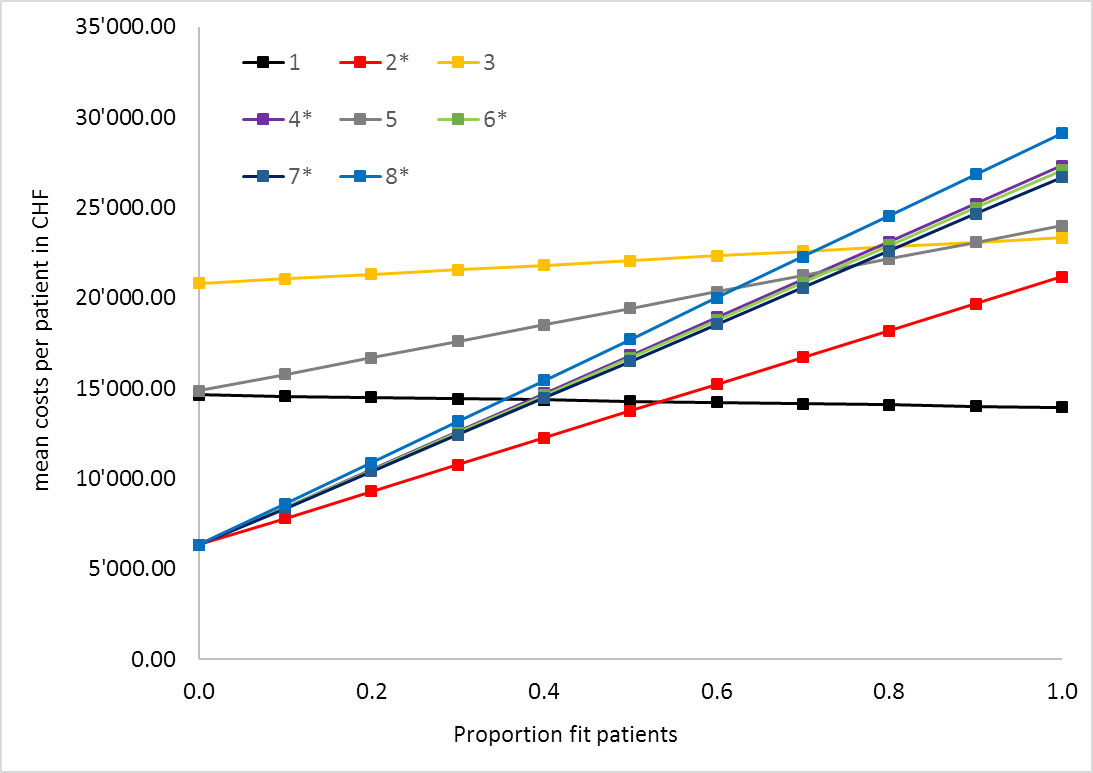
Figure 1 One-way sensitivity analysis expected base case mean costs per patient and centre by proportion of fit patients.
* Centres were unfit patients receive best supportive care only
DOI: https://doi.org/10.4414/smw.2019.20153
Glioblastoma multiforme (GBM) is the most common primary brain tumour in adults, with an annual incidence of about three per 100,000 persons [1]. Despite recent progress in multimodality treatment [2, 3] and molecular classification [4], its overall prognosis remains poor with a median survival of 15 months for patients receiving surgery followed by chemoradiation and temozolomide therapy [2]. Despite extensive surgery and completion of adjuvant therapy, the vast majority of patients will experience tumour recurrence [5].
There is currently no standard therapy for recurrent GBM, although several approaches have been investigated in clinical trials [6, 7].
Temozolomide, which has become standard-of-care for patients receiving long-course chemoradiation in the adjuvant setting [2], has been investigated in various treatment schedules for recurrent glioblastoma without clear benefit with dose-intensified schedules [6, 8, 9]. Alternatively, nitrosoureas such as lomustine or fotemustine, as well as the monoclonal antibody bevacizumab targeting the vascular endothelial growth factor-A, have been tested in recurrent GBM [6, 10, 11].
Local therapies such as re-irradiation and repeat surgery have been used for recurrent GBM with controversial results [12, 13]. More extensive tumour resection has been associated with improved survival in some studies [14, 15], but no association between repeat surgery at recurrence and improved progression-free or overall survival could be evidenced in a pooled analysis of eight prospective European Organisation for Research and Treatment of Cancer (EORTC) trials [16]. Retrospective data support the use of re-irradiation of recurrent glioma in selected patients, but prospective data on this approach are still lacking [13, 17].
Overall, the deduction of obvious treatment recommendations from the available clinical trials and patient series is hampered by small sample sizes, the lack of control arms and potential selection bias [6, 18].
In view of the lack of an evidence-based standard therapy, we conducted a survey in 2015 among eight Swiss neuro-oncology centres in order to determine consensus and controversies in their treatment recommendations for any scenario of recurrent GBM [19] using the objective consensus methodology [20]. We found a range of treatment recommendations implemented based on a variety of decision criteria, with barely any consensus among centres [19].
Health economics in GBM are a subject of rising interest, as in many other cancers [21], but there is only limited knowledge on cost effectiveness and other economic aspects of different therapies for recurrent glioblastoma [22, 23]. The aim of this study was therefore to deduce the average treatment costs for recurrent GBM from previously assessed institutional treatment decision algorithms [19] in order to assess the financial impact of institutional therapeutic strategies in Switzerland.
The health economics analysis was based on a recent review of treatment algorithms for recurrent GBM among eight Swiss neuro-oncology centres [19]. We investigated decision criteria for re-treatment and therapeutic pathways using the objective consensus methodology [20] in order to determine consensus and controversies by means of an automatic software-based comparison of individual decision trees. The following decision criteria were named: patient fitness, the presence of a symptomatic recurrence, unifocal recurrence, lesion size, time of recurrence, resectability, O6-methylguanine DNA methyltransferase (MGMT) methylation status. Similar criteria were fused into new categories with the agreement of all centres to increase comparability [19]. For instance, “patient fitness” was a combination of “age”, “performance status” and “co-morbidities” without fixed cut-off values. We identified a total of 16 different treatment recommendations, which resulted in a variety of up to six different treatment recommendations among the eight centres for a specific clinical situation of tumour recurrence. Areas of consensus were identified in only a few situations, such as in the recommendation of best supportive care for unfit patients. Recommendations supported by the majority of centres were bevacizumab for inoperable, large early recurrences with unmethylated MGMT promoter status, and temozolomide for fit inoperable MGMT-promoter-methylated late recurrences. There was no majority recommendation for any other scenario [19].
In order to estimate minimum, maximum and average costs per patient given the treatment pathways for each centre, a decision analytic model was built using the TreeAge Pro 2017 software (Tree-Age Software, Williamstown, MA). For every single centre the treatment pathways for patients, depending on several serial clinical criteria and resulting in various treatment choices, were developed based on previously published decision tree data [19].
Probabilities for decision criteria were not available from the literature or real clinical data, therefore in the base case analysis all decision probabilities were set to 0.5. Costs for treatments were assessed from the Swiss healthcare payer perspective based on the Swiss outpatient tariff (Tarmed [24]), the Swiss inpatient tariff (SwissDRG [25]) or the Swiss drug price list [26] that were valid at the time of the original study [19], and the number of treatment cycles according to the literature based on an average patient with a body surface [10, 27] of 1.77 m2 and a weight of 70 kg (see table 1 below). Treatment costs for temozolomide chemotherapy for recurrent GBM were calculated based on a schedule of 200 mg/m2 on days 1–5 of a 28-day cycle, which has become a widely accepted schedule in Switzerland in view of the lack of clear benefit of more dose-intensified schedules [6]. Treatment costs of bevacizumab were based on a 2-weekly administration of 10 mg/kg/m2 with a median of six administered cycles and of lomustine on administration of single dose of 90–110 mg/m2 with a median duration of one cycle [10].
As there is no established schedule for re-irradiation, we calculated costs based either on a 5-fraction course of stereotactic radiotherapy or a 15-fraction course of hypofractionated radiotherapy, which corresponds well to current practice [13]. Radiotherapy costs were assessed as an outpatient procedure according to the Swiss outpatient tariff [24]. Costs for hypofractionated radiotherapy or stereotactic radiotherapy were calculated based on the individual institutional recommendation [19] of each centre.
Surgery costs were based on the inpatient costs according to the average Swiss disease-related group (DRG) for brain tumour resection [25].
On the basis of our institutional practice, costs for clinical controls, imaging and supportive medication were added to each treatment strategy (see table 1). End-of-life care was considered to be equal in all treatment strategies and was not included into the analysis. Potential third-line therapies or costs of treatment of side effects were not included into the analysis because of insufficient data.
All tariffs were calculated with the version valid in May 2017. Costs were not discounted owing to the poor prognosis of the patient population investigated.
We did not have clinical data from all centres concerning the frequency of specific patient and tumour characteristics that influenced treatment decision making [19], such as patient performance (fit vs unfit), MGMT promoter methylation or resectability. Therefore, we performed a series of sensitivity analyses to test a wide variety of these factors and their impact on treatment costs. Univariate sensitivity analysis was used to test for the effect on costs of the probabilities in a range of 0–1 for all decision steps. A probabilistic sensitivity analysis including uncertainty in all decision probabilities as well as several treatment cycles for medical treatments and costs for procedures was performed (see table 1). In addition, a Pearson’s correlation coefficient between mean costs and number of different possible pathways per centre was calculated, to estimate if complex treatment decision algorithms are correlated to higher or lower costs.
Our decision analytic model, modelling expected costs for patients with recurrent GBM in eight different neuro-oncology centres in Switzerland demonstrated profound cost differences when analysed from the Swiss healthcare payer perspective with current pricing (May 2017, table 1).
Table 1 Costs, treatment duration and decision probability input parameters.
| Treatment | Scheme | Unit price | Absolute dose | Costs per cycle |
Cycle length
(weeks) |
Number of cycles | Distribution | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Bevacizumab | 10 mg/kg every 2 weeks | CHF 4.354/mg | 700 mg (patient 70 kg) |
CHF 3048 + CHF 498 infusion | 2 | Median = 6 (range 1–30) [10] |
Gamma | SL [26] | |
| Clinical checks | Blood count 1×/cycle Urine 1× / 2 cycles MRI 1× every 3 months |
||||||||
| Temozolomide | 200 mg/m2 days 1–5 | CHF 0.6166/mg | 1800 mg (patient m2 = 1.77) |
CHF 1110 | 4 | Median = 3 (range 1–6) [10] |
Gamma | SL [26] | |
| Antiemetic (ondansetron) | 1 tablet days 1–6 | CHF 62.45 | SL [26] | ||||||
| Clinical checks | Clinical visit 1×/cycle Blood count 4×/cycle Visit for blood count 3×/cycle MRI 1× every 3 months |
||||||||
| Lomustine | 4×40 mg | CHF 0.897/mg | 160 mg | CHF 143.5 | 6 | Median = 1 (range 1–6) [10] |
Gamma | SL [26] | |
| Antiemetic (ondansetron) | 1 tablet/cycle | 8 mg | CHF 10.40 | SL [26] | |||||
| Clinical checks | Blood count 6×/cycle Visit for blood count 5×/cycle Clinical visit 1×/cycle MRI 1× every 3 months |
||||||||
| Repeat surgery | CHF 25,840 | Triangular* | Swiss DRG [25] | ||||||
| Hypofractionated radiotherapy | One course of 15×2.66 Gy over 3 weeks | CHF 9981 | Triangular* | Tarmed† [24] | |||||
| Stereotactic fractionated radiotherapy | One course of 6×5 Gy over 1 week |
CHF 16,073 | Triangular* | Tarmed† [24] | |||||
| Best supportive care | CHF 250/week | 4 | Median = 6 (range 1–12) |
Triangular | Clinical estimate | ||||
| Clinical checks | Clinical Visit | CHF 379.00 | Tarmed† [24] | ||||||
| MRI 1× every 3 cycles | CHF 965.00 | Tarmed† [24] | |||||||
| Blood count | CHF 15.00 | Tarmed† [24] | |||||||
| Urine test | CHF 20.00 | Tarmed† [24] | |||||||
| Visit for blood count | CHF 69.00 | Tarmed†
[24] |
|||||||
| Probabilities for all decisions | Mean = 0.5 95% CI 0.2–0.8 |
Beta | |||||||
CI = confidence interval; MRI = magnetic resonance imaging; SL = Spezialitätenliste (official Swiss medication prices) * Triangular distribution with min = 0.75 and max = 1.25; † Tarmed 1.08 BR version of Tarmed
In the base case analysis, mean costs per patient were distributed over a range from CHF 13,748 to CHF 22,072, being the lowest in centre 2 (CHF 13,748, 95% confidence interval [CI] 9868–17,089) and the highest in centre 3 (CHF 22,072, 95% CI 21,414–22,639), which is 1.6 times higher than in centre 2 (table 2).
Table 2 Base case mean cost/patient in CHF.
| Strategy |
Mean
(95% CI) |
Incremental value | 100% patients fit† | 0% patients fit† |
|---|---|---|---|---|
| Centre 2* | 13,748 (9868–17,089) |
0 | 21,162 | 6333 |
| Centre 1 | 14,292 (14,136–14,474) |
545 | 13,945 | 14,639 |
| Centre 7* | 16,508 (11,184–21,206) |
2216 | 26,682 | 6333 |
| Centre 6* | 16,700 (11,275–21,384) |
192 | 27,066 | 6333 |
| Centre 4* | 16,825 (11,335–21,566) |
125 | 27,316 | 6333 |
| Centre 8* | 17,726 (11,764–22,874) |
901 | 29,118 | 6333 |
| Centre 5 | 19,436 (17,047–21,500) |
1710 | 24,002 | 14,870 |
| Centre 3 | 22,072 (21,414–22,639) |
2636 | 23,328 | 20,815 |
CI = confidence interval * Centres where unfit patients receive best supportive care only; † all other decision probabilities = 0.5
Of the eight participating centres, five treated unfit patients only with best supportive care. These centres showed the largest difference in treatment costs, with a varying prevalence of fit versus unfit patients (fig. 1): Centres recommending only best supportive care to unfit patients showed an increase of mean costs per patient of CHF 14,829 to CHF 22,785 for 0% versus 100% fit patients in the univariate sensitivity analysis, whereas centres with tumour-specific treatment options for unfit patients showed a cost difference of CHF 694 to CHF 9132 between fit and unfit patients (fig. 1). Interestingly, centre 1 had higher costs for unfit patients than for fit patients. This can be explained by the use of bevacizumab in unfit patients, whereas fit patients received only radiotherapy or surgery without systemic therapy. Another factor strongly influencing overall treatment costs in the strategy of the centres was the proportion of patients with resectable tumours (fig. 2): sensitivity analysis demonstrated differences in mean costs per patient of up to CHF 17,120 depending on the prevalence of 0% versus 100% resectable tumours.

Figure 1 One-way sensitivity analysis expected base case mean costs per patient and centre by proportion of fit patients.
* Centres were unfit patients receive best supportive care only
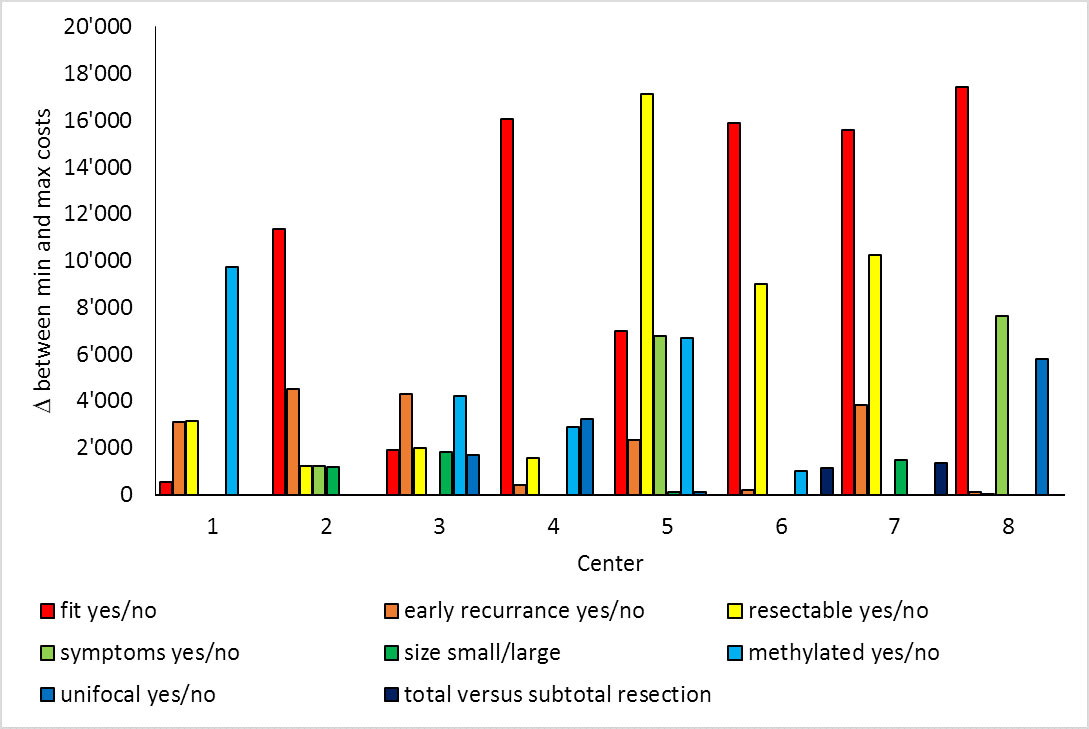
Figure 2 Univariate sensitivity analysis for all decision probabilities separately.
The probabilistic sensitivity analyses showed that there were considerable differences in the magnitude of cost distributions among the centres with centre 3 not only having the highest costs but also the largest 95% CI and spread in costs (figs 3 and 4).
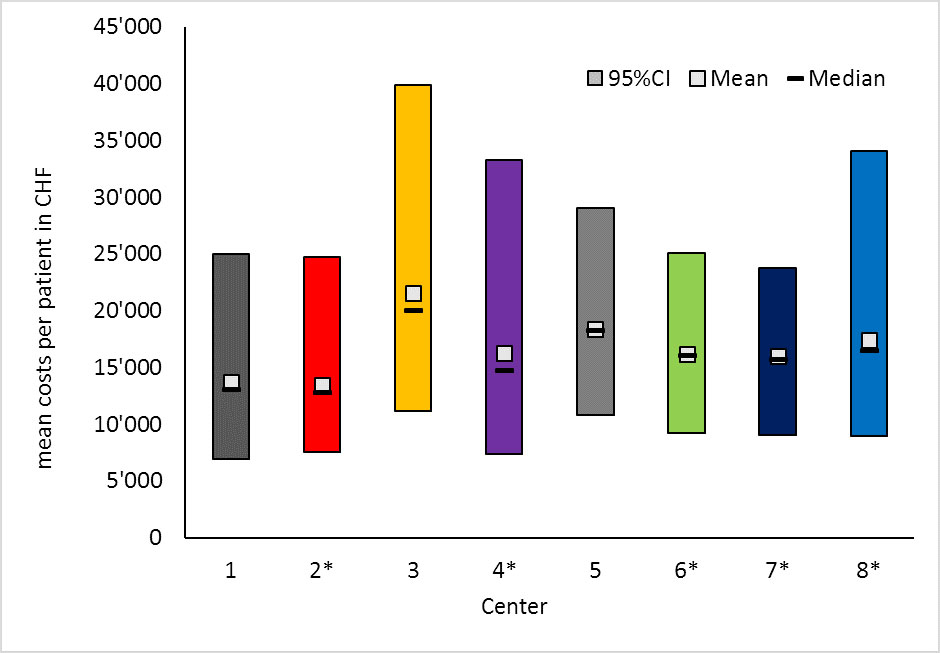
Figure 3 Probabilistic sensitivity analysis I, probable mean, median and 95% confidence interval costs per patient and centre.
There was a significant correlation (R2 = 0.793, p = 0.019) between the number of different treatment options at a centre and the average treatment costs, meaning that the more complex the decision pathway and treatment options a centre provided, the more costly the average treatment was (see supplementary fig. S1 in appendix 1).
Recurrent or progressive GBM after primary therapy remains a therapeutic challenge and there is currently no standard of care [6], although various treatment options have been developed in parallel [28]. We recently performed a survey among eight Swiss neuro-oncology centres to assess and compare institutional treatment algorithms for recurrent GBM [19], and for the vast majority of scenarios a consensus was not identified.
Health economics is a topic of rising importance in oncology [29] and has also been studied in the case of GBM [21, 30]. The introduction of novel therapeutic agents such as temozolomide and bevacizumab increased treatment costs from 2004 to 2008 in a French cohort study, but these increases were considered cost-effective owing to improved oncological outcome [31]. Likewise, temozolomide was shown to be cost-effective in the treatment of newly diagnosed GBM [32, 33]. A Swiss study demonstrated that the use of temozolomide in recurrent glioma is cost-effective [22], which was confirmed by a systematic review of the cost-effectiveness of temozolomide [34]. In contrary, a retrospective patient cohort analysis from Canada suggested that lomustine might be more cost-effective than temozolomide in the treatment of recurrent GBM as a result of lower treatment costs and similar outcomes [35]. Bevacizumab has been shown not to be cost-effective in the treatment of newly diagnosed GBM [36], which can be primarily explained by the lack of overall survival benefit in newly diagnosed GBM as demonstrated in the AVAglio and RTOG 0825 trials [37, 38]. Based on the recently presented data of the EORTC 26101 trial, which showed a moderate benefit in progression-free, but not overall, survival by adding bevacizumab to lomustine in the treatment of recurrent GBM [11], it is also doubtful that its use may achieve cost-effectiveness for recurrent disease. For repeat surgery and re-irradiation of recurrent glioma, there are no cost-effectiveness data available.
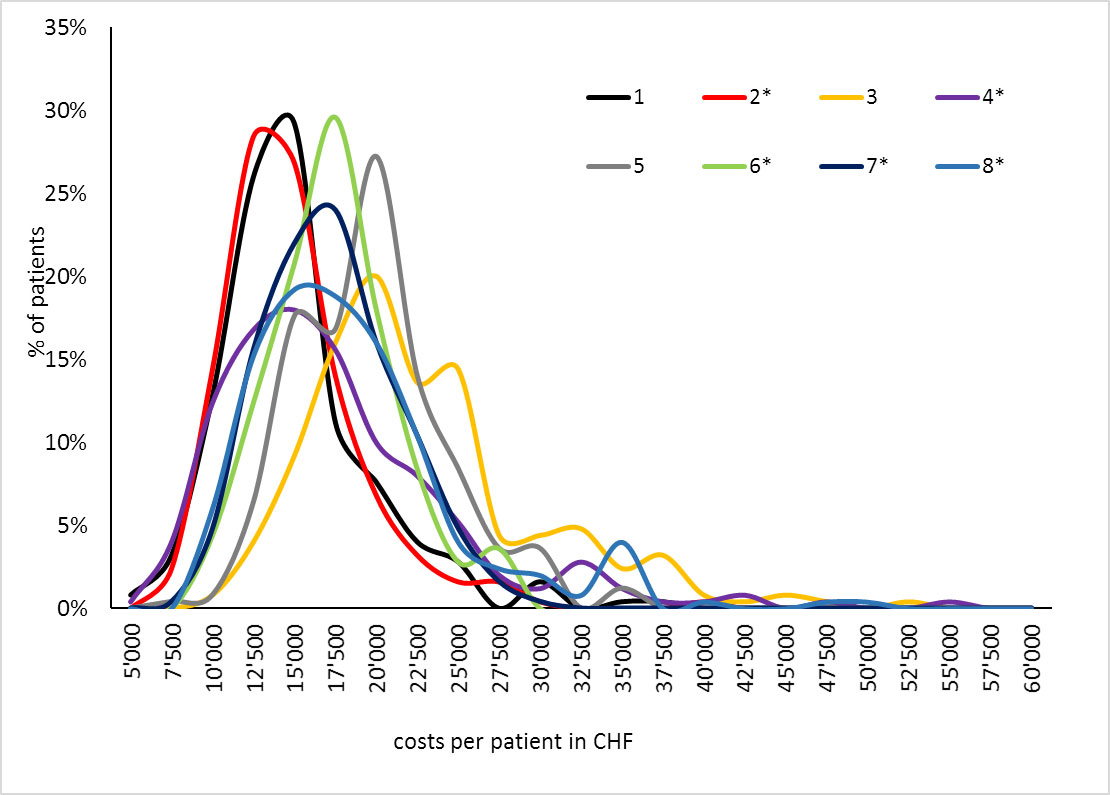
Figure 4 Probabilistic sensitivity analysis II, proportion of patients by costs.
Although there are very limited comparative outcome data for the available therapies in recurrent GBM [6], we found considerable cost differences among these treatment options in Switzerland (see table 1). In the base case scenario, treatment costs varied between the centres by a factor of 1.6 from CHF 13,748 to CHF 22,072 (approximately USD 13,760 to 22,090 in May 2018) depending on the individual treatment algorithm.
Sensitivity analysis revealed that the distribution of two patient characteristics particularly influenced the overall institutional treatment costs: the proportion of patients deemed “unfit” due to age, poor performance status or comorbidities [19], and the resectability of the tumour recurrence. Given the poor prognosis of elderly patients and patients with poor performance status [39], it may be justified to withhold extensive tumour-directed therapies. Treatment costs and potential side effects may outweigh the benefits of any therapeutic intervention in this situation on a group level from the view of the healthcare insurer. Because of the considerable contribution of repeated surgery to overall treatment costs, careful patient selection is paramount: treatment decisions may be facilitated by scores as published by Park et al. [40] taking into account tumour volume, performance status, tumour location in non-eloquent areas and ependymal involvement. As there is very little data directly comparing different treatment strategies for GBM, it is not possible to correlate the varying treatment costs with outcome. Our data show, however, the considerable health economic consequences of different interpretations of the available evidence and consequent treatment choices. Additionally, our study serves as proof-of-principle that centre-specific decision trees obtained by the objective consensus methodology [20, 41] can be used to estimate and to compare the average treatment costs of different therapeutic algorithms. Especially in clinical scenarios such as recurrent GBM where there is no high-level evidence for a superior oncological outcome with any therapeutic strategy, treatment costs can be an important factor in the medical decision making process.
However, there are several limitations to the study. First, the analysis was based on the treatment recommendations of the participating neuro-oncological centre without data on the distribution of patient characteristics [19]. Consequently, we performed a probabilistic sensitivity analysis over a wide range of possible distributions of each patient characteristic to investigate the possible cost distribution. Centre-specific patient characteristics are needed for a more accurate analysis of the economic impact of institutional treatment algorithms.
Additionally, the survey did not include further therapies after progression from second-line therapy or inclusion into a prospective study as further treatment option. Subsequent treatments after further progression and experimental therapies are consequently not included into our analysis, as they were not part of our survey [19] and high-quality data were lacking [42]. However, different distributions of subsequent therapy lines depending on the treatment at first recurrence could affect overall treatment costs for each strategy and should therefore be reported for series of patients treated for recurrent glioblastoma. Moreover, we did not include costs for ambulatory care for outpatients with poor performance as we assumed they might be similar in all patients. Finally, we did not perform a cost-effectiveness analysis owing to the lack of comparable, unbiased outcome data for the different treatment options [6], as well as to the limited literature on health state utilities in GBM, particularly for recurrent disease [43, 44].
In conclusion, our analysis of institutional treatment algorithms of eight Swiss neuro-oncology centres study was able to estimate the economic impact of individual therapeutic strategies for recurrent GBM and demonstrated a high variability in treatment costs among the centres.
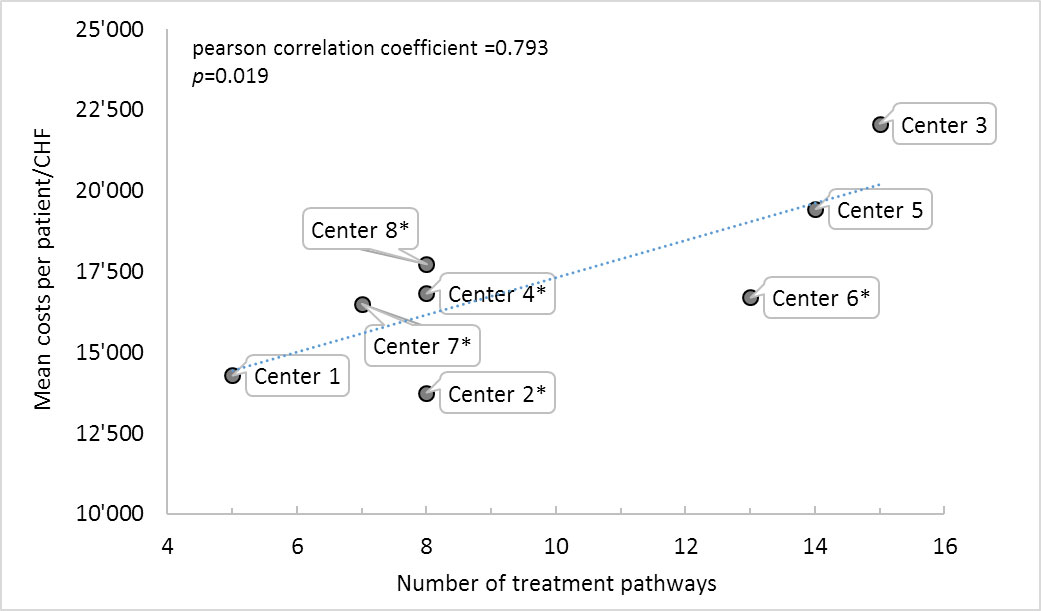
Figure S1 Correlation between base case costs and number of treatment pathways.
* Centres where unfit patients receive best supportive care only.
There was no financial support for this study.
PMP obtained an institutional grant from Roche for a previous study that provided the data for this analysis. TH recreived advisory board compensation and/or travel grants from Roche, MSD and Novocure. GP received travel grants from Abbvie and Brainlab. All other authors declare that they have no conflict of interest. All other authors declare that they have no conflict of interest.
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
1 Ostrom QT , Gittleman H , Truitt G , Boscia A , Kruchko C , Barnholtz-Sloan JS . CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro-oncol. 2018;20(suppl_4):iv1–86. doi:.https://doi.org/10.1093/neuonc/noy131
2 Stupp R , Mason WP , van den Bent MJ , Weller M , Fisher B , Taphoorn MJ , et al.; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. doi:.https://doi.org/10.1056/NEJMoa043330
3 Perry JR , Laperriere N , O’Callaghan CJ , Brandes AA , Menten J , Phillips C , et al.; Trial Investigators. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N Engl J Med. 2017;376(11):1027–37. doi:.https://doi.org/10.1056/NEJMoa1611977
4 Louis DN , Perry A , Reifenberger G , von Deimling A , Figarella-Branger D , Cavenee WK , et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–20. doi:.https://doi.org/10.1007/s00401-016-1545-1
5 Weller M , Cloughesy T , Perry JR , Wick W . Standards of care for treatment of recurrent glioblastoma--are we there yet? Neuro-oncol. 2013;15(1):4–27. doi:.https://doi.org/10.1093/neuonc/nos273
6 Seystahl K , Wick W , Weller M . Therapeutic options in recurrent glioblastoma--An update. Crit Rev Oncol Hematol. 2016;99:389–408. doi:.https://doi.org/10.1016/j.critrevonc.2016.01.018
7 Birk HS , Han SJ , Butowski NA . Treatment options for recurrent high-grade gliomas. CNS Oncol. 2017;6(1):61–70. doi:.https://doi.org/10.2217/cns-2016-0013
8 Weller M , Tabatabai G , Kästner B , Felsberg J , Steinbach JP , Wick A , et al.; DIRECTOR Study Group. MGMT Promoter Methylation Is a Strong Prognostic Biomarker for Benefit from Dose-Intensified Temozolomide Rechallenge in Progressive Glioblastoma: The DIRECTOR Trial. Clin Cancer Res. 2015;21(9):2057–64. doi:.https://doi.org/10.1158/1078-0432.CCR-14-2737
9 Brada M , Stenning S , Gabe R , Thompson LC , Levy D , Rampling R , et al. Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high-grade glioma. J Clin Oncol. 2010;28(30):4601–8. doi:.https://doi.org/10.1200/JCO.2009.27.1932
10 Taal W , Oosterkamp HM , Walenkamp AM , Dubbink HJ , Beerepoot LV , Hanse MC , et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–53. doi:.https://doi.org/10.1016/S1470-2045(14)70314-6
11 Wick W , Gorlia T , Bendszus M , Taphoorn M , Sahm F , Harting I , et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N Engl J Med. 2017;377(20):1954–63. doi:.https://doi.org/10.1056/NEJMoa1707358
12 Robin AM , Lee I , Kalkanis SN . Reoperation for Recurrent Glioblastoma Multiforme. Neurosurg Clin N Am. 2017;28(3):407–28. doi:.https://doi.org/10.1016/j.nec.2017.02.007
13 Howard SP , Krauze A , Chan MD , Tsien C , Tomé WA . The evolving role for re-irradiation in the management of recurrent grade 4 glioma. J Neurooncol. 2017;134(3):523–30. doi:.https://doi.org/10.1007/s11060-017-2392-1
14 Suchorska B , Weller M , Tabatabai G , Senft C , Hau P , Sabel MC , et al. Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neuro-oncol. 2016;18(4):549–56. doi:.https://doi.org/10.1093/neuonc/nov326
15 Yong RL , Wu T , Mihatov N , Shen MJ , Brown MA , Zaghloul KA , et al. Residual tumor volume and patient survival following reoperation for recurrent glioblastoma. J Neurosurg. 2014;121(4):802–9. doi:.https://doi.org/10.3171/2014.6.JNS132038
16 Gorlia T , Stupp R , Brandes AA , Rampling RR , Fumoleau P , Dittrich C , et al. New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: a pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur J Cancer. 2012;48(8):1176–84. doi:.https://doi.org/10.1016/j.ejca.2012.02.004
17 Hundsberger T , Brügge D , Putora PM , Weder P , Weber J , Plasswilm L . Re-irradiation with and without bevacizumab as salvage therapy for recurrent or progressive high-grade gliomas. J Neurooncol. 2013;112(1):133–9. doi:.https://doi.org/10.1007/s11060-013-1044-3
18 Panje CM , et al. Treatment Options in Oncology. JCO Clinical Cancer Inform. 2018;(2):1–10.
19 Hundsberger T , Hottinger AF , Roelcke U , Roth P , Migliorini D , Dietrich PY , et al. Patterns of care in recurrent glioblastoma in Switzerland: a multicentre national approach based on diagnostic nodes. J Neurooncol. 2016;126(1):175–83. doi:.https://doi.org/10.1007/s11060-015-1957-0
20 Putora PM , Panje CM , Papachristofilou A , Dal Pra A , Hundsberger T , Plasswilm L . Objective consensus from decision trees. Radiat Oncol. 2014;9(1):270. doi:.https://doi.org/10.1186/s13014-014-0270-y
21 Messali A , Villacorta R , Hay JW . A review of the economic burden of glioblastoma and the cost effectiveness of pharmacologic treatments. Pharmacoeconomics. 2014;32(12):1201–12. doi:.https://doi.org/10.1007/s40273-014-0198-y
22 Wasserfallen JB , Ostermann S , Leyvraz S , Stupp R . Cost of temozolomide therapy and global care for recurrent malignant gliomas followed until death. Neuro-oncol. 2005;7(2):189–95. doi:.https://doi.org/10.1215/S1152851704000687
23 Henaine AM , Paubel N , Ducray F , Diebold G , Frappaz D , Guyotat J , et al. Current trends in the management of glioblastoma in a French University Hospital and associated direct costs. J Clin Pharm Ther. 2016;41(1):47–53. doi:.https://doi.org/10.1111/jcpt.12346
24Tarmed, Swiss National Tariff Catalogue. Available at: www.tarmed.ch [Accessed 23 July 2017].
25Swiss DRG. SwissDRG System. Available at: http://www.swissdrg.org/de. [Accessed 23July 2017].
26BundesamtfürGesundheit. Swiss Specialty List. 2019; Available from: www.listedesspecialites.ch [accessed on 22 Feb. 2019].
27 Chang SM , Theodosopoulos P , Lamborn K , Malec M , Rabbitt J , Page M , et al. Temozolomide in the treatment of recurrent malignant glioma. Cancer. 2004;100(3):605–11. doi:.https://doi.org/10.1002/cncr.11949
28 Panje CM , Glatzer M , Sirén C , Plasswilm L , Putora PM . Treatment Options in Oncology. JCO Clin Cancer Inform. 2018;1:1–10. doi:.https://doi.org/10.1200/CCI.18.00017
29 Taylor D . The reality of economics for oncologists. Breast. 2017;33:183–90. doi:.https://doi.org/10.1016/j.breast.2017.03.014
30 Cagney DN , Alexander BM . The cost and value of glioblastoma therapy. Expert Rev Anticancer Ther. 2017;17(8):657–9. doi:.https://doi.org/10.1080/14737140.2017.1351355
31 Diebold G , Ducray F , Henaine AM , Frappaz D , Guyotat J , Cartalat-Carel S , et al. Management of glioblastoma: comparison of clinical practices and cost-effectiveness in two cohorts of patients (2008 versus 2004) diagnosed in a French university hospital. J Clin Pharm Ther. 2014;39(6):642–8. doi:.https://doi.org/10.1111/jcpt.12199
32 Lamers LM , Stupp R , van den Bent MJ , Al MJ , Gorlia T , Wasserfallen JB , et al.; EORTC 26981/22981 NCI-C CE3 Intergroup Study. Cost-effectiveness of temozolomide for the treatment of newly diagnosed glioblastoma multiforme: a report from the EORTC 26981/22981 NCI-C CE3 Intergroup Study. Cancer. 2008;112(6):1337–44. doi:.https://doi.org/10.1002/cncr.23297
33 Wasserfallen JB , Ostermann S , Pica A , Mirimanoff RO , Leyvraz S , Villemure JG , et al. Can we afford to add chemotherapy to radiotherapy for glioblastoma multiforme? Cost-identification analysis of concomitant and adjuvant treatment with temozolomide until patient death. Cancer. 2004;101(9):2098–105. doi:.https://doi.org/10.1002/cncr.20619
34 Dinnes J , Cave C , Huang S , Major K , Milne R . The effectiveness and cost-effectiveness of temozolomide for the treatment of recurrent malignant glioma: a rapid and systematic review. Health Technol Assess. 2001;5(13):1–73. doi:.https://doi.org/10.3310/hta5130
35 Mabasa VH , Taylor SC . Re-evaluation of the cost effectiveness of temozolomide for malignant gliomas in British Columbia. J Oncol Pharm Pract. 2006;12(2):105–11. doi:.https://doi.org/10.1177/1078155206069161
36 Kovic B , Xie F . Economic Evaluation of Bevacizumab for the First-Line Treatment of Newly Diagnosed Glioblastoma Multiforme. J Clin Oncol. 2015;33(20):2296–302. doi:.https://doi.org/10.1200/JCO.2014.59.7245
37 Chinot OL , Wick W , Mason W , Henriksson R , Saran F , Nishikawa R , et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–22. doi:.https://doi.org/10.1056/NEJMoa1308345
38 Gilbert MR , Dignam JJ , Armstrong TS , Wefel JS , Blumenthal DT , Vogelbaum MA , et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. doi:.https://doi.org/10.1056/NEJMoa1308573
39 Roth P , Gramatzki D , Weller M . Management of Elderly Patients with Glioblastoma. Curr Neurol Neurosci Rep. 2017;17(4):35. doi:.https://doi.org/10.1007/s11910-017-0740-3
40 Park CK , Kim JH , Nam DH , Kim CY , Chung SB , Kim YH , et al. A practical scoring system to determine whether to proceed with surgical resection in recurrent glioblastoma. Neuro-oncol. 2013;15(8):1096–101. doi:.https://doi.org/10.1093/neuonc/not069
41 Panje CM , Glatzer M , von Rappard J , Rothermundt C , Hundsberger T , Zumstein V , et al. Applied Swarm-based medicine: collecting decision trees for patterns of algorithms analysis. BMC Med Res Methodol. 2017;17(1):123. doi:.https://doi.org/10.1186/s12874-017-0400-y
42 Tosoni A , Franceschi E , Poggi R , Brandes AA . Relapsed Glioblastoma: Treatment Strategies for Initial and Subsequent Recurrences. Curr Treat Options Oncol. 2016;17(9):49. doi:.https://doi.org/10.1007/s11864-016-0422-4
43 Bernard-Arnoux F , Lamure M , Ducray F , Aulagner G , Honnorat J , Armoiry X . The cost-effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma. Neuro-oncol. 2016;18(8):1129–36. doi:.https://doi.org/10.1093/neuonc/now102
44 Garside R , Pitt M , Anderson R , Rogers G , Dyer M , Mealing S , et al. The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation. Health Technol Assess. 2007;11(45):iii–iv, ix-221. doi:.https://doi.org/10.3310/hta11450
There was no financial support for this study.
PMP obtained an institutional grant from Roche for a previous study that provided the data for this analysis. TH recreived advisory board compensation and/or travel grants from Roche, MSD and Novocure. GP received travel grants from Abbvie and Brainlab. All other authors declare that they have no conflict of interest. All other authors declare that they have no conflict of interest.