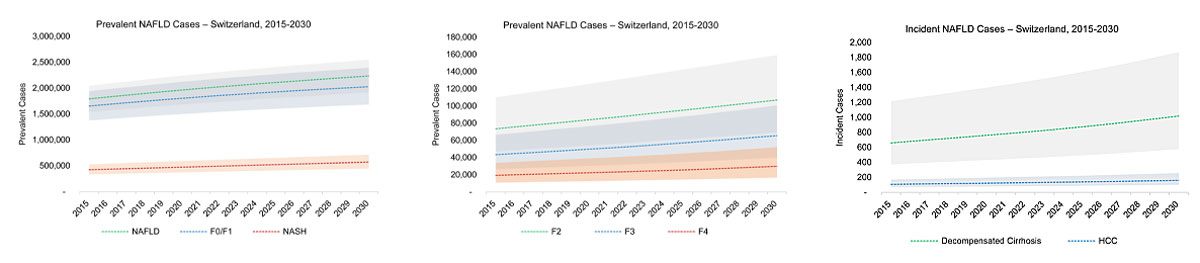
Figure 1 Model estimated cases by disease stage with 95% uncertainty interval – Switzerland 2015–2030. NAFLD = nonalcoholic fatty liver disease.
DOI: https://doi.org/10.4414/smw.2019.20152
Nonalcoholic fatty liver is increasingly recognised as a cause of liver disease in Europe, where prevalence of was estimated at 23.71% (95% confidence interval [CI] 16.12–33.45%) during 2000–2015 [1]. Globally, nonalcoholic fatty liver disease (NAFLD) now accounts for greater numbers of advanced liver disease cases, including hepatocellular carcinoma (HCC) and liver transplantation [2–5], with a substantial economic impact [6]. As with other chronic lifestyle conditions, it is increasing in Switzerland with the epidemics of obesity [7, 8] and type 2 diabetes mellitus (DM) [9]. NAFLD is reported to account for increasing numbers of cases of advanced liver disease in Europe [10], including liver cancer [11]. Morbidity related to NAFLD and nonalcoholic steatohepatitis (NASH) is likely to increase in the coming decades, especially in countries with aging populations [12, 13] that experience higher rates of chronic lifestyle-related conditions. In this analysis, a Markov model was built using data from Switzerland for obesity and HCC to predict and validate changes in the burden of NAFLD-related liver disease up to 2030.
NAFLD is characterised by excessive liver fat in the absence of other causes [14, 15], with overweight/obesity, DM and metabolic syndrome identified as important risk factors [14, 16]. In the current analysis, NAFLD cases were tracked by fibrosis stage and further classified as NAFL (NAFLD cases with steatosis only) or NASH, where inflammation can progress to liver fibrosis, and advanced liver disease and related mortality [17, 18]. Increased age, in combination with obesity, DM and metabolic syndrome are reported to be predictive for progression of disease and cirrhosis [16, 19]. Advanced liver disease usually occurs after a period of significant fibrosis and cirrhosis, whereas HCC often occurs in patients with NASH-related cirrhosis; however, HCC can occur in non-cirrhotic NASH patients [20, 21].
There is currently a pressing need to understand the future burden of NAFLD-related liver disease. Use of a disease burden modelling framework allows more efficient distribution of limited resources, while assisting in the development of strategies to mitigate further growth in morbidity and mortality.
Recent reports analysed the disease and cost burden related to NASH [1, 22, 23] based on existing literature. In addition, a recent dynamic model of NAFLD progression overcomes several limitations in data availability [24, 25]. In this analysis, we report the development of such a model for Switzerland, based on the trend for adult obesity in the country [7, 8].
To estimate changes over time in NAFLD disease burden, a Markov model was constructed. The model calculated the NAFLD population by fibrosis stage and NASH status (NAFL or NASH). Progression of disease through fibrosis and liver disease stages (fig. 1) was calculated with adjustment for all-cause mortality, including liver-related mortality (supplementary table S1 in appendix 1), background mortality (table S2) and excess cardiovascular disease (CVD) mortality (table S3), with fibrosis progression rates (table S1) back-calculated using surveillance data [24]. The total population by age group and gender was tracked over time, with new NAFLD cases entering the model based on adult obesity prevalence trends in Switzerland. Fibrosis transition rates were varied by gender (males experiencing faster progression), with rates that also increased with advanced age (table S1). Results from the analysis were compared with estimates for the incidence of liver cancer, after accounting for liver cancer under-reporting, cancer morphology (HCC), and the estimated aetiology of incident HCC cases (NAFLD, viral hepatitis, etc.)

Figure 1 Model estimated cases by disease stage with 95% uncertainty interval – Switzerland 2015–2030. NAFLD = nonalcoholic fatty liver disease.
In addition, model results were compared with studies in European populations that estimated the prevalence of advanced fibrosis and incidence of liver cancer attributable to NAFLD [11, 13, 26, 27]. Progression rates to end-stage disease and liver-related death were based on reported estimates [24] (table S1). Indexed articles and non-indexed sources including national data reports were utilised to identify reported estimates of NAFLD or NASH prevalence, including reports of advanced liver disease attributable to NAFLD or NASH. Estimates for adult prevalence of obesity (body mass index [BMI] ≥30 kg/m2) and DM at the national level were included in the analysis, where increases in NAFLD prevalence were assumed to follow the change in adult obesity over time [28]. In addition to a literature review, a Delphi process was used to incorporate expert input based on interviews to identify key model inputs and review outputs against available estimates of disease burden, as shown in figure S1 in appendix 1.
The total population of Switzerland was organised by year (1950–2030), gender, and 5-year age groups based on historical estimates and projections from the Swiss Federal Statistical Office ‘Reference’ scenario A-00-2015 [29, 30]. Population data were reported for the specific age groups and years included in the model, so no further adjustments were applied. Annual background deaths (supplementary table S2 in appendix 1) were calculated by year (1950–2030), gender and 5-year age groups based on rates reported in two files (MORT/17-2 and MORT/17-3) from the United Nations World Population Prospects 2017 database [12]. Background rates were adjusted to account for incrementally increased mortality related to CVD [31–33]. A standard mortality ratio 1.15 (uncertainty range 1.00–1.31) was applied to all background mortality rates in all years of the model [31–33]. Incremental background mortality rates are reported in table S3. Incremental CVD mortality may increase with severity of liver disease and vary by age group; however, data were largely unavailable and a constant multiplier was applied. Progression to decompensated cirrhosis [34] and rates of liver-related deaths among prevalent HCC, decompensated cirrhosis and liver transplant cases [3, 24, 35, 36] were based on published estimates (table S1).
The annual number of new cases was back-calculated for each year based on the adult prevalence of obesity, for which more robust data exist than those for NAFLD. Trends for adult prevalence of obesity (BMI ≥30 kg/m2) were used to estimate the annual number of new NAFLD cases over time, with the fastest growth in prevalence occurring simultaneously with the fastest increase in NAFLD prevalence. All NAFLD cases were then tracked by fibrosis stage, with progression to advanced liver disease and liver-related death (supplementary fig. S2 in appendix 1). Relative changes in adult obesity were estimated using data reported from the Swiss National Health Survey [37, 38] and the NCD Risk Factor Collaboration (NCD-RisC) estimates for Switzerland [7, 8, 39]. Variation between the two sources for reported magnitude of obesity increase after 2015 were included as part of uncertainty analysis.
Among individuals ≥15 years of age in 2015, there was an assumed NAFLD prevalence rate of 25% (uncertainty range 20–30%). After adjustment for lower prevalence among persons aged <15 years, prevalence among all ages was estimated to be 21.6% in 2015. The age and gender distribution of prevalent NAFLD cases was based on data from general population studies where prevalence increases with age and overall prevalence is higher among males [40–42]. Prevalence among younger age groups not usually included in general population studies was assumed to decline with decreasing age. Because of elevated mortality, it was assumed that prevalence would naturally decline among the oldest age groups (≥80 years), with peak prevalence occurring in late middle age.
NASH prevalence was calculated based on fibrosis progression among the total NAFLD population. NASH diagnosis is based on results of liver biopsy, which have serious limitations in terms of reliably sampling and measuring the features of NASH [43]. Furthermore, NASH-related fibrosis can regress in NAFLD patients [17]; the model included an assumption that ≤5% of NAFLD cases without NASH could be experiencing NASH regression, including a portion with fibrotic changes. Because the features of NASH have reduced, but fibrosis still remains, these cases were classified as regressed NASH. Increasing fibrosis stage was assumed to result in lower probability of experiencing regressed NASH. Overall, a relatively small number of ≥F2 cases were classified as non-NASH NAFLD.
Fibrosis progression and NASH status were initially calibrated to surveillance data for NASH-related HCC [24], with adjustments between countries based on relative rates of overweight (BMI >25 and ≤30 kg/m2) and obesity [8] and published odds of disease progression to advanced fibrosis [16]. A higher proportion of advanced fibrosis cases results from increasing age of the overall population, and timing of the obesity epidemic where large increases occurred in earlier decades.
Total annual liver transplants are reported by Swisstransplant [44]. Using total annual transplant data, with expert input for total transplants [45], we assumed that up to 20% of current liver transplants could be attributable to NASH. These estimates were used to validate the model outputs and informed by studies showing that a portion of transplants indicated for cryptogenic or idiopathic cirrhosis are potentially related to NAFLD, based on high obesity rates among the recipients [46, 47]. Given the uncertainties around transplant demand and availability, it was assumed that the annual number of NAFLD-related transplants would remain constant until 2030. However, this was a conservative estimate, as data already suggest that the proportion of NAFLD-related transplants is increasing [47].
Uncertainty analyses were conducted for the models. Beta-PERT distributions [48] were defined for key model inputs, including total NAFLD prevalence, excess background mortality multipliers and fibrosis transition probabilities. Monte Carlo simulation was conducted using Crystal Ball® (11.1.3708.0 by Oracle®), an Excel® add-in, to estimate 95% uncertainty intervals. Sensitivity analyses were conducted to identify the inputs that accounted for the greatest variation in modelled outcomes.
Surveillance data for liver cancer were used to validate the results of the model. The National Institute for Cancer Epidemiology and Registration reports incident liver cancers by 5-year period in Switzerland with 4035 total incident liver cancers reported during 2010–2014 [49]. Assumptions were applied to these data for cancer under-reporting, cancer morphology and the proportion of cancers that could be NAFLD-related. These assumptions were applied to incident liver cancer data to develop a range for the potential number of annual liver cancers attributable to NAFLD. For under-reporting, it was conservatively assumed that 10% of cancers may not be reported to the registry with a range from 0 to 100% [50]. For cancer morphology, an estimated 72% [51] of liver cancers were assumed to be HCC, with a range of 65% [52] to 90%, based on the Geneva Tumour Registry. The estimated proportion of NAFLD-related HCC was 17% based on risk factors for HCC surveillance at University Hospital of Bern during 2010–2011 [53]. The conceivable range for NAFLD-related HCC was estimated at 4% (low) [54] to 29% (high) [53]. This wide range was utilised owing to uncertainty and changes over time in the aetiology of liver cancer, with viral hepatitis expected to contribute relatively fewer cases in the future. The outcomes of this analysis for 1990–2014 were compared to model predicted incident HCC (see fig. 3 below) to ensure that the model was predictive based on surveillance data for advanced disease.
Total prevalent NAFLD cases in 2018 were estimated to be 1,898,000 (1,626,000–2,167,000) (22.2% [19.0–25.4%] prevalence among all ages). By 2030, the total NAFLD population was projected to increase 18% to 2,234,000 (1,914,000–2,550,000) cases (fig. 1), with overall prevalence rates estimated at 24.3% (20.827.7%). Likewise, F0/F1 cases would increase 16% from 1,748,000 (1,451,600–2,053,000) to 2,028,000 (1,683,000–2,393,000) during 2018–2030. Even greater relative increases were projected for later disease stages; F2 cases would increase 35% during 2018–2030, from 79,700 (51,200–119,000) to 107,000 (69,000–159,000). F3 cases would increase from 47,100 (28,600–72,400) to 65,500 (39,700–101,000), a change of 40%. F4 (compensated cirrhosis) cases would increase 40% from 21,100 (11,800–36,900) to 29,800 (16,700–52,100).
Prevalent NAFLD cases by age group and gender were compared with the distribution of the non-NAFLD Swiss population (fig. 2). Among modelled NAFLD cases in 2018, there were 15% more cases among males (1,015,000 cases as compared with 884,000 cases among females). The age group with the largest number of NAFLD cases was estimated to be the 50- to 54-year-old group with 224,400 cases in 2018 (fig. 2). By 2030, the largest prevalent NAFLD age group will be aged 60–64 years, with 228,000 NAFLD cases. Overall NAFLD cases will number 1,204,000 among males, 17% greater than total female cases (1,030,000 cases) (fig. 2).
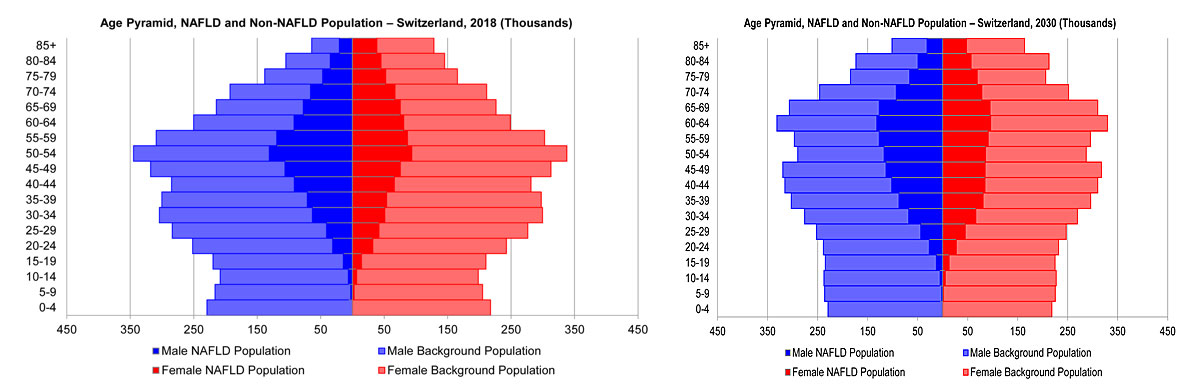
Figure 2 Model age distribution of nonalcoholic fatty liver disease (NAFLD) and background population by sex and age group (thousands) – Switzerland 2018 and 2030.
The NAFL population was assumed to be cases with steatosis only that never progressed to NASH, in addition to a relatively small number of cases that were formerly NASH and experienced disease regression. In 2018, the NAFL population was estimated to be 1,444,000 (1,272,000–1,601,000) in Switzerland (76.1% of all NAFLD cases) and will increase to 1,664,000 (1,469,000–1,835,000) cases in 2030 (74.5% of all NAFLD cases), a 15% increase. NAFL classified as ≥F2 fibrosis were estimated at 710 cases in 2018, with a projected 960 cases in 2030.
The number of NASH cases in 2018 was estimated to be 454,000 (356,000–566,000) in Switzerland, equivalent to 23.9% of all NAFLD cases. General population NASH prevalence in 2018 was estimated to be 5.3% (4.2–6.6%). In Switzerland, NASH cases were projected to increase 26% to 570,000 (446,000–714,000) cases in 2030 (see fig. 1), when they will comprise 25.5% of all NAFLD cases. Overall prevalence of NASH in 2030 was estimated to be 6.2% (4.8–7.8%) (table 1).
Table 1 Model estimates of NAFLD disease burden – Switzerland 2015–2030.
| 2015 | 2020 | 2025 | 2030 | |||
|---|---|---|---|---|---|---|
| Prevalent cases | NAFLD cases | 1,792,000 (1,535,000–2,045,000) |
1,964,000 (1,683,000–2,241,000) |
2,108,000 (1,806,000–2,406,000) |
2,234,000 (1,914,000–2,550,000) |
|
| NAFLD prevalence rate (all ages) | 21.5% (18.4%–24.6%) |
22.7% (19.4%–25.8%) |
23.5% (20.2%–26.9%) |
24.3% (20.8%–27.7%) |
||
| F0 | 1,519,000 (1,286,000–1,749,000) |
1,653,000 (1,400,000–1,906,000) |
1,759,000 (1,490,000–2,031,000) |
1,845,000 (1,564,000–2,132,000) |
||
| F1 | 135,000 (87,800–191,000) |
152,000 (98,900–216,000) |
168,000 (109,000–239,000) |
183,000 (119,000–261,000) |
||
| F2 | 73,400 (47,200–110,000) |
84,000 (54,000–126,000) |
95,100 (61,300–142,000) |
107,000 (69,000–159,000) |
||
| F3 | 43,300 (26,300–66,600) |
49,700 (30,200–76,500) |
57,000 (34,600–87,700) |
65,500 (39,700–101,000) |
||
| Compensated cirrhosis | 19,300 (10,800–33,700) |
22,300 (12,500–38,900) |
25,600 (14,300–44,700) |
29,800 (16,700–52,100) |
||
| NASH cases | 423,000 (332,000–528,000) |
473,000 (371,000–592,000) |
522,000 (408,000–653,000) |
570,000 (446,000–714,000) |
||
| NASH prevalence rate (all ages) | 5.1% (4.0%–6.3%) |
5.5% (4.3%–6.8%) |
5.8% (4.6%–7.3%) |
6.2% (4.8%–7.8%) |
||
| Incident cases | Decompensated cirrhosis | 660 (370–1,200) |
760 (430–1,400) |
870 (490–1,600) |
1000 (570–1,900) |
|
| HCC | 110 (70–170) |
120 (80–200) |
140 (90–220) |
160 (100–260) |
||
| Liver death | 530 (300–980) |
610 (350–1,100) |
700 (400–1,300) |
820 (470–1,500) |
||
HCC = hepatocellular carcinoma; NAFLD = nonalcoholic fatty liver disease; NASH = nonalcoholic steatohepatitis
Among NASH cases in 2018, 46,900 (28,500–72,200) and 21,000 (11,800–36,800) were classified as F3 and F4 (compensated cirrhosis), respectively. F3 cases encompassed approximately 10.4% of all NASH cases and 0.5% (0.3–0.8%) of the total Swiss population (all ages), whereas F4 cases accounted for 4.6% of all NASH cases and 0.2% (0.1–0.4%) of the total population. By 2030, the numbers of F3 and F4 cases were both expected to increase 40% to 65,300 (39,600–100,000) cases and 29,700 (16,600–52,000) cases, respectively. In 2030, F3 cases would account for 11.4% of all NASH cases whereas F4 cases would comprise 5.2% of the total NASH population. Prevalence of NASH-related F3 was estimated to be 0.7% (0.4–1.1%) in the total population in 2030, and F4 cases were estimated at 0.3% (0.2–0.6%) of the total population.
In Switzerland, incident decompensated cirrhosis was projected to increase by 41%, from 720 (410–1300) cases in 2018 to 1000 (580–1800) cases in 2030; cumulative incidence during 2018–2030 was estimated to be 11,140 cases (fig. 1). Incident HCC cases would increase by 39% during 2018–2030 from 120 (70–180) to 160 (100–250) cases (fig. 1). Cumulative incidence of HCC during 2018–2030 was estimated to be 1760 cases in Switzerland.
In the total NASH population in Switzerland in 2018, there were 9500 background deaths (including 1200 deaths classified as excess cardiovascular) and 580 (340–1000) liver-related deaths. By 2030, annual liver-related deaths were estimated to be 820 (480–1500) deaths in Switzerland (40% increase from 2018) (fig. 1).
By 2030, it was projected that liver-related mortality will comprise 2.8% of all deaths in the total NAFLD population in Switzerland, as compared with 2.3% of deaths in 2018. Liver deaths comprised 5.7% of liver-related deaths in the entire NASH population in 2018, and will increase to 6.7% of deaths in 2030. Cumulative liver deaths were estimated to be 9000 deaths during 2018–2030.
Model estimates for HCC incidence during 1990–2014 were within the range expected based upon reported estimates of liver cancer incidence, HCC morphology and the proportion of HCCs that are potentially NAFLD-related. Model NAFLD-related HCC incidence in 1990 comprised 8.0% of all incident HCC cases in Switzerland, increasing to 16.2% of cases by 2014 (fig. 3).
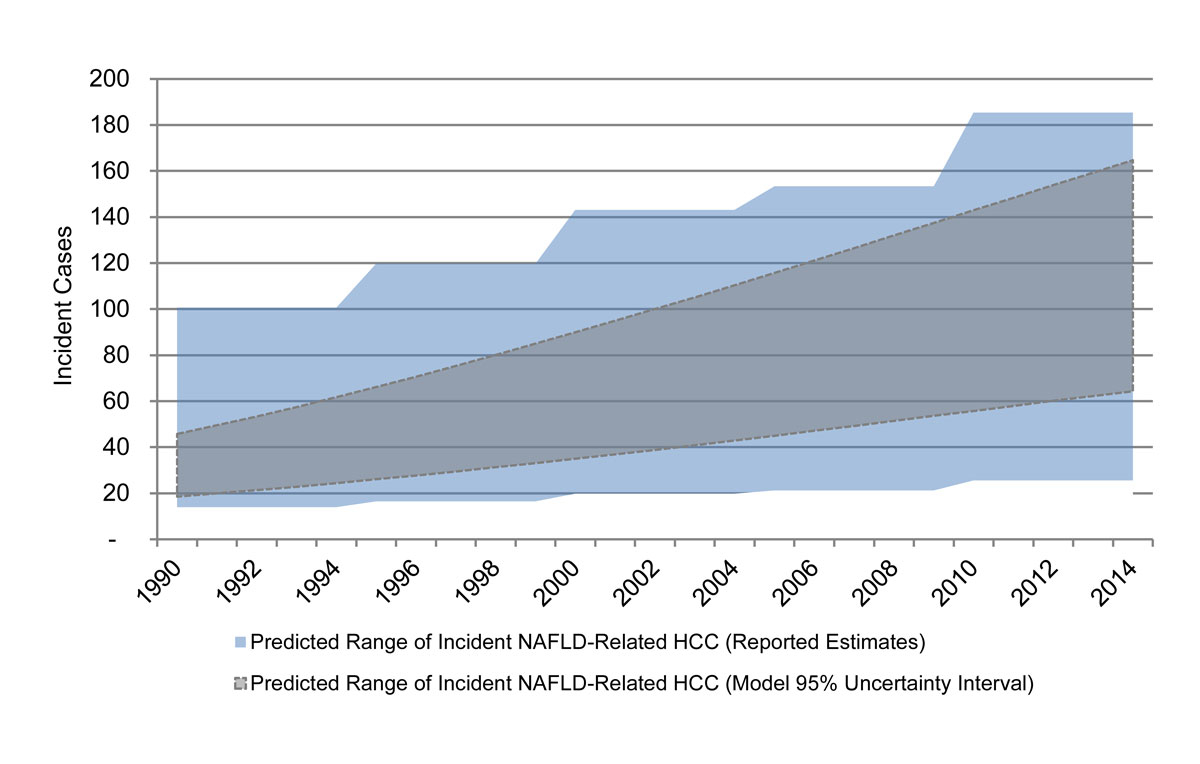
Figure 3 Predicted range of incident nonalcoholic fatty liver (NAFLD)-related hepatocellular carcinoma (HCC), reported estimates and model 95% uncertainty interval – Switzerland 1990–2014.
There were 2192 total incident liver cancer cases during 1990–1994, increasing to 4035 by 2010–2014. After an estimated rate of incident liver cancer underreporting of 10% was applied, annual incident liver cancers were estimated to be 482 cases annually during 1990–1994, increasing to 888 cancers annually during 2010–2014. There are a range of estimates for cancer morphology, but for the base estimate, 72% of liver cancers were assumed to be HCC, meaning that annual HCC cases increased from 347 during 1990–1994 to 639 annual cases by 2010–2014. Finally, a wide range was considered for the proportion of HCC cases that could be NAFLD related. These cases were estimated at 59 (14–101) cases during 1990–1994, increasing to 109 (26–185) cases during 2010–2014.
Levels of liver disease in Switzerland are estimated to be similar to those in other countries in Western Europe based on rates of cirrhosis and liver-related mortality reported by the European Association for the Study of the Liver HEPAHEALTH project [2]. The population of Switzerland is aging [12], resulting in potentially larger increases in disease burden in the coming decades. Model results demonstrated that the burden of advanced disease among the NAFLD population will increase in tandem with increases in average age and the ongoing increases in the obesity epidemic. Paediatric and adolescent populations experience high rates of obesity in Switzerland [37] and are susceptible to developing NAFLD [55]. There may be an impact of NAFLD in young populations with progression to advanced disease at earlier ages [56]. In the coming decade, NASH will likely be a leading indication for liver transplantation in Western Europe, as a result of a reduced burden of viral hepatitis in combination with growing obesity rates [10].
The current analysis utilised both literature review and expert interviews to design the model and validate model outputs. For longitudinal trends in adult obesity levels, estimates from the Swiss National Health Survey and NCD-RisC study were used [7, 8, 38, 39]. In Switzerland, it was assumed that an eventual plateauing of obesity prevalence would result in a similar slowing in the growth of the NAFLD epidemic. It was assumed that long-term trends in NAFLD prevalence follow in tandem with trends in adult obesity prevalence. However, more studies of NAFLD prevalence in general populations should be conducted in Switzerland. With the availability of more published data, it will become possible to develop reliable trends for NAFLD incidence and prevalence over longer time periods. Likewise, a starting NAFLD prevalence of 25% among persons aged ≥15 years in 2015 represents a most-likely case scenario for Switzerland, but high and low prevalence estimates of 20–30% were used to capture a potential range of prevalence, and to calculate 95% uncertainty intervals for model outputs.
Studies of general European populations in multiple regions have reported rates of advanced fibrosis based on liver stiffness measurement (LSM) with transient elastography that was not attributable to viral hepatitis or excess alcohol consumption [13, 26, 27]. Over 5% of adults were estimated to have LSM ≥8.0 kPa [27], and 0.6% of adults aged ≥45 years were reported to have LSM ≥13.0 kPa [13]. LSM cut-off values for determining fibrosis stage vary between studies [57]. Results of the current analysis are broadly consistent with estimates in general European adult populations. As compared with results of NAFLD modelling in the United States [25], Switzerland has a lower overall disease burden. The assumed base prevalence was lower in the Switzerland model as compared with the United States, and fibrosis progression was slower based on reported odds for advanced fibrosis by BMI class [16]. These revisions to model inputs resulted in lower disease burden. Such outcomes are consistent with reported data showing higher levels of obesity and diabetes in the United States [7, 58]; however, there is a need for further study of NAFLD-related liver disease burden in Switzerland.
Because of the long course of the disease, the burden of NAFLD-related morbidity and mortality will increase for decades, even if increases in obesity are halted; increased age is a risk factor for advanced liver disease [16] and progression to advanced stages of NASH [14, 17]. The portion of NASH among total NAFLD cases will increase, along with the portion of NASH cases with advanced fibrosis. Model results demonstrate a rapid increase in the number of individuals with cirrhosis, which drives increases in decompensation and liver-related death, and the proportion of HCC attributed to NAFLD is already reported to be growing rapidly [3, 59]. These outcomes will increase the demand for NAFLD-related liver transplantation. The future availability of donor organs and changes in other transplant indicators, such as viral hepatitis, are unknown. However, any increase in transplant demand incurs a high level of disability [60] and economic costs [61]. In addition, liver transplants may be limited as a treatment option among patients with multiple comorbidities. Model results emphasise the necessity of identifying NAFLD cases, especially persons with clinically significant fibrosis, who may be candidates for therapy, before progression to advanced liver disease.
A limitation of modelling is a scarcity of data from general population studies measuring hepatic steatosis and fibrosis with consistent methods [57]. Estimates rely on historical data, and may not reflect the current disease burden owing to ongoing increases in obesity and DM. Furthermore, assumptions of the future growth in obesity are based on current trends, which contributes to the uncertainty around future disease burden. The analysis also does not consider the potential impact of interventions to decrease the growth in obesity and other risk factors for NAFLD, or of interventions focused on reducing the progression of disease among populations at risk. The model adjusted for the increasing prevalence of obesity, but there has been a dramatic increase in childhood obesity in recent decades [62], possibly leading to a longer course of disease, on average, with potentially a greater risk to develop end-stage liver disease [16].
A lack of consistent diagnostic measures means that reported NAFLD prevalence rates vary between studies [1], and NASH can be histologically detected in some NAFLD cases with normal liver enzyme levels [63]. Staging of fibrosis alone can be a predictor of long-term outcomes [64, 65]; however, there is considerable uncertainty around reported fibrosis progression rates among studied NAFLD and NASH populations [66]. This analysis stratified fibrosis progression by gender and two age groups, ages <40 years and ≥40 years (see table S1). Although male gender and increased age are reported to result in faster progression, these differences have not been fully quantified across the lifespan.
The results demonstrated a growing disease burden associated with NAFLD and NASH, following the trajectory of increasing obesity in Switzerland [37]. Modelling has the potential to inform healthcare systems. The World Health Organization has called for increases in diabetes and obesity to be stopped [67], with sustainable development goal 3.4 outlining a goal to reduce premature mortality from noncommunicable diseases by one third before 2030. There are increasing needs for strategies that address factors contributing to obesity, and identify and provide therapeutic options for those with NAFLD [67] to avert further increases in advanced liver disease and related mortality.
The appendix is available as a separate file at: https://smw.ch/article/doi/smw.2019.20152.
Funding for this project was provided by Gilead Sciences. The funders had no role in the study design, data collection, analysis, interpretation of data, or preparation of the manuscript. The experts consulted in the Delphi process did not receive financial compensation.
JFD: Bayer AG, BMS, Eli Lilly, Intercept, Jansen-Cilag, Merck, Novartis, Roche. BM has received speaking and/or consulting fees from Intercept and Gilead, and research support from Gilead. CE reports grants from Gilead Sciences, during the conduct of the study; grants from Pfizer, Gilead Sciences, AbbVie outside the submitted work.
1 Younossi ZM , Koenig AB , Abdelatif D , Fazel Y , Henry L , Wymer M . Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi:.https://doi.org/10.1002/hep.28431
2 Pimpin L , Cortez-Pinto H , Negro F , Corbould E , Lazarus JV , Webber L , et al.; EASL HEPAHEALTH Steering Committee. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69(3):718–35. doi:.https://doi.org/10.1016/j.jhep.2018.05.011
3 Younossi ZM , Otgonsuren M , Henry L , Venkatesan C , Mishra A , Erario M , et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62(6):1723–30. doi:.https://doi.org/10.1002/hep.28123
4 Sanyal A , Poklepovic A , Moyneur E , Barghout V . Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26(9):2183–91. doi:.https://doi.org/10.1185/03007995.2010.506375
5 Loomba R , Sanyal AJ . The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686–90. doi:.https://doi.org/10.1038/nrgastro.2013.171
6 Huber CA , Schwenkglenks M , Rapold R , Reich O . Epidemiology and costs of diabetes mellitus in Switzerland: an analysis of health care claims data, 2006 and 2011. BMC Endocr Disord. 2014;14(1):44. doi:.https://doi.org/10.1186/1472-6823-14-44
7 NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387(10026):1377–96. doi:.https://doi.org/10.1016/S0140-6736(16)30054-X
8 Abarca-Gómez L , Abdeen ZA , Hamid ZA , Abu-Rmeileh NM , Acosta-Cazares B , Acuin C , et al.; NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–42. doi:.https://doi.org/10.1016/S0140-6736(17)32129-3
9 Federal Statistical Office. Diabetes 2014 [Available from: https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/gesundheitszustand/krankheiten/diabetes.html.
10 Williams R , Alexander G , Armstrong I , Baker A , Bhala N , Camps-Walsh G , et al. Disease burden and costs from excess alcohol consumption, obesity, and viral hepatitis: fourth report of the Lancet Standing Commission on Liver Disease in the UK. Lancet. 2018;391(10125):1097–107. doi:.https://doi.org/10.1016/S0140-6736(17)32866-0
11 Dyson J , Jaques B , Chattopadyhay D , Lochan R , Graham J , Das D , et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60(1):110–7. doi:.https://doi.org/10.1016/j.jhep.2013.08.011
12United Nations. Department of Economic Social Affairs Population Division. World population prospects: The 2017 revision. New York: United Nations; 2018.
13 Koehler EM , Plompen EP , Schouten JN , Hansen BE , Darwish Murad S , Taimr P , et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: The Rotterdam study. Hepatology. 2016;63(1):138–47. doi:.https://doi.org/10.1002/hep.27981
14 Chalasani N , Younossi Z , Lavine JE , Diehl AM , Brunt EM , Cusi K , et al.; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterologyh. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592–609. doi:.https://doi.org/10.1053/j.gastro.2012.04.001
15 European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59(6):1121–40. doi:.https://doi.org/10.1007/s00125-016-3902-y
16 Angulo P , Hui JM , Marchesini G , Bugianesi E , George J , Farrell GC , et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846–54. doi:.https://doi.org/10.1002/hep.21496
17 Singh S , Allen AM , Wang Z , Prokop LJ , Murad MH , Loomba R . Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13(4):643–54.e9, quiz e39–40. doi:.https://doi.org/10.1016/j.cgh.2014.04.014
18 Dulai PS , Singh S , Patel J , Soni M , Prokop LJ , Younossi Z , et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65(5):1557–65. doi:.https://doi.org/10.1002/hep.29085
19 McPherson S , Hardy T , Henderson E , Burt AD , Day CP , Anstee QM . Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148–55. doi:.https://doi.org/10.1016/j.jhep.2014.11.034
20 White DL , Kanwal F , El-Serag HB . Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10(12):1342–1359.e2. doi:.https://doi.org/10.1016/j.cgh.2012.10.001
21 Piscaglia F , Svegliati-Baroni G , Barchetti A , Pecorelli A , Marinelli S , Tiribelli C , et al.; HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63(3):827–38. doi:.https://doi.org/10.1002/hep.28368
22 Younossi ZM , Henry L . Economic and Quality-of-Life Implications of Non-Alcoholic Fatty Liver Disease. Pharmacoeconomics. 2015;33(12):1245–53. doi:.https://doi.org/10.1007/s40273-015-0316-5
23 Younossi ZM , Zheng L , Stepanova M , Henry L , Venkatesan C , Mishra A . Trends in outpatient resource utilizations and outcomes for Medicare beneficiaries with nonalcoholic fatty liver disease. J Clin Gastroenterol. 2015;49(3):222–7. doi:.https://doi.org/10.1097/MCG.0000000000000071
24 Estes C , Razavi H , Loomba R , Younossi Z , Sanyal AJ . Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–33.
25 Estes C , Anstee QM , Arias-Loste MT , Bantel H , Bellentani S , Caballeria J , et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69(4):896–904. doi:.https://doi.org/10.1016/j.jhep.2018.05.036
26 Petta S , Di Marco V , Pipitone RM , Grimaudo S , Buscemi C , Craxì A , et al. Prevalence and severity of nonalcoholic fatty liver disease by transient elastography: Genetic and metabolic risk factors in a general population. Liver Int. 2018;38(11):2060–8. doi:.https://doi.org/10.1111/liv.13743
27 Caballería L , Pera G , Arteaga I , Rodríguez L , Alumà A , Morillas RM , et al. High Prevalence of Liver Fibrosis Among European Adults With Unknown Liver Disease: A Population-Based Study. Clin Gastroenterol Hepatol. 2018;16(7):1138–1145.e5. doi:.https://doi.org/10.1016/j.cgh.2017.12.048
28 Lonardo A , Ballestri S , Marchesini G , Angulo P , Loria P . Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. 2015;47(3):181–90. doi:.https://doi.org/10.1016/j.dld.2014.09.020
29Federal Statistical Office. Permanent and non permanent resident population by canton, sex, residence permit, place of birth and age. (Population and Households Statistics STATPOP) STAT-TAB - interactive tables (FSO) 2016 [Available from: https://www.pxweb.bfs.admin.ch/pxweb/en/px-x-0103010000_102/px-x-0103010000_102/px-x-0103010000_102.px.
30Federal Statistical Office. Population change scenarios for Switzerland 2017 [Available from: https://www.media-stat.admin.ch/animated/chart/01pyramid/ga-q-01.03.02-dashboard.html.
31 Stepanova M , Rafiq N , Makhlouf H , Agrawal R , Kaur I , Younoszai Z , et al. Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD). Dig Dis Sci. 2013;58(10):3017–23. doi:.https://doi.org/10.1007/s10620-013-2743-5
32 Byrne CD , Targher G . NAFLD: a multisystem disease. J Hepatol. 2015;62(1, Suppl):S47–64. doi:.https://doi.org/10.1016/j.jhep.2014.12.012
33 Targher G , Byrne CD , Lonardo A , Zoppini G , Barbui C . Nonalcoholic Fatty Liver Disease and Risk of Incident Cardiovascular Disease: A Meta-Analysis. J Hepatol. 2016;65(3):589–600. doi:. https://doi.org/10.1016/j.jhep.2016.05.013
34 Sanyal AJ , Banas C , Sargeant C , Luketic VA , Sterling RK , Stravitz RT , et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43(4):682–9. doi:.https://doi.org/10.1002/hep.21103
35 Bernfort L , Sennfält K , Reichard O . Cost-effectiveness of peginterferon alfa-2b in combination with ribavirin as initial treatment for chronic hepatitis C in Sweden. Scand J Infect Dis. 2006;38(6-7):497–505. doi:.https://doi.org/10.1080/00365540500532803
36Ries L, Young G, Keel G, Eisner M, Lin Y, Horner M. SEER survival monograph: Cancer survival among adults: U.S. SEER program, 1988-2001, patient and tumor characteristics. Bethesda, MD: National Cancer Institute, SEER Program; 2007.
37Federal Statistical Office. Swiss Health Survey 2012 Overview 2013 [Available from: https://www.bfs.admin.ch/bfsstatic/dam/assets/349060/master.
38Federal Statistical Office. Health Determinants 2018 [Available from: https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/determinanten/uebergewicht.assetdetail.6466132.html
39Country Profile - Switzerland [Internet]. 2018. Available from: http://www.ncdrisc.org/downloads/country-pdf/country-profile-Switzerland.pdf.
40 Lazo M , Hernaez R , Eberhardt MS , Bonekamp S , Kamel I , Guallar E , et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178(1):38–45. doi:.https://doi.org/10.1093/aje/kws448
41 Caballería L , Pera G , Auladell MA , Torán P , Muñoz L , Miranda D , et al. Prevalence and factors associated with the presence of nonalcoholic fatty liver disease in an adult population in Spain. Eur J Gastroenterol Hepatol. 2010;22(1):24–32. doi:.https://doi.org/10.1097/MEG.0b013e32832fcdf0
42 Fan JG , Farrell GC . Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol. 2009;50(1):204–10. doi:.https://doi.org/10.1016/j.jhep.2008.10.010
43 Ratziu V , Charlotte F , Heurtier A , Gombert S , Giral P , Bruckert E , et al.; LIDO Study Group. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128(7):1898–906. doi:.https://doi.org/10.1053/j.gastro.2005.03.084
44Swisstransplant. Swisstransplant Preliminary statistics 2017. Available from: https://www.swisstransplant.org/fileadmin/user_upload/Infos_und_Material/Statistiken/Jahreszahlen/Preliminary_statistics_2017.pdf
45IRODaT. International Registry on Organ Donation and Transplantation 2017 [cited 2017. Available from: http://www.irodat.org/?p=database.
46Organ Procurement and Transplantation Network (OPTN). OPTN data as of October 28. 2016 2016 [updated 2016. Available from: https://optn.transplant.hrsa.gov/data/.
47 Wong RJ , Cheung R , Ahmed A . Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59(6):2188–95. doi:.https://doi.org/10.1002/hep.26986
48 Malcolm DG , Roseboom JH , Clark CE , Fazar W . Application of a Technique for Research and Development Program Evaluation. Oper Res. 1959;7(5):646–69. doi:.https://doi.org/10.1287/opre.7.5.646
49National Institute for Cancer Epidemiology and Registration. Prevalence of liver cancer in Switzerland. 2014. Cancer data extracted from the Swiss national dataset managed by the Foundation National Institute for Cancer Epidemiology and Registration (NICER). Available from http://www.nicer.org/.
50 Hong TP , Gow P , Fink M , Dev A , Roberts S , Nicoll A , et al. Novel population-based study finding higher than reported hepatocellular carcinoma incidence suggests an updated approach is needed. Hepatology. 2016;63(4):1205–12. doi:.https://doi.org/10.1002/hep.28267
51 Altekruse SF , Henley SJ , Cucinelli JE , McGlynn KA . Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109(4):542–53. doi:.https://doi.org/10.1038/ajg.2014.11
52Robert Koch Institute and the Association of Population-based Cancer Registries in Germany. Cancer in Germany 2009/2010. Berlin; 2014.
53 Al Hasani F , Knoepfli M , Gemperli A , Kollar A , Banz V , Kettenbach J , et al. Factors affecting screening for hepatocellular carcinoma. Ann Hepatol. 2014;13(2):204–10. doi:.https://doi.org/10.1016/S1665-2681(19)30883-X
54 Weinmann A , Alt Y , Koch S , Nelles C , Düber C , Lang H , et al. Treatment and survival of non-alcoholic steatohepatitis associated hepatocellular carcinoma. BMC Cancer. 2015;15(1):210. doi:.https://doi.org/10.1186/s12885-015-1197-x
55 Wiegand S , Keller KM , Röbl M , L’Allemand D , Reinehr T , Widhalm K , et al.; APV-Study Group and the German Competence Network Adipositas. Obese boys at increased risk for nonalcoholic liver disease: evaluation of 16,390 overweight or obese children and adolescents. Int J Obes. 2010;34(10):1468–74. doi:.https://doi.org/10.1038/ijo.2010.106
56 Ratziu V , Marchesini G . When the journey from obesity to cirrhosis takes an early start. J Hepatol. 2016;65(2):249–51. doi:.https://doi.org/10.1016/j.jhep.2016.05.021
57 European Association for Study of LiverAsociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–64. doi:.https://doi.org/10.1016/j.jhep.2015.04.006
58 Zhou B , Lu Y , Hajifathalian K , Bentham J , Di Cesare M , Danaei G , et al.; NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–30. doi:.https://doi.org/10.1016/S0140-6736(16)00618-8
59 Weinmann A , Koch S , Niederle IM , Schulze-Bergkamen H , König J , Hoppe-Lotichius M , et al. Trends in epidemiology, treatment, and survival of hepatocellular carcinoma patients between 1998 and 2009: an analysis of 1066 cases of a German HCC Registry. J Clin Gastroenterol. 2014;48(3):279–89. doi:.https://doi.org/10.1097/MCG.0b013e3182a8a793
60 Chong CA , Gulamhussein A , Heathcote EJ , Lilly L , Sherman M , Naglie G , et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol. 2003;98(3):630–8. doi:.https://doi.org/10.1111/j.1572-0241.2003.07332.x
61 Pfeil AM , Reich O , Guerra IM , Cure S , Negro F , Müllhaupt B , et al. Cost-effectiveness analysis of sofosbuvir compared to current standard treatment in Swiss patients with chronic hepatitis C. PLoS One. 2015;10(5):e0126984. doi:.https://doi.org/10.1371/journal.pone.0126984
62 Alberti G , Zimmet P , Shaw J , Bloomgarden Z , Kaufman F , Silink M ; Consensus Workshop Group. Type 2 diabetes in the young: the evolving epidemic: the international diabetes federation consensus workshop. Diabetes Care. 2004;27(7):1798–811. doi:.https://doi.org/10.2337/diacare.27.7.1798
63 Fracanzani AL , Valenti L , Bugianesi E , Andreoletti M , Colli A , Vanni E , et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48(3):792–8. doi:.https://doi.org/10.1002/hep.22429
64 Angulo P , Bugianesi E , Bjornsson ES , Charatcharoenwitthaya P , Mills PR , Barrera F , et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145(4):782–9.e4. doi:.https://doi.org/10.1053/j.gastro.2013.06.057
65 Angulo P , Kleiner DE , Dam-Larsen S , Adams LA , Bjornsson ES , Charatcharoenwitthaya P , et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149(2):389–97.e10. doi:.https://doi.org/10.1053/j.gastro.2015.04.043
66 Singh S , Venkatesh SK , Loomba R , Wang Z , Sirlin C , Chen J , et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol. 2016;26(5):1431–40. doi:.https://doi.org/10.1007/s00330-015-3949-z
67World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Geneva: World Health Organization; 2013.
Funding for this project was provided by Gilead Sciences. The funders had no role in the study design, data collection, analysis, interpretation of data, or preparation of the manuscript. The experts consulted in the Delphi process did not receive financial compensation.
JFD: Bayer AG, BMS, Eli Lilly, Intercept, Jansen-Cilag, Merck, Novartis, Roche. BM has received speaking and/or consulting fees from Intercept and Gilead, and research support from Gilead. CE reports grants from Gilead Sciences, during the conduct of the study; grants from Pfizer, Gilead Sciences, AbbVie outside the submitted work.