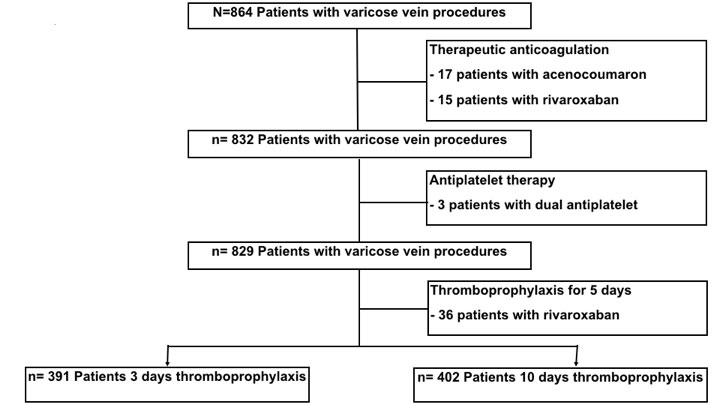
Figure 1 Study cohort selection.
DOI: https://doi.org/10.4414/smw.2019.20166
Endovenous thermal ablation (ETA) of truncal varicose veins was introduced in 1999 and its use has been rapidly growing in recent decades [1, 2]. In 2011, the Society for Vascular Surgery and the American Venous Forum recommended ETA as the preferred treatment option for symptomatic varicose veins [3]. In 2013, the UK National Institute for Health and Care Excellence (NICE) also recommended ETA as the preferred treatment option for symptomatic varicose veins [4]. And in 2015, the European Society for Vascular Surgery recommended ETA as the preferred treatment option over surgery for symptomatic great saphenous vein reflux [5]. Thus, it is likely that the use of ETA will continue to increase owing to its recommendation in evidence-based guidelines. This minimally invasive technique immediately improves quality of life without the morbidity associated with saphenous crossectomy and stripping [6]. However, with the growing popularity of this technique, there have been numerous reports on complications associated with this treatment [7, 8]. Patients may develop deep venous thrombosis (DVT) at the saphenofemoral junction (SFJ) or saphenopopliteal junction (SPJ) [9]. This complication is known as endovenous heat-induced thrombosis (EHIT). EHIT refers to the extension of thrombus from the ablated superficial vein into the deep venous system at or proximal to a site of recent thermoablation [10]. EHIT has been categorised in four classes. EHIT class 1 is when the thrombus extends to the SFJ or SPJ. Thrombus extension into the deep venous system with a cross-sectional obstruction area of less than 50% is considered EHIT class 2. EHIT class 3 is defined as more than 50% cross-sectional area obstruction. EHIT class 4 is complete occlusion of the common femoral or popliteal vein [11]. DVT at any site in the deep venous system and pulmonary embolism (PE) – although rare – may also develop during the procedure or thereafter, with deaths of relatively young patients being reported in the press [12, 13]. Published rates of EHIT vary between 0% and 8% and the reported incidence of DVT is 0.85% [9, 14–17].
Occurrence of these complications suggests the need for early postprocedural duplex scanning to detect potential thrombotic events, as recommended by the Society for Vascular Surgery [16]. Given the growing numbers of ETA procedures performed worldwide and the potential for severe complications, some form of thromboprophylaxis needs to be considered. Current guidelines recommend early ambulation after ETA for thromboprophylaxis; however, to date no internationally accepted recommendations regarding pharmacological thromboprophylaxis exist to guide clinical practice [18–20]. Many centres in Europe, including several in Switzerland, do use pharmacological thromboprophylaxis after thermal ablation [10, 21, 22]. Some centres prefer single-dose low molecular weight heparin or direct oral anticoagulants as their primary choice for thromboprophylaxis, whereas other centres use prolonged thromboprophylaxis for up to 10 days. Epidemiological evidence from the Million Women Study suggests that the postprocedural venous thromboembolism incidence may be increased in the first 6 weeks after operation as a day case [23]. Thus, single-dose or short-term thromboprophylaxis might be ineffective. However, the optimal duration of pharmacological thromboprophylaxis after ETA is currently not defined. In the absence of good evidence, we decided to pool and analyse the prospectively collected quality and outcome date of four vein centres to assess the efficacy and safety of two pharmacological thromboprophylaxis regimens (3 vs 10 days) after outpatient endovenous laser ablation (EVLA) of truncal and accessory saphenous veins.
At all participating centres, patient, procedural and follow-up data were prospectively obtained as part of the local quality and outcome assessment process and reviewed in accordance with the various reporting standard guidelines [24]. In this retrospective study, we pooled and reviewed the medical records of all consecutive patients with truncal or accessory saphenous veins treated with EVLA as an outpatient between February 2009 and December 2015. The study followed the principles outlined in the Declaration of Helsinki and was approved by the local ethics committee. This manuscript was prepared in compliance with the STROBE checklist [25].
All patients signed an informed consent form before the EVLA procedure, agreeing to use of their medical data anonymously for publication. A waiver for additional formal informed consent to retrospectively review charts of subjects meeting the inclusion criteria was granted by the ethics committee. All patients who had EVLA of the great saphenous vein (GSV), accessory saphenous vein (ASV), or small saphenous vein (SSV) using a 1470-nm wavelength radial laser (ELVeS; Biolitec, Vienna, Austria; VenaCure, AngioDynamics, Inc, Queensbury, NY, USA) at the University Hospital Basel, the regional hospital of Limmattal, and private practices GefässmedizinRapperswil and the Vascular Institute Aarau were included in this analysis. Patients were not included in the analysis if they had an endovenous ablation technique other than EVLA; veins other than the GSV, ASV, or SSV treated with EVLA (i.e., perforators); and postinterventional EHIT prophylaxis other than a prophylactic dose (10 mg/d) of oral rivaroxaban (Bayer AG, Zurich, Switzerland) or fondaparinux 2.5 mg subcutaneously.
Demographic data, vein characteristics, procedural data including concomitant phlebectomies and sclerotherapy, and outcome data including ultrasound findings and complications were assessed. All data were routinely collected after each procedure/follow-up appointment and entered into a local Excel database by a dedicated study nurse at the University Hospital Basel or the interventionist performing EVLA at the other centres. Anonymised data were pooled and checked for data integrity and data selection according to the inclusion/exclusion criteria by the first and last author. All patients were diagnosed preoperatively with superficial venous insufficiency by means of duplex ultrasound. Vein incompetence was assessed as reflux in response to manual calf compression or Valsalva manoeuvre with the patient standing. Reflux was defined as evidence of reverse flow >500 ms in a vein segment [26].
EVLA was performed by experienced vascular specialists. Bilateral treatment was allowed. Tumescent anaesthesia was used in all cases of EVLA as a walk-in, walk-out procedure. No sedation was routinely given.
The GSV was cannulated percutaneously at the distal point of insufficiency under ultrasound guidance using the Seldinger technique. The access point was mostly infragenual. After insertion of the laser fibre through the sheath, the fibre tip was advanced to the SFJ or SPJ, then positioned 1–2 cm distal to the SFJ/SPJ with ultrasound guidance and connected to a 1470 nm radial diode laser device. For local tumescent anaesthesia, a volume of 0.5 l was prepared using 500 ml 0.9% saline, 50 ml of 2% rapidocaine and 5 ml of 8.4% sodium bicarbonate. This was then infiltrated in the perivenous space under high-resolution ultrasound guidance. After tumescent anaesthesia was administered, the position of the laser tip was again verified before activating the laser. Laser energy was released at 8–10 W power using a continuous mode, aiming for a linear endovenous energy delivery target of 50–100 J/cm. After activation, the laser fibre was slowly and continuously pulled back during ablation. Treatment length and vein diameter were not available from all centres. In general, we ablated the refluxing vein segments completely from below the knee for the GSV and from the distal third of calf for the SSV. After the EVLA, refluxing tributaries were removed by phlebectomy or closed with sclerotherapy during the same procedure. After tumescent anaesthesia alongside the tributaries, 1- to 3-mm incisions over varicosities were made and varicose tributaries were removed using a hook (Oesch; Salzmann AG, S. Gallen, Switzerland). Concomitant foam sclerotherapy was given alone or in addition to phlebectomy with up to 10 ml of 1% to 3% aethoxysklerol mixed 1:4 with air in patients with neovascularisation or tributaries of perforators.
After the treatment, the legs were wrapped in sterile absorbent bandages and, in those patients who had concomitant phlebectomy, covered with a compressive cohesive bandage. After 24 to 72 hours, the patients removed the bandage and were told to wear a class 2 compression stocking during the day for at least 1 week. We did not monitor compliance regarding the use of the stockings.
Any patients undergoing EVLA procedures routinely received thromboprophylaxis with doses of 10 mg/d of rivaroxaban (Bayer AG, Zurich, Switzerland) or fondaparinux 2.5 mg subcutaneously (Sanofi-Aventis, Vernier, Switzerland) for 3 to 10 days at the discretion of the operator. The duration of thromboprophylaxis was somewhat arbitrary – as the standard pack size of rivaroxaban contains 10 pills in Switzerland a 10-day regimen was chosen by most operators, whereas the 3-day regime was arbitrary and based on the believe that after 3 days the patient is fully recovered and fully mobilised. The first dose of antithrombotic agent was administered immediately postoperatively and the remaining doses were given to the patients to take at home. Routine mobilisation was encouraged for the postoperative period. We did not monitor the adherence to drug intake or the compliance with wearing the compression stockings.
In all participating centres standardised there were follow-up examinations according to the Swiss Tect Registry [27] at 7 ± 2 and at 30 ± 2 days postoperatively. These included a short medical history and clinical examination with assessment of any procedure-related complication (especially pain, bleeding, medication side effects, signs of infection). At all follow-up appointments, a dedicated duplex ultrasound examination of the deep and superficial leg veins was performed by experienced vascular physicians, to assess the treated vein segment and to check for EHIT and deep vein thrombosis (DVT), with special attention paid to the SFJ and SPJ and calf muscle veins.
The primary efficacy endpoint was a composite of EHIT class 2–4, DVT diagnosed with duplex ultrasound and PE diagnosed with computed tomography (CT) in cases of clinical suspicion. Cumulative secondary endpoints were major bleeding, skin burns, infection and complete abolition of the treated veins. The distance of the occluded vein or thrombus in relation to the SFJ or SPJ recorded during the follow-up duplex ultrasound examinations was reviewed and classified according to Kabnick classification [11]. The secondary endpoint of major bleeding was defined as fatal bleeding, bleeding in critical sites such as retroperitoneal, intracranial and spinal cord bleeds, bleeding leading to operation, significant bleeding leading to a 20 g/l or greater fall in haemoglobin, or a transfusion requirement of more than two units of whole blood / red blood cells [28]. Skin burn was defined as redness and pain alongside the treated varicose veins and infection was defined as the need for an antibiotic in association with the procedure. Complete ablation was defined as absence of flow in the treated vein on ultrasound imaging. Partial ablation was defined as complete recanalisation of 5 cm or more and complete recanalisation as reflux in the treated varicose vein.
Categorical data are presented as frequency and percentage, continuous data reported as mean and standard deviation (SD). Chi-squared and Fisher’s exact tests were used for comparisons of categorical data. Logistic regression models were used to assess the association between efficacy outcome and the thromboprophylaxis groups. As adjusting factors, we used sex, age and miniphlebectomy.
The command in STATA is as follows: logistic depvar indepvars. Thereafter propensity score matching was applied to reduce the effect of treatment selection bias and potential confounding effect. The propensity score (PS) was calculated using a logistic regression model. The 10-day regimen group defined as group 2 versus the 3-day regimen group defined as group 1 were matched using the nearest neighbour-matching method. Average treatment effect on the treated (ATT) with nearest neighbour matching method was used to analyse the outcome variables in Stata version 15 (command pscore/ attnd). We defined sex, age and phlebectomy as potential confounders and used these variables in our PS-matched analysis. We did not match for total energy applied and total time applied because the balancing property was not satisfied. We did not match for sclerotherapy as the cases were to low. We also did not match for CEAP classification as CEAP class has not been shown to be related to EHIT and DVT.
A two-sided p-value of <0.05 was considered statistically significant. Data analyses were performed using Stata software version 15 (Stata, Inc. Stata Statistical Software Release 10, College Station, TX, USA).
From February 2009 to December 2015, 864 patients were treated with EVLA of truncal varicose veins. Thirty-five cases were excluded as the patients were on therapeutic dose anticoagulation with acenocoumaron (n = 17) and rivaroxaban (n = 15) or on dual antiplatelet therapy (n = 3). Of the remaining 829 cases, another 36 were excluded from our analysis as the patients received pharmacological thromboprophylaxis for 5 days and the case number was too low for analysis.
In total, we included 793 patients in this study (Vascular Centre Rapperswil n = 188, Limmattal Hospital n = 123, Vascular Institute Aarau n = 391 and University Hospital Basel n = 91). Figure 1 is the flow diagram of patients who were finally included in the analysis.

Figure 1 Study cohort selection.
Power calculation indicated that the sample size was sufficient to detect a between-group difference of 3.1% with 80% power. Of the study population, 75.2% were female and the mean age was 55.2 ± 14.8 years. Of the total cohort, 225 patients (28.4%) received fondaparinux 2.5 mg subcutaneously as thromboprophylaxis for 3 days, 166 patients (20.9%) received rivaroxaban 10 mg orally for 3 days and 402 patients (50.7%) received rivaroxaban 10 mg for 10 days. Detailed demographic characteristics for the total population and for group 1 (treated for 3 days) and group 2 (treated for 10 days) are shown in table 1.
| Table 1: Patient demographics. | |||||
| Variable | Total (n = 793) | Group 1 (n = 391) | Group 2 (n = 402) | p-value | |
| Female sex, n (%) | 596 (75.2) | 302 (77.2) | 294 (73.1) | 0.759 | |
| Age, mean (SD), year | 55.2 (14.8) | 56.7 (14.1) | 73.7 (15.4) | <0.005 | |
| CEAP classification, n (%) | C2 | 275 (34.7) | 59 (15.1) | 216 (53.7) | <0.001 |
| C3 | 267 (33.7) | 156 (39.9) | 111 (27.6) | <0.001 | |
| C4 | 209 (26.3) | 146 (37.3) | 63 (15.7) | <0.001 | |
| C5 | 15 (1.9) | 8 (2.1) | 7 (1.7) | 0.753 | |
| C6 | 27 (3.4) | 22 (5.6) | 5 (1.2) | 0.001 | |
| CEAP = clinical,(a)etiology, anatomy, pathophysiology; SD = standard deviationGroup 1 received thromboprophylaxis for 3 days. Group 2 received thromboprophylaxis for 10 days. | |||||
Characteristics of the treated veins and procedural data are given in table 2.
| Table 2: Lesion characteristics and procedural data. | |||||
| Characteristic | Total (n = 793) | Group 1 (n = 391) | Group 2 (n = 402) | p-value | |
| Treated vein, n (%) | GSV right | 381 (48.1) | 229 (58.6) | 152 (37.8) | <0.001 |
| SSV right | 81 (10.2) | 15 (3.8) | 66 (16.4) | <0.001 | |
| GSV left | 388 (48.9) | 224 (57.3) | 164 (40.8) | <0.001 | |
| SSV left | 56 (7.1) | 20 (5.1) | 36 (8.9) | 0.038 | |
| Anterior ASV right or left | 13 (1.6) | 0 | 13 (3.2) | <0.001 | |
| Posterior ASV right or left | 2 (0.2) | 0 | 2 (0.5) | 0.499 | |
| Length, mean (SD), cm* | 52.1 (15.3) | 52.1 (15.3) | |||
| Diameter, mean (SD), cm† | 7.4 (2.8) | 7.4 (2.8) | |||
| Applied energy, mean (SD), J | 2855 (1155) | 2959 (972) | 2754 (1302) | 0.013 | |
| Application time, mean (SD), s | 329 (160) | 293 (97) | 364 (198) | <0.001 | |
| Concomitant phlebectomy, n (%) | 368 (46.4) | 47 (12.0) | 321 (79.9) | <0.001 | |
| Concomitant sclerotherapy, n (%) | 20 (2.5) | 0 | 20 (4.9) | <0.001 | |
| ASV = accessory saphenous vein; GSV = great saphenous vein; SSV = small saphenous vein; SD = standard deviationGroup 1 received thromboprophylaxis for 3 days. Group 2 received thromboprophylaxis for 10 days.* data available n = 367; † data available n = 394 | |||||
Group 1 included significantly younger patients than group 2 (56.7 ± 14.1 years vs 73.7 ± 15.3, p <0.005). CEAP clinical scores C2, C3 and C4 were highly prevalent in the entire population and accounted for 94.7% of all treated varicose veins. However, group 1 showed a higher prevalence of stages C3, C4 and C6 in total: 82.8 vs 44.5% in group 2 (p <0.001). Total energy administered for ablating the truncal veins differed significantly between groups, as did application time, concomitant phlebectomy and concomitant sclerotherapy (table 2).
Detailed cumulative primary efficacy and safety outcomes of all patients and separated by group 1 and group 2 are given in table 3. In the total cohort, EHIT was identified in 42 patients (5.3%) and DVT in 3 (0.4%) at the 1-week follow-up. At 4 weeks follow-up cumulative total number of cases of EHIT was 43 (5.4%) and of DVT was 3 (0.4%). All cases of DVT were in the calf. EHIT level 1 was identified in 37 (4.7%) patients and EHIT class 2 in 6 (0.8%). Of note, one of six (16.7%) of the EHIT class 2 complications was observed at the 4-week follow-up but not earlier. All other complications were observed at 1-week follow-up.
| Table 3: Cumulative primary efficacy endpoint and cumulative secondary outcomes for all patients receiving thromboprophylaxis for 3 days (group 1) and 10 days (group 2) after endovenous laser ablation. | ||||||||
| Variables | Total (n = 793) | Group 1 (n = 391) | Group 2 (n = 402) | OR (95% CI) | p-value | PS-matched ATT | PS-matched 95% CI | |
| Highest EHIT class during follow-up | EHIT class 1 | 37 (4.7) | 20 (5.1) | 17 (4.2) | 0.82 (0.42 to 1.59) | 0.555 | 0.005 | −0.033 to 0.043 |
| EHIT class 2 | 6 (0.8) | 5 (1.3) | 1 (0.3) | 0.19 (0.02 to 1.66) | 0.133 | −0.021 | −0.066 to 0.023 | |
| Deep vein thrombosis | 3 (0.4) | 3 (0.8) | 0 | n.a. | 0.119* | −0.003 | −0.008 to 0.002 | |
| Composite endpoint | 9 (1.1) | 8 (2.1) | 1 (0.3) | 0.12 (0.01 to 0.96) | 0.046 | −0.024 | −0.074 to 0.26 | |
| Occlusion rate | Complete occlusion | 785 (99.0) | 388 (99.2) | 397 (98.8) | 0.61 (0.15 to 2.59) | 0.506 | −0.007 | −0.022 to 0.007 |
| Partial occlusion | 6 (0.8) | 2 (0.5) | 4 (1.0) | 1.95 (0.36 to 10.73) | 0.440 | 0.005 | −0.007 to 0.018 | |
| Complete recanalisation | 2 (0.3) | 1 (0.3) | 1 (0.3) | 0.97 (0.06 to 15.60) | 0.984 | 0.002 | −0.003 to 0.007 | |
| Paraesthesia | 86 (10.8) | 49 (12.5) | 37 (9.2) | 0.71 (0.45 to 1.11) | 0.133 | −0.037 | −0.135 to 0.062 | |
| Infection | 4 (0.5) | 4 (1.0) | 0 | n.a. | 0.059* | −0.003 | −0.007 to 0.002 | |
| ATT = average treatment effect on treated; CI = confidence interval; EHIT = endovenous heat-induced thrombosis; EVLA = endovenous laser ablation; n.a. = not applicable; PS-matched = propensity score-matched* Fisher’s exact test. After propensity score-matched analysis the comparison of the two groups showed 95% confidence interval crosses zero, meaning that the comparison is not significant at p <0.05 | ||||||||
There were no cases of EHIT class 3 and 4. The incidence of all EHIT in group 1 and group 2 were 6.4% (25/391) and 4.5% (18/402), respectively, and the difference between the groups was not statistically significant. No patient in either group experienced symptomatic PE. The incidence of DVT was similar in both groups and did not show any significant difference (0.8% in group 1 vs 0% in group 2, p = 0.119).
PS-matched analysis using nearest neighbour matching identified 255 patients from group 1 as control. In the PS-matched analysis we found no difference between the PS-matched group 1 versus group 2 for sex (p = 0.447) or age (p = 0.363). For phlebectomy there was a statistical significance between PS-matched group 1 versus group 2 (p <0.001), as well as for the unmatched group. As shown in table 3, PS-matched analysis showed no difference for the combined endpoint or for efficacy outcome variables. Differences in secondary outcome variables such as occlusion rate were not significant in the unmatched and PS-matched group. Complete recanalisation of the treated veins occurred in only one patient (0.3%) in each group and was statistically not significant.
There were no major bleeding events in group 1 or group 2. No episodes of fatal bleeding occurred. Infection at the puncture site needing antibiotic treatment occurred in four patients in group 1 (1.0%) and none in group 2 (p = 0.059, table 3). No skin burn occurred in either groups during EVLA treatment.
EHIT and venous thromboembolism (VTE), consisting of DVT and PE, are well recognised complications of endovenous thermal ablation [10, 29, 30]. In our multicentre registry, thromboprophylaxis was given routinely after EVLA, as with minor orthopaedic surgery [31, 32] where it has been effectively used to reduce VTE. Although the duration of thromboprophylaxis after thermal ablation was variable, we set out to analyse whether a 3-day or a 10-day course of thromboprophylaxis after outpatient thermal ablation would reduce the incidence of EHIT and VTE.
We demonstrated in a PS-matched analysis that extended thromboprophylaxis (10-day regimen) was equally effective as a shorter regimen (3 days), and occlusion rates and safety profile remained similar. An interesting observation was that group 1 consisted of younger patients with more advanced disease, which could not be fully explained. This could suggest that vascular specialists in peripheral centres may be more selective in performing procedures on patients with venous disease. And thus conservative treatment may be more frequently offered to patients with CEAP class C2. Overall, our findings do not support the arguments for prolonged thromboprophylaxis from haematologists, which were based on epidemiological evidence from the Million Women Study [23]. This study assessed time since surgery and the relative risk of VTE after inpatient surgery and day surgery [23]. They demonstrated that both types of surgery were associated with a significantly increased risk of postoperative DVT, starting on the day of surgery and remained increased for weeks into the postoperative period.
Pharmacological thromboprophylaxis and graduated compression stockings are used to reduce the risk of perioperative VTE in patients undergoing general or open varicose vein surgery. Vascular specialists have adapted and extended this practice to patients undergoing endovenous thermal interventions. Compression stockings have been shown to reduce the incidence of VTE by approximately 44% and low molecular weight heparin by 76% for patients undergoing general or open varicose vein surgery [33]. Compared with standard anticoagulation therapy, rivaroxaban, a factor Xa inhibitor, has been shown to be safe and effective for thromboprophylaxis after orthopaedic procedures [31, 32]. It has also been effectively used for thromboprophylaxis after thermal ablation as compared with fondaparinux, a low molecular weight heparin with a long half-life [34]. The authors demonstrated that rivaroxaban 10 mg once daily orally after thermal ablation is safe and effective compared with low molecular weight heparin. Also the multicentre RITE registry showed an effective use of rivaroxaban for VTE prophylaxis [35].
The extent to which graduated compression stockings, low molecular weight heparin or direct acting oral anticoagulants confer additional benefit over mobilisation following outpatient endovenous treatment is not known. Thus, some vascular specialists question the need for any pharmacological thromboprophylaxis at all, as outpatient endovenous treatment using modern 1470-nm ring laser fibres causes a relatively minor trauma and patients return almost immediately to preoperative levels of mobility. Relevant evidence is virtually nonexistent because of the difficulties of conducting high-quality research in this area. Nonetheless, in the absence of good evidence and limited professional consensus, EHIT and VTE after endovenous treatment should be of significant concern given the potential for litigation. In a poll at the 2014 UK-Venous Forum meeting, a clear majority of specialists favoured single-dose low molecular weight heparin after thermal ablation [36]. Thus, at least some form of thromboprophylaxis seems to be favoured by the vascular interventionists.
The wavelength and the forward firing mode of the energy of the laser fibre might impact on EHIT formation as the EHIT rate for the 810-nm laser tip has been reported to be 4% [37]. For the EVLA procedure in our cohort, a 1470-nm wavelength targeting water in the vein wall and emitting energy circumferentially was used. This release of energy might partially contribute to the low overall EHIT rate. The management of EHIT has changed from operative thrombectomy with or without saphenofemoral ligation to anticoagulation [38, 39]. Nowadays, most authors agree that treatment with low molecular weight heparin or direct acting oral anticoagulants is adequate for most cases, although firm evidence is lacking [9, 29]. Overall, the EHIT rate in this study was low, which might be the result of the thromboprophylaxis given. EHIT class 2 and calf DVT in our study were managed with therapeutic anticoagulation with direct acting oral anticoagulants until resolution on ultrasound, in accordance with the treatment algorithm suggested by Kabnick et al., which was introduced with his classification system [11]. Resolution was achieved between 4 and 6 weeks of therapeutic anticoagulation.
This study has several limitations. First, it is limited by its nonrandomised, observational character and, thus, selection bias cannot be excluded or fully adjusted for: the documentation of established risk factors for VTE was not part of the quality and outcome assessment protocol in all centres, thus a dedicated matching/adjustment for these risk factors was not possible. Furthermore, we did not monitor adherence to drug intake and compression stockings, and thus cannot exclude that a greater malcompliance with the “long-term” pharmacological thromboprophylaxis may have biased our results. Certainly, this major methodological limitation limits the significance of the present investigation. However, given the multicentre character, the large number of patients and the propensity score analysis, risk of potential biases is considered to be low. As all participating centres prescribed pharmacological thromboprophylaxis after EVLA we were not able to include a group of patients with no thromboprophylaxis in our analysis. Thus, we cannot make any estimates on the effect of rivaroxaban on the frequency of EHIT/DVT and bleeding events as compared with no pharmacological thromboprophylaxis. Furthermore, the overall incidence of EHIT was low in our study, and so a much larger cohort would be required to detect a significant difference between group differences (approximately 4600 patients to detect a between group difference of 1%). Ultimately, we cannot exclude the possibility that, by using high-definition ultrasound guidance and modern 1470-nm ring laser fibres thromboprophylaxis is still required for EHIT prevention. However, many experienced operators nowadays no longer respect the “safety” ablation distance of >2cm to the SFJ, but rather aim for a “flush” ablation to reduce the rate of subsequent varicose recurrence due to untreated SFJ tributaries. Especially in this setting with thermal ablation close to the SFJ, it is reasonable to believe that some pharmacological thromboprophylaxis is useful.
Despite these limitations, this study provides valuable information on outpatient EVLA and thromboprophylaxis given the lack of guidelines and the limited data currently available. A much larger registry or a randomised clinical trial is clearly warranted, to further investigate the safety and efficacy of outpatient EVLA and its thromboprophylaxis management. Unless such data are available, we administer thromboprophylaxis given the high acceptance and zero major bleeding rate.
This is the first report to compare different durations of pharmacological thromboprophylaxis after outpatient EVLA. PS-matched analysis indicated that extended thromboprophylaxis for 10 days is equally effective when compared with a 3-day regimen with excellent ablation rates, low EHIT/VTE rates and no major bleeding in both groups. Undoubtedly, randomised controlled data, including a group without any pharmacological thromboprophylaxis at all, are needed to draw more definitive conclusions on the optimal duration of pharmacological post-EVLA thromboprophylaxis.
No funding and no other potential conflict of interest relevant to this article was reported.
1. Bonè C . Tratamiento endoluminal de las varices con laser de Diodo: estudio preliminary. Rev Patol Vasc. 1999;(5):35–46.
2. Weiss RA , Munavalli G . Endovenous ablation of truncal veins. Semin Cutan Med Surg. 2005 Dec;24(4):193–9. https://doi.org/10.1016/j.sder.2005.10.006
3. Gloviczki P , Comerota AJ , Dalsing MC , Eklof BG , Gillespie DL , Gloviczki ML , et al.; Society for Vascular Surgery; American Venous Forum . The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011 May;53(5 Suppl):2S–48S. https://doi.org/10.1016/j.jvs.2011.01.079
4. National Institute for Health and Care Excellence . Available at http://guidance.nice.org.uk/CG168/NICEGuidance/pdf/English
5. Oni OO , Mahabir JP , Iqbal SJ , Gregg PJ . Serum osteocalcin and total alkaline phosphatase levels as prognostic indicators in tibial shaft fractures. Injury. 1989 Jan;20(1):37–8. https://doi.org/10.1016/0020-1383(89)90042-9
6. Kane K , Fisher T , Bennett M , Shutze W Jr , Hicks T , Grimsley B , et al. The incidence and outcome of endothermal heat-induced thrombosis after endovenous laser ablation. Ann Vasc Surg. 2014 Oct;28(7):1744–50. https://doi.org/10.1016/j.avsg.2014.05.005
7. Boersma D , Kornmann VN , van Eekeren RR , Tromp E , Ünlü Ç , Reijnen MM , et al. Treatment Modalities for Small Saphenous Vein Insufficiency: Systematic Review and Meta-analysis. J Endovasc Ther. 2016 Feb;23(1):199–211. https://doi.org/10.1177/1526602815616375
8. Malgor RD , Gasparis AP , Labropoulos N . Morbidity and mortality after thermal venous ablations. Int Angiol. 2016 Feb;35(1):57–61.
9. Puggioni A , Kalra M , Carmo M , Mozes G , Gloviczki P . Endovenous laser therapy and radiofrequency ablation of the great saphenous vein: analysis of early efficacy and complications. J Vasc Surg. 2005 Sep;42(3):488–93. https://doi.org/10.1016/j.jvs.2005.05.014
10. Marsh P , Price BA , Holdstock J , Harrison C , Whiteley MS . Deep vein thrombosis (DVT) after venous thermoablation techniques: rates of endovenous heat-induced thrombosis (EHIT) and classical DVT after radiofrequency and endovenous laser ablation in a single centre. Eur J Vasc Endovasc Surg. 2010 Oct;40(4):521–7. https://doi.org/10.1016/j.ejvs.2010.05.011
11. Kabnick L . Ombrellino M Agis Hea. Endovenous heat induced thrombus (EHIT) at the superficial-deep venous junction: a new post-treatment clinical entity, classification and potential treatment strategies. 18th Annual Meeting of the American Venous Forum, Miami, FL, USA. February 2006.
12. The Daily Mail . Mother-of-two's death after varicose vein surgery 'could have been prevented', her husband claims. Accessed at at http://dailymial.co.uk/health/article-2980924/Mother-of-two's death after varicose vein surgery 'could have been prevented', her husband claims.html (accessed date 21.3.2019). Published 12:20 GMT, 5 March 2015.
13. News RT . Inquest into the death of Karen McCabe adjourned until May. http://www.rte.ie/news/2016/0218/769058-inquest-varicose-veins-surgery-death. Date accessed 21.03.2019).
14. Ash JL , Moore CJ . Laser treatment of varicose veins: order out of chaos. Semin Vasc Surg. 2010 Jun;23(2):101–6. https://doi.org/10.1053/j.semvascsurg.2010.01.005
15. Spreafico G , Kabnick L , Berland TL , Cayne NS , Maldonado TS , Jacobowitz GS , et al. Laser saphenous ablations in more than 1,000 limbs with long-term duplex examination follow-up. Ann Vasc Surg. 2011 Jan;25(1):71–8. https://doi.org/10.1016/j.avsg.2010.09.005
16. Gloviczki P , Gloviczki ML . Guidelines for the management of varicose veins. Phlebology. 2012 Mar;27(1_suppl Suppl 1):2–9. https://doi.org/10.1258/phleb.2012.012s28
17. Knipp BS , Blackburn SA , Bloom JR , Fellows E , Laforge W , Pfeifer JR , et al.; Michigan Venous Study Group . Endovenous laser ablation: venous outcomes and thrombotic complications are independent of the presence of deep venous insufficiency. J Vasc Surg. 2008 Dec;48(6):1538–45. https://doi.org/10.1016/j.jvs.2008.07.052
18. Gloviczki P , Comerota AJ , Dalsing MC , Eklof BG , Gillespie DL , Gloviczki ML , et al.; Society for Vascular Surgery; American Venous Forum . The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011 May;53(5 Suppl):2S–48S. https://doi.org/10.1016/j.jvs.2011.01.079
19. http://www.nice.org.uk/guidance/cg168/chapter/1-Recommendations. Accessed Jan. 30. 2013.
20. Kahn SR , Lim W , Dunn AS , Cushman M , Dentali F , Akl EA , et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e195S-e226S.
21. Christenson JT , Gueddi S , Gemayel G , Bounameaux H . Prospective randomized trial comparing endovenous laser ablation and surgery for treatment of primary great saphenous varicose veins with a 2-year follow-up. J Vasc Surg. 2010 Nov;52(5):1234–41. https://doi.org/10.1016/j.jvs.2010.06.104
22. Schwarz T , von Hodenberg E , Furtwängler C , Rastan A , Zeller T , Neumann FJ . Endovenous laser ablation of varicose veins with the 1470-nm diode laser. J Vasc Surg. 2010 Jun;51(6):1474–8. https://doi.org/10.1016/j.jvs.2010.01.027
23. Sweetland S , Green J , Liu B , Berrington de González A , Canonico M , Reeves G , et al.; Million Women Study collaborators . Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ. 2009 Dec;339 dec03 1:b4583. https://doi.org/10.1136/bmj.b4583
24. Porter JM , Moneta GL ; International Consensus Committee on Chronic Venous Disease . Reporting standards in venous disease: an update. J Vasc Surg. 1995 Apr;21(4):635–45. https://doi.org/10.1016/S0741-5214(95)70195-8
25. von Elm E , Altman DG , Egger M , Pocock SJ , Gøtzsche PC , Vandenbroucke JP ; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008 Apr;61(4):344–9. https://doi.org/10.1016/j.jclinepi.2007.11.008
26. Labropoulos N , Tiongson J , Pryor L , Tassiopoulos AK , Kang SS , Ashraf Mansour M , et al. Definition of venous reflux in lower-extremity veins. J Vasc Surg. 2003 Oct;38(4):793–8. https://doi.org/10.1016/S0741-5214(03)00424-5
27. http://www.uvs.ch/; accessed January 15th, 2017.
28. Kaatz S , Ahmad D , Spyropoulos AC , Schulman S ; Subcommittee on Control of Anticoagulation . Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015 Nov;13(11):2119–26. https://doi.org/10.1111/jth.13140
29. Hingorani AP , Ascher E , Markevich N , Schutzer RW , Kallakuri S , Hou A , et al. Deep venous thrombosis after radiofrequency ablation of greater saphenous vein: a word of caution. J Vasc Surg. 2004 Sep;40(3):500–4. https://doi.org/10.1016/j.jvs.2004.04.032
30. Carradice D , Leung C , Chetter I . Laser; best practice techniques and evidence. Phlebology. 2015 Nov;30(2 Suppl):36–41. https://doi.org/10.1177/0268355515591440
31. Akin M , Schäfer A , Akin I , Widder J , Brehm M . Use of New Oral Anticoagulants in the Treatment of Venous Thromboembolism and Thrombotic Prophylaxis. Cardiovasc Hematol Disord Drug Targets. 2015;15(2):92–6. https://doi.org/10.2174/1871529X1502151209110620
32. Ageno W , Mantovani LG , Haas S , Kreutz R , Monje D , Schneider J , et al. Safety and effectiveness of oral rivaroxaban versus standard anticoagulation for the treatment of symptomatic deep-vein thrombosis (XALIA): an international, prospective, non-interventional study. Lancet Haematol. 2016 Jan;3(1):e12–21. https://doi.org/10.1016/S2352-3026(15)00257-4
33. Chapter JJ . 41: Thrombotic Risk Assessment: A Hybrid Aproach. the Vein Book (ed. Bergan JJ). 2007 Elselvier Inc.
34. Keo HH , Baumann F , Diehm N , Regli C , Staub D . Rivaroxaban versus fondaparinux for thromboprophylaxis after endovenous laser ablation. J Vasc Surg Venous Lymphat Disord. 2017 Nov;5(6):817–23. https://doi.org/10.1016/j.jvsv.2017.04.017
35. Uthoff H , Holtz D , Broz P , Staub D , Spinedi L . Rivaroxaban for thrombosis prophylaxis in endovenous laser ablation with and without phlebectomy. J Vasc Surg Venous Lymphat Disord. 2017 Jul;5(4):515–23. https://doi.org/10.1016/j.jvsv.2016.12.002
36. Goodyear SJ . A W K NI. Chapter 387: Anti-venous thrombosis protocol - a proposed scoring system. Charing Cross 1978-2017. Vascular and Endovasclar Consensus Update (ed. Greenhalgh RM). 2017 BIBA Publishing.
37. Rhee SJ , Cantelmo NL , Conrad MF , Stoughton J . Factors influencing the incidence of endovenous heat-induced thrombosis (EHIT). Vasc Endovascular Surg. 2013 Apr;47(3):207–12. https://doi.org/10.1177/1538574413478494
38. Manfrini S , Gasbarro V , Danielsson G , Norgren L , Chandler JG , Lennox AF , et al.; Endovenous Reflux Management Study Group . Endovenous management of saphenous vein reflux. J Vasc Surg. 2000 Aug;32(2):330–42. https://doi.org/10.1067/mva.2000.107573
39. Merchant R Jr , Kistner RL , Kabnick LS . Is there an increased risk for DVT with the VNUS closure procedure? J Vasc Surg. 2003 Sep;38(3):628. https://doi.org/10.1016/S0741-5214(03)00322-7