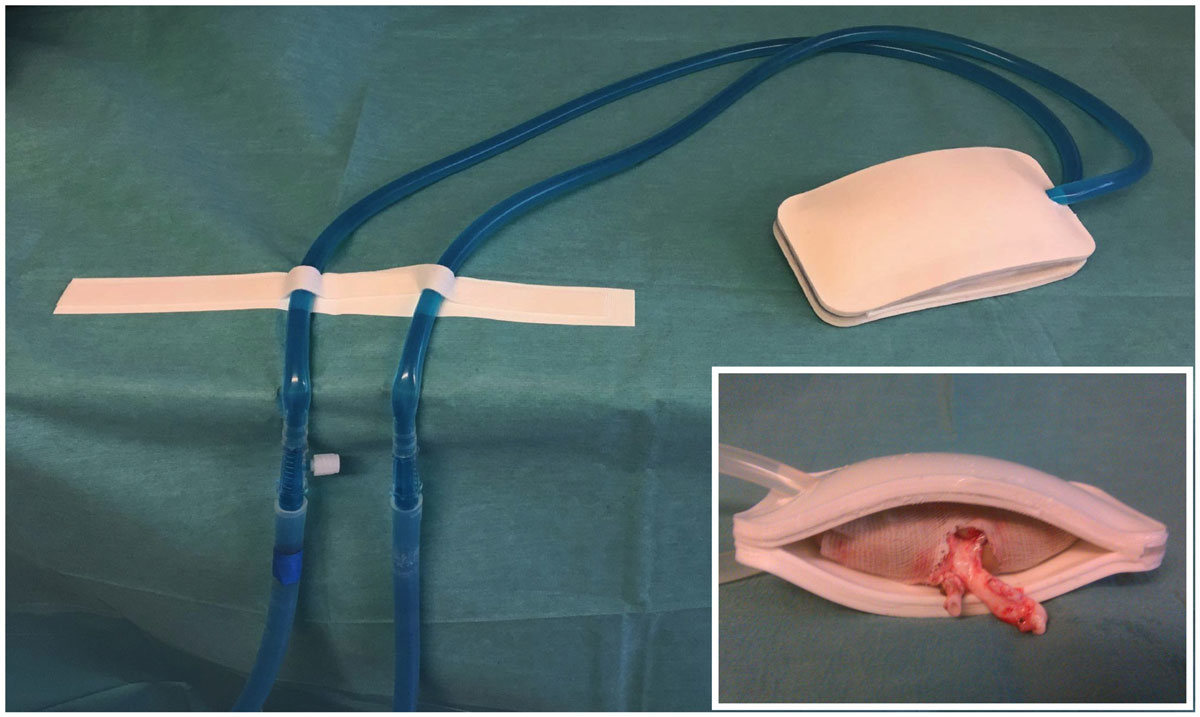
Figure 1 The cooling device. The cooling device is a watertight double sheath of silicone surrounding the kidney and continuously perfused via a tubing system with ethanol and methylene blue at 4°C. Inset: kidney with the perfused cooling device.
DOI: https://doi.org/10.4414/smw.2019.20143
Kidney transplantation improves survival, is cost effective and offers the highest quality of life to patients with end-stage renal disease [1–3]. Kidney transplantation outcomes are variable, and depend on nonmodifiable factors such as donor and recipient age, pre-existing diseases or time on dialysis of the recipient, as well as modifiable factors such as graft warm ischaemic time [4].
The current consensus is that longer ischemia time primes the tissue for the generation of oxygen reactive species and damage upon reperfusion [5]. Hypothermia decreases metabolic rate and preserves adenosine triphosphate levels. In kidneys, temperatures below 18°C prevent ischaemic damage [6, 7]. Prolonged second warm ischaemic time (defined as the duration of the implantation) is a risk factor for delayed graft function [8]. Similarly, longer anastomosis times are associated with a higher rate of delayed graft function, acute and chronic rejection, and shorter graft survival [9, 10]. In human, anastomosis times above 45 minutes independently increased the risk of delayed graft function and impaired allograft function 3 years after transplantation [11]. Importantly, kidneys from marginal donors, which are increasingly being used, are less resistant to warm ischaemia [12]. Any delayed function has important implications, as it prolongs length of stay, increases the need for dialysis and adversely affects long-term survival [13–15].
During the operation, temperature is not monitored, and typically surgeons intermittently splash the kidney with cold saline or microparticulated ice. Experimental devices have been developed to maintain a cold environment during transplantation, including stockinettes or a net holding ice, with or without a cold preservation chemical [16]. None of these systems can achieve controlled or homogeneous cooling [17]. Moreover, none have translated to daily clinical practice, despite encouraging results. Recently, we developed a cooling device made of a double layer of silicone in which ethanol and methylene blue continuously circulate. In the setting of robot-assisted kidney transplantation, this device allowed strict temperature control, which prevented kidney ischaemia-reperfusion injuries [18]. During a standard open kidney transplant, vascular anastomoses are easier and faster, and thus second warm ischaemia is relatively short. Therefore, in the setting of open transplantation, we questioned if our cooling device was similarly efficient at reducing ischaemia reperfusion injury (IRI) and improving short-term outcomes.
The study was approved by the University of Geneva animal ethics committee (protocol number: GE/53/14/22826). Five-month-old female pigs (n = 13) with an average weight of 44.7 ± 2.3 kg were obtained from the animal facility of Arare, Switzerland. All pigs were maintained under standard conditions. Water and food were provided ad libitum. Animals were allocated to two groups: conventionally performed open auto-transplant without (control, n = 6) or with (cooling, n = 7) the cooling system.
Animals were first premedicated with azaperone (2.2 mg/kg i.m.), midazolam (1.6 mg/kg i.m.), and atropine (0.02 mg/kg i.m.), and anaesthetised with ketamine (2–6 mg/kg/h), fentanyl (4–6 µg/kg/h), midazolam (0.2–0.4 mg/kg/h) and atracurium (1 mg/kg/h). Animals were then intubated and ventilated before a nasogastric tube was placed. The arterial line was placed in the internal carotid artery. Monitoring included heart rate, systemic blood pressure, pulse oximetry and end-tidal CO2.
Surgery was as described [18]. Briefly, following a midline incision, the peritoneal cavity was opened and the bowels were reclined. First, the aorta, vena cava and renal vessels were prepared. The pigs received 300 UI/kg heparin intravenously. Kidney temperature was measure every 10 sec using a thermal probe. The kidneys were explanted and instantly flushed with 4°C Institut Georges Lopez-1 preservation solution. After 1 hour on ice, both kidneys were transplanted sequentially onto the vena cava and aorta using a 6-0 running suture. In the cooling system group, the device surrounded the kidney from the back table to kidney reperfusion in vivo. The pigs were maintained under general anaesthesia for 2 hours after kidney reperfusion, and urine output was recorded. After 2 hours, pigs were sacrificed using 100 mEq of potassium chloride intravenously.
The cooling system is described elsewhere [18]. It is a double silicone sheath, continuously perfused with 4°C ethanol and methylene blue (see fig. 1C). External and internal thicknesses are 5 and 0.8 mm, respectively.
Sections 3 μm thick were prepared from formalin-fixed kidney biopsies, and stained with silver Jones and periodic acid-Schiff (PAS). The histopathological scores were based on those described by Goujon et al. [18, 19] and were made using Osirix software. Four different representative fields were assessed without knowledge of the group assignment. Lesion severity was graded 0 to 5 according to the following criteria: no abnormality (0), mild lesions affecting 1–10% of the sample surface (1), 10–25% (2), 25–50% (3), 50–75% (4) and >75% (5). The final score for each biopsy ranged from 0 to 40, a higher score corresponding to more severe ischaemia.
Results were expressed as mean values ± standard deviation (SD). Differences between groups were analysed using a two-sided Student t-test or Mann-Whitney U-test. A p value <0.05 was considered statistically significant. Computations were performed using Prism 7 (GraphPad Softwares, San Diego, CA, USA).
We tested the potential of a cooling device (fig. 1) to reduce second warm ischaemia time during vascular anastomosis and protect from IRI. The cooling device was connected to a tubing system allowing continuous circulation of ethanol and methylene blue at 4°C. In the control group, the kidney temperature was poorly controlled, reaching 26.5 ± 5.5°C before reperfusion. The cooling device maintained the temperature at reperfusion at 4.3 ± 1.1°C (p <0.001, fig. 2). Similarly, the temperature slope from the start of the anastomosis to reperfusion was significantly steeper in the cooling group (fig. 2). The temperature drop during flushing and the temperature on the back table (static, cold ischaemia) were similar between the two groups (fig. 2). Overall operative time was similar: 382 vs 353 minutes in the control vs cooling group (p >0.05, fig. 3A). A similar surgical technique was used, and the time between kidney artery clamping and flush with cold IGL-1 was 3.5 (control) and 3.6 (cooling) minuntes (p >0.05, fig. 3B). To mimic the clinical scenario, after cold flushing the kidneys were kept on ice for 128 (control) and 129 (cooling) minutes (p >0.05, fig. 3C). Importantly, the device did not prolong the anastomosis time (45 and 43 minutes in the control and cooling groups, respectively, p >0.05, fig. 3D) or increase technical difficulties.

Figure 1 The cooling device. The cooling device is a watertight double sheath of silicone surrounding the kidney and continuously perfused via a tubing system with ethanol and methylene blue at 4°C. Inset: kidney with the perfused cooling device.
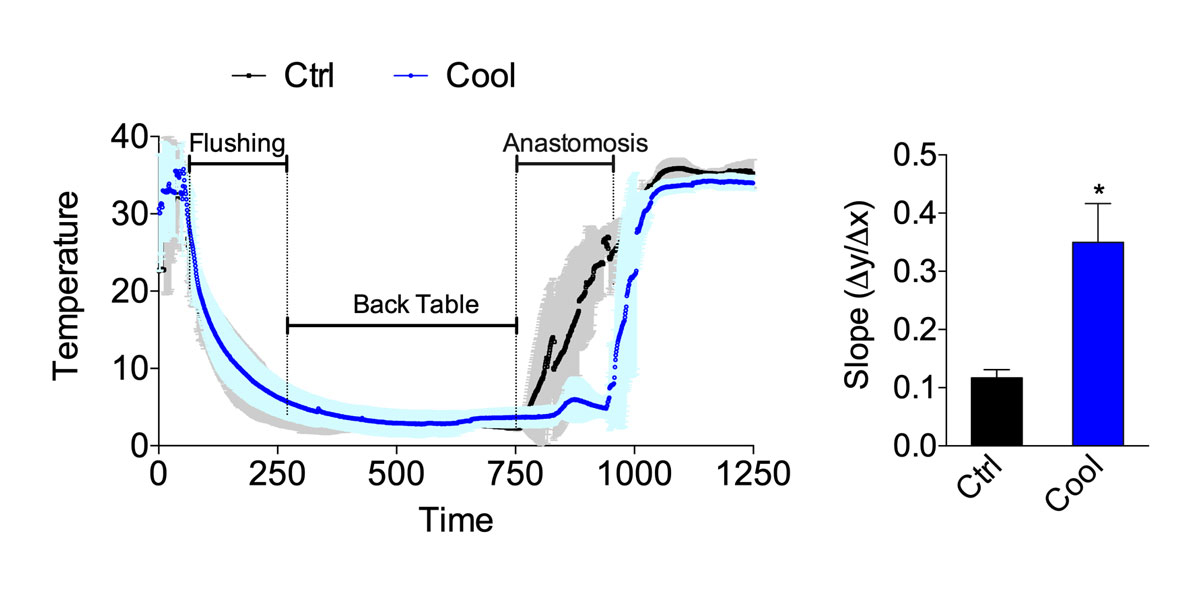
Figure 2 Temperature curve from procurement to reperfusion. Curve (left) and slope during anastomosis (right) in the control or cooling group (n = 6–7). Errors bars indicate the standard error of the mean. * p <0.05; Ctrl = control
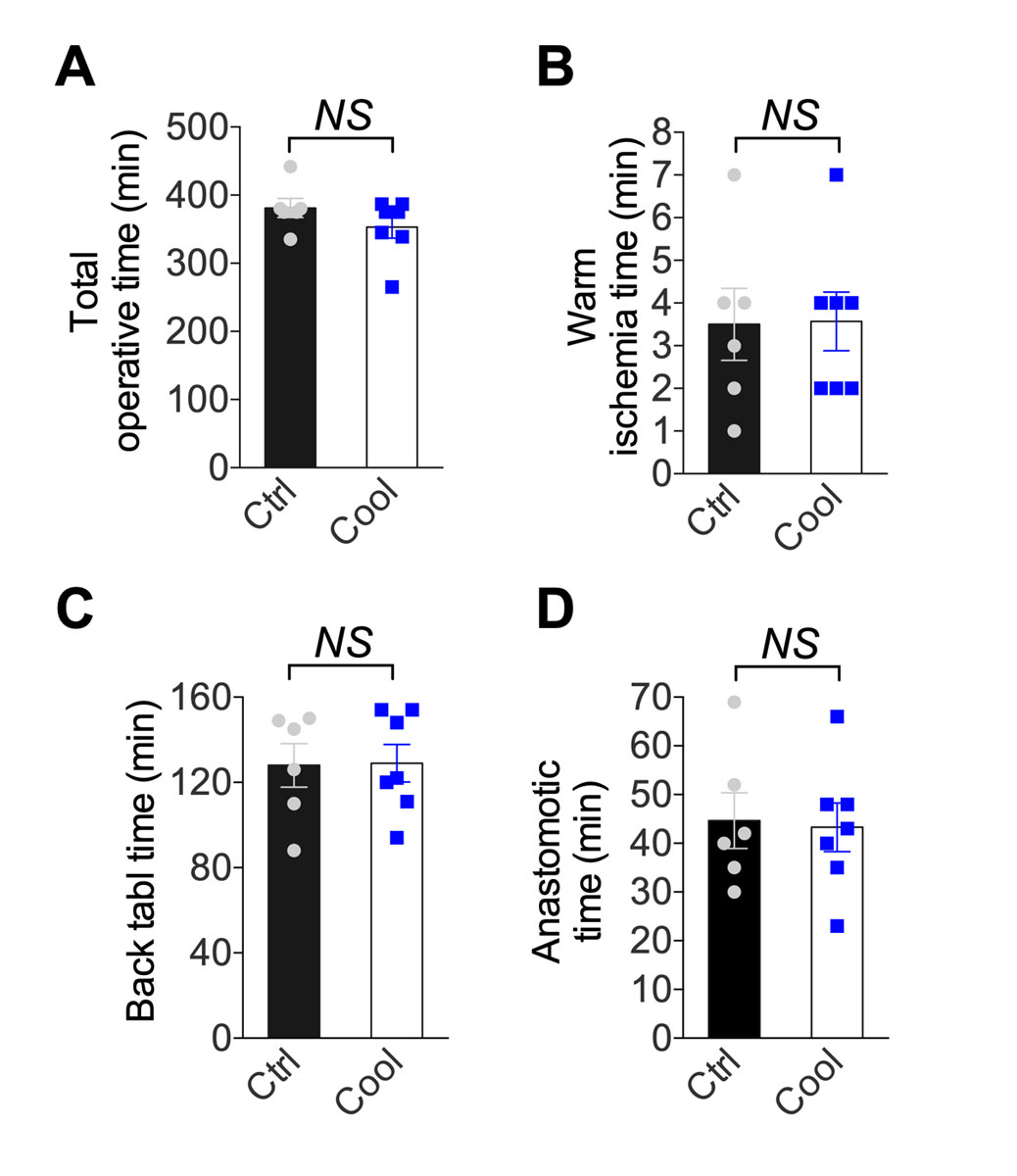
Figure 3 Intra-operative data. (A) Total operative time, (B) warm ischaemia time, (C) back table time, (D) anastomotic time from control and cooling kidney (n = 6–7). Errors bars indicate the standard error of the mean. NS = p >0.05; Ctrl = control
Following ischaemia and reperfusion, kidneys are at risk of delayed function, characterised by a reduction in urine output. On a cellular level, IRI induces glomerular floculus retraction, brush border loss and tubular dilatation. IRIs injuries were slightly reduced in the group with cooling as compared with the control group (fig. 4), but this was not statistically significant. In the cooled group, the percentage of glomeruli with Bowman’s spaces showing a retraction of the flocculus was lower than in the control group. However, the amount of cellular debris, brush border loss and dilated tubules were similar. When these changes were analysed independently, no significant difference was observed (fig. 4A and B). The Goujon score [18], which combines all the previous histopathological observations into a score, suggested that cooling protected from IRI (fig. 4). Urine output within the first days after transplantation defines graft function and thus allows functional assessment of kidney function following IRI. Within the first hour after reperfusion, urine output was higher in cooled kidneys than in the controls (fig. 5). This is consistent with a protective effect of continuous intra-abdominal cooling during transplantation.
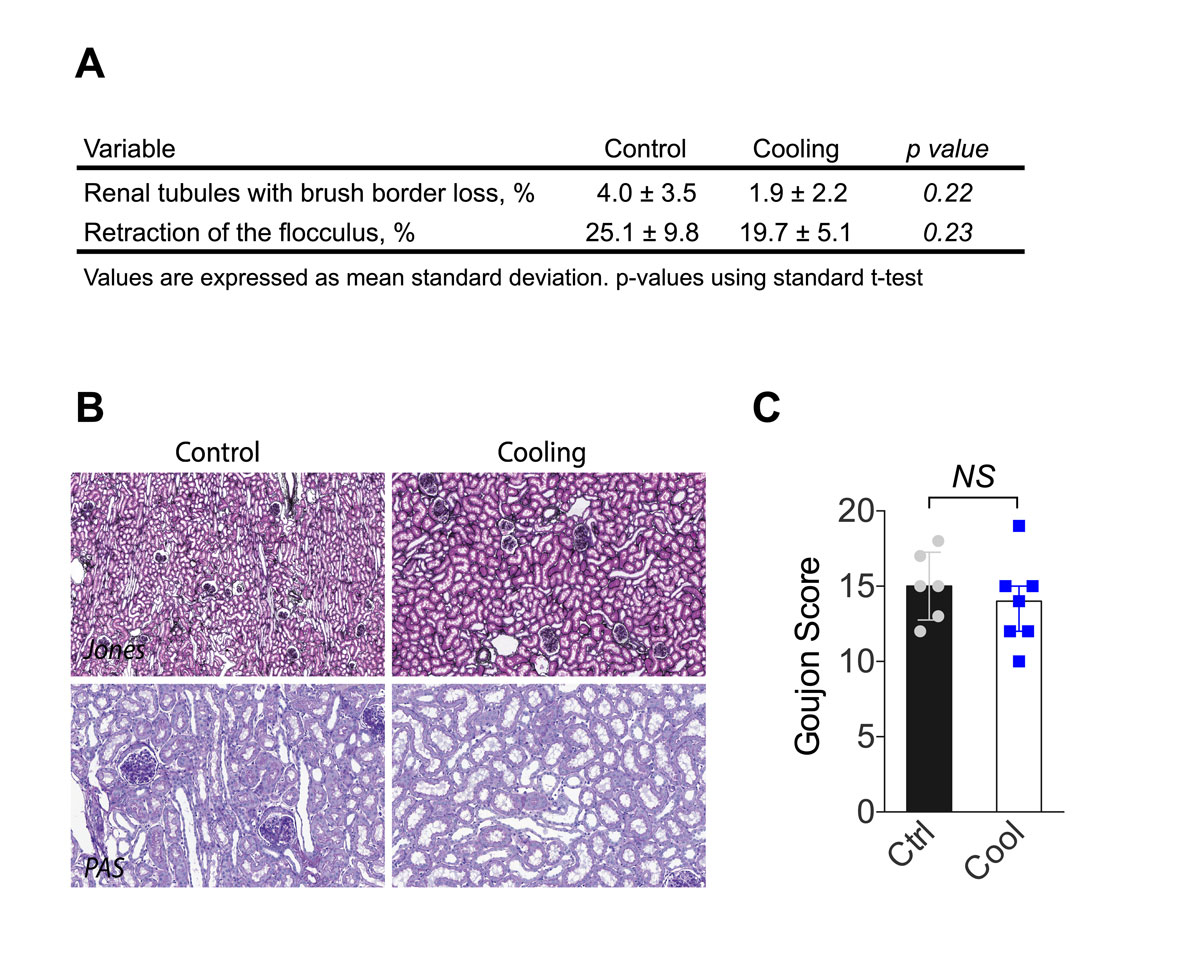
Figure 4 Histological analyses following kidney reperfusion. (A) Percentage of lumina of tubules with cellular debris, the percentage of brush border loss, tubular dilatation, and percentage of floculus retraction in Bowman's space. (B) Representative kidney histological sections stained with periodic acid-Schiff (proximal tubular lesions) and Jones (glomerular injury). (C) Goujon score (lesion severity) was graded 0 to 10 for each type of lesion (n = 6–7). Error bars indicate the standard error of the mean. NS = p >0.05; Ctrl = control.
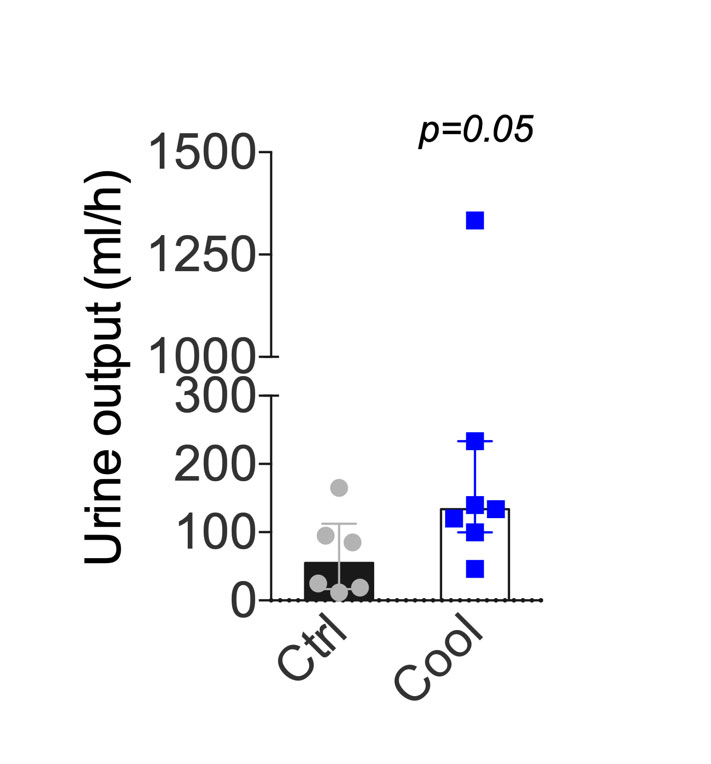
Figure 5 Urine output following transplantation. Mean urine output during the first hour after revascularisation. Errors bars indicate the interquartile range. Ctrl = control
Solid organ transplantation would greatly benefit from measures that attenuate ischaemia and reperfusion injury. Here, we present experimental evidence of a cooling device that significantly reduced graft rewarming, and allowed efficient, controlled and continuous cooling of the kidney. Although short-term histological measures of injury were not statistically different between the groups, cooling of the kidney improved immediate graft function. The device was safe and did not prolong the surgical time.
The histology of kidneys collected 2 hours after reperfusion showed consistent ischaemia-reperfusion lesions. The most significant lesion was retraction of the floculus, which was found in 25.1% and 19.7% of the control and cooled kidneys, respectively. In pigs, we demonstrated that during robotic transplantation with anastomotic time of 70 minutes, continuous cooling of the kidney led to a 25% reduction in histopathological damage as compared with open standard kidney transplantation [18]. In humans, warm ischaemia time longer than 60 minutes is associated with a 25% higher risk of death or graft failure [5]. In our study, anastomosis time was similar between groups (43.9 ± 13 minutes). We believe that the cooling device would provide more benefits in the context of longer anastomoses, such as laparoscopic [20] or robot-assisted renal transplantation [18]. Its benefits might also depend on factors that complicate or lengthen the surgery, such as pre-existing vascular diseases, vessel length or recipient body mass. Also, the absence of time constraint might facilitate the teaching of young trainees. Histological lesions were not statistically different between groups, but urinary output and thus immediate renal function was improved in the cooling group. Altogether, this supports the utility of an efficient and continuous cooling device, especially in humans, when a technically demanding anastomosis is foreseen.
Early graft dysfunction is initiated by IRI [21, 22]. Thus, to ameliorate after kidney transplantation, several clinical trials are investigating measures to reduce IRI (e.g., by perioperative oestrogen administration NCT03663543). It is unlikely that a single intervention suffices. Complementary strategies, in addition to those that reduce warm ischaemia time might have more meaningful benefits. A group that would considerably benefit from shorter warm ischemia time are recipients of organs from expanded criteria donors (ECDs), defined as donors older than 60 years, or older than 50 years with associated vascular comorbidities [23]. ECDs represent half of kidney donors, and are utilised more and more frequently in our aging population [24, 25]. Importantly, ECD organs are more prone to IRI, and both graft and patient survival are reduced compared with organs from healthy young donors [26]. It is possible that the slight benefits observed here with controlled, continuous organ cooling would have significant impact on patient survival, with the most benefits in the most marginal organs.
Our study has several limitations. We chose an auto-transplant experimental model to avoid alloimmunity, potentially inadequate immunosuppressive regimens, toxicity and acute rejection [27, 28]. Furthermore, the relatively short cold ischaemia time corresponded mainly to a living donor situation, where IRI is limited. The current experiments were performed on healthy, young adult pigs, more resistant to stress than humans (e.g., IRI). In addition, we only examined short-term outcomes, as pigs could not be kept alive for survival surgery at our establishment.
In conclusion, our results indicate that the intra-abdominal cooling device significantly reduces warm ischaemic time during transplantation, is technically safe and does not prolong anastomotic time. A randomised, prospective clinical trial using the cooling device is warranted to evaluate the benefits in human.
We thank Jean-Pierre Gilberto and Raphael Ruttiman for their excellent technical assistance.
This work was supported by grants from the Swiss National Science Foundation to JMC and FL (SNSF 320030_182658), and from The Mendez National Institute of Transplantation and the Leenards Foundation to AL.
No potential conflict of interest relevant to this article was reported.
1 Garfield SS , Poret AW , Evans RW . The cost-effectiveness of organ preservation methods in renal transplantation: US projections based on the machine preservation trial. Transplant Proc. 2009;41(9):3531–6. doi:.https://doi.org/10.1016/j.transproceed.2009.10.004
2 Laupacis A , Keown P , Pus N , Krueger H , Ferguson B , Wong C , et al. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996;50(1):235–42. doi:.https://doi.org/10.1038/ki.1996.307
3 Wolfe RA , Ashby VB , Milford EL , Ojo AO , Ettenger RE , Agodoa LY , et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–30. doi:.https://doi.org/10.1056/NEJM199912023412303
4 Legendre C , Canaud G , Martinez F . Factors influencing long-term outcome after kidney transplantation. Transpl Int. 2014;27(1):19–27. doi:.https://doi.org/10.1111/tri.12217
5 Tennankore KK , Kim SJ , Alwayn IP , Kiberd BA . Prolonged warm ischemia time is associated with graft failure and mortality after kidney transplantation. Kidney Int. 2016;89(3):648–58. doi:.https://doi.org/10.1016/j.kint.2015.09.002
6 Ward JP . Determination of the Optimum temperature for regional renal hypothermia during temporary renal ischaemia. Br J Urol. 1975;47(1):17–24. doi:.https://doi.org/10.1111/j.1464-410X.1975.tb03913.x
7 Feuillu B , Cormier L , Frimat L , Kessler M , Amrani M , Mangin P , et al. Kidney warming during transplantation. Transpl Int. 2003;16(5):307–12. doi:.https://doi.org/10.1111/j.1432-2277.2003.tb00305.x
8 Creagh TA , McLoughlin F , Broe PJ , McLean PA , Murphy DM , Bouchier-Hayes DJ . A novel method of induced renal hypothermia. J Urol. 1992;147(1):249–52. doi:.https://doi.org/10.1016/S0022-5347(17)37207-5
9 Marzouk K , Lawen J , Alwayn I , Kiberd BA . The impact of vascular anastomosis time on early kidney transplant outcomes. Transplant Res. 2013;2(1):8. doi:.https://doi.org/10.1186/2047-1440-2-8
10 Canadian Multicentre Transplant Study Group. A randomized clinical trial of cyclosporine in cadaveric renal transplantation. N Engl J Med. 1983;309(14):809–15. doi:.https://doi.org/10.1056/NEJM198310063091401
11 Heylen L , Naesens M , Jochmans I , Monbaliu D , Lerut E , Claes K , et al. The effect of anastomosis time on outcome in recipients of kidneys donated after brain death: a cohort study. Am J Transplant. 2015;15(11):2900–7. doi:.https://doi.org/10.1111/ajt.13397
12 Dittrich S , Groneberg DA , von Loeper J , Lippek F , Hegemann O , Grosse-Siestrup C , et al. Influence of cold storage on renal ischemia reperfusion injury after non-heart-beating donor explantation. Nephron, Exp Nephrol. 2004;96(3):e97–102. doi:.https://doi.org/10.1159/000076751
13 Belzer FO , Ashby BS , Dunphy JE . 24-hour and 72-hour preservation of canine kidneys. Lancet. 1967;200(7515):536–9. doi:.https://doi.org/10.1016/S0140-6736(67)90498-9
14 Davison JM , Uldall PR , Taylor RM . Relation of immediate post-transplant renal function to long-term function in cadaver kidney recipients. Transplantation. 1977;23(4):310–5. doi:.https://doi.org/10.1097/00007890-197704000-00003
15 Forsythe JL , Dunnigan PM , Proud G , Lennard TW , Taylor RM . Reducing renal injury during transplantation. Br J Surg. 1989;76(10):999–1001. doi:.https://doi.org/10.1002/bjs.1800761004
16 Hameed AM , Yuen L , Pang T , Rogers N , Hawthorne WJ , Pleass HC . Techniques to Ameliorate the Impact of Second Warm Ischemic Time on Kidney Transplantation Outcomes. Transplant Proc. 2018;50(10):3144–51. doi:.https://doi.org/10.1016/j.transproceed.2018.09.003
17 Karipineni F , Campos S , Parsikia A , Durinka JB , Chang PN , Khanmoradi K , et al. Elimination of warm ischemia using the Ice Bag Technique does not decrease delayed graft function. Int J Surg. 2014;12(6):551–6. doi:.https://doi.org/10.1016/j.ijsu.2014.04.002
18 Meier RPH , Piller V , Hagen ME , Joliat C , Buchs JB , Nastasi A , et al. Intra-Abdominal Cooling System Limits Ischemia-Reperfusion Injury During Robot-Assisted Renal Transplantation. Am J Transplant. 2018;18(1):53–62. doi:.https://doi.org/10.1111/ajt.14399
19 Goujon JM , Hauet T , Menet E , Levillain P , Babin P , Carretier M . Histological evaluation of proximal tubule cell injury in isolated perfused pig kidneys exposed to cold ischemia. J Surg Res. 1999;82(2):228–33. doi:.https://doi.org/10.1006/jsre.1998.5526
20 Modi P , Pal B , Modi J , Singla S , Patel C , Patel R , et al. Retroperitoneoscopic living-donor nephrectomy and laparoscopic kidney transplantation: experience of initial 72 cases. Transplantation. 2013;95(1):100–5. doi:.https://doi.org/10.1097/TP.0b013e3182795bee
21 Azuma H , Nadeau K , Takada M , Mackenzie HS , Tilney NL . Cellular and molecular predictors of chronic renal dysfunction after initial ischemia/reperfusion injury of a single kidney. Transplantation. 1997;64(2):190–7. doi:.https://doi.org/10.1097/00007890-199707270-00002
22 Salvadori M , Rosso G , Bertoni E . Update on ischemia-reperfusion injury in kidney transplantation: Pathogenesis and treatment. World J Transplant. 2015;5(2):52–67. doi:.https://doi.org/10.5500/wjt.v5.i2.52
23 Port FK , Bragg-Gresham JL , Metzger RA , Dykstra DM , Gillespie BW , Young EW , et al. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors. Transplantation. 2002;74(9):1281–6. doi:.https://doi.org/10.1097/00007890-200211150-00014
24 Tullius SG , Rabb H . Improving the Supply and Quality of Deceased-Donor Organs for Transplantation. N Engl J Med. 2018;378(20):1920–9. doi:.https://doi.org/10.1056/NEJMra1507080
25 Aubert O , Reese PP , Audry B , Bouatou Y , Raynaud M , Viglietti D , et al. Disparities in Acceptance of Deceased Donor Kidneys Between the United States and France and Estimated Effects of Increased US Acceptance. JAMA Intern Med. 2019. doi:.https://doi.org/10.1001/jamainternmed.2019.2322
26 Sung RS , Guidinger MK , Leichtman AB , Lake C , Metzger RA , Port FK , et al. Impact of the expanded criteria donor allocation system on candidates for and recipients of expanded criteria donor kidneys. Transplantation. 2007;84(9):1138–44. doi:.https://doi.org/10.1097/01.tp.0000287118.76725.c1
27 Halloran PF , Venner JM , Madill-Thomsen KS , Einecke G , Parkes MD , Hidalgo LG , et al. Review: The transcripts associated with organ allograft rejection. Am J Transplant. 2018;18(4):785–95. doi:.https://doi.org/10.1111/ajt.14600
28 Seyda M , Quante M , Uehara H , Slegtenhorst BR , Elkhal A , Tullius SG . Immunosenescence in renal transplantation: a changing balance of innate and adaptive immunity. Curr Opin Organ Transplant. 2015;20(4):417–23. doi:.https://doi.org/10.1097/MOT.0000000000000210
Contributed equally
This work was supported by grants from the Swiss National Science Foundation to JMC and FL (SNSF 320030_182658), and from The Mendez National Institute of Transplantation and the Leenards Foundation to AL.
No potential conflict of interest relevant to this article was reported.