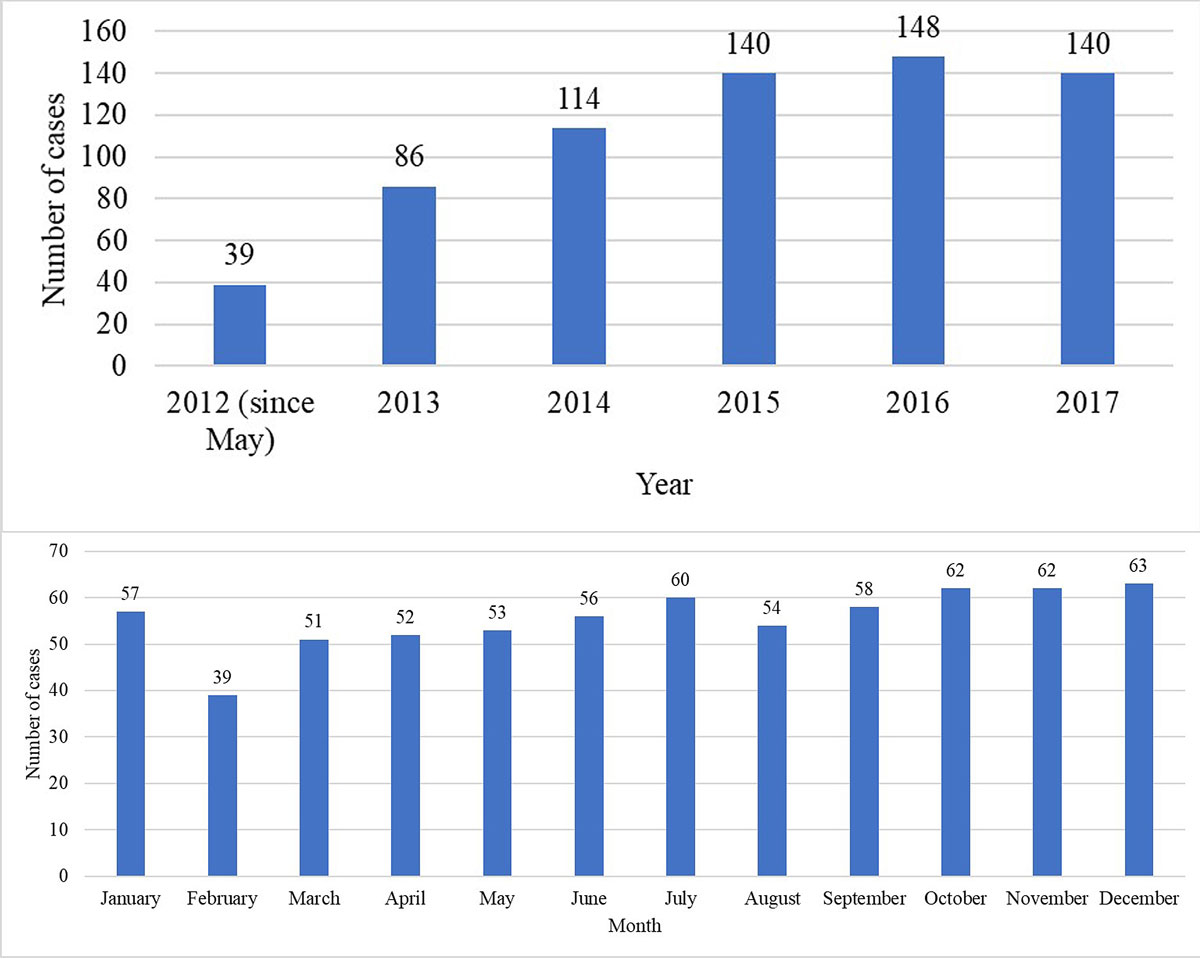
Figure 1 Annual (a) and monthly (b) distributions of the cases for which contact with the Poisons Information Centre was undertaken at the emergency department (N = 667).
DOI: https://doi.org/10.4414/smw.2019.20164
Poisoning is a common reason for patients to present at the emergency department (ED). According to data from European hospitals [1, 2], about 1% of all ED admissions are related to poisoning. Although this does not seem very high at first glance, it constitutes an important component when the risk of a potentially fatal outcome in many of these cases is considered. Poison information centres (PICs) can provide valuable support to ED personnel and help to optimise patient management in such cases. In Switzerland, the official information point for all poisoning cases is Tox Info Suisse. Tox Info Suisse has extensive documentation on chemicals, drugs, poisonous plants and animals, food and other products, and offers 24-hour telephone information. PICs are contacted in about one out of every four cases of poisoning [3], with lower rates in fatal cases [4, 5].
The treatment of poisoning depends on the specific case. In some cases, solely supportive measures (e.g., administration of oxygen, intravenous fluids) may be sufficient to adequately treat the patient, whereas in other cases the administration of a specific antidote (e.g., naloxone in the case of opioid poisoning [6]) and/or decontamination measures (e.g., administration of activated charcoal to adsorb toxins in the digestive tract) are indicated. For certain common agents (e.g., paracetamol [7]), the indicated immediate measures are often part of the hospital’s emergency protocols – in the form of specific schemes, treatment algorithms or nomograms [8] – and are widely known among physicians and ED personnel, as they are part of basic medical education. However, for many other less common agents, or if the toxicant is not known to the attending physician, the procedure is less systematic. Since time is a factor of great importance in poisoning cases, consultation of PIC specialists in such cases can be lifesaving.
The aim of the present study was to describe the characteristics of cases presenting at the ED due to poisoning for which consultation of the national PIC on patient management was needed. Our findings should help to optimise the management of poisoned patients at the ED by providing an overview of cases which required consultation of a toxicology specialist and a basis for the updating of emergency treatment protocols.
This was a retrospective single centre study at the ED of the University Hospital of Bern, Switzerland, from May 2012 to December 2017. The ED of the University Hospital of Bern serves as both a primary care facility (walk-in patients) and a tertiary referral centre for other hospitals in the region (for patients ≥16 years of age). It serves a catchment area of approximately two million people and has about 48,000 emergency admissions a year (2018).
All patient data collected during routine clinical care at the ED is stored in the electronic database E.care. In order to identify relevant patients for whom the ED contacted the PIC, E.care was searched using appropriate full-text search terms (e.g., poison centre, poison information centre, Tox Info Suisse). Each identified case was reviewed by one of the study authors. We included all cases of patients presenting at the ED due to (suspected) poisoning during the study period and for whom the PIC was contacted by the ED. We excluded cases in which the PIC was contacted by an external hospital or by the patients themselves, as well as cases in which the PIC was not contacted, cases where the patient had not given general consent to the use of their medical data for research purposes, or cases for which insufficient information was available.
The following data were exported (if available) from E.care for the analyses: sex, age, type of transport to the ED (e.g., by ambulance), date and time of presentation and onset of symptoms, clinical symptoms and severity of poisoning, type of treatment (inpatient versus outpatient), toxicological laboratory values, documented specific recommendations of PIC specialists, and management before and after contact with the PIC. The category “diagnostic decision” included PIC recommendations for blood tests, drug concentration measurements, toxicological screening and computed tomography scans. Patients admitted at the ED at the same time due to exposure to the same agent(s) were regarded as forming a single cluster. Night arrival was defined as ED presentation between 20:00 and 08:00, and weekend arrival as presentation between 17:00 on Friday and 08:00 on Monday. Based on the definitions used by Tox Info Suisse and the American Association of Poison Control Centers [9, 10], acute exposure was defined as single, repeated or continuous exposure occurring over eight hours or less, and chronic exposure as continuous or repeated exposure occurring over >8 hours. The classification of the involved agents into groups was also based on the substance categories used by the American Association of Poison Control Centers [9]. Urine drug screening (when available) was performed using an immunoassay test (Triage® TOX Drug Screen, Alere, Cologne, Germany) to screen for amphetamines, barbiturates, benzodiazepines, cocaine, methadone, methamphetamines (including MDMA), opiates, paracetamol, phencyclidine (PCP), tricyclic antidepressants and tetrahydrocannabinol (cannabis). The severity of poisoning was assessed using the Poisoning Severity Score to grade acute poisoning [11].
A total of 821 potential cases were initially retrieved using the full-text search terms. Of these, 154 cases had to be excluded, either because they failed to meet our inclusion criteria (n = 124) or because the patient’s general consent to use their medical data for research purposes was absent (n = 30). Finally, 667 cases were included in the final analysis, including 21 patients in nine clusters.
The median age of the study population was 32 years (range 16–94). Of the 667 patients, 405 (61%) were female and 262 (39%) male. The majority of the patients were Swiss (n = 513, 77%) and the mean (±SD) duration of stay at the ED was 7 ± 7 hours. The annual and monthly distributions of the cases over the study period are shown in figure 1. Table 1 shows the characteristics of the cases. In 53 of the 667 cases (8%), the incident was an occupational accident. In 470 cases (70%), a psychiatric disease was documented in the medical history. Figure 2 shows the agents/substances involved. The category “others (medication)” included the following agents/substances: allopurinol, antidiabetic, antimalarial and anti-Parkinson agents, chlorhexidine, clomethiazole, colchicine, disulfiram, fluorouracil, immunosuppressants, memantine, oxybutynin, thyroid and other hormones, Fenipic® (an over-the-counter product for insect bites or other skin irritations), a product sold online for penis enlargement, and a veterinary drug. The category “others (non-medication)” included ammonia, batteries, caffeine, diesel, kerosene, mercury, nicotine, pork liver, potassium cyanide, potassium permanganate and propane. Common clinical features of the cases are shown in table 2. Table 3 presents an overview of patient management before and after contacting the PIC, as well as the specific recommendations of the consultation.

Figure 1 Annual (a) and monthly (b) distributions of the cases for which contact with the Poisons Information Centre was undertaken at the emergency department (N = 667).
Table 1 Case characteristics (N = 667).
| n (%) | |||
|---|---|---|---|
| Time of admission | Night arrival | 296 (44) | |
| Weekend arrival | 236 (35) | ||
| Type of admission | By ambulance | 436 (65) | |
| Self-admission | 210 (31) | ||
| By Swiss air-ambulance | 21 (3) | ||
| Route of exposure | Ingestion | 587 (88) | |
| Inhalation/nasal | 44 (7) | ||
| Transdermal | 26 (4) | ||
| Bite/sting | 10 (1) | ||
| Ocular | 8 (1) | ||
| Injection | 7 (1) | ||
| Unknown | 2 (<1) | ||
| Circumstances of exposure | Intentional | 505 (76) | |
| Suicidal | 427 (64) | ||
| Misuse | 46 (7) | ||
| Abuse | 32 (5) | ||
| Unintentional | 162 (24) | ||
| Accident | 116 (17) | ||
| Therapeutic error | 42 (6) | ||
| Adverse reaction | 1 (<1) | ||
| Unknown | 3 (<1) | ||
| Chronicity | Acute | 631 (95) | |
| Chronic | 36 (5) | ||
| Number of agents involved | Multiple | 345 (52) | |
| Single | 321 (48) | ||
| Unknown | 1 (<1) | ||
| Time from exposure until ED admission | <1 hour | 67 (10) | |
| 1–4 hours | 279 (42) | ||
| 5–12 hours | 98 (15) | ||
| 13–23 hours | 29 (4) | ||
| >24 hours | 19 (3) | ||
| Unknown | 175 (26) | ||
| Poisoning Severity Score | Minor | 359 (54) | |
| Moderate | 142 (21) | ||
| Severe | 56 (8) | ||
| Fatal | 0 (0) | ||
| Not applicable (no symptoms) | 110 (16) | ||
| Outcome | Admitted to psychiatric hospital | 247 (37) | |
| Discharged home | 212 (32) | ||
| Admitted to ICU | 171 (26) | ||
| Admitted to ward other than ICU | 37 (6) | ||
ED = emergency department; ICU = intensive care unit
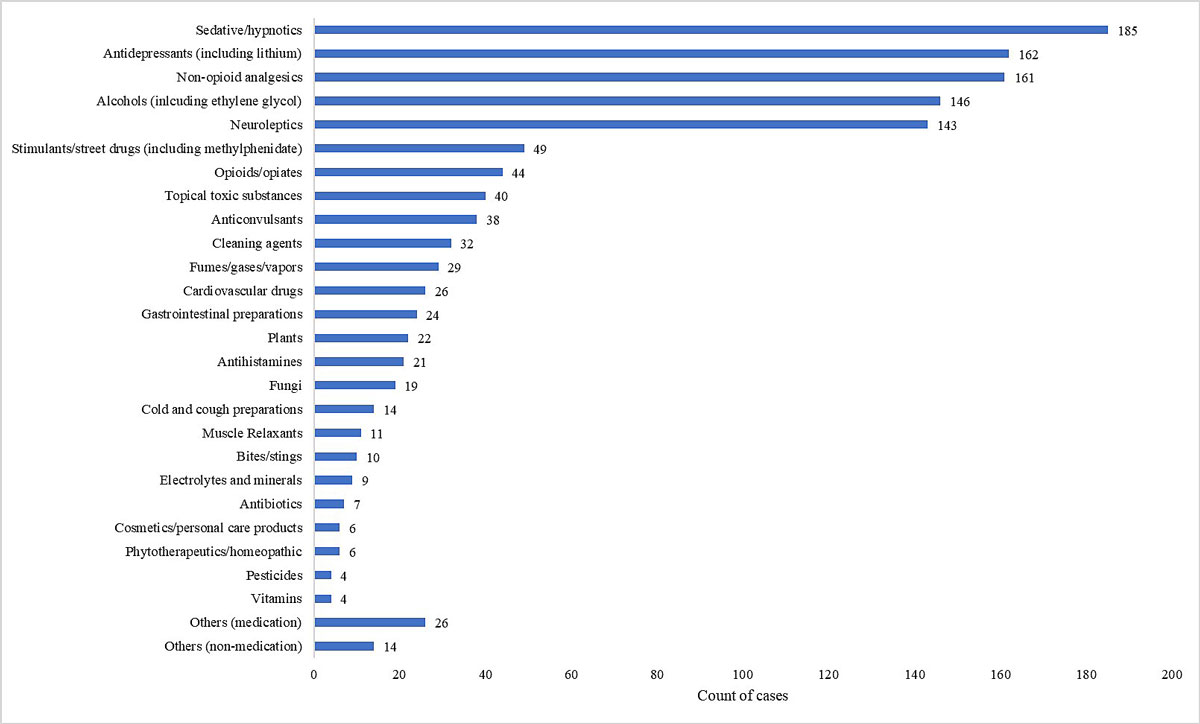
Figure 2 Involved agents/substances by number of cases (more than one agent involved in some cases).
Table 2 Common clinical features in cases in which the emergency department contacted the poison information centre (N = 667).
| n (%) | ||
|---|---|---|
| Nervous system | Drowsiness / GCS 8–14 | 230 (34) |
| Coma / GCS 3–7 | 69 (10) | |
| Mydriasis | 61 (9) | |
| Miosis | 54 (8) | |
| Vertigo | 47 (7) | |
| Agitation | 28 (4) | |
| Seizures | 15 (2) | |
| Cardiovascular system | Tachycardia (heart rate >100 bpm) | 152 (23) |
| ECG abnormalities | 127 (19) | |
| Bradycardia (heart rate <60 bpm) | 16 (2) | |
| Hypotension (systolic blood pressure <90 mm Hg) | 16 (2) | |
| Hypertension (systolic blood pressure >180 mm Hg) | 10 (1) | |
| Gastrointestinal tract | Nausea/vomiting | 128 (19) |
| Stomach ache | 38 (6) | |
| Diarrhoea | 14 (2) | |
| Respiratory system | Hyperventilation (respiratory rate >20/min) | 58 (9) |
| Hypoventilation | 24 (4) | |
| Dyspnoea | 11 (2) | |
| Other | Local irritation/swelling | 57 (9) |
| Anticholinergic toxidrome | 11 (2) | |
| Hyposecretion | 10 (1) | |
| Hypersecretion | 8 (1) | |
| Urinary retention | 8 (1) | |
| Extrapyramidal symptoms | 3 (<1) | |
| Hyperthermia (>39°C) | 2 (<1) | |
| Cholinergic toxidrome | 2 (<1) | |
| Serotonin syndrome | 1 (<1) | |
ECG = electrocardiogram; GCS = Glasgow Coma Scale
Table 3 Specific recommendations of the poison information centre and patient management before and after the consultation: n (%); N = 667.
| Measure | Management prior to consultation | Specific recommendations | Management after consultation |
|---|---|---|---|
| Only clinical examination | 68 (10) | ||
| Monitoring/ECG | 427 (64) | 84 (13) | 77 (12) |
| Blood test(s) | 580 (87) | ||
| Urine tox screen | 237 (36) | ||
| Blood drug concentration(s) | 58 (9) | ||
| No specific treatment | 547 (82) | 132 (20) | |
| Specific antidote/agent | 77 (12) | 87 (13) | 86 (13) |
| Activated charcoal | 32 (5) | 87 (13) | 78 (12) |
| Intubation | 31 (5) | 16 (2) | |
| Topical decontamination | 9 (1) | 6 (1) | 5 (1) |
| Gastroscopy | 6 (1) | 30 (4) | 31 (5) |
| Whole bowel irrigation | 7 (1) | 6 (1) | |
| Haemodialysis | 3 (<1) | 1 (<1) | |
| Information regarding possible symptoms | 469 (70) | ||
| Symptomatic therapy/ treatment if symptoms | 203 (30) | 144 (22) | |
| Observation | 295 (44) | at ED: 306 (46) at ICU: 165 (25) |
|
| Diagnostic decision | 140 (21) | 165 (25) | |
| Stop specific treatment | 14 (2) | 21 (3) | |
| No change in management | 109 (16) |
ECG = electrocardiogram; ED = emergency department; ICU = intensive care unit
In 237 cases, toxicological drug screening was performed before contact with Tox Info Suisse. This was positive for benzodiazepines in 87, cannabis in 40, paracetamol in 37, opiates in 24, cocaine in 15, tricyclic antidepressants in 15, methadone in 7, methamphetamine in 4 and barbiturates in 4 cases (some cases were positive for more than one substance). The test was negative in 71 cases, and in 27 cases the results were not specified in the report.
Table 4 shows the specific antidotes and therapeutic agents administered at the ED prior to contacting the PIC, as well as the specific antidotes/agents recommended by Tox Info Suisse and those administered after the consultation without specific recommendation by the PIC. The specific agents were administered as recommended by Tox Info Suisse in 70 cases. In 17 cases, the specific agents were not administered as recommended by the Tox Info Suisse because either the measures already taken were sufficient to lead to clinical improvement (n = 11), the therapy was stopped on the basis of laboratory results (e.g., drug concentrations) (n = 3), the therapy was refused by the patient (n = 2), or a mushroom was identified by a mushroom expert as one that does not contain amatoxin (n = 1).
Table 4 Specific antidotes and therapeutic agents (left column) administered prior to consulting the poison centre, as well as those recommended by the poison centre or administered without specific recommendation after the consultation (right column: number of cases for causative agents/symptoms).
| Specific antidote/ therapeutic agent | Causative agent/symptom (n) | |
|---|---|---|
| Flumazenil | Administered prior to consultation | Benzodiazepines (n = 30), other (n = 4) |
| Recommended by the poison centre | Benzodiazepines (n = 1; already administered prior to consultation) | |
| Administered after consultation without specific recommendation | Benzodiazepines (n = 5) | |
| Acetylcysteine | Administered prior to consultation | Paracetamol (n = 16), mushrooms (n = 2), other (n = 2) |
| Recommended by the poison centre | Paracetamol (n = 33; already administered prior to consultation in 7 of these cases), mushrooms (n = 10), other (n = 1) | |
| Administered after consultation without specific recommendation | Paracetamol (n = 2) | |
| Naloxone | Administered prior to consultation | Opioids (n = 7), other (n = 3) |
| Recommended by the poison centre | Opioids (n = 4; already administered prior to consultation in 2 of these cases) | |
| Administered after consultation without specific recommendation | Opioids (n = 2) | |
| Calcium gluconate | Administered prior to consultation | Hydrofluoric acid (n = 4) |
| Recommended by the poison centre | Hydrofluoric acid (n = 1) | |
| Sodium bicarbonate | Administered prior to consultation | Analgesic agent/acidosis (n = 2), cleaning agent/acidosis (n = 1) |
| Recommended by the poison centre | Acetylsalicylic acid/coma (n = 3), acidosis (n = 3; already administered prior to consultation in all 3 cases) | |
| Ethanol | Administered prior to consultation | Ethylene glycol (n = 2) |
| Phytomenadione (vitamin K) | Administered prior to consultation | Anticoagulant (n = 1), rodenticide (n = 1) |
| Recommended by the poison centre | Anticoagulant (n = 1) | |
| Atropine | Administered prior to consultation | Pesticide/cholinergic toxidrome (n = 1) |
| Recommended by the poison centre | Pesticide/cholinergic toxidrome (n = 2; already administered prior to consultation in 1 case), mushrooms/coma (n = 1), other (n = 1) | |
| Simeticone | Administered prior to consultation | Cleaning agent (n = 1) |
| Recommended by the poison centre | Cleaning agent (n = 10) | |
| Hypertonic amphoteric solution (Diphoterin®) | Administered prior to consultation | Alkaline solution (n = 1) |
| Magnesium | Administered prior to consultation | Antidepressants and neuroleptic agents / torsade de pointes (n = 1) |
| Administered after consultation without specific recommendation | Neuroleptic agents / prolonged QTc (n = 2), acetylsalicylic acid/hypomagnesaemia (n = 1) | |
| Proton-pump inhibitor | Administered prior to consultation | Cleaning agent (n = 2) |
| Recommended by the poison centre | Cleaning agent (n = 1) | |
| Corticosteroids | Administered prior to consultation | Gastrointestinal swelling (n = 2) |
| Benzodiazepines | Administered prior to consultation | Caffeine (n = 1), analgesic agent/seizures (n = 1) |
| Recommended by the poison centre | Illicit drugs/agitation (n = 2), antidepressants / anticholinergic toxidrome (n = 1) | |
| Fomepizole | Recommended by the poison centre | Ethylene glycol (n = 4) |
| Biperiden | Recommended by the poison centre | Neuroleptic agent / extrapyramidal symptoms (n = 1) |
| Administered after consultation without specific recommendation | Neuroleptic agent / extrapyramidal symptoms (n = 1) | |
| Silibinin | Recommended by the poison centre | Mushrooms (n = 10) |
| Deferoxamine | Recommended by the poison centre | Iron (n = 1) |
| Leucovorin | Recommended by the poison centre | Methotrexate (n = 1) |
| Dimercaptopropanesulfonic acid | Recommended by the poison centre | Mercury (n = 1) |
| Hydroxocobalamin | Recommended by the poison centre | Potassium cyanide / status epilepticus (n = 1) |
| Oxygen | Recommended by the poison centre | Dichlormethane (n = 1) |
| Physostigmine | Administered after consultation without specific recommendation | Atropa belladonna / anticholinergic toxidrome (n = 1) |
Table 5 shows an overview of the decontamination procedures that were recommended and/or performed before or after contacting the PIC. Administration of activated charcoal was recommended by the PIC in 87 cases and was administered after the consultation in 78 cases (table 5). In seven cases in which it was recommended by the PIC, activated charcoal had already been administered prior to consultation. A gastroscopy was recommended by the PIC in 30 cases. This had already been performed prior to contact in one of these cases. Gastroscopy was performed after consultation in 23 of the cases in which it was recommended by the PIC, as well as in eight additional cases. Whole bowel irrigation was performed in five of the seven cases in which it was recommended, as well as in one additional case (tables 3 and 5 ).
Table 5 Decontamination procedures by agent (number of cases; more than one agent involved in some cases).
| Involved agent | Activated charcoal | Gastroscopy | Whole bowel irrigation | Haemodialysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prior (n = 32) |
Rec. (n = 87) |
After (n = 78) |
Prior (n = 6) |
Rec. (n = 30) |
After (n = 31) |
Prior (n = 0) |
Rec. (n = 7) |
After (n = 6) |
Prior (n = 0) |
Rec. (n = 3) |
After (n = 1) |
|
| Analgesics | 14 | 16 | 14 | 2 | 6 | 4 | 2 | 1 | 2 | 1 | ||
| Antidepressants | 9 | 19 | 18 | 6 | 7 | 3 | 2 | 1 | ||||
| Sedatives | 7 | 17 | 15 | 1 | 10 | 11 | 2 | 2 | 2 | |||
| Anticonvulsants | 6 | 6 | 6 | 2 | 3 | 1 | 1 | 1 | ||||
| Neuroleptics | 6 | 25 | 23 | 1 | 9 | 11 | 2 | 2 | ||||
| Alcohol | 5 | 18 | 13 | 1 | 3 | 3 | 2 | 2 | ||||
| Fungi | 4 | 10 | 9 | 1 | 1 | |||||||
| Cardiovascular drugs | 3 | 5 | 2 | 2 | 1 | 1 | 1 | 1 | ||||
| Opioids | 2 | 7 | 6 | 2 | 2 | 1 | ||||||
| Antihistamines | 1 | 2 | 2 | |||||||||
| Muscle Relaxants | 1 | 2 | 2 | |||||||||
| Cleaning agents | 1 | 2 | 5 | 5 | ||||||||
| Plants | 1 | 14 | 12 | 3 | 7 | 7 | ||||||
| Dextromethorphan | 2 | 1 | ||||||||||
| Laxatives | 1 | |||||||||||
| Illicit drugs | 2 | 1 | 1 | 1 | ||||||||
| Lithium | 2 | 1 | 4 | 3 | ||||||||
| Cosmetics | 1 | |||||||||||
| Ammonia | 1 | 2 | ||||||||||
| Other | 3 | 10 | 7 | 4 | 3 | 2 | 3 | |||||
After = after consultation; Prior = before consultation; Rec. = recommended
The PIC recommended cessation of the specific treatment based on the paracetamol blood concentration in six cases, on the seizure threshold in four cases with benzodiazepine poisoning, on the opinion of the mushroom expert in one case, and on the gastroscopy findings in one case of ammonia poisoning. Of the 21 cases in which the specific treatment was stopped after consulting the PIC (table 3), this was based on the recommendation of the PIC in eight cases. The specific treatment was continued despite the recommendation to stop in six cases. These cases included four for which flumazenil was continued because the patient was non-responsive and one case of acetylcysteine treatment due to clinical suspicion of poisoning with mushrooms containing amatoxin (reason unclear in one case). There were 13 additional cases in which the specific therapy was stopped without an explicit recommendation from the PIC. These included nine cases of paracetamol poisoning (acetylcysteine stopped on the basis of the paracetamol blood concentration), four cases of mushroom poisoning (acetylcysteine (n = 2) and silibinin (n = 2) stopped on the basis of negative amatoxin laboratory results), one case of acetylsalicylic acid poisoning (sodium bicarbonate stopped on the basis of the acetylsalicylic acid blood concentration), and one case of hydrofluoric acid in which calcium gluconate was stopped due to the low concentration of the solution.
In this retrospective study at a large ED in Switzerland, most cases requiring contact with the PIC involved relatively young (median age 32 years) female patients with known psychiatric co-morbidities presenting within four hours after intentional ingestion of prescription drugs. Approximately half of the cases were of minor severity and there were no fatalities (table 1). Approximately one third of the patients were discharged home directly from the ED, one third were admitted to a psychiatric hospital and one fourth to the intensive care unit (table 1). In most cases, therapeutic and/or diagnostic measures were initiated prior to contacting the PIC. Decontamination measures and antidotes undertaken/administered only after contacting the PIC included whole bowel irrigation, haemodialysis, fomepizole, biperiden, silibinin, deferoxamine, leucovorin, dimercaptopropanesulfonic acid and hydroxocobalamin. Only in 16% of cases was patient management unchanged after contacting the PIC (table 3).
A previous study in Switzerland identified the following factors as being significantly associated with ED physicians contacting the PIC [12]: female sex, ingestion of more than one substance, and intentional poisoning. This is consistent with our finding that most cases involved female patients and intentional exposure (e.g., suicide attempts; table 1). Greater doses are usually ingested in cases of intentional poisoning [12], and most cases of attempted suicide in Switzerland involve women and poisoning [13]. However, we found similar numbers of cases involving a single and multiple substances (table 1). As in the previous study [12], the PIC was sometimes contacted even though the patients were asymptomatic (table 1). Reasons for this might be exposure to uncommon agent(s), reassurance that it was acceptable to discharge the patients [12], or as precaution when it was expected that the patient would subsequently develop symptoms (e.g., with presentation immediately or very soon after exposure). In our study, most patients presented on weekdays and during the day (table 1). Possible reasons for this might be that night-time and weekend poisonings are often related to recreational drugs and alcohol [12] and would not therfore require the consultation of a specialist. Alternatively, it may be more difficult for the ED team to contact the PIC during off-peak times, as fewer medical staff are available.
In contrast to what one might have expected, most cases for which the PIC was contacted involved commonly used prescription drugs for which poisoning management is often known (e.g. flumazenil for benzodiazepine poisoning [14], acetylcysteine for paracetamol poisoning [15]), rather than unusual or less familiar substances or chemicals (fig. 2). It is possible that this is because these prescription drugs are used very widely and are thus more likely to cause poisoning than more rarely used agents such as chemicals or plants. Another factor might be that in many cases the clinical presentation, rather than the agent itself, is the most important trigger for contacting the PIC. Our findings indicate that the decision to contact the PIC is more often influenced by impaired consciousness than by, for example, sympathomimetic symptoms (impaired consciousness (GCS <15) in 44% of cases in our study vs., e.g., agitation in 4%; table 2). The importance of the clinical presentation is also highlighted by the cases in which the specific antidotes and therapies were not administered as recommended by the PIC, mainly due to clinical improvement.
Therapies/measures that were undertaken only after contacting the PIC included decontamination procedures (whole bowel irrigation [16], haemodialysis) and specific antidotes (fomepizole, biperiden, silibinin, deferoxamine, leucovorin, dimercaptopropanesulfonic acid and hydroxocobalamin; tables 3 and 5 ). The procedures associated with such measures are more complicated compared to alternatives such as the administration of activated charcoal, and the possible contraindications (e.g., ileus, haemodynamic instability in the case of whole bowel irrigation [17]) and complications might be among the reasons that these decontamination measures were not performed prior to contacting the PIC. Among the antidotes, the alcohol dehydrogenase inhibitor fomepizole is the preferred therapy in cases of methanol and ethylene glycol poisoning, although ethanol might also be used and was indeed administered prior to contacting the PIC in two of the ethylene glycol poisoning cases in our study. Antidotes such as the milk thistle extract silibinin [18, 19], the chelating agents deferoxamine and dimercaptopropanesulfonic acid, and hydroxocobalamin [20, 21] are less well known than, for example, acetylcysteine for paracetamol or naloxone for opioid poisoning. They are, however, among the most important antidotes for acute poisoning [21]. It is thus an advantage if these substances are readily available in the ED and if physicians are familiar with their use. The anticholinergic agent biperiden can be useful against extrapyramidal symptoms after poisoning with neuroleptics (however, alternative treatments such as amantadine have been shown to be as effective as anticholinergic agents, but with fewer adverse effects [22]). The reduced folate leucovorin (also called folinic acid) can be administered to decrease the toxic effects of high-dose therapy with the folate antimetabolite methotrexate. Since leucovorin can only rescue cells that do not already have lethal DNA damage, treatment must be initiated within 24 to 36 hours to be effective [23]. Antidotes administered prior to contact with the PIC included well known substances/antidotes for common agents (e.g. flumazenil for benzodiazepines, acetylcysteine for paracetamol, naloxone for opioids and vitamin K for anticoagulant poisoning), but also some less commonly used and often not readily available substances, such as calcium gluconate (used as treatment for hydrofluoric acid burns; calcium ions complex free fluoride ions) and the antiflatulent simeticone (anti-foaming use in cases involving surfactants, such as shampoo) [21]. In one case with anticholinergic toxidrome, the acetylcholinesterase inhibitor physostigmine was administered after contacting the PIC but without an explicit recommendation (table 4). Physostigmine (in contrast to other carbamates such as neostigmine and pyridostigmine) crosses the blood-brain barrier and is thus indicated in cases with both peripheral and central anticholinergic toxicity. Although infrequent, adverse effects include seizures and arrhythmias [24].
The limitations of our study include its retrospective design, missing information regarding the exact time lag between first contact at the ED and PIC contact, and missing information on the long-term outcomes, especially for cases admitted to a psychiatric hospital shortly after arrival at the ED. Furthermore, in some cases specific decontamination measures were recommended or indicated for some but not all the agents involved, and some cases were not included due to the lack of a general consent form or if the PIC was contacted by an external hospital or by the patients themselves. Also, data from one ED may not be representative of the whole country. It cannot be excluded that the call to the PIC might not have been recorded in some cases. although this is not expected since all measures regarding patients’ management and medical procedures (including external consultations and treatment decisions) are documented in detail in E.care for all cases presenting at the ED. The strengths of this study include the sensitive search and the individual review of each case. Although previous studies from the United States have investigated the impact of PIC consultation on the reduction of health-care costs and length of hospital stay [25–27], this is to our knowledge the first study addressing the impact of PIC consultation on ED patient management in Switzerland, and it could thus contribute to the improvement of ED procedures and patient care and provide a basis for future prospective studies investigating not only specific medical aspects, but also economic ones.
In conclusion, cases requiring contact with the PIC involved mainly acute, intentional poisoning with prescription drugs, and the patients often presented with impaired consciousness. Although in most cases therapeutic and/or diagnostic measures were initiated prior to contacting the PIC, there were also decontamination measures and antidotes undertaken or administered only after PIC consultation. In cases of severe poisoning, it is important that ED physicians should contact the PIC. This helps them to implement specific treatment measures and avoid fatalities. Moreover, it reduces the likelihood of unnecessary treatment measures and provides a source of information and/or confirmation – particularly in less severe cases involving more common agents such as paracetamol and benzodiazepines.
We thank Rodney Yeates for his editorial assistance.
No financial support and no other potential conflict of interest relevant to this article were reported.
1 Burillo-Putze G , Munne P , Dueñas A , Pinillos MA , Naveiro JM , Cobo J , et al., Clinical Toxicology Working Group, Spanish Society of Emergency Medicine (SEMESTOX). National multicentre study of acute intoxication in emergency departments of Spain. Eur J Emerg Med. 2003;10(2):101–4. doi:.https://doi.org/10.1097/00063110-200306000-00006
2 Maignan M , Pommier P , Clot S , Saviuc P , Debaty G , Briot R , et al. Deliberate drug poisoning with slight symptoms on admission: are there predictive factors for intensive care unit referral? A three-year retrospective study. Basic Clin Pharmacol Toxicol. 2014;114(3):281–7. doi:.https://doi.org/10.1111/bcpt.12132
3 Harchelroad F , Clark RF , Dean B , Krenzelok EP . Treated vs reported toxic exposures: discrepancies between a poison control center and a member hospital. Vet Hum Toxicol. 1990;32(2):156–9.
4 Linakis JG , Frederick KA . Poisoning deaths not reported to the regional poison control center. Ann Emerg Med. 1993;22(12):1822–8. doi:.https://doi.org/10.1016/S0196-0644(05)80408-1
5 Blanc PD , Kearney TE , Olson KR . Underreporting of fatal cases to a regional poison control center. West J Med. 1995;162(6):505–9.
6 Kim HK , Nelson LS . Reversal of Opioid-Induced Ventilatory Depression Using Low-Dose Naloxone (0.04 mg): a Case Series. J Med Toxicol. 2016;12(1):107–10. doi:.https://doi.org/10.1007/s13181-015-0499-3
7 Blieden M , Paramore LC , Shah D , Ben-Joseph R . A perspective on the epidemiology of acetaminophen exposure and toxicity in the United States. Expert Rev Clin Pharmacol. 2014;7(3):341–8. doi:.https://doi.org/10.1586/17512433.2014.904744
8 Levine M , Stellpflug S , Pizon AF , Traub S , Vohra R , Wiegand T , et al. Estimating the impact of adopting the revised United Kingdom acetaminophen treatment nomogram in the U.S. population. Clin Toxicol (Phila). 2017;55(6):569–72. doi:.https://doi.org/10.1080/15563650.2017.1291945
9 Gummin DD , Mowry JB , Spyker DA , Brooks DE , Osterthaler KM , Banner W . 2017 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila). 2018;56(12):1213–415. doi:.https://doi.org/10.1080/15563650.2018.1533727
10Tox Info Suisse. Jahresbericht 2017 Tox Info Suisse. 2017; Available from: https://toxinfo.ch/customer/files/691/9181408_Tox_JB-2017_DE_Website.pdf
11 Persson HE , Sjöberg GK , Haines JA , Pronczuk de Garbino J . Poisoning severity score. Grading of acute poisoning. J Toxicol Clin Toxicol. 1998;36(3):205–13. doi:.https://doi.org/10.3109/15563659809028940
12 Schurter D , Rauber-Lüthy C , Jahns M , Haberkern M , Kupferschmidt H , Exadaktylos A , et al. Factors that trigger emergency physicians to contact a poison centre: findings from a Swiss study. Postgrad Med J. 2014;90(1061):139–43. doi:.https://doi.org/10.1136/postgradmedj-2013-132242
13Bundesamt für Gesundheit BAG. Suizidprävention in der Schweiz. Ausgangslage, Handlungsbedarf und Aktionsplan. 2016; Available from: https://www.bag.admin.ch/bag/de/home/strategie-und-politik/politische-auftraege-und-aktionsplaene/aktionsplan-suizidpraevention.html.
14 An H , Godwin J . Flumazenil in benzodiazepine overdose. CMAJ. 2016;188(17-18):E537. doi:.https://doi.org/10.1503/cmaj.160357
15 Heard KJ . Acetylcysteine for acetaminophen poisoning. N Engl J Med. 2008;359(3):285–92. doi:.https://doi.org/10.1056/NEJMct0708278
16 Tenenbein M . Whole bowel irrigation for toxic ingestions. J Toxicol Clin Toxicol. 1985;23(2-3):177–84. doi:.https://doi.org/10.3109/15563658508990626
17 Thanacoody R , Caravati EM , Troutman B , Höjer J , Benson B , Hoppu K , et al. Position paper update: whole bowel irrigation for gastrointestinal decontamination of overdose patients. Clin Toxicol (Phila). 2015;53(1):5–12. doi:.https://doi.org/10.3109/15563650.2014.989326
18 Mengs U , Pohl RT , Mitchell T . Legalon® SIL: the antidote of choice in patients with acute hepatotoxicity from amatoxin poisoning. Curr Pharm Biotechnol. 2012;13(10):1964–70. doi:.https://doi.org/10.2174/138920112802273353
19 Faulstich H , Jahn W , Wieland T . Silybin inhibition of amatoxin uptake in the perfused rat liver. Arzneimittelforschung. 1980;30(3):452–4.
20 Thompson JP , Marrs TC . Hydroxocobalamin in cyanide poisoning. Clin Toxicol (Phila). 2012;50(10):875–85. doi:.https://doi.org/10.3109/15563650.2012.742197
21 Schaper A , Ebbecke M . Intox, detox, antidotes - Evidence based diagnosis and treatment of acute intoxications. Eur J Intern Med. 2017;45:66–70. doi:.https://doi.org/10.1016/j.ejim.2017.10.019
22 König P , Chwatal K , Havelec L , Riedl F , Schubert H , Schultes H . Amantadine versus biperiden: a double-blind study of treatment efficacy in neuroleptic extrapyramidal movement disorders. Neuropsychobiology. 1996;33(2):80–4. doi:.https://doi.org/10.1159/000119254
23 Ackland SP , Schilsky RL . High-dose methotrexate: a critical reappraisal. J Clin Oncol. 1987;5(12):2017–31. doi:.https://doi.org/10.1200/JCO.1987.5.12.2017
24 Arens AM , Kearney T . Adverse Effects of Physostigmine. J Med Toxicol. 2019;15(3):184–91.
25 Vassilev ZP , Marcus SM . The impact of a poison control center on the length of hospital stay for patients with poisoning. J Toxicol Environ Health A. 2007;70(2):107–10. doi:.https://doi.org/10.1080/15287390600755042
26 Spiller HA , Griffith JR . The value and evolving role of the U.S. Poison Control Center System. Public Health Rep. 2009;124(3):359–63. doi:.https://doi.org/10.1177/003335490912400303
27 Bunn TL , Slavova S , Spiller HA , Colvin J , Bathke A , Nicholson VJ . The effect of poison control center consultation on accidental poisoning inpatient hospitalizations with preexisting medical conditions. J Toxicol Environ Health A. 2008;71(4):283–8. doi:.https://doi.org/10.1080/15287390701738459
No financial support and no other potential conflict of interest relevant to this article were reported.