Cochlear implants in single-sided deafness – clinical results of a Swiss multicentre study
DOI: https://doi.org/10.4414/smw.2019.20171
Nicole
Petera, Tobias
Kleinjunga, Rudolf
Probsta, Colette
Hemsleya, Dorothe
Veragutha, Alexander
Hubera, Marco
Caversacciobc, Martin
Kompisb, Georgios
Mantokoudisb, Pascal
Sennd, Wilhelm
Wimmerbc
aDepartment of Otorhinolaryngology and Head and Neck Surgery, University Hospital Zurich, and University of Zurich, Switzerland
bDepartment of ENT, Head and Neck Surgery, Inselspital, Bern University Hospital, University of Bern, Switzerland
cHearing Research Laboratory, ARTORG Centre for Biomedical Engineering Research, University of Bern, Switzerland
dENT Department, Geneva University Hospitals, Switzerland
Summary
AIM OF THE STUDY
The aim of this multicentre, prospective, open, nonrandomised clinical trial was to demonstrate the clinical efficiency and outcomes of cochlear implants (CIs) in adult patients with post-lingual single-sided deafness (SSD).
METHODS
A group of five left and five right SSD participants were investigated with various clinical tests and questionnaires before and 12 months after CI activation. Changes in hearing thresholds, speech understanding in noise, sound localisation, tinnitus (Tinnitus Handicap Inventory; THI), subjective hearing ability (Speech, Spatial and Qualities of Hearing Scale; SSQ), and quality of life (WHOQOL-BREF) were assessed. In addition, the pre- and postoperative results of the SSD patients were compared with an age- and gender-matched normal hearing control group.
RESULTS
Surgery was uncomplicated in all patients. Two years after implantation, 9 of the 10 patients used their CI regularly for an average of more than 11 hours a day. A significant improvement in speech understanding in noise measured in the sound field using the Oldenburg sentence test could be demonstrated in the two situations in which patients with SSD experience the greatest difficulty: speech from the front and noise at the healthy ear, and speech to the implanted ear and noise from the front. The sound localisation test showed significant improvement of the mean localisation error and the root mean square error after CI activation. Furthermore, a significant reduction of the THI was measured, and the SSQ showed a significant improvement in the subscale speech comprehension and in the subscale spatial hearing. Also, quality of life measured with the WHOQOL-BREF showed a general improvement, which was significant in the global subscale. For this questionnaire, there was no significant difference between the normal-hearing control group and the patients after 12 months of CI use.
CONCLUSION
This study confirmed the clinical benefit of cochlear implantation in patients with SSD. The significant improvement of speech understanding in noise, sound localisation, tinnitus perception, subjective hearing ability, and in particular the improved quality of life support the recommendation that patients with recently acquired SSD should be offered a CI. (Clinical trial registration number on clinicaltrial.gov: NCT01749592)
Introduction
Single-sided deafness (SSD) is a type of hearing impairment in which the patient has normal hearing in one ear and severely impaired hearing in the other ear. By consensus, SSD is defined as a mean pure-tone hearing threshold (averaged over 0.5, 1, 2 and 4 kHz) of at least 70 dB hearing level (HL) in the poorer and not more than 30 dB HL in the better ear [1]. Post-lingual SSD can occur for different reasons. A common cause is sudden sensorineural hearing loss (SSNHL). Other causes, such as Menière’s disease, unilateral vestibular schwannoma, or infections (e.g., labyrinthitis, mumps, meningitis), and trauma such as temporal bone fractures, iatrogenic hearing loss after ear surgery or unilateral noise damage are more rarely involved [2].
SSD often has a significant impact in communication skills owing to impaired sound localisation and decreased speech understanding in noise. Hearing loss is also a general risk factor for the development of tinnitus [3–7], and 73 to 84% of patients with SSNHL experience it [8–13], with 29% having a moderate to severe level of tinnitus handicap [9]. The traditional rehabilitation of SSD consists of hearing systems with routing of acoustic signals from the deaf ear to the healthy normal hearing ear, such as hearing aids with contralateral routing of signals (CROS) or bone-anchored hearing aids (BAHAs). These hearing systems overcome the head shadow, but they do not reliably improve sound localisation [14]. Contradictory findings with small effects on speech understanding in noise were reported in the literature [15–22]. In addition, they do not usually reduce or suppress the perception of tinnitus [15].
Several theoretical models assume that a lesion of the cochlear hair cells induces a suboptimal or maladaptive plasticity of the central nervous system, which also induces the perception of tinnitus [23–29]. Consequently, restoring hearing by cochlear implantation may affect tinnitus perception [30]. Under this assumption, Van de Heyning et al. were the first to provide a group of patients with post-lingual SSD and severe to profound tinnitus with cochlear implants (CIs) [31]. This and following studies demonstrated that CI implantation is an effective treatment for tinnitus in patients with SSD [15, 30–39], even though a small portion of patients experienced deterioration of tinnitus after CI surgery [30, 40]. Further studies demonstrated an improvement of speech understanding in noise and sound localisation after CI implantation in patients with SSD along with the tinnitus reduction [15–17, 22, 33–35, 39, 41–50]. Many additional studies have demonstrated the clinical benefits of CI implantation in patients with SSD by using questionnaires [33, 34, 44, 45, 51–54]. However, some studies have included not only patients with SSD but also those with asymmetrical hearing loss [30, 43, 45, 51]. Additional studies with carefully selected and well documented patients are still needed to substantiate the benefit of CI in SSD. Such studies may also contribute to reliable meta-analyses in the future.
The aim of this study was to replicate, investigate and document the effects of CI implantation in adults with post-lingual SSD in Switzerland, and to evaluate the functional adaptions using speech understanding in noise, sound localisation, tinnitus assessment and quality of life questionnaires. In addition, the pre- and postoperative results of the SSD patients were compared with an age- and gender-matched normal hearing control group. Our hypothesis was that these patients profit consistently from CI provision through improved hearing, tinnitus reduction and improved quality of life.
Materials and methods
Study design and participants
From 2012 to 2016, we performed a multicentre (University Otorhinolaryngology Departments of Zurich and Bern), prospective, open, nonrandomised clinical trial with the sequential enrolment of five patients with left and five patients with right SSD. SSD was defined as a hearing loss of ≥70 dB HL in the mean thresholds of 0.5, 1, 2 and 4 kHz in the affected ear, and 25 dB HL or better in the frequencies from 125 to 2 kHz and 35 dB HL or better from 4 to 8 kHz in the normally hearing contralateral ear. The patients had to fulfil the following inclusion criteria: age between 18 and 70 years; acquired SSD due to cochlear damage; normal structure of the cochlea and the cochlear nerve on magnetic resonance imaging (MRI); onset of SSD within 6 months to 10 years before study inclusion; impairment of daily life as a consequence of SSD; and unsatisfactory benefit from a trial of a conventional acoustic hearing aid (CROS or BAHA). Patients were excluded when they had middle ear pathology of one ear, psychiatric comorbidity such as depression or cognitive deficits, or severe coexisting illness. To compare the 10 patients with a normal hearing control group, 10 age-, and gender-matched, healthy participants were included in the study. These participants had normal hearing in both ears (hearing thresholds from 0.125 to 2 kHz 25 dB HL or better and from 4 to 8 kHz 35 dB HL or better). All study participants gave their written informed consent before undergoing the study procedure. The study was approved by the local institutional review boards (reference numbers KEK-ZH 2012-0034 and KEK-BE 233/12).
Outcome measures
Pure-tone and sound field audiometry
Hearing thresholds were measured before CI implantation using insert earphones at 0.125, 0.25, 0.5, 1, 2, 3, 4, 6 and 8 kHz for air conduction, and at 0.25, 0.5, 1, 2, 3 and 4 kHz for bone conduction. Appropriate masking of the healthy ear was applied for threshold measurements of the deaf ear. Thresholds exceeding the assessment limits of the audiometer were recorded as 120 dB HL. Binaural hearing thresholds were measured using wobble tone stimuli in the sound field. Twelve months after CI activation, pure-tone audiometry was repeated using insert earphones in the normal hearing ear. For the implanted ear, the sound field hearing thresholds were assessed with the normal hearing ear plugged and muffed. Pure-tone audiometry was performed only once in the normal hearing control participants.
Transiently evoked otoacoustic emissions
Transiently evoked otoacoustic emissions (TEOAE) were measured on both ears before implantation using standard clinical equipment. The measurements were repeated 3 months after the CI activation. Normal hearing participants were tested only once.
Speech understanding in noise
All sound field experiments were performed inside an acoustically treated chamber (6 × 4 × 2 m3) with a reverberation time of approximately 200 ms for frequencies between 0.25 and 10 kHz. We assessed speech understanding in noise using the Oldenburg sentence test, a standardised adaptive German matrix test [55]. The speech reception threshold, expressed as the signal-to-noise ratio at which 50% of the words are correctly understood, was measured with 30 sentences per trial. The background noise (speech babble noise) was presented at a fixed level of 65 dB sound pressure level (SPL) and the level of the speech signals was adapted according to the participant’s response. The tests were performed in five different spatial configurations (fig. 1): (i) speech and noise from the front (S0N0), (ii) speech from the front, noise to the contralateral (normal hearing) ear (S0NCL), (iii) speech to the ipsilateral (implanted) ear, noise from the front (SIPN0), (iv) speech from the front, noise to the ipsilateral (implanted) ear (S0NIP), and (v) speech to the contralateral (normal hearing) ear and noise from the front (SCLN0). These test situations were chosen to enable the assessment of the binaural summation effect (S0N0 unaided vs aided condition), the squelch effect (S0NIP unaided vs aided condition), the head shadow effect (SIPN0 unaided vs aided condition) and spatial release from masking (S0N0 aided vs S0NIP aided) [44]. For these measures, positive values indicate binaural benefit. Before testing, two training tests were performed. The order of the test conditions, spatial configurations and test lists were systematically varied to minimise bias. The tests were performed preoperatively and 12 months after the CI activation on all patients and once on the normal hearing participants. Additionally, the S0N0 test was performed 3 and 6 months after CI activation.
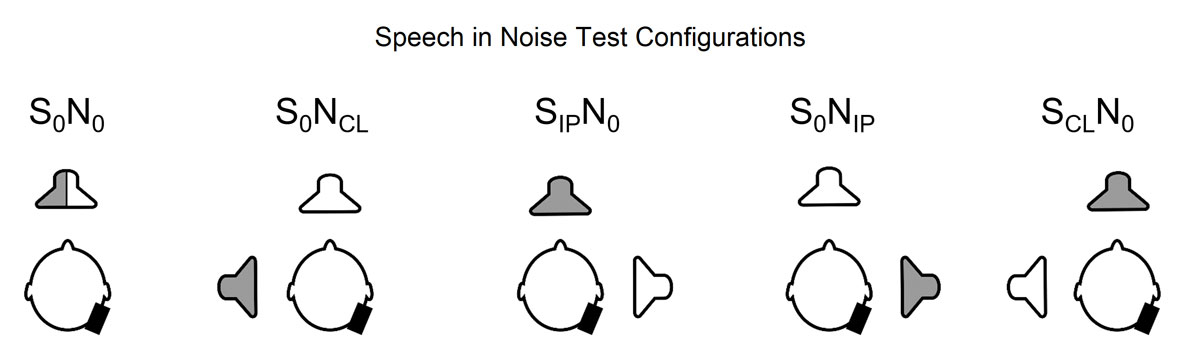
Figure 1 Test settings for speech understanding in noise. S0N0: speech and noise from the front; S0NCL: speech from the front, noise to the contralateral (normal hearing) ear; SIPN0: speech to the ipsilateral (implanted) ear, noise from the front; S0NIP: speech from the front, noise to the ipsilateral ear; SCLN0: speech to the contralateral ear and noise from the front. Gray speaker = noise, white speaker = speech, black rectangle = implant position.
Sound localisation
Sound localisation was assessed using 12 speakers arranged in a horizontal circle at 1.2 m height with a radius of 1 m and an angular resolution of 30°. Participants were seated in the centre of the array. Three white noise stimuli of 200-ms duration were presented in a random order from each speaker in a roving pattern (levels between 65 and 70 dB SPL), totalling 36 stimuli in each test condition. Roving was used to reduce any hints for localisation that could be caused by acoustic characteristics of the loudspeakers. Each of the loudspeakers were labelled with a number according to a clock face. The participants verbally indicated the number associated with the location of the loudspeaker that was the presumed source of the stimulus. Before testing, the participants underwent a 5-minute training session. The participants were instructed to keep their head fixed to the frontal direction during stimulus presentation and correct head position was monitored by the examiner. No feedback on results was provided during or after the test procedures. The mean absolute localisation error (in degrees) and the root mean square error (in degrees) between the azimuthal positions of the stimulus speaker and the indicated speaker were assessed. This test was performed preoperatively and 12 months after the CI activation on all patients and once on the normal hearing participants. The chance level of this test setup was 90° for the mean absolute localisation error corresponding to 104.6° when expressed as root mean square error.
Questionnaires
To assess tinnitus severity, we used the Tinnitus Handicap Inventory (THI) [56]. The validated German version [57] contains 25 questions with three response options (yes – 4 points; sometimes – 2 points; no – 0 points). Consequently, the THI total score ranges from zero to 100 and indicates the subject’s overall handicap related to tinnitus [58]. The total score can be divided into five categories of tinnitus severity: slight (0–16), mild (18–36), moderate (38–56), severe (58–76), and catastrophic (78–100) [59].
The validated questionnaire World Health Organization Quality of Life Short Form Survey (WHOQOL-BREF) was used to measure health-related quality of life [60]. The WHOQOL-BREF consists of 26 items assessing the following broad domains: physical health, psychological health, social relationships, environment and global quality of life [58]. Generally, a higher value in every domain represents a better quality of life.
The German version of the standardised Speech, Spatial and Qualities of Hearing Scale questionnaire (SSQ, Version 5.6 [61–63]) was used to measure the improvement of hearing ability in various environmental conditions of everyday life after CI implantation. The questionnaire contains three parts in which speech comprehension, spatial hearing and qualities of hearing are measured. The questionnaire consists of 19 questions, which are answered on a Likert scale from 0 (complete disability) to 10 (complete ability). It follows that the higher the score in each part of the SSQ, the better the respective ability.
The patients were asked to fill in all questionnaires preoperatively and 12 months after activation. Normal hearing participants answered the SSQ and WHOQOL-BREF questionnaire only once.
Statistical analysis
Descriptive statistics were calculated and mean values with standard deviations (SDs) are presented. Two-sided paired t-tests were used to compare the following preoperative and postoperative aided outcome measures: speech reception thresholds for each speech in noise configuration, mean absolute and root mean square localisation errors, and minimum audible angle. To test whether speech reception threshold changed over time in the S0N0 situation, a repeated measures analysis of variance (ANOVA) was performed. WHOQOL-BREF scores were compared using paired t-tests with adjusted p-values using a Bonferroni correction. Differences in audiological outcomes between the implanted subjects and the normal hearing group were not statistically tested. The level of significance was set at p ≤0.05. Graphical representations and statistical analyses of the data were performed using the Prism software package (GraphPad Software Inc, US; version 6.0).
Results
Participants
From November 2012 to May 2016, 54 candidates with post-lingual SSD fulfilling the inclusion criteria were asked to participate in the study. Forty-four of them declined to take part, mostly because of the invasiveness of cochlear implantation, too little impairment in daily life, aesthetic concerns with the clearly visible speech processor, lack of motivation for hearing rehabilitation, or sufficient benefit with a conventional hearing aid fitting (CROS or BAHA). The 10 patients (4 women) included had a mean age of 47 years (range 27.8–62.7, SD 10.2). The mean duration of SSD before the inclusion date was 1.3 years (range 0.5–4.3, SD 1.1) and before CI implantation 1.7 years (range 0.8–4.6, SD 1.1). Five patients had involvement on the left side (two women; mean age 44.3 years, SD 10.3) and five on the right (two women; mean age 49.6 years, SD 10.4). The mean time between CI implantation and CI activation was 4 weeks (SD 1 week). The cause of the SSD was SSNHL in nine cases and herpes zoster oticus in the other.
Preoperative measurements of TEOAE confirmed the unilateral deafness. The mean total otoacoustic response amplitude for the healthy right ears was 11.3 dB SPL and 7.8 dB SPL for the healthy left ears. The normal hearing control group showed mean total otoacoustic response amplitudes of 7.6 dB SPL for the right ears and of 7.5 dB SPL for the left. The mean preoperative pure-tone threshold results of the patients and the normal hearing control group are displayed in figure 2
.
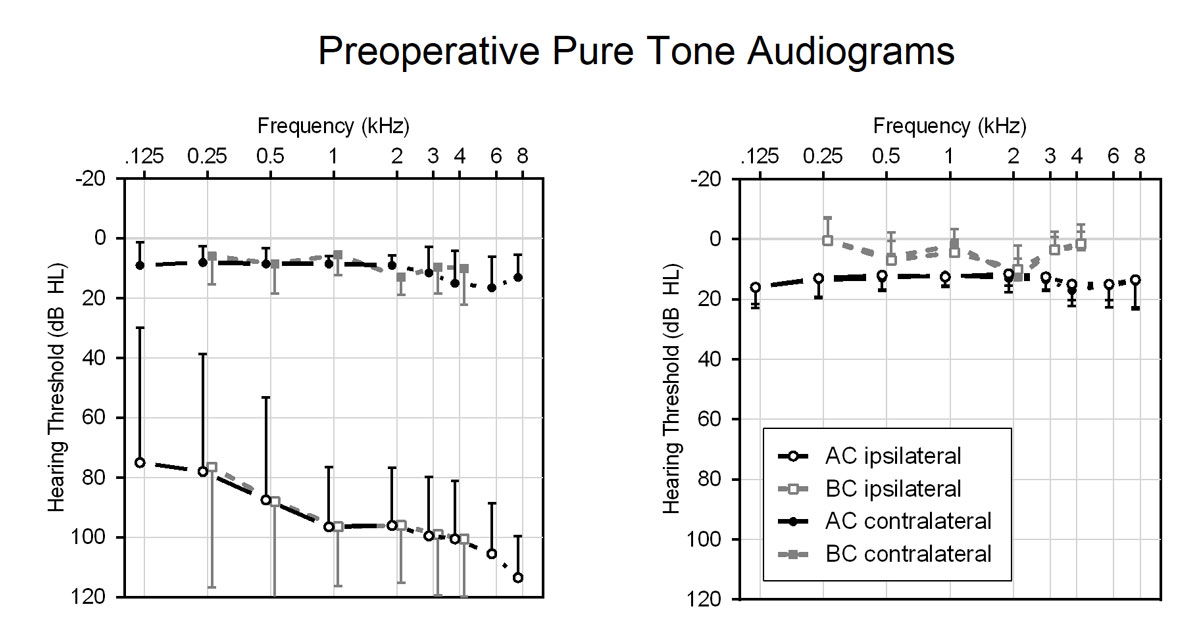
Figure 2 Averaged preoperative masked pure-tone thresholds of the patients (left panel) and normal hearing control participants (right panel). Data are shown as mean and standard deviation.
Surgical treatment and fitting of CI
Before inclusion, every patient underwent an MRI to confirm normal structure of the cochlea and the cochlear nerve. Surgery was uneventful in all patients. The surgeries were performed by three experienced surgeons. A standard retroauricular incision and a limited mastoidectomy with a posterior tympanotomy were used for the CI surgery. The round window was exposed and perforated either with a needle or by an extended round window approach. A Nucleus CI422 implant with Slim Straight electrode (Cochlear Ltd.) was introduced. The subcutaneous stimulator/receiver unit was fixed retroauricularly. The implant function was confirmed after the electrode insertion using telemetric assessment of electrically evoked nerve potentials. There were no major surgical complications after the CI implantations. The correct localisation of the electrode array was confirmed with a postoperative X-ray or computed tomography scan.
The CI was activated 31 days (SD 7.3) after surgery. The CI fitting was performed by behavioural measurements following standard clinical procedures. Initially, threshold and comfortable levels were assessed individually for all electrodes. When a stable map was achieved after several months of CI use, the stimulation settings were verified using a sweep at comfortable levels over several adjacent electrodes and at levels of typically 25% of the dynamic range.
The objective data logging of the CI system showed that 9 of the 10 patients were using their CIs frequently 24 months or later after the CI activation for a mean daily duration of 11.2 hours (SD 2.5). One female patient used the CI only sporadically for 14 months and later discontinued its use completely because of insufficient benefit.
Pure-tone and sound field audiometry
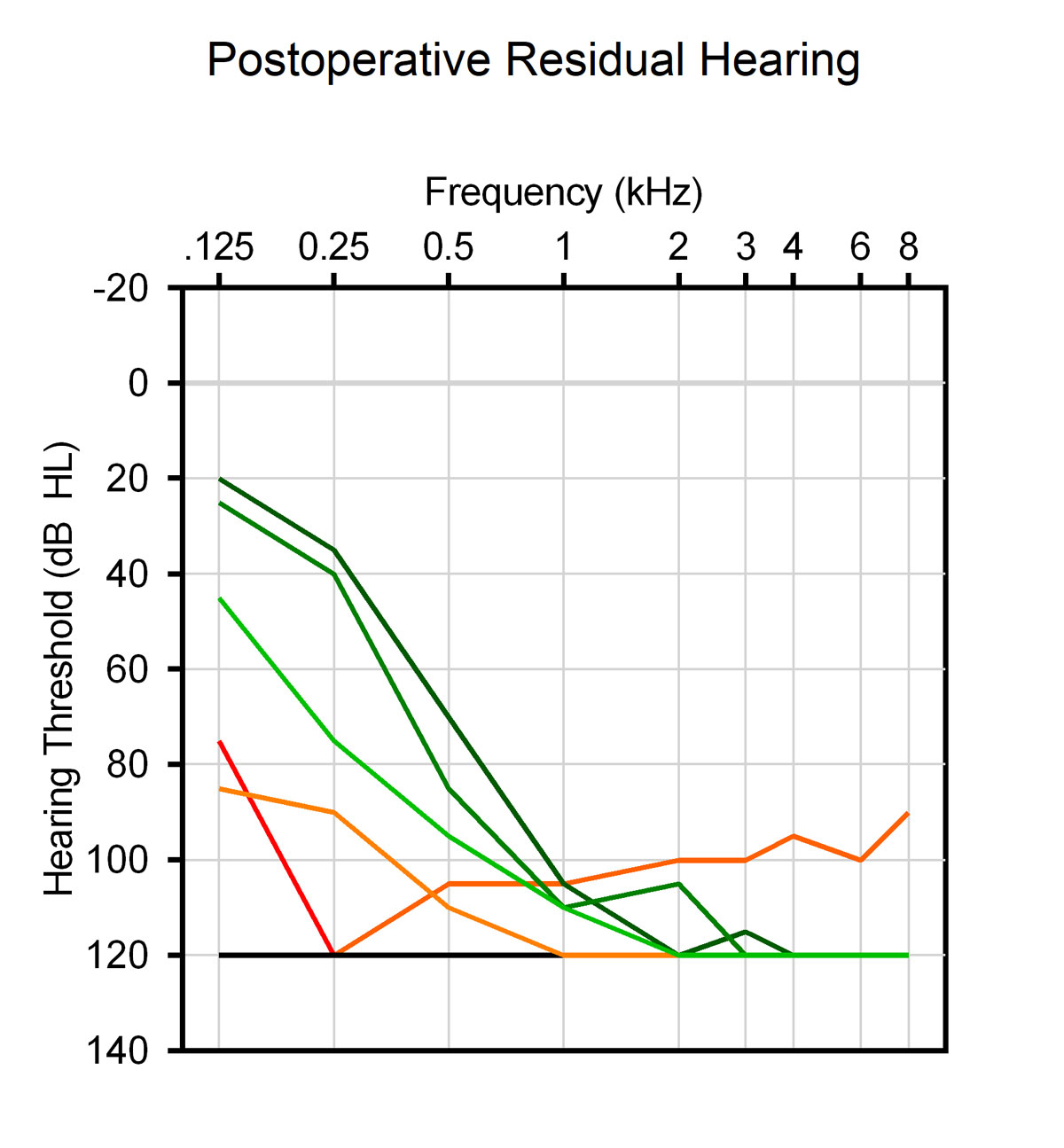
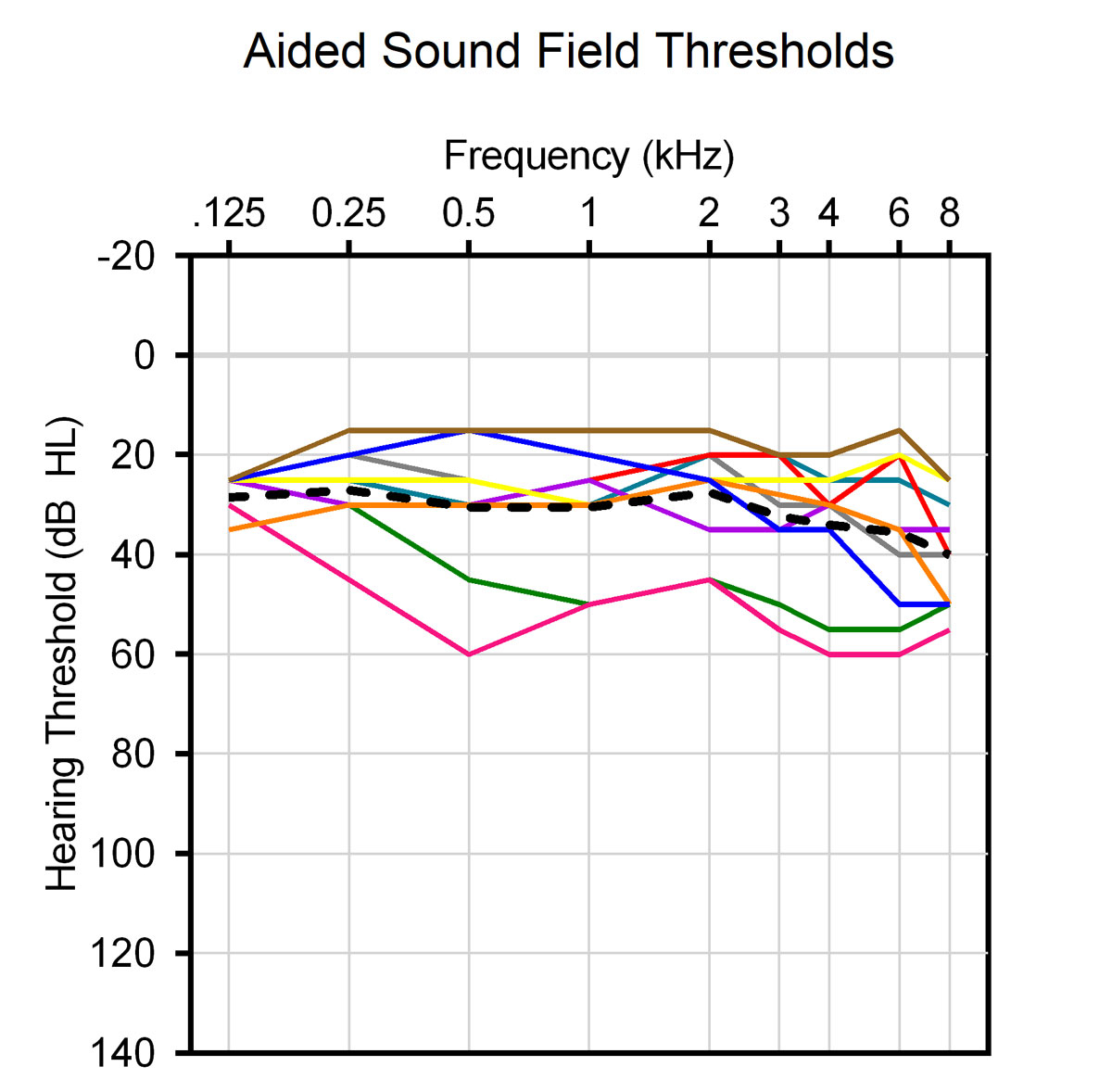
No significant changes of the preoperative pure-tone air conduction hearing thresholds of the healthy ears were noted postoperatively. Six patients had residual hearing of the affected ear preoperatively that was fully preserved in three and partially preserved in two of them after surgery (fig. 3). Sound field CI-aided thresholds (normal hearing ear plugged and muffed) were substantially improved when compared with the preoperative pure-tone air conduction hearing thresholds of the SSD ears (fig. 4).
Speech understanding in noise
Figure 5 illustrates the speech reception thresholds (SRTs) for the test situation S0N0 measured preoperatively, as well as 3, 6, and 12 months post-activation. There were no statistically significant differences between group means as determined by one-way ANOVA (F(3,27) = 0.451, p = 0.72). We did not observe an improvement of the SRTs by binaural summation between the preoperative and the 12-month measurements (mean difference: −0.3 dB, SD 1.2 dB).
Twelve months after CI activation, a significant improvement in SRT in noise could be demonstrated in the two situations in which patients with SSD experience the most difficulties (fig. 6): speech from the front and noise at the healthy ear (S0NCL, 2.7 dB improvement, p = 0.0029); and speech to the implanted ear and noise from the front (SIPN0, 1.5 dB improvement, p = 0.018). Use of CI resulted in an average squelch effect of 1.5 dB (SD 1.0), improvement of the head shadow effect of 1.5 dB (SD 1.6), and spatial release from masking of 3.0 dB (SD 1.9).

Figure 6 Speech reception thresholds (SRTs) in noise measured in the sound field preoperatively and 12 months after CI activation. See figure 1 for a description of the tested spatial configurations. * p <0.05; ** p <0.01.
The participants in the normal hearing control group achieved an average SRT of –8.9 dB (SD 2.2) in the S0N0, –13.0 dB (SD 1.7) in the S0NCL, –13.0 dB (SD 2.0) in the SIPN0, −12.3 dB (SD 2.2) in the S0NIP, and −12.8 dB (SD: 1.7) in the SCLN0 conditions.
Sound localisation
The mean localisation error of all participants was improved by 10.2° (p = 0.030), and the root mean square error by 12.2° (p= 0.029) 12 months after CI activation (fig. 7). One participant lost low-frequency residual hearing from the surgery, resulting in poorer localisation performance after implantation with an increased error of 11.3°. All other participants either showed no differences or improvement of their localisation accuracy. As expected, the participants of the normal hearing control group had clearly higher localisation accuracy with a mean absolute localisation error of 2.9° and root mean square error of 10.9°.
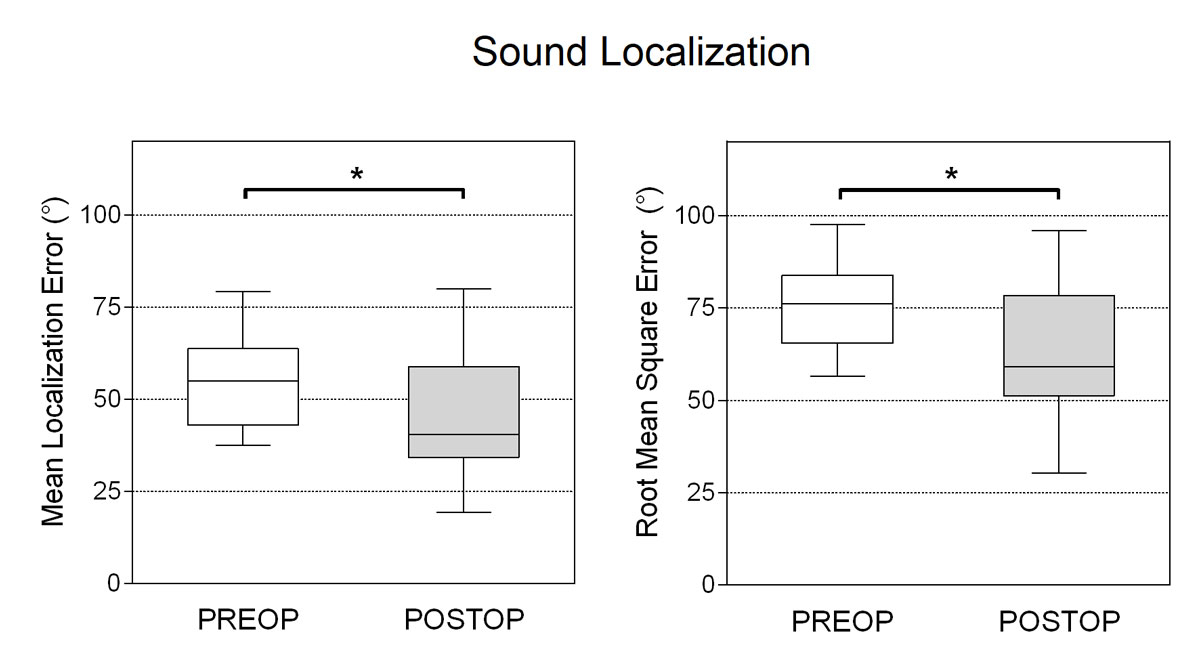
Figure 7 Mean absolute (left) and root mean square (right) localisation errors assessed preoperatively and 12 months post activation. * p <0.05.
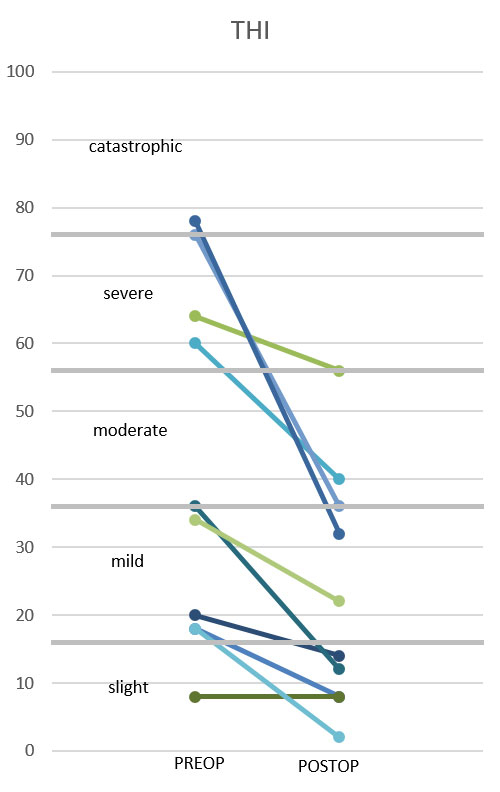
Tinnitus handicap
There was a significant reduction of tinnitus severity 12 months after CI activation. The mean THI value dropped from 41.2 points (SD 26.5) preoperatively to 23.0 (SD 17.5) 12 months after CI activation. The paired t-test showed a highly significant reduction of the THI (p = 0.004). The individual pre- and postoperative THI values are displayed in figure 8. Four of the 10 patients suffered from severe or catastrophic tinnitus preoperatively. They all improved to a mild to moderate tinnitus postoperatively. The values of patients with slight or mild tinnitus preoperatively showed a smaller postoperative improvement of the tinnitus handicap. In summary, one patient demonstrated complete tinnitus suppression, seven patients reported an improvement, and two patients had no change in tinnitus handicap. None experienced an increase of the perception of their tinnitus or its severity.
Quality of life
Quality of life also showed a general improvement, which was significant in the global subscale of the WHOQOL-BREF questionnaire (p = 0.007; fig. 9). In contrast to the preoperative finding, there was no significant difference between the normal hearing control group and the patients after CI implantation (p = 0.71).
Speech, spatial and qualities of hearing scale
The SSQ results showed a significant improvement from 4.2 preoperatively to 6 postoperatively (p = 0.004) in the subscale speech comprehension and from 3 to 5.3 (p = 0.009) in the subscale spatial hearing. There was no significant change in the subscale qualities of hearing from 6.2 preoperatively to 6.9 postoperatively (p = .13). The scores of the patients on the three subscales were significantly lower than for the normal hearing control group, with an average speech comprehension of 8.7 (difference to the patient group p = 0.001), an average spatial hearing of 8.6 (difference p <0.001), and an average qualities of hearing of 9.1 (difference p = 0.005).
Discussion
Recent literature has demonstrated that CI implantation in SSD patients is a suitable hearing rehabilitation option. It is the only option to restore binaural hearing [52]. This study aimed to replicate the widely reported benefits of CI implantation in adult patients with post-lingual SSD in Switzerland and to investigate detailed objective and subjective aspects using speech understanding in noise, sound localisation, tinnitus assessment, quality of life and hearing ability questionnaires. In addition, results of the SSD population were compared with an age- and gender-matched normal hearing cohort. Only a few studies have compared selective results of speech understanding in noise [34, 39, 43, 64], sound localisation [34, 43, 52, 64], and subjective hearing ability [39] with a normal hearing cohort, mostly without control for age or gender matching.
Generally, our patients with SSD had a significant improvement in their quality of life 12 months after CI activation. Furthermore, we could demonstrate that their global score on the WHOQOL-BREF questionnaire became comparable to that of the normal hearing control group. This finding suggests that the patients with SSD had impaired quality of life, which could be reversed by CI implantation. Other studies used specific questionnaires regarding hearing or hearing aids for the evaluation of quality of life [33, 45, 52, 54]. Härkönnen et al. [34] and Sladen et al. [54] also used general health related quality of life questionnaires. Like us, Härkönnen et al. [34] showed significant improvement of quality of life 6 months after CI activation using the Glasgow Benefit Inventory. This study [34] also demonstrated a significant improvement in the subscale general health only. In contrast, the study by Sladen et al. [54] showed no significant improvement in quality of life through CI implantation in SSD patients using the SF-36 (Medical Outcomes Study Questionnaire Short Form 36) questionnaire. An often-used questionnaire regarding quality of life in CI implantation is the Njimegen Cochlear Implant Questionnaire (NCIQ), which has also shown a significant improvement when applied to CI for SSD [45, 54]. Improvement in quality of life is likely to be a result of the reduction in perceived tinnitus severity, and to improvements in speech understanding in noise, spatial hearing, and listening effort.
As with other studies [33, 34, 39, 49, 51, 52, 54, 65], we used the SSQ to measure the improvement of hearing ability in various environmental conditions. Several studies [16, 34, 65] have shown comparable results with significant improvements in the subscale speech comprehension and spatial hearing, and nonsignificant improvement in the subscale qualities of hearing. Other studies were able to document a significant improvement in this subscale as well [33, 39, 49, 52]. The reason for a less frequently significantly documented improvement in the qualities of hearing subscale may be the normal hearing in the contralateral ear.
As described for the first time by Van de Heyning et al. [31], we could also demonstrate a significant reduction of tinnitus severity 12 months after CI activation in our SSD patients. Particularly, SSD patients with a high degree of tinnitus severity improved considerably. Overall, eight patients reported a tinnitus reduction, two patients had stable tinnitus, and none experienced an increase in the perception of their tinnitus. These results are comparable to other studies investigating tinnitus reduction after CI implantation in SSD patients, as summarised in a recent review [27].
A significant improvement of speech understanding in noise could be demonstrated in two conditions 12 months after CI activation: speech from the front and noise to the healthy ear (S0NCL), and speech to the implanted ear and noise from the front (SIPN0). Patients with SSD experience the most difficulties in understanding speech in noise in these situations. Cochlear implantation can improve these conditions. Equally important, we did not observe a deterioration of speech comprehension in the situations where noise was closer to the implanted ear (S0NIP and SCLN0). In a cohort of 45 SSD patients with CI assessed with the Oldenburg sentence test, Arndt et al. [16] reported an improvement of approximately 7 dB in the test situation with speech presented to the SSD side and noise to the normal hearing side in a ±45° configuration. Our participants experienced an improvement of 1.5 to 2 dB in a 0/90° configuration (S0NCL and SIPN0). Since the same test material and procedural parameters were used, we assume that the different test configuration (i.e., speakers from ±45° instead of ±90°) was contributing to the difference in outcomes. In addition, Arndt et al. [16] observed a small but statistically significant summation effect of approximately 1 dB. A similar result was reported by Mertens et al. [45] with a summation effect of 1.3 dB in a 12-month follow-up of SSD patients. We did not reproduce these results in our smaller cohort. We observed a small and statistically insignificant squelch effect of 1.5 dB, which reproduces the findings of Mertens et al. [45]. They found an effect size of 0.3 at the 12-month follow-up. The head shadow effect was smaller (1.5 dB) than reported by Mertens et al. (3.0 dB) [45]. We attribute this difference mainly to our test configuration, in which we presented noise closer to the normal hearing ear than did Mertens et al. [45]. The result may reflect a partial bypass of the head shadow. Spatial release of masking (3.0 dB) was similar to the effect measured by Mertens et al. (4.7 dB) [45], indicating that our participants could benefit from separated sound sources when using the implant.
Finally, CIs significantly improved the sound localisation abilities of the patients 12 months after activation, demonstrating binaural information integration of the normal hearing and the implanted ear. Impeded spatial hearing abilities are usually among the major complaints of patients with SSD. A comparison with previous studies is difficult since widely variable test conditions have been used with different numbers and spatial arrangements of loudspeakers, different test stimulus levels and types, and different error quantification. The majority of previous studies have demonstrated a partial restoration of sound localisation [32, 33, 36, 42, 43, 51]. However, comparisons are difficult because of the above-mentioned differences, and because patients’ characteristics, including the extent of hearing preservation, can influence outcomes. In the following discussion, we will focus on relative improvements of the localisation errors. The study of Hoth et al. [43] used an identical measurement setup and showed a root mean square localisation improvement of 25°, which is twice as much as in our study (12.2°). We believe that the higher improvement reported by Hoth et al. [43] was because speech material was used to assess localisation accuracy, which can be considered easier to localise (longer stimulus duration and stimuli with semantic meaning vs short-time broad band noise). Galvin et al. [33] used a 12-speaker array for sound localisation arranged behind the subjects and observed an improvement between 6.7 and 11.5° after CI activation. Easier test conditions can be expected with semicircular setups and fewer speakers that are positioned in the front of the subjects. With a setup consisting of 11 speakers, an improvement of 37° was observed by Dillon et al. [32]. Grossmann et al. [42] and Mertens et al. [36] used nine speakers and reported an improvement of 35.6° and 44.1°, respectively. Arndt et al. [51] tested 45 subjects with SSD in a semicircular setup consisting of seven loudspeakers positioned in front of the subjects. The mean absolute error improved by 13.5° after CI activation (compared with 10.2° in our study). We could not see a clear tendency for better localisation performance in participants with preserved residual hearing compared with participants with no residual hearing. However, localisation accuracy decreased after implantation in just one patient who lost residual hearing, demonstrating the importance of persevering residual hearing capabilities during surgery. All other participants improved.
Even though speech understanding in noise and sound localisation after CI implantation were not comparable to the abilities of the normal hearing control participants, the global improvement and the regular use of the CI for more than 11 hours per day in 9 of 10 patients demonstrates that the majority of patients with SSD profit from it.
Limitations of this study included an unexpectedly long recruiting duration because of refusal of many patients to participate, and an imbalance between the participant centres. Bern contributed one patient only. The major limitation of this study may have been that only five patients per deaf side could be included. We will investigate further the effect of the laterality, but preliminary results did not reveal side difference in our small sample. Deafness of one ear induces multiple changes of neural plasticity in central auditory pathways. As the left and right hemispheres are specialised for different auditory tasks, a right- or left-sided deafness could have different consequences. The left auditory cortex and hemisphere show advantages for speech understanding, whereas the right auditory cortex and hemisphere are more involved in music perception and sound localisation. Additional studies investigating the laterality of deafness with respect to speech understanding in noise and sound localisation may be needed. To enable effective data collection and comparability of outcome measures, and thus to get firm statements ensuring clinical quality control in Switzerland, we suggest implementing standardised minimum outcome measures for SSD patients undergoing CI surgery [1]. This applies in particular for sound field audiometry, including speech in noise and sound localisation tests, where the broad range of measurement conditions and experimental setups impedes data pooling. The successfully operating Swiss CI database (CICH) [66] will provide an efficient and solid framework for such data collection.
To conclude, our SSD patients showed a significant improvement in quality of life, tinnitus perception, speech understanding in noise and sound localisation. Our results confirmed those reported previously in the literature. Additionally, we found that the general quality of life of patients with SSD significantly increased after CI implantation to a level comparable to the normal hearing control group. Cochlear implantation should be offered to adult patients with recently acquired SSD. However, the benefit of CI implantations in congenital and early acquired SSD, or SSD of long-time duration (more than 10 years) [67] is still matter of debate.
Acknowledgments
Staff of Audiology Section and CI-Centre at the University Hospital Zurich and Berne, in particular Wai Kong Lai and Michael Büchler for CI device programming.
References
1
Van de Heyning
P
,
Távora-Vieira
D
,
Mertens
G
,
Van Rompaey
V
,
Rajan
GP
,
Müller
J
, et al.
Towards a Unified Testing Framework for Single-Sided Deafness Studies: A Consensus Paper. Audiol Neurotol. 2016;21(6):391–8. doi:.https://doi.org/10.1159/000455058
2
Holder
JT
,
O’Connell
B
,
Hedley-Williams
A
,
Wanna
G
. Cochlear implantation for single-sided deafness and tinnitus suppression. Am J Otolaryngol. 2017;38(2):226–9. doi:.https://doi.org/10.1016/j.amjoto.2017.01.020
3
Baguley
D
,
McFerran
D
,
Hall
D
. Tinnitus. Lancet. 2013;382(9904):1600–7. doi:.https://doi.org/10.1016/S0140-6736(13)60142-7
4
Kompis
M
,
Neuner
NT
,
Hemmeler
W
,
Häusler
R
. [Tinnitus]. Ther Umsch. 2004;61(1):15–20. doi:.https://doi.org/10.1024/0040-5930.61.1.15
5
Langguth
B
,
Kreuzer
PM
,
Kleinjung
T
,
De Ridder
D
. Tinnitus: causes and clinical management. Lancet Neurol. 2013;12(9):920–30. doi:.https://doi.org/10.1016/S1474-4422(13)70160-1
6
Nondahl
DM
,
Cruickshanks
KJ
,
Huang
GH
,
Klein
BE
,
Klein
R
,
Nieto
FJ
, et al.
Tinnitus and its risk factors in the Beaver Dam offspring study. Int J Audiol. 2011;50(5):313–20. doi:.https://doi.org/10.3109/14992027.2010.551220
7
Tunkel
DE
,
Bauer
CA
,
Sun
GH
,
Rosenfeld
RM
,
Chandrasekhar
SS
,
Cunningham
ER, Jr
, et al.
Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(2, Suppl):S1–40. doi:.https://doi.org/10.1177/0194599814545325
8
Chandrasekhar
SS
,
Tsai Do
BS
,
Schwartz
SR
,
Bontempo
LJ
,
Faucett
EA
,
Finestone
SA
, et al.
Clinical Practice Guideline: Sudden Hearing Loss (Update) Executive Summary. Otolaryngol Head Neck Surg. 2019;161(2):195–210. doi:.https://doi.org/10.1177/0194599819859883
9
Chiossoine-Kerdel
JA
,
Baguley
DM
,
Stoddart
RL
,
Moffat
DA
. An investigation of the audiologic handicap associated with unilateral sudden sensorineural hearing loss. Am J Otol. 2000;21(5):645–51.
10
Klemm
E
,
Bepperling
F
,
Burschka
MA
,
Mösges
R
. Hemodilution therapy with hydroxyethyl starch solution (130/0.4) in unilateral idiopathic sudden sensorineural hearing loss: a dose-finding, double-blind, placebo-controlled, international multicenter trial with 210 patients. Otol Neurotol. 2007;28(2):157–70. doi:.https://doi.org/10.1097/01.mao.0000231502.54157.ad
11
Mühlmeier
G
,
Baguley
D
,
Cox
T
,
Suckfüll
M
,
Meyer
T
. Characteristics and Spontaneous Recovery of Tinnitus Related to Idiopathic Sudden Sensorineural Hearing Loss. Otol Neurotol. 2016;37(6):634–41. doi:.https://doi.org/10.1097/MAO.0000000000001081
12
Nosrati-Zarenoe
R
,
Hultcrantz
E
. Corticosteroid treatment of idiopathic sudden sensorineural hearing loss: randomized triple-blind placebo-controlled trial. Otol Neurotol. 2012;33(4):523–31. doi:.https://doi.org/10.1097/MAO.0b013e31824b78da
13
Rauch
SD
,
Halpin
CF
,
Antonelli
PJ
,
Babu
S
,
Carey
JP
,
Gantz
BJ
, et al.
Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA. 2011;305(20):2071–9. doi:.https://doi.org/10.1001/jama.2011.679
14
Newman
CW
,
Sandridge
SA
,
Wodzisz
LM
. Longitudinal benefit from and satisfaction with the Baha system for patients with acquired unilateral sensorineural hearing loss. Otol Neurotol. 2008;29(8):1123–31. doi:.https://doi.org/10.1097/MAO.0b013e31817dad20
15
Arndt
S
,
Aschendorff
A
,
Laszig
R
,
Beck
R
,
Schild
C
,
Kroeger
S
, et al.
Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol Neurotol. 2011;32(1):39–47. doi:.https://doi.org/10.1097/MAO.0b013e3181fcf271
16
Arndt
S
,
Laszig
R
,
Aschendorff
A
,
Hassepass
F
,
Beck
R
,
Wesarg
T
. Cochleaimplantatversorgung bei einseitiger Taubheit oder asymmetrischem Hörverlust [Cochlear implant treatment of patients with single-sided deafness or asymmetric hearing loss. German version]. HNO. 2017;65(7):586–98. doi:.https://doi.org/10.1007/s00106-016-0294-8
17
Hol
MK
,
Kunst
SJ
,
Snik
AF
,
Bosman
AJ
,
Mylanus
EA
,
Cremers
CW
. Bone-anchored hearing aids in patients with acquired and congenital unilateral inner ear deafness (Baha CROS): clinical evaluation of 56 cases. Ann Otol Rhinol Laryngol. 2010;119(7):447–54. doi:.https://doi.org/10.1177/000348941011900704
18
Kompis
M
,
Wimmer
W
,
Caversaccio
M
. Long term benefit of bone anchored hearing systems in single sided deafness. Acta Otolaryngol. 2017;137(4):398–402. doi:.https://doi.org/10.1080/00016489.2016.1261410
19
Laske
RD
,
Röösli
C
,
Pfiffner
F
,
Veraguth
D
,
Huber
AM
. Functional Results and Subjective Benefit of a Transcutaneous Bone Conduction Device in Patients With Single-Sided Deafness. Otol Neurotol. 2015;36(7):1151–6. doi:.https://doi.org/10.1097/MAO.0000000000000791
20
Salcher
R
,
Zimmermann
D
,
Giere
T
,
Lenarz
T
,
Maier
H
. Audiological Results in SSD With an Active Transcutaneous Bone Conduction Implant at a Retrosigmoidal Position. Otol Neurotol. 2017;38(5):642–7. doi:.https://doi.org/10.1097/MAO.0000000000001394
21
Schmerber
S
,
Deguine
O
,
Marx
M
,
Van de Heyning
P
,
Sterkers
O
,
Mosnier
I
, et al.
Safety and effectiveness of the Bonebridge transcutaneous active direct-drive bone-conduction hearing implant at 1-year device use. Eur Arch Otorhinolaryngol. 2017;274(4):1835–51. doi:.https://doi.org/10.1007/s00405-016-4228-6
22
Wimmer
W
,
Kompis
M
,
Stieger
C
,
Caversaccio
M
,
Weder
S
. Directional Microphone Contralateral Routing of Signals in Cochlear Implant Users: A Within-Subjects Comparison. Ear Hear. 2017;38(3):368–73. doi:.https://doi.org/10.1097/AUD.0000000000000412
23
Eggermont
JJ
,
Roberts
LE
. The neuroscience of tinnitus. Trends Neurosci. 2004;27(11):676–82. doi:.https://doi.org/10.1016/j.tins.2004.08.010
24
Hoare
DJ
,
Adjamian
P
,
Sereda
M
. Electrical Stimulation of the Ear, Head, Cranial Nerve, or Cortex for the Treatment of Tinnitus: A Scoping Review. Neural Plast. 2016;2016:5130503. doi:.https://doi.org/10.1155/2016/5130503
25
Kaltenbach
JA
,
Afman
CE
. Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res. 2000;140(1-2):165–72. doi:.https://doi.org/10.1016/S0378-5955(99)00197-5
26
Mühlnickel
W
,
Elbert
T
,
Taub
E
,
Flor
H
. Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci USA. 1998;95(17):10340–3. doi:.https://doi.org/10.1073/pnas.95.17.10340
27
Peter
N
,
Kleinjung
T
. Neuromodulation for tinnitus treatment: an overview of invasive and non-invasive techniques. J Zhejiang Univ Sci B. 2019;20(2):116–30. doi:.https://doi.org/10.1631/jzus.B1700117
28
Salvi
RJ
,
Wang
J
,
Ding
D
. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147(1-2):261–74. doi:.https://doi.org/10.1016/S0378-5955(00)00136-2
29
Vanneste
S
,
De Ridder
D
. The auditory and non-auditory brain areas involved in tinnitus. An emergent property of multiple parallel overlapping subnetworks. Front Syst Neurosci. 2012;6:31. doi:.https://doi.org/10.3389/fnsys.2012.00031
30
Peter
N
,
Liyanage
N
,
Pfiffner
F
,
Huber
A
,
Kleinjung
T
. The Influence of Cochlear Implantation on Tinnitus in Patients with Single-Sided Deafness: A Systematic Review. Otolaryngol Head Neck Surg. 2019;161(4):576–88. doi:.https://doi.org/10.1177/0194599819846084
31
Van de Heyning
P
,
Vermeire
K
,
Diebl
M
,
Nopp
P
,
Anderson
I
,
De Ridder
D
. Incapacitating unilateral tinnitus in single-sided deafness treated by cochlear implantation. Ann Otol Rhinol Laryngol. 2008;117(9):645–52. doi:.https://doi.org/10.1177/000348940811700903
32
Dillon
MT
,
Buss
E
,
Anderson
ML
,
King
ER
,
Deres
EJ
,
Buchman
CA
, et al.
Cochlear Implantation in Cases of Unilateral Hearing Loss: Initial Localization Abilities. Ear Hear. 2017;38(5):611–9. doi:.https://doi.org/10.1097/AUD.0000000000000430
33
Galvin
JJ, 3rd
,
Fu
QJ
,
Wilkinson
EP
,
Mills
D
,
Hagan
SC
,
Lupo
JE
, et al.
Benefits of Cochlear Implantation for Single-Sided Deafness: Data From the House Clinic-University of Southern California-University of California, Los Angeles Clinical Trial. Ear Hear. 2019;40(4):766–81. doi:.https://doi.org/10.1097/AUD.0000000000000671
34
Härkönen
K
,
Kivekäs
I
,
Rautiainen
M
,
Kotti
V
,
Sivonen
V
,
Vasama
JP
. Single-Sided Deafness: The Effect of Cochlear Implantation on Quality of Life, Quality of Hearing, and Working Performance. ORL J Otorhinolaryngol Relat Spec. 2015;77(6):339–45. doi:.https://doi.org/10.1159/000439176
35
Kitoh
R
,
Moteki
H
,
Nishio
S
,
Shinden
S
,
Kanzaki
S
,
Iwasaki
S
, et al.
The effects of cochlear implantation in Japanese single-sided deafness patients: five case reports. Acta Otolaryngol. 2016;136(5):460–4. doi:.https://doi.org/10.3109/00016489.2015.1116046
36
Mertens
G
,
Desmet
J
,
De Bodt
M
,
Van de Heyning
P
. Prospective case-controlled sound localisation study after cochlear implantation in adults with single-sided deafness and ipsilateral tinnitus. Clin Otolaryngol. 2016;41(5):511–8. doi:.https://doi.org/10.1111/coa.12555
37
Punte
AK
,
Vermeire
K
,
Hofkens
A
,
De Bodt
M
,
De Ridder
D
,
Van de Heyning
P
. Cochlear implantation as a durable tinnitus treatment in single-sided deafness. Cochlear Implants Int. 2011;12(sup1, Suppl 1):S26–9. doi:.https://doi.org/10.1179/146701011X13001035752336
38
Ramos
Á
,
Polo
R
,
Masgoret
E
,
Artiles
O
,
Lisner
I
,
Zaballos
ML
, et al.
Implante coclear en pacientes con hipoacusia súbita unilateral y acúfeno asociado [Cochlear implant in patients with sudden unilateral sensorineural hearing loss and associated tinnitus]. Acta Otorrinolaringol Esp. 2012;63(1):15–20. doi:.https://doi.org/10.1016/j.otorri.2011.07.004
39
Távora-Vieira
D
,
Marino
R
,
Acharya
A
,
Rajan
GP
. The impact of cochlear implantation on speech understanding, subjective hearing performance, and tinnitus perception in patients with unilateral severe to profound hearing loss. Otol Neurotol. 2015;36(3):430–6. doi:.https://doi.org/10.1097/MAO.0000000000000707
40
Kompis
M
,
Pelizzone
M
,
Dillier
N
,
Allum
J
,
DeMin
N
,
Senn
P
. Tinnitus before and 6 months after cochlear implantation. Audiol Neurotol. 2012;17(3):161–8. doi:.https://doi.org/10.1159/000335126
41
Friedmann
DR
,
Ahmed
OH
,
McMenomey
SO
,
Shapiro
WH
,
Waltzman
SB
,
Roland
JT, Jr
. Single-sided Deafness Cochlear Implantation: Candidacy, Evaluation, and Outcomes in Children and Adults. Otol Neurotol. 2016;37(2):e154–60. doi:.https://doi.org/10.1097/MAO.0000000000000951
42
Grossmann
W
,
Brill
S
,
Moeltner
A
,
Mlynski
R
,
Hagen
R
,
Radeloff
A
. Cochlear Implantation Improves Spatial Release From Masking and Restores Localization Abilities in Single-sided Deaf Patients. Otol Neurotol. 2016;37(6):658–64. doi:.https://doi.org/10.1097/MAO.0000000000001043
43
Hoth
S
,
Rösli-Khabas
M
,
Herisanu
I
,
Plinkert
PK
,
Praetorius
M
. Cochlear implantation in recipients with single-sided deafness: Audiological performance. Cochlear Implants Int. 2016;17(4):190–9. doi:.https://doi.org/10.1080/14670100.2016.1176778
44
Mertens
G
,
De Bodt
M
,
Van de Heyning
P
. Cochlear implantation as a long-term treatment for ipsilateral incapacitating tinnitus in subjects with unilateral hearing loss up to 10 years. Hear Res. 2016;331:1–6. doi:.https://doi.org/10.1016/j.heares.2015.09.016
45
Mertens
G
,
De Bodt
M
,
Van de Heyning
P
. Evaluation of Long-Term Cochlear Implant Use in Subjects With Acquired Unilateral Profound Hearing Loss: Focus on Binaural Auditory Outcomes. Ear Hear. 2017;38(1):117–25. doi:.https://doi.org/10.1097/AUD.0000000000000359
46
Rahne
T
,
Plontke
SK
. Functional Result After Cochlear Implantation in Children and Adults With Single-sided Deafness. Otol Neurotol. 2016;37(9):e332–40. doi:.https://doi.org/10.1097/MAO.0000000000000971
47
Sladen
DP
,
Frisch
CD
,
Carlson
ML
,
Driscoll
CL
,
Torres
JH
,
Zeitler
DM
. Cochlear implantation for single-sided deafness: A multicenter study. Laryngoscope. 2017;127(1):223–8. doi:.https://doi.org/10.1002/lary.26102
48
Tokita
J
,
Dunn
C
,
Hansen
MR
. Cochlear implantation and single-sided deafness. Curr Opin Otolaryngol Head Neck Surg. 2014;22(5):353–8. doi:.https://doi.org/10.1097/MOO.0000000000000080
49
Vermeire
K
,
Van de Heyning
P
. Binaural hearing after cochlear implantation in subjects with unilateral sensorineural deafness and tinnitus. Audiol Neurotol. 2009;14(3):163–71. doi:.https://doi.org/10.1159/000171478
50
Zeitler
DM
,
Dorman
MF
,
Natale
SJ
,
Loiselle
L
,
Yost
WA
,
Gifford
RH
. Cochlea-Implantat-Versorgung von Patienten mit einseitiger Taubheit oder asymmetrischem Hörverlust [Sound Source Localization and Speech Understanding in Complex Listening Environments by Single-sided Deaf Listeners After Cochlear Implantation]. Otol Neurotol. 2015;36(9):1467–71. doi:.https://doi.org/10.1097/MAO.0000000000000841
51
Arndt
S
,
Laszig
R
,
Aschendorff
A
,
Hassepass
F
,
Beck
R
,
Wesarg
T
. Cochlea-Implantat-Versorgung von Patienten mit einseitiger Taubheit oder asymmetrischem Hörverlust [Cochlear implant treatment of patients with single-sided deafness or asymmetric hearing loss]. HNO. 2017;65(S2, Suppl 2):98–108. doi:.https://doi.org/10.1007/s00106-016-0297-5
52
Dillon
MT
,
Buss
E
,
Rooth
MA
,
King
ER
,
Deres
EJ
,
Buchman
CA
, et al.
Effect of Cochlear Implantation on Quality of Life in Adults with Unilateral Hearing Loss. Audiol Neurotol. 2017;22(4-5):259–71. doi:.https://doi.org/10.1159/000484079
53
Finke
M
,
Strauß-Schier
A
,
Kludt
E
,
Büchner
A
,
Illg
A
. Speech intelligibility and subjective benefit in single-sided deaf adults after cochlear implantation. Hear Res. 2017;348:112–9. doi:.https://doi.org/10.1016/j.heares.2017.03.002
54
Sladen
DP
,
Carlson
ML
,
Dowling
BP
,
Olund
AP
,
Teece
K
,
DeJong
MD
, et al.
Early outcomes after cochlear implantation for adults and children with unilateral hearing loss. Laryngoscope. 2017;127(7):1683–8. doi:.https://doi.org/10.1002/lary.26337
55
Wagener
K
,
Brand
T
,
Kollmeier
B
. Development and evaluation of a German sentence test. Part III: Evaluation of the Oldenburg sentence test. Zeitschr Audio. 1999;38:86–95.
56
Newman
CW
,
Jacobson
GP
,
Spitzer
JB
. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996;122(2):143–8. doi:.https://doi.org/10.1001/archotol.1996.01890140029007
57
Kleinjung
T
,
Fischer
B
,
Langguth
B
,
Sand
PG
,
Hajak
G
,
Dvorakova
J
, et al.
Validierung einer deutschsprachigen Version des Tinnitus Handicap Inventory [Validation of the German-version Tinnitus Handicap Inventory (THI)]. Psychiatr Prax. 2007;34:S140–2. doi:.https://doi.org/10.1055/s-2006-940218
58
Peter
N
,
Kleinjung
T
,
Horat
L
,
Schmidt-Weitmann
S
,
Meyer
M
,
Büchi
S
, et al.
Validation of PRISM (Pictorial Representation of Illness and Self Measure) as a novel visual assessment tool for the burden of suffering in tinnitus patients. Health Qual Life Outcomes. 2016;14(1):47. doi:.https://doi.org/10.1186/s12955-016-0454-2
59
Zeman
F
,
Koller
M
,
Langguth
B
,
Landgrebe
M
; Tinnitus Research Initiative database study group. Which tinnitus-related aspects are relevant for quality of life and depression: results from a large international multicentre sample. Health Qual Life Outcomes. 2014;12(1):7. doi:.https://doi.org/10.1186/1477-7525-12-7
60
The WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998;28(3):551–8. doi:.https://doi.org/10.1017/S0033291798006667
61
Gatehouse
S
,
Noble
W
. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int J Audiol. 2004;43(2):85–99. doi:.https://doi.org/10.1080/14992020400050014
62
Noble
W
,
Gatehouse
S
. Interaural asymmetry of hearing loss, Speech, Spatial and Qualities of Hearing Scale (SSQ) disabilities, and handicap. Int J Audiol. 2004;43(2):100–14. doi:.https://doi.org/10.1080/14992020400050015
63
Kiessling
J
,
Grugel
L
,
Meister
H
,
Meis
M
. German translations of questionnaires SADL, ECHO and SSQ and their evaluation. Zeitschrift für Audiologie.
2011;50(1):6–16.
64
Buss
E
,
Dillon
MT
,
Rooth
MA
,
King
ER
,
Deres
EJ
,
Buchman
CA
, et al.
Effects of Cochlear Implantation on Binaural Hearing in Adults With Unilateral Hearing Loss. Trends Hear. 2018;22:2331216518771173. doi:.https://doi.org/10.1177/2331216518771173
65
Mertens
G
,
Kleine Punte
A
,
De Bodt
M
,
Van de Heyning
P
. Binaural auditory outcomes in patients with postlingual profound unilateral hearing loss: 3 years after cochlear implantation. Audiol Neurotol. 2015;20(Suppl 1):67–72. doi:.https://doi.org/10.1159/000380751
66Stieger C. Die einzigartige Schweizer Datenbank. Sonos. 2016;6??
67
Cohen
SM
,
Svirsky
MA
. Duration of unilateral auditory deprivation is associated with reduced speech perception after cochlear implantation: A single-sided deafness study. Cochlear Implants Int. 2019;20(2):51–6. doi:.https://doi.org/10.1080/14670100.2018.1550469