily representative of everyday practice at that establishment.
In conclusion, there is a lack of consensus on various aspects of red blood cell transfusion in neonatal units in Switzerland and wide variability in practice. A paucity of evidence, particularly regarding haemoglobin transfusion thresholds, explains the diversity in local guidelines and illustrates the need for further studies. Conscious of the various practices around RBCT at regional and local levels, we would welcome the development of Swiss-wide recommendations, particularly in the absence of good evidence. This would foster a “unité de doctrine” among Swiss neonatologists and support the establishment of a national database enabling the development of studies and comparison with international literature. Our sentiment is shared by the neonatology community, with all but two Swiss neonatal units stating that they would welcome Swiss RBCT guidelines.
The questionnaires are available in separate files at: https://smw.ch/article/doi/smw.2020.20178.
We would like to acknowledge all neonatologists and laboratory technicians who kindly participated in the study. Special thanks go to Dr Brigitte Brand and to Ruth Luginbühl, consultant haematologist and head of the laboratory technicians, respectively, of the University Hospital Zurich for their help in developing the questionnaire for the blood transfusion services.
CR received an internal research grant (protected time) from the University Hospital Zurich.
The authors declare no potential conflicts of interest.
1 Fabres J , Wehrli G , Marques MB , Phillips V , Dimmitt RA , Westfall AO , et al. Estimating blood needs for very-low-birth-weight infants. Transfusion. 2006;46(11):1915–20. doi:.https://doi.org/10.1111/j.1537-2995.2006.00997.x
2 dos Santos AMN , Guinsburg R , Procianoy RS , Sadeck LS , Netto AA , Rugolo LM , et al.; Brazilian Network on Neonatal Research. Variability on red blood cell transfusion practices among Brazilian neonatal intensive care units. Transfusion. 2010;50(1):150–9. doi:.https://doi.org/10.1111/j.1537-2995.2009.02373.x
3 Patel RM , Meyer EK , Widness JA . Research Opportunities to Improve Neonatal Red Blood Cell Transfusion. Transfus Med Rev. 2016;30(4):165–73. doi:.https://doi.org/10.1016/j.tmrv.2016.06.005
4 ETTNO Investigators. The ‘Effects of Transfusion Thresholds on Neurocognitive Outcome of Extremely Low Birth-Weight Infants (ETTNO)’ Study: Background, Aims, and Study Protocol. Neonatology. 2012;101(4):301–5. doi:.https://doi.org/10.1159/000335030
5 Whyte RK , Jefferies AL ; Canadian Paediatric Society, Fetus and Newborn Committee. Red blood cell transfusion in newborn infants. Paediatr Child Health. 2014;19(4):213–17. doi:.https://doi.org/10.1093/pch/19.4.213
6 Paul DA , Leef KH , Locke RG , Stefano JL . Transfusion volume in infants with very low birth weight: a randomized trial of 10 versus 20 ml/kg. J Pediatr Hematol Oncol. 2002;24(1):43–6. doi:.https://doi.org/10.1097/00043426-200201000-00012
7 Patterson JA , Bowen JR , Francis S , Ford JB . Comparison of neonatal red cell transfusion reporting in neonatal intensive care units with blood product issue data: a validation study. BMC Pediatr. 2018;18(1):86. doi:.https://doi.org/10.1186/s12887-018-1005-2
8 Howarth C , Banerjee J , Aladangady N . Red Blood Cell Transfusion in Preterm Infants: Current Evidence and Controversies. Neonatology. 2018;114(1):7–16. doi:.https://doi.org/10.1159/000486584
9 von Lindern JS , Khodabux CM , Hack KEA , van Haastert IC , Koopman-Esseboom C , van Zwieten PHT , et al. Long-term outcome in relationship to neonatal transfusion volume in extremely premature infants: a comparative cohort study. BMC Pediatr. 2011;11(1):48. Available at: http://www.biomedcentral.com/1471-2431/11/48. doi:.https://doi.org/10.1186/1471-2431-11-48
10 Fergusson DA , Hébert P , Hogan DL , LeBel L , Rouvinez-Bouali N , Smyth JA , et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308(14):1443–51. doi:.https://doi.org/10.1001/2012.jama.11953
11Haute autorité de santé HAS. Recommandation de bonne pratique: Transfusion de globules rouges homologues: produits, indications, alternatives. Méthode Recommandations pour la pratique clinique. www.has-sante.fr/novembre 2014:46-60.
12 Querschnitts-Leitlinien (BÄK) zur Therapie mit Blutkomponenten und Plasmaderivaten. 4. überarbeitete und aktualisierte Auflage 2014. http://www.bundesaerztekammer.de/aerzte/medizin-ethik/wissenschaftlicher-beirat/veroeffentlichungen/haemotherapie-transfusionsmedizin/querschnitt-leitlinie.
13 New HV , Berryman J , Bolton-Maggs PHB , Cantwell C , Chalmers EA , Davies T , et al.; British Committee for Standards in Haematology. Guidelines on transfusion for fetuses, neonates and older children. Br J Haematol. 2016;175(5):784–828. doi:.https://doi.org/10.1111/bjh.14233
14National Blood Authority Australia. Patient Blood Management Guidelines: Module 6, Neonatal and Paediatrics 2016. https://www.blood.gov.au/pbm-guidelines.
15Blutspende SRK Schweiz. Transfusionsmedizin, Vorschriften. http://www.blutspende.ch/de/medizin/transfusionsmedizin/vorschriften.
16Blutspende SRK Schweiz. Transfusionsmedizinische Laboruntersuchungen an Patientenproben TL. 6.0 ed. Bern: Blutspende SRK Schweiz; 2017.
17Blutspende SRK Schweiz. Regionale Blutspendedienste. http://www.blutspende.ch/de/blutspende/kontakt/blutspende_regionale_blutspendedienste.
18Swissmedic, by arrangement with the Council of Europe. Optimaler Einsatz von Blut, Zusammenfassung der Produkteigenschaften. Swissmedic. 2016. https://www.swissmedic.ch/marktueberwachung/00138/00982/index.html.
19 Roth-Kleiner M , Pfister R . Einteilung der neonatologischen Abteilungen in der Schweiz. Paediatrica. 2013;24(1):31.
20Swiss Society of Neonatology. Swiss neonatology units accredited to train neonatologists. http://www.neonet.ch/en/unit accreditation/accredited neonatology units 2018.
21Derek N. Handbook of Transfusion Medicine. 5th edition ed. Norwich: United Kingdom Blood Services; 2013: pp 120–4.
22European Committee on Blood Transfusion. Guide to the preparation, use and quality assurance of blood components, 18th Edition. Strasbourg: European Directorate for the Quality of Medicines and HealthCare; 2015; pp 118–22,343–61.
23 Guillén U , Cummings JJ , Bell EF , Hosono S , Frantz AR , Maier RF , et al. International survey of transfusion practices for extremely premature infants. Semin Perinatol. 2012;36(4):244–7. doi:.https://doi.org/10.1053/j.semperi.2012.04.004
24 Carson JL , Guyatt G , Heddle NM , Grossman BJ , Cohn CS , Fung MK , et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316(19):2025–35. doi:.https://doi.org/10.1001/jama.2016.9185
25 Venkatesh V , Khan R , Curley A , Hopewell S , Doree C , Stanworth S . The safety and efficacy of red cell transfusions in neonates: a systematic review of randomized controlled trials. Br J Haematol. 2012;158(3):370–85. doi:.https://doi.org/10.1111/j.1365-2141.2012.09180.x
26 Kirpalani H , Whyte RK , Andersen C , Asztalos EV , Heddle N , Blajchman MA , et al., for the PINT investigators. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149(3):301–7.e3. doi:.https://doi.org/10.1016/j.jpeds.2006.05.011
27 Whyte RK , Kirpalani H , Asztalos EV , Andersen C , Blajchman M , Heddle N , et al.; PINTOS Study Group. Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics. 2009;123(1):207–13. doi:.https://doi.org/10.1542/peds.2008-0338
28 Whyte R , Kirpalani H . Low versus high haemoglobin concentration threshold for blood transfusion for preventing morbidity and mortality in very low birth weight infants. Cochrane Database Syst Rev. 2011;(11):CD000512. doi:.https://doi.org/10.1002/14651858.CD000512.pub2
29Kirpalani H, Bell E, D'Angio C, Hintz S, Kennedy K, Ohls R, et al. Protocol: Transfusion of Prematures (TOP) trial. Does a liberal red blood cell transfusion strategy improve neurologically-intact survival of extremely-low-birth-weight-infants as compared to a restrictive strategy? ClinicalTrials.gov;2012:NCT01702805
30 Stainsby D , Jones H , Wells AW , Gibson B , Cohen H ; SHOT Steering Group. Adverse outcomes of blood transfusion in children: analysis of UK reports to the serious hazards of transfusion scheme 1996-2005. Br J Haematol. 2008;141(1):73–9. doi:.https://doi.org/10.1111/j.1365-2141.2008.07022.x
CR received an internal research grant (protected time) from the University Hospital Zurich.
The authors declare no potential conflicts of interest.