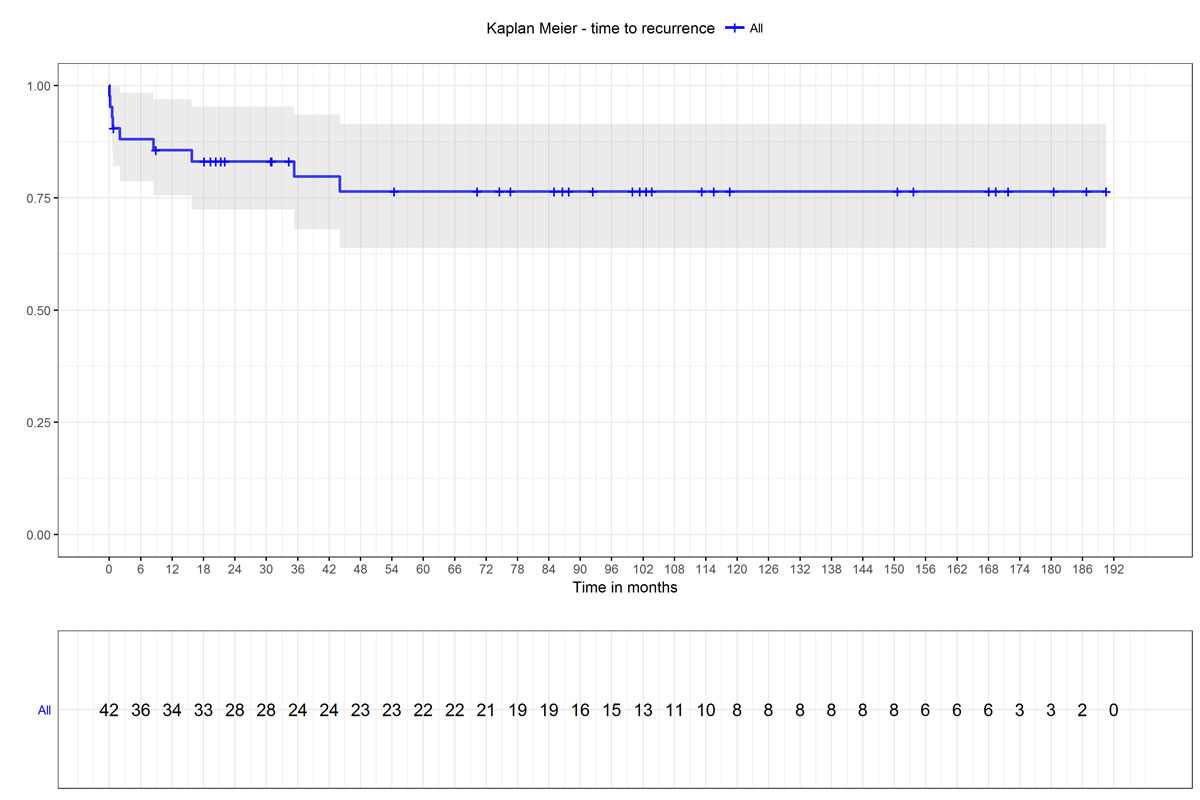
Figure 1 The Kaplan-Meier plot shows the probability of remaining recurrence free until a given point in time. The shaded areas represent the 95% confidence interval.
DOI: https://doi.org/10.4414/smw.2019.20160
More than 85% of all patients with end-stage chronic kidney disease (CKD) suffer from secondary hyperparathyroidism. CKD leads to the dysregulation of mineral metabolism, including hypocalcaemia, hyperphosphataemia, calcitriol (1,25-dihydroxycholecalciferol) deficiency, elevated levels of the bone-derived hormone fibroblast growth factor 23 (FGF-23), and elevated secretion of parathyroid hormone (PTH) [1, 2]. The stimulation of PTH secretion leads to hyperplasia of all parathyroid glands [3]. Severe secondary hyperparathyroidism can lead to pruritus, muscular pain, tissue calcifications, osteitis fibrosa and fractures [4]. Elevated PTH levels are also associated with a higher risk of cardiovascular mortality. The underlying pathophysiological mechanisms involve elevated serum concentrations of calcium (Ca) and Ca × PO4 product, which lead to vascular and myocardial calcification [5–7].
Most patients with secondary hyperparathyroidism can be successfully treated with calcium and vitamin D substitution and reduction of phosphate intake. If necessary, drug treatment can be extended to include phosphate binders and calcimimetics. If secondary hyperparathyroidism persists for many years, it can progress to tertiary hyperparathyroidism as PTH overproduction becomes autonomous and independent of serum calcium levels, and therefore refractory to drug treatment [8]. According to the KDIGO (Kidney Disease: Improving Global Outcomes) guidelines, the target PTH levels in patients with CKD stages 3 and 4 are 35–70 ng/l and 70–110 ng/l, respectively. In patients with CKD stage 5 who are on dialysis (stage 5D), PTH levels between two and nine times higher than normal are recommended. In patients with CKD stages 3 to 5D and with severe secondary hyperparathyroidism who fail to respond to drug therapy, surgery is indicated [9]. Despite improved drug treatment, 5–10% of all long-term haemodialysis patients need parathyroid surgery to control the disease [10]. Year by year, about 1% of patients with secondary hyperparathyroidism need surgical therapy [11]. Surgery aims to reduce the hyperplastic parathyroid gland mass and therefore the PTH levels. Reduction of PTH levels has been shown to reduce cardiovascular mortality in haemodialysis patients [12].
Three different surgical options are available to treat secondary hyperparathyroidism: subtotal parathyroidectomy, total parathyroidectomy with autotransplantation (PTX-AT), and total parathyroidectomy (PTX) without autotransplantation (AT). The KDIGO guidelines recommend subtotal parathyroidectomy or PTX-AT, depending on the surgeon’s preference [9]. Subtotal parathyroidectomy is associated with a higher risk of recurrence, and in the event of recurrence a more difficult second neck exploration is necessary [13]. If secondary hyperparathyroidism recurs after PTX-AT, the site of recurrence can be determined with a non-invasive Casanova test [14]. In most cases, the recurrence is caused by hyperplasia of the gland tissue reimplanted in the forearm, which can then be removed under local anaesthetic [15, 16].
Recurrence of secondary hyperparathyroidism is important because it implies recurrence of the above-mentioned symptoms and consequences of elevated PTH levels, including the elevated risk of cardiovascular mortality. Our goal was to analyse the long-term outcomes of patients undergoing PTX-AT in a single-centre patient cohort. We especially wanted to focus on the recurrence rate and time to recurrence of secondary hyperparathyroidism after PTX-AT. The study sought to evaluate the recurrences in detail in order to develop strategies to prevent them in the future.
For this retrospective study, we retrieved medical records on all patients who underwent PTX-AT for secondary hyperparathyroidism and tertiary hyperparathyroidism at the University Hospital in Basel, Switzerland between January 2000 and December 2013. All these patients were either on dialysis or had a functioning kidney transplant. In accordance with the KDIGO guidelines, the indication for surgery was either severe secondary hyperparathyroidism with PTH values that were uncontrollable under best drug therapy or tertiary hyperparathyroidism [9]. In primary operations, our standard workup did not include imaging studies because we always performed a complete four-gland exploration during the operation. In the case of re-operation in patients with previous subtotal parathyroidectomy, our standard preoperative imaging studies included neck sonography combined with 99mTc-sestamibi scintigraphy. In all cases, the operation performed was total PTX (or excision of the remaining parathyroid tissue in the case of previous subtotal parathyroidectomy) and AT of parathyroid tissue into the forearm.
Due to the retrospective nature of this study, without any patient involvement, no ethics approval was required.
Through a standard collar incision, bilateral four-gland exploration with identification of the recurrent laryngeal nerve on each side was performed. After resection, one or two glands were placed in cold saline solution for later AT, and all resected gland tissue was sent for histological workup. We did not cryopreserve parathyroid glands, as this is not considered necessary when parathyroid tissue is autotransplanted into the forearm [16]. In any case, our institution does not have the facilities for cryopreservation. If necessary, an intraoperative PTH assay was performed to confirm a drop in serum PTH level. After 2006, intraoperative PTH measurement was introduced as standard for all operations. An intraoperative PTH level of 15% of the preoperative baseline concentration 20 minutes after resection was considered adequate, and if this was achieved, no further exploration was performed [17]. If the intraoperative PTH level was more than 15% of the preoperative baseline level, the operation was continued. Cervical bilateral thymectomy was performed only if we did not find all four glands or if intraoperative PTH levels did not decrease adequately after the resection of all four glands. In addition, intraoperative histological examination was available to confirm complete removal of all glands. The non-shunt or non-dominant forearm was chosen for AT. Around 50 mg of gland tissue was cut into fragments and implanted in small pouches of the brachioradial muscle marked with titanium clips.
The postoperative follow-up comprised of regular visits to the Department of Nephrology at the University Hospital Basel between January 2000 and January 2018. Blood serum samples were taken to monitor the postoperative progression of PTH and calcium levels. The samples were drawn by nurses using vacuum extraction systems in accordance with clinical routine procedures and the World Health Organization’s guidelines on drawing blood [18]. PTH levels were quantified by immunochemiluminometric assay (IMMULITE Turbo intact PTH, Diagnostic Products Corporation, Los Angeles, USA). Our laboratory’s reference range for normal PTH values was 10–73 ng/l.
We calculated the median PTH and calcium levels at specific points in time during follow-up: one day, six months, one year and five years after operation. Recurrence of secondary hyperparathyroidism was defined as three or more postoperative PTH values above 200 ng/l. Recurrence detected within the first six months after surgery was defined as persistence [15]. Due to different target PTH levels depending on renal function, we analysed persistence and recurrence rates separately in patients who were on dialysis and patients who had a functioning kidney transplant. As secondary outcomes, we analysed the intraoperative PTH measurements and the peri- and postoperative morbidity and mortality.
For the descriptive statistics of the study population, we use medians and interquartile ranges (IQR) for continuous variables, and numbers and percentages for categorical variables. To describe the time to recurrence or persistence, we derived a Kaplan-Meier curve. As we were not testing any formal hypothesis, we did not perform power and sample size calculations. All statistical analyses were performed with the R software version 3.4.2 [19].
Over the course of the study period, 42 patients (20 women and 22 men) underwent PTX-AT at the University Hospital in Basel. Their median age at the time of operation was 53.6 years (42.1–62.4 years). Thirty-three patients (79%) were on dialysis and nine (21%) had a functioning kidney transplant. These nine patients all had tertiary hyperparathyroidism, as did five (15%) of the patients on dialysis. Relevant comorbidities at the time of operation were arterial hypertension in 20 patients (48%), anaemia in 13 patients (31%), diabetes mellitus in four patients (10%), obesity in four patients (10%), and calciphylaxis in two patients (5%).
Table 1 shows the patient characteristics, including the median preoperative PTH level, calcium level, and glomerular filtration rate (GFR), which was estimated using the Modification of Diet in Renal Disease (MDRD) formula.
Table 1 Patient characteristics.
|
All patients
(n = 42) |
On dialysis
(n = 33) |
Functioning KT
(n = 9) |
|
|---|---|---|---|
| Age, years* | 53.6 (42.1–62.4) | 53.0 (38.5–62.6) | 57.8 (47.2–61.9) |
| Female, n (%) | 20 (48%) | 14 (42%) | 6 (67%) |
| Tertiary hyperparathyroidism, n (%) | 14 (33%) | 5 (15%) | 9 (100%) |
| GFR, ml/min/1.73m2* | 5.6 (4.7–9.0) | 5.1 (4.4–6.1) | 44.4 (20.6–68.8) |
| PTH level, ng/l* | 780.8 (522.8–1,361.6) | 952.5 (635.0–1,555.3) | 427.8 (316.3–476.8) |
| Calcium level, mmol/l* | 2.46 (2.29–2.65) | 2.39 (2.28–2.62) | 2.88 (2.63–2.99) |
GFR = glomerular filtration rate; KT = kidney transplant; PTH = parathyroid hormone * Median (interquartile range)
Intraoperative measurements showed that serum PTH decreased to 6.9% (3.3–15.0%) of the preoperative baseline level. In five patients (11.9%), fewer than four glands (with histological confirmation) were found during the operation, despite performing thymectomy.
The median duration of postoperative hospitalisation was 4 days (2.8–6.0 days). Two patients (5%) suffered transient hoarseness, while laryngoscopy showed no abnormalities. No cases of postoperative paralysis of the recurrent laryngeal nerve were reported. Postoperative hypocalcaemia was reported in 24 patients (57%), but only three patients (7%) were symptomatic. All patients with hypocalcaemia were treated with oral calcium substitution.
A median of 13 (7–25) postoperative PTH measurements per patient were available for analysis. The median PTH level on the first postoperative day was 9.8 ng/l (3.0–42.2 ng/l), representing a decrease to 1.3% (0.3–5.2%) of preoperative baseline levels. After a median follow-up of 89.5 months (31.9–152.9 months), secondary hyperparathyroidism persisted in five patients (11.9%) and had recurred in four patients (9.5%), giving a total rate of recurrence or persistence of 21.4% (9/42). Of the five patients with persistence, three presented values higher than 200 ng/l on the first postoperative day, and the other two did so during the first six months. In the four patients with a recurrence, the median time to recurrence was 25.6 months (13.9–37.5 months), and all recurrences occurred within 48 months.
As shown in the Kaplan-Meier plot (fig. 1), the probability of remaining recurrence-free for six months was 0.88 (95% confidence interval [CI] 0.79–0.98); for one year, 0.86 (95% CI 0.76–0.97); and for five years, 0.76 (95% CI 0.64–0.92).

Figure 1 The Kaplan-Meier plot shows the probability of remaining recurrence free until a given point in time. The shaded areas represent the 95% confidence interval.
Of the 33 patients on dialysis, four (12.1%) had persistence, and three (9.1%) recurrence of secondary hyperparathyroidism (total n = 7, 21.2%). Of the nine patients with a functioning kidney transplant, we found one case of persistence (11.1%) and one of recurrence (11.1%) (total n = 2, 22.2%). Evaluating the postoperative calcium levels of the patients with a functioning kidney transplant, we did not find more cases of recurrence when recurrence was defined by hypercalcaemia.
Table 2 shows the recurrence/persistence rate and the median PTH and calcium levels at one day, six months, one year and five years after PTX-AT separately for the patients on dialysis and the patients with a functioning kidney transplant.
Table 2 Postoperative follow-up.
|
All patients
(n = 42) |
On dialysis
(n = 33) |
Functioning KT
(n = 9) |
||
|---|---|---|---|---|
| Recurrence/persistence, n (%) | 1 day | 3 (7.1) | 3 (9.1) | 0 (0.0) |
| 6 months | 5 (11.9) | 4 (12.1) | 1 (11.1) | |
| 1 year | 6 (14.3) | 4 (12.1) | 2 (22.2) | |
| 5 years | 9 (21.4) | 7 (21.2) | 2 (22.2) | |
| PTH level, ng/l* | 1 day | 9.8 (3.0–42.2) | 10.1 (3.0–66.2) | 8.5 (3.2–26.8) |
| 6 months | 42.2 (9.2–126.3) | 40.3 (6.7–74.5) | 120.0 (33.0–152.5) | |
| 1 year | 49.0 (23.7–151.0) | 57.0 (22.7–151.0) | 38.5 (34.5–93.0) | |
| 5 years | 67.5 (30.1–113) | 51.2 (27.5–113.0) | 73.9 (67.8–117.0) | |
| Calcium level, mmol/l* | 1 day | 1.98 (1.66–2.14) | 1.94 (1.64–2.14) | 2.04 (1.97–2.12) |
| 6 months | 2.12 (1.97–2.44) | 2.12 (1.87–2.41) | 2.42 (2.10–2.48) | |
| 1 year | 2.20 (2.10–2.32) | 2.21 (2.09–2.32) | 2.16 (2.13–2.27) | |
| 5 years | 2.25 (2.15–2.37) | 2.25 (2.10–2.36) | 2.29 (2.21–2.40) | |
KT = kidney transplant; PTH = parathyroid hormone * Median (interquartile range)
In three of the five patients who suffered persistence, no intraoperative PTH-measurements were performed, as this was not standard before 2006. In the other two patients, fewer than four glands were found despite intraoperative PTH-measurements and thymectomy. No persistence was observed among the 16 patients treated after the introduction of intraoperative PTH measurement as standard in 2006.
The nine patients with persistence or recurrence were managed as follows: five received a kidney transplant, two were treated with medication only, and two underwent re-operation, with the removal of parathyroid tissue from the neck in one patient and from the AT site on the forearm in the other.
Ten (24%) of the 42 patients died during follow-up. The median time from PTX to death was 53.6 months (25.2–89.8 months), and the one-year postoperative mortality was 4.8% (two patients). Three (33%) of the nine patients with persistence or recurrence died during follow-up.
More than seven years after PTX-AT, we found a total recurrence rate of 21.4%. More than half of the recurrences occurred during the first six months after surgery and were thus defined as persisting secondary hyperparathyroidism. The recurrence rate in our study was in line with those reported in the international literature (8–38%) [10, 12, 20, 21]. Interestingly, our patient cohort showed more cases of persistence (n = 5) than of recurrence (n = 4). All operations were performed by the same surgeon, who had extensive expertise in the field. Three of the five patients with persistence had no intraoperative measurement of PTH. After the introduction of intraoperative PTH measurement as standard procedure in 2006, no further cases of persistence were observed. We therefore consider intraoperative PTH measurement an important tool for preventing inadequate surgery in PTX-AT.
In the other two patients with secondary hyperparathyroidism persistence, intraoperative PTH measurements were made, but fewer than four glands were found despite PTX-AT with thymectomy being performed. Due to the glands’ embryological migration, ectopic parathyroid tissue can be found at sites other than the four classic gland locations, so preoperative diagnostic imaging might have led to a better outcome in these patients [22]. However, routine preoperative diagnostic imaging in secondary hyperparathyroidism remains controversial because it has low sensitivity for localising ectopic glands; it is mainly recommended for re-operations [5, 23].
Even in experienced hands, the intraoperative identification of parathyroid tissue remains challenging. Therefore, real-time intraoperative parathyroid autofluorescence detection using near-infrared fluorescence spectroscopy might be a promising new tool to help surgeons detect adenomatous and hyperplastic parathyroid glands with high sensitivity [24–26].
Although PTX ameliorates the symptoms of secondary hyperparathyroidism and increases patients’ quality of life and survival, the surgery is associated with perioperative risks [23, 27]. However, PTX is considered a low-risk procedure, with similar morbidity to thyroid surgery. The specific risks of PTX are recurrent laryngeal nerve palsy and postoperative hypocalcaemia. None of our patients suffered from postoperative paralysis of the recurrent laryngeal nerve. Although postoperative hypocalcaemia was reported in 24 patients, only three patients were symptomatic and required oral calcium substitution. Notably, none of the patients experienced major complications and there were no treatment-associated deaths. However, other studies have reported perioperative mortality rates during hospitalisation and up to 30 days after discharge of up to 2% for PTX [28]. Therefore, individual risk-benefit evaluations must be performed and indications for surgery should be discussed in a multidisciplinary setting. Our patients were treated according to the KDIGO guidelines, where surgery is reserved for patients who either have severe secondary hyperparathyroidism with high PTH levels or have tertiary hyperparathyroidism. The risk of cardiovascular events is relevant in such patients, and surgery offers a definitive treatment option.
According to the KDIGO guidelines, the targeted “ideal” PTH levels differ between patients on dialysis and patients with a functioning kidney transplant. The PTH levels, and especially the calcium levels, of patients with a functioning kidney transplant should be controlled more strictly than those of patients on dialysis. Using a uniform threshold of 200 ng/l as the definition of recurrence for all patients is therefore clearly a limitation of our study. However, this definition has been used before [15, 29], while others have defined a slightly higher cut-off of PTH values three times greater than normal [30]. According to the KDIGO guidelines, higher PTH levels can be tolerated in patients with end-stage CKD. For this group, the guidelines recommend that PTH levels are maintained at between “two to nine times higher than normal”, which would correspond to an upper threshold of 657 ng/l if calculated with our laboratory’s reference range of 10–73 ng/l. As a consequence, the recurrence rate may have been overestimated in the dialysis group of our study. On the other hand, the recurrence rate in the transplant group may have been underestimated because of the use of a uniform PTH threshold, as these patients had tertiary hyperparathyroidism, with lower PTH levels but clinically relevant hypercalcaemia, which may be considered recurrence. However, we evaluated the postoperative calcium levels of all the patients who had a functioning kidney transplant and found no more recurrences when recurrence was defined by hypercalcaemia [9].
Furthermore, our study was limited by the relatively small sample size. Nevertheless, we would like to emphasise that it is the largest study on recurrence of secondary hyperparathyroidism after PTX performed in Switzerland. Switzerland has a large number of hospitals relative to its population, so collecting the limited number of cases of PTX for secondary hyperparathyroidism together in a prospective national registry would aid outcome analysis and future high-quality research.
Even in experienced hands, recurrence of secondary hyperparathyroidism after PTX remains a serious problem, occurring in every fifth patient. In our experience, the introduction of intraoperative PTH measurement has helped to lower the rates of persistence and recurrence. Further reductions in the recurrence rate might be achieved with novel, more accurate pre- and intraoperative imaging techniques.
We would like to thank Prof. Dr. med. Jürg Steiger, Head of the Department of Nephrology and Transplantation Immunology at the University Hospital Basel, Switzerland, for granting us access to patient files. We would also like to thank Prof. Dr. med. Heiner C. Bucher MD MPH, Director of the Basel Institute for Clinical Epidemiology and Biostatistics, Department of Clinical Research, University Hospital Basel, Switzerland, for his comments on the manuscript.
No financial support and no potential conflict of interest relevant to this article was reported.
1 Silver J , Naveh-Many T . FGF-23 and secondary hyperparathyroidism in chronic kidney disease. Nat Rev Nephrol. 2013;9(11):641–9. doi:.https://doi.org/10.1038/nrneph.2013.147
2 Vervloet M . Renal and extrarenal effects of fibroblast growth factor 23. Nat Rev Nephrol. 2019;15(2):109–20. doi:.https://doi.org/10.1038/s41581-018-0087-2
3 Levin A , Bakris GL , Molitch M , Smulders M , Tian J , Williams LA , et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71(1):31–8. doi:.https://doi.org/10.1038/sj.ki.5002009
4 Block GA , Klassen PS , Lazarus JM , Ofsthun N , Lowrie EG , Chertow GM . Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–18. doi:.https://doi.org/10.1097/01.ASN.0000133041.27682.A2
5 Lorenz K , Bartsch DK , Sancho JJ , Guigard S , Triponez F . Surgical management of secondary hyperparathyroidism in chronic kidney disease--a consensus report of the European Society of Endocrine Surgeons. Langenbecks Arch Surg. 2015;400(8):907–27. doi:.https://doi.org/10.1007/s00423-015-1344-5
6 Ganesh SK , Stack AG , Levin NW , Hulbert-Shearon T , Port FK . Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12(10):2131–8.
7 Rostand SG , Drüeke TB . Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int. 1999;56(2):383–92. doi:.https://doi.org/10.1046/j.1523-1755.1999.00575.x
8 Pitt SC , Sippel RS , Chen H . Secondary and tertiary hyperparathyroidism, state of the art surgical management. Surg Clin North Am. 2009;89(5):1227–39. doi:.https://doi.org/10.1016/j.suc.2009.06.011
9 Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;(113):S1–130 .
10 Skinner KA , Zuckerbraun L . Recurrent secondary hyperparathyroidism. An argument for total parathyroidectomy. Arch Surg. 1996;131(7):724–7. doi:.https://doi.org/10.1001/archsurg.1996.01430190046012
11 Triponez F , Clark OH , Vanrenthergem Y , Evenepoel P . Surgical treatment of persistent hyperparathyroidism after renal transplantation. Ann Surg. 2008;248(1):18–30. doi:.https://doi.org/10.1097/SLA.0b013e3181728a2d
12 Iwamoto N , Sato N , Nishida M , Hashimoto T , Kobayashi H , Yamazaki S , et al. Low parathyroid hormone levels after parathyroidectomy reduce cardiovascular mortality in chronic hemodialysis patients. Clin Exp Nephrol. 2016;20(5):808–14. doi:.https://doi.org/10.1007/s10157-015-1208-x
13 Rothmund M , Wagner PK , Schark C . Subtotal parathyroidectomy versus total parathyroidectomy and autotransplantation in secondary hyperparathyroidism: a randomized trial. World J Surg. 1991;15(6):745–50. doi:.https://doi.org/10.1007/BF01665309
14 Casanova D , Sarfati E , De Francisco A , Amado JA , Arias M , Dubost C . Secondary hyperparathyroidism: diagnosis of site of recurrence. World J Surg. 1991;15(4):546–9, discussion 549–50. doi:.https://doi.org/10.1007/BF01675660
15 Chou F-F , Lee C-H , Chen H-Y , Chen J-B , Hsu K-T , Sheen-Chen S-M . Persistent and recurrent hyperparathyroidism after total parathyroidectomy with autotransplantation. Ann Surg. 2002;235(1):99–104. doi:.https://doi.org/10.1097/00000658-200201000-00013
16 Tominaga Y , Matsuoka S , Uno N , Tsuzuki T , Hiramitsu T , Goto N , et al. Removal of autografted parathyroid tissue for recurrent renal hyperparathyroidism in hemodialysis patients. World J Surg. 2010;34(6):1312–7. doi:.https://doi.org/10.1007/s00268-010-0412-9
17 Beck TM , Huber PR , Oertli D . Intraoperative parathormone measurement in patients with primary hyperparathyroidism: a prospective clinical study. Swiss Med Wkly. 2003;133(13-14):206–9.
18World Health Organization. WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy. Geneva; 2010.Available from: http://www.ncbi.nlm.nih.gov/books/NBK138650/
19R Core Team. A language and environment for statistical computing, R.C. Team, Editor 2015, R Foundation for Statistical Computing, Vienna, Austria.
20 Agha A , Loss M , Schlitt HJ , Scherer MN . Recurrence of secondary hyperparathyroidism in patients after total parathyroidectomy with autotransplantation: technical and therapeutic aspects. Eur Arch Otorhinolaryngol. 2012;269(5):1519–25. doi:.https://doi.org/10.1007/s00405-011-1776-7
21 Kievit AJ , Tinnemans JGM , Idu MM , Groothoff JW , Surachno S , Aronson DC . Outcome of total parathyroidectomy and autotransplantation as treatment of secondary and tertiary hyperparathyroidism in children and adults. World J Surg. 2010;34(5):993–1000. doi:.https://doi.org/10.1007/s00268-010-0446-z
22 Pattou FN , Pellissier LC , Noël C , Wambergue F , Huglo DG , Proye CA . Supernumerary parathyroid glands: frequency and surgical significance in treatment of renal hyperparathyroidism. World J Surg. 2000;24(11):1330–4. doi:.https://doi.org/10.1007/s002680010220
23 Xu D , Yin Y , Hou L , Dai W . Surgical management of secondary hyperparathyroidism: how to effectively reduce recurrence at the time of primary surgery. J Endocrinol Invest. 2016;39(5):509–14. doi:.https://doi.org/10.1007/s40618-015-0410-8
24 McWade MA , Paras C , White LM , Phay JE , Mahadevan-Jansen A , Broome JT . A novel optical approach to intraoperative detection of parathyroid glands. Surgery. 2013;154(6):1371–7, discussion 1377. doi:.https://doi.org/10.1016/j.surg.2013.06.046
25 Wolf HW , Grumbeck B , Runkel N . Intraoperative verification of parathyroid glands in primary and secondary hyperparathyroidism using near-infrared autofluorescence (IOPA). Updates Surg. 2019;71(3):579–85. doi:.https://doi.org/10.1007/s13304-019-00652-1
26 Ladurner R , Sommerey S , Arabi NA , Hallfeldt KKJ , Stepp H , Gallwas JKS . Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg Endosc. 2017;31(8):3140–5. doi:.https://doi.org/10.1007/s00464-016-5338-3
27 Komaba H , Taniguchi M , Wada A , Iseki K , Tsubakihara Y , Fukagawa M . Parathyroidectomy and survival among Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int. 2015;88(2):350–9. doi:.https://doi.org/10.1038/ki.2015.72
28 Ishani A , Liu J , Wetmore JB , Lowe KA , Do T , Bradbury BD , et al. Clinical outcomes after parathyroidectomy in a nationwide cohort of patients on hemodialysis. Clin J Am Soc Nephrol. 2015;10(1):90–7. doi:.https://doi.org/10.2215/CJN.03520414
29 Chen H-H , Lin C-J , Wu C-J , Lai C-T , Lin J , Cheng S-P , et al. Chemical ablation of recurrent and persistent secondary hyperparathyroidism after subtotal parathyroidectomy. Ann Surg. 2011;253(4):786–90. doi:.https://doi.org/10.1097/SLA.0b013e318211ccc2
30 Rajeev P , Lee KY , Tang XJ , Goo TT , Tan WB , Ngiam KY . Outcomes of parathyroidectomy in renal hyperparathyroidism in patients with No access to renal transplantation in Singapore. Int J Surg. 2016;25:64–8. doi:.https://doi.org/10.1016/j.ijsu.2015.11.005
Shared senior authorship
Study conception and design: LS, DO. Acquisition of data: LS, DO. Analysis and interpretation of data: all authors. Special statistical analysis of data: LS, GM. Writing the manuscript: all authors. Critical revision and final approval of the manuscript: all authors
No financial support and no potential conflict of interest relevant to this article was reported.