
Figure 1 Indication for WCD use (n = 207). Data are expressed as percentage of total population (n = 207). EF = ejection fraction; PCI = percutaneous coronary intervention; CABG = coronary artery bypass graft; HTX = heart transplantation
DOI: https://doi.org/10.4414/smw.2019.20136
Sudden cardiac death (SCD) is a catastrophic event caused by malignant ventricular tachyarrhythmias. Implantable cardioverter-defibrillator (ICD) therapy is indicated in selected patients for the prevention of SCD. Selection is mainly based on left ventricular function, namely systolic left ventricular ejection fraction (EF). Landmark trials showed that patients with severely reduced EF (<35%) and symptomatic heart failure have a survival benefit after implantation of an ICD [1, 2]. Too early implantation, on the other hand, has shown no benefit and is contraindicated [3, 4]. Therefore, 40- to 90-day waiting periods are recommended in patients after myocardial infarction (MI), revascularisation and newly diagnosed cardiomyopathy. Uncertainty remains as to which of these patients with reduced EF are nevertheless at elevated risk for SCD within this 40 to 90 days period.
In certain cases the risk of SCD is estimated to be high and temporary protection is deemed necessary. The wearable cardioverter-defibrillator (WCD, LifeVest, ZOLL Pittsburgh, PA) can provide temporary protection in such cases. Another possible indication for its use is the necessity for ICD explantation due to lead infection or malfunction, until re-implantation can ensue. The 2016 European guidelines outline the recommended use of the WCD in such selected cases [5]. Nonetheless, randomised controlled data are scarce in this area. The recently published VEST trial is to date the only randomised controlled trial investigating WCD use, although only patients with ischaemic heart disease (ischaemic cardiomyopathy; ICM) were investigated [6]. Therefore, contemporary real-world data including also non-ischaemic cardiomyopathy (NICM) patients may provide helpful information to close an apparent knowledge gap.
The WCD was approved in Switzerland in July 2014. The aim of this study was to provide first insights into the use of the WCD in Switzerland, with an emphasis on patient outcome.
This was a retrospective observational study reporting on initial experience with the use of the WCD in Switzerland. We report data collected at a tertiary medical care centre, the University Hospital Zurich, and its affiliated teaching hospital, the GZO Regional Health Centre in Wetzikon, Switzerland. All patients who received a WCD between its approval in June 2014 and February 2018 were screened for inclusion. Patients with incomplete data on WCD use or refusal to participate were excluded. The study was approved by the local ethics committee and conforms to the Declaration of Helsinki as revised in 2013. This study was conducted according to the STROBE statement.
The technical and practical aspects of the WCD have previously been reported [7]. In brief, arrhythmia recognition is based on an algorithm analysing heart rate and QRS morphology. If ventricular tachycardia or ventricular fibrillation detected, sequential acoustic and vibrational alarms are activated and an automatic event is registered and sent to the LifeVest Network via wireless connection. In the event of a haemodynamically stable tachyarrhythmia or a false alarm (absence of tachycardia), patients are instructed to activate the device’s response button to withhold shock administration. If the patient fails to push the response button (i.e., loss of consciousness), up to five biphasic shocks (75 to 150 Joules) may be delivered after detection of a 30-second arrhythmia and confirmation period. The default settings for the detection of ventricular tachycardia or fibrillation are 150 and 200 beats per minute, respectively. The device also has a bradycardia/asystole detection function, but provides no pacing capabilities.
Data were entered using the REDCap software (Vanderbilt, Nashville, TN, USA); access was provided by the Clinical Trials Centre Zurich. The time period for data collection was between May and November 2018. The electronic clinical records of all included patients were reviewed. Baseline characteristics included age, body mass index (BMI), sex, underlying heart disease, indication for WCD use, EF (measured with either echocardiography or magnetic resonance imaging), medical therapy and history of supraventricular tachycardia before first wearing the WCD. Underlying heart disease was categorised as ischaemic cardiomyopathy (ICM), non-ischaemic cardiomyopathy (NICM) and congenital/inherited heart disease according to the diagnosis made by the treating physicians. The indication for WCD use was categorised as follows:
The manufacturer provided data on WCD use from the WCD database. This included average wear-time calculated as the cumulative wear hours divided by the wear duration, total wear-days, number of events (automatic, manual), number of registered asystoles, number of (appropriate or inappropriate) therapies and the rhythm tracing of every event.
Patient outcome data were collected from electronic medical records. EF measurements with either echocardiography or magnetic resonance imaging after cessation of WCD use, reason for WCD discontinuation, incidence and type of device implantation after WCD use including devices without a defibrillator function (categorised as single-chamber ICD [SC-ICD]; dual-chamber ICD [DC-ICD]; subcutaneous ICD [S-ICD]; cardiac resynchronisation therapy [CRT] pacemaker [CRT-P] and CRT defibrillator [CRT-D]), time to device implantation after WCD discontinuation, number of appropriate and inappropriate therapies by the ICD, incidence of heart transplantation and number of patients deceased at the time of data collection of any cause. Clinical records were reviewed to determine the reason if no ICD was implanted.
Assessment of EF after WCD use was not routinely performed in patients with a normal EF before WCD use in the two institutions. The reason for WCD discontinuation was categorised as: (1) implantation of an ICD, (2) improvement of EF obviating ICD implantation, (3) termination of risk stratification without an indication for ICD implantation, (4) comfort issues and patient choice and (5) other reasons.
This was primarily a descriptive statistical analysis. Categorical variables were reported as frequencies (percentage), continuous variables as means ± standard deviation or as medians (interquartile range [IQR], range). Exploratory statistical analysis was performed by comparing continuous data using Student’s t test or the Mann-Whitney U test, as appropriate, and categorical data using Fishers exact test or the chi-square test depending on the number of groups. Correlation was calculated using Pearson’s coefficient. Cases with missing data were excluded from statistical analysis. A two sided p value <0.05 was considered statistically significant. All statistical analyses were conducted using SPSS version 21 (IBM Corp., Armonk, NY, USA).
A total of 227 patients wore a WCD between July 2014 and February 2018 and were screened for inclusion and exclusion criteria. A total of 207 patients were finally selected for our primary analysis. Five patients refused participation and 15 patients had incomplete WCD data because they were still wearing the device at the time of data collection, leading to their exclusion. The mean age of the study population was 58 ± 13 years, and 86% were male. Further baseline characteristics are shown in table 1.
Table 1 Baseline patient characteristics (n = 207).
| Variable | ||
|---|---|---|
| Age (years) | 58 ± 13 | |
| Women | 29 (14) | |
| Body mass index (kg/m2) | 27 ± 6 | |
| Baseline ejection fraction (%) | 32 ± 13 | |
| Primary underlying heart disease | Ischaemic | 124 (60) |
| Non-ischaemic | 74 (35) | |
| Congenital/inherited | 9 (5) | |
| Cardiovascular medication | Beta-blocker | 185 (89) |
| ACE inhibitor or ARB | 176 (85) | |
| Aldosterone antagonist | 114 (55) | |
| Sacubitril/Valsartan | 9 (4) | |
| Diuretic | 152 (73) | |
| Antiplatelet therapy | 128 (62) | |
| Vitamin K antagonist | 64 (31) | |
| Non-vitamin K antagonist oral anticoagulant | 32 (16) | |
| Statin | 135 (65) | |
| Amiodarone | 45 (22) | |
| Calcium channel blocker | 16 (8) | |
| Supraventricular tachycardia | Atrial fibrillation | 59 (29) |
| Atrial flutter | 21 (10) | |
| Other* | 6 (3) | |
| ACE = angiotensin-converting enzyme; ARB = angiotensin II receptor blocker Data are expressed as mean ± standard deviation or n (percentage). * Other supraventricular tachycardia: ectopic atrial tachycardia: atrioventricular (nodal) re-entry tachycardia. |
||
The most frequent indications for WCD use were either newly diagnosed ICM with EF ≤35% or NICM with EF ≤35%, followed by bridging either due to infection, planned heart transplantation or other reasons, and risk stratification in patients at presumed increased risk for SCD (fig. 1).

Figure 1 Indication for WCD use (n = 207). Data are expressed as percentage of total population (n = 207). EF = ejection fraction; PCI = percutaneous coronary intervention; CABG = coronary artery bypass graft; HTX = heart transplantation
Patients wore the WCD for a median of 62 days (IQR 35–95, range 1–355) with a median daily wear-time of 22.6 hours (IQR 19.9–23.2, range 2.3–23.8). There was a weak positive correlation between the number of days that the WCD was worn and the daily wear-time (r = 0.179, p = 0.01). The mean number of automatic events (thus an alarm) per patient was 23 ± 56. Patients with ICM had significantly more automatic alarms than the rest of the study population (31 vs 12, p = 0.017). There was no association between the mean number of automatic events and the prevalence of supraventricular tachycardia (18 vs 30, p = 0.197). The device registered a total of 86 asystole events in 5 patients. Of all asystole alarms registered in the cohort, 85 (99%) were due to undersensing of QRS complexes. The remaining one alarm was triggered by severe bradycardia. Eighty-one of these (94%) were registered in a single patient.
None of the patients had a fatal outcome during WCD use. Three patients (1.4%) received an appropriate shock by the WCD. The underlying arrhythmia was ventricular tachycardia in two patients (patients 59 and 205) and ventricular fibrillation in the third patient (patient 207), with successful termination by the first shock after 215, 89 and 63 seconds, respectively. The clinical characteristics and outcomes of these patients are shown in table 2. No inappropriate shocks were administered in our study population.
Table 2 Characteristics of patients with appropriate therapy by the WCD.
| Patient | Age (years) | Sex | Heart disease | Indication for WCD | EF (%) | Time to therapy (days) | Antiarrhythmic medication | Device implanted | Duration of follow-up (days) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 59 | 64 | Male | HNOCM | Device-explantation due to infection | 25 | 30 | Amiodarone | S-ICD | 713 | Deceased, no further therapies |
| 205 | 72 | Male | ICM, VHD | Device-explantation due to infection | 27 | 4 | Amiodarone, beta blocker | S-ICD | 45 | Appropriate shock by S-ICD |
| 207 | 60 | Male | DCM | EF <35% | 13 | 66 | Amiodarone, beta blocker | CRT-D | 546 | No further therapies |
DCM = dilated cardiomyopathy; HNOCM = hypertrophic non-obstructive cardiomyopathy; ICM = ischaemic cardiomyopathy; VHD = valvular heart disease; S-ICD = subcutaneous implantable cardioverter-defibrillator; CRT-D = cardiac resynchronisation therapy with defibrillator
Fifty-nine patients (28%) wore the device longer than the standard 90 days, with a median duration of 119 days (IQR 100–137, range 91–355). The most common underlying heart disease in these patients was ICM (34 patients, 57%). No shocks were given in any of these patients with prolonged WCD use.
Reasons for WCD discontinuation were implantation of an ICD (87 patients, 42%), improvement in EF obviating the indication for ICD implantation (64 patients, 31%), patient discomfort (17 patients, 8%) and other reasons (39 patients, 19%, including four patients for whom no follow-up was available). Patients stopping WCD use because of discomfort (n = 17, 8%) wore the device for a significantly shorter period (42 vs 72 days, p = 0.013), had a lower average daily wear-time (18 vs 21 hours per day, p = 0.003; fig. 2) and less often underwent ICD implantation (3/99 vs 13/104, p = 0.017), although there was no significant difference between the groups in baseline characteristics, in EF at follow-up (35 vs 38%, p = 0.34), or the number of automatic events (alarms) (19 vs 23, p = 0.781).
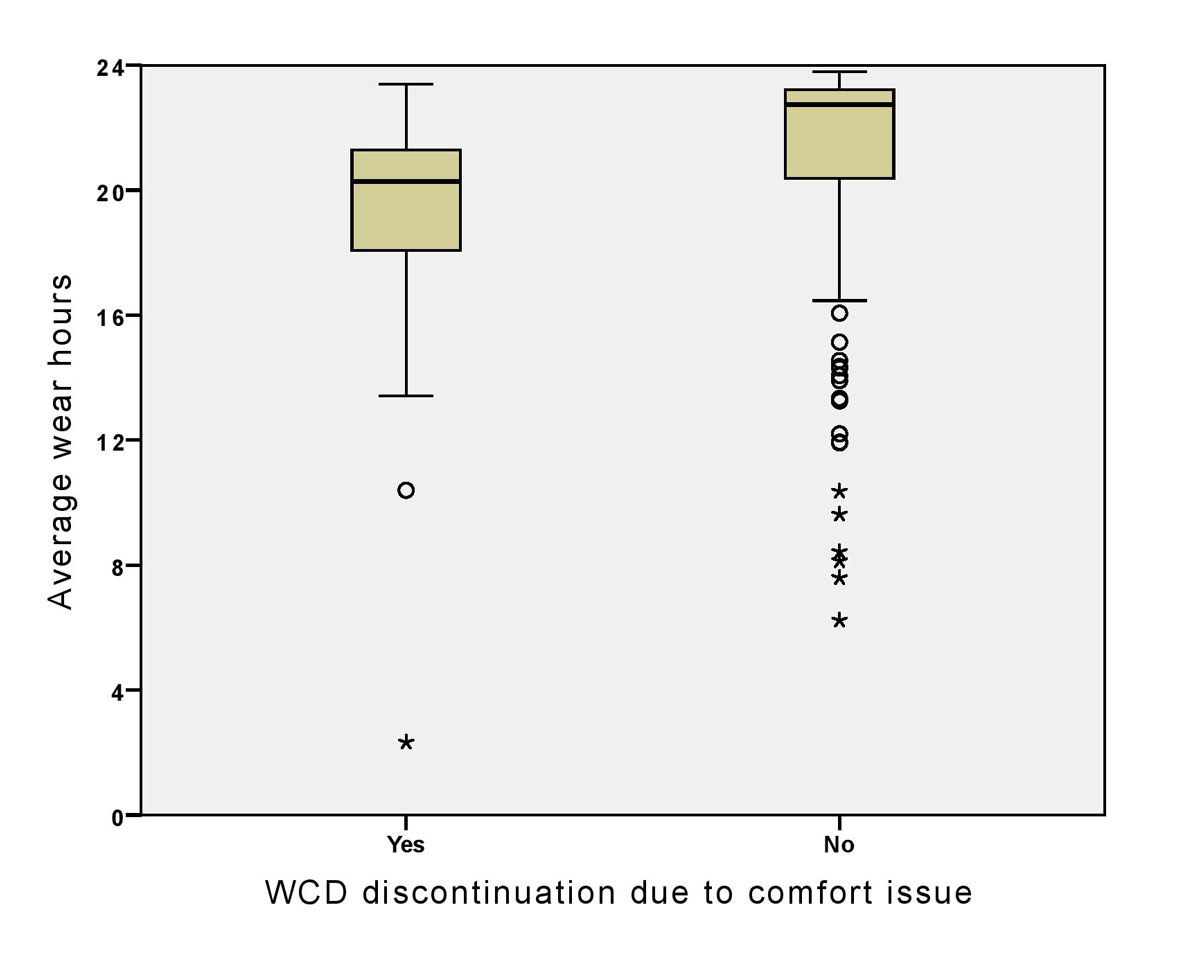
Figure 2 Distribution of mean wear hours by WCD discontinuation because of wear discomfort or other reasons.
At the time of WCD discontinuation, EF had improved by a mean of 7 ± 10% in patients for whom follow-up measurements were available (184 patients, 89% of total study population). Patients with ICM had significantly less improvement in EF than the rest of the study population (5 vs 9%, p = 0.012).
One hundred patients (48%) of the total population were implanted with a device (in one case without defibrillator function) after a median of 0 days (IQR 0–0, range 0–585) following WCD discontinuation. Thirteen patients (6%) stopped WCD use for reasons other than ICD implantation, but were still implanted with a device after a median of 71 days (IQR 21–144, range 0–585). The implanted devices were: 29 (14%) SC-ICD, 29 (14%) DC-ICD, 21 (10%) S-ICD, 20 (10%) CRT-D and 1 (0.5%) CRT-P. Median follow-up was 431 days (IQR 204–707, range 8–1237) for patients with device implantation. During this period, five patients (2.4%) had appropriate shocks, three patients appropriate antitachycardia pacing (ATP) intervention (1.4%) and two patients had inappropriate shocks (1%), Median time to ICD therapy was 110 days (IQR 23–421, range 6–1035).
Of the 103 patients who were not implanted with any device, 82 (79%) had no indication for device implantation due to EF recovery above 35% or completed risk stratification with no elevated arrhythmogenic risk, refused ICD implantation (n = 11, 10%) or had various reasons for not receiving an ICD such as technical reasons, or low life-expectancy (n = 10, 10%). Patients implanted with an ICD were older (60 vs 56 years, p = 0.024), had a lower EF at the time of WCD discontinuation (34 vs 42%, p <0.001) and significantly less improvement in EF from baseline (3 vs 11%, p <0.001). The remaining four patients of the total cohort (n = 207) had an unknown outcome after discontinuing WCD use and device implantation status was therefore missing.
During the observational period, three patients (5.3%) received a heart transplant for ICM, cardiac sarcoidosis and ICM/valvular heart disease. Of these patients, one received the WCD as bridge to heart transplantation, one as bridge after explantation of an infected ICD and one because of newly diagnosed ICM with EF <35%. Of these patients, one received an appropriate shock by the WCD and afterwards by the implanted S-ICD (patient 205 in table 2).
Fifteen patients of the total cohort (7.2%) died during the study period. The underlying heart disease was ICM in nine patients (60%), NICM in five patients (33%) and congenital heart disease in one patient (6.6%). Only one of these patients had appropriate WCD therapy (patient 205 in table 2). Ten out of these 15 patients (66%) had an ICD implanted as compared with the remaining patients (89/188, 47%), which did not significantly differ (p = 0.184). Eight of these ten had no treatment by the implanted device, one patient had an appropriate shock (patient 205 in table 2), and one patient had an appropriate ATP therapy. Exploratory statistical analysis revealed that deceased patients were less likely to take beta-blockers (10/15 vs 171/188, p = 0.004), wore the WCD for a shorter duration (46 vs 72 days, p = 0.046), and had a lower improvement in EF at follow-up (0.3. vs 7.8%, p = 0.011), but were otherwise similar in clinical characteristics to the patients still alive at the time of data collection.
This study has following main characteristics: it is the first report on the use of the WCD in Switzerland with a study population comparable to other larger studies in this topic; the data suggest that the WCD is safe and effective in the treatment of life-threatening ventricular arrhythmias; comfort issues with the use of the WCD affect a relevant subgroup of patients who possibly are at a higher risk for therapy non-adherence.
The most common indication for WCD use was low EF due to either newly diagnosed ICM (60%) or NICM (35%) in our study population. At the time of starting WCD use, guideline recommended medical therapy of heart failure had begun in the majority of patients (table 1) and EF had improved, obviating the need to implant an ICD in 39% of all patients at follow-up. No patients had a fatal outcome while using the WCD and no inappropriate shocks were administered. Furthermore, an appropriate shock rate of only 1.4% with effective termination of tachycardia suggests that the WCD is efficacious. This low rate of therapy, on the other hand, suggests that patient selection is key. Randomised controlled data are necessary to determine which patients truly benefit from the use of a WCD, and which patients’ risk of SCD is effectively elevated. The only randomised controlled trial investigating its use to date is the VEST trial [6]. It failed to show a significant reduction in arrhythmic death in patients with severely reduced EF during the early post-myocardial infarction phase with use of the WCD. Of note, the VEST study reported a similar appropriate shock rate to our study (1.9%). Appropriate shock rates reported in real-world registries are also similar, ranging from 1.5 to 4.7% [8–11]. These findings suggest that our Swiss study population is representative of an “average” WCD-wearing population.
In our cohort a high adherence (>22 hours per day) to WCD use was detected over a median wear-time of 2 months. The VEST trial reported a lower compliance rate at a median of 18 hours (IQR 3.8–22.7). Wässnig et al. reported on the largest European population with a history of WCD use to date [8]. They included 6043 patients with a history of WCD use for any indication. The proportion of patients with ICM was 26.9%. Adherence to the prescribed WCD was excellent at 23.1 hours (IQR 21–23.7). The discrepancy in adherence rates reported by Olgin et al. and Wässnig at al. and our findings is most likely due to the study design of the VEST trial. Compliance with WCD therapy is understandably higher in patients actively choosing this therapy option than in patients randomised to its use. In the real world, WCD use is often terminated in patients who do not wear the device owing to futility. Another finding of our study was that patients with longer WCD wear-times had a significantly higher therapy compliance, including permanent ICD implantation. This is in line with previous findings [8, 9, 12, 13] and may indicate a subgroup of patients who have a higher adherence to prescribed therapy. These patients may actually benefit from prolonged bridging with the goal of up-titration of optimal medical therapy to obviate the need for ICD implantation [14–17].
A clinically relevant issue with the use of the WCD is the discomfort reported by patients. In the literature, comfort issues commonly referred to skin rash caused by the vest or false alarms due to automatic event detection [18]. This can lead to reduced compliance and premature interruption of therapy. Published data on the comfort issue are scarce and reported rates of WCD discontinuation due to discomfort ranged from 7 to 22% [13, 18–21]. This is the first study to report on ICD implantation after discontinuation of WCD due to patient discomfort. Comfort issues were the primary cause of stopping WCD use in 8% of our total study population. These patients also wore the device for a shorter period and had significantly lower wear compliance (fig. 2). Furthermore, we found that significantly fewer of these patients were implanted with an ICD in spite of similar underlying heart disease. Although none of these patients died during follow-up, the lower rate of ICD implantation is noteworthy. A third of them were not implanted as a result of patient choice, despite an existing indication according to current guidelines. Effort should be made to identify such patients early as they may be at increased risk for adverse outcome. This subset may especially benefit from proper patient education and support. In our opinion, beside the underlying cardiac condition, assessment of patients’ adherence prior to WCD use is key for selection. A benefit from its use is only present when patients truly wear it and, according to these findings, this wear rate could have an influence on further management.
To our knowledge, our study is the largest to date reporting on both ICD implantation rate and the type of device implanted. Reported implantation rates after WCD use ranged from 32 to 55% [9, 10, 16, 17, 22]. In our study, 48% of all patients were eventually implanted with an ICD. Over half of these patients were implanted with a transvenous ICD, a quarter received an S-ICD and another quarter a CRT-D. In comparison, Duncker et al. reported a lower (37%) implantation rate, 29% of which were CRT-D (the remaining were ICDs of any type) [16]. Interestingly, Röger at al. reported a similar 51% implantation rate, of which 22% were CRT-D. They however also chose an S-ICD in 55% of implanted cases [10]. They justified the high rate of S-ICD implantation by analysing the rate of bradyarrhythmias with the WCD. Even though the baseline characteristics in both studies were similar, it is unclear how many patients in the study of Röger et al. were implanted owing to an elevated risk of bloodstream infections, which is a common reason for subcutaneous device implantation. Ultimately, the most probable reason for the different ICD implantation rates is institutional and reimbursement differences. Although the S-ICD is a valuable alternative with probably fewer lead-associated complications such as blood stream infections, the lack of brady- or tachypacing can be a disadvantage of this device [23]. In our study population, 19% of S-ICDs were implanted specifically after a lead-associated infection.
Patients with a fatal outcome during follow-up wore the WCD for a significantly shorter time. They had a rate of administered WCD and ICD therapies comparable to those of patients without a fatal outcome. These findings suggest a high portion of non-arrhythmic deaths, similar to reports in the VEST trial. The significance of non-arrhythmic death in these severely ill patients has previously been examined in the DINAMIT and IRIS studies for ICM, and in the DEFINITE trial in NICM [3, 4, 24]. This again highlights the necessity of more randomised controlled trials to facilitate patient selection for the use of the WCD. Predictors of mortality have recently been identified by Kutyifa et al. [25]. They found that a history of syncope, lack of beta-blocker use and congenital/inherited heart disease vs other indications were associated with an increased mortality. Our findings were similar only with regard to beta-blocker use, since fewer patients with fatal outcomes took beta-blockers at baseline. The reason for the shorter WCD wear-time in these patients in our cohort, however, is unclear.
The major limitation of this study was that cause of death was often not available and hence not systematically assessed. This is largely attributed to the retrospective study design. Furthermore, for the same reason, loss of data is a possibility although patients prescribed a WCD are usually subject to close and regular follow-up. For the unconfounded assessment of mortality in patients using a WCD, more randomised controlled data are needed.
In conclusion, although the WCD is safe and effective in terminating malignant ventricular arrhythmias, a substantial subgroup of patients discontinued WCD use prematurely owing to comfort issues. This subset of patients may have an increased risk for adverse outcome. Efforts should be made to identify these patients so that early interventions through patient education and close follow-up can prevent a deleterious outcome.
The authors thank for the support of ZOLL in providing data on device usage from the LifeVest Network.
No external funding was received for this study. None of the authors have any conflict of interests related to this study to declare.
1 Moss AJ , Zareba W , Hall WJ , Klein H , Wilber DJ , Cannom DS , et al.; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–83. doi:.https://doi.org/10.1056/NEJMoa013474
2 Bardy GH , Lee KL , Mark DB , Poole JE , Packer DL , Boineau R , et al.; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–37. doi:.https://doi.org/10.1056/NEJMoa043399
3 Hohnloser SH , Kuck KH , Dorian P , Roberts RS , Hampton JR , Hatala R , et al.; DINAMIT Investigators. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351(24):2481–8. doi:.https://doi.org/10.1056/NEJMoa041489
4 Steinbeck G , Andresen D , Seidl K , Brachmann J , Hoffmann E , Wojciechowski D , et al.; IRIS Investigators. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361(15):1427–36. doi:.https://doi.org/10.1056/NEJMoa0901889
5 Priori SG , Blomström-Lundqvist C , Mazzanti A , Blom N , Borggrefe M , Camm J , et al.; ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36(41):2793–867. doi:.https://doi.org/10.1093/eurheartj/ehv316
6 Olgin JE , Pletcher MJ , Vittinghoff E , Wranicz J , Malik R , Morin DP , et al.; VEST Investigators. Wearable Cardioverter-Defibrillator after Myocardial Infarction. N Engl J Med. 2018;379(13):1205–15. doi:.https://doi.org/10.1056/NEJMoa1800781
7 Kovacs B , Reek S , Krasniqi N , Eriksson U , Duru F . Die tragbare Defibrillatorweste. Swiss Med Forum. 2018;18:509–12. doi:https://doi.org/10.4414/smf.2018.03301
8 Wäßnig NK , Günther M , Quick S , Pfluecke C , Rottstädt F , Szymkiewicz SJ , et al. Experience With the Wearable Cardioverter-Defibrillator in Patients at High Risk for Sudden Cardiac Death. Circulation. 2016;134(9):635–43. doi:.https://doi.org/10.1161/CIRCULATIONAHA.115.019124
9 Leyton-Mange JS , Hucker WJ , Mihatov N , Reynolds M , Albert C , Lubitz SA , et al. Experience With Wearable Cardioverter-Defibrillators at 2 Academic Medical Centers. JACC Clin Electrophysiol. 2018;4(2):231–9. doi:.https://doi.org/10.1016/j.jacep.2017.09.180
10 Röger S , Rosenkaimer SL , Hohneck A , Lang S , El-Battrawy I , Rudic B , et al. Therapy optimization in patients with heart failure: the role of the wearable cardioverter-defibrillator in a real-world setting. BMC Cardiovasc Disord. 2018;18(1):52. doi:.https://doi.org/10.1186/s12872-018-0790-8
11 Barsheshet A , Kutyifa V , Vamvouris T , Moss AJ , Biton Y , Chen L , et al. Study of the wearable cardioverter defibrillator in advanced heart-failure patients (SWIFT). J Cardiovasc Electrophysiol. 2017;28(7):778–84. doi:.https://doi.org/10.1111/jce.13229
12 Kutyifa V , Moss AJ , Klein H , Biton Y , McNitt S , MacKecknie B , et al. Use of the wearable cardioverter defibrillator in high-risk cardiac patients: data from the Prospective Registry of Patients Using the Wearable Cardioverter Defibrillator (WEARIT-II Registry). Circulation. 2015;132(17):1613–9. doi:.https://doi.org/10.1161/CIRCULATIONAHA.115.015677
13 Chung MK , Szymkiewicz SJ , Shao M , Zishiri E , Niebauer MJ , Lindsay BD , et al. Aggregate national experience with the wearable cardioverter-defibrillator: event rates, compliance, and survival. J Am Coll Cardiol. 2010;56(3):194–203. doi:.https://doi.org/10.1016/j.jacc.2010.04.016
14 Kovacs B , Reek S , Krasniqi N , Eriksson U , Duru F . Extended Use of the Wearable Cardioverter-Defibrillator: Which Patients Are Most Likely to Benefit? Cardiol Res Pract. 2018;2018:7373610. doi:.https://doi.org/10.1155/2018/7373610
15 Kutyifa V , Vermilye K , Daimee UA , McNitt S , Klein H , Moss AJ . Extended use of the wearable cardioverter-defibrillator in patients at risk for sudden cardiac death. Europace. 2018;20(FI2):f225–32. doi:.https://doi.org/10.1093/europace/euy091
16 Duncker D , König T , Hohmann S , Bauersachs J , Veltmann C . Avoiding Untimely Implantable Cardioverter/Defibrillator Implantation by Intensified Heart Failure Therapy Optimization Supported by the Wearable Cardioverter/Defibrillator-The PROLONG Study. J Am Heart Assoc. 2017;6(1):e004512. doi:.https://doi.org/10.1161/JAHA.116.004512
17 Duncker D , König T , Hohmann S , Bauersachs J , Veltmann C . Ventricular arrhythmias in patients with newly diagnosed nonischemic cardiomyopathy: Insights from the PROLONG study. Clin Cardiol. 2017;40(8):586–90. doi:.https://doi.org/10.1002/clc.22706
18 Ettinger S , Stanak M , Szymański P , Wild C , Tandara Haček R , Erčević D , et al. Wearable cardioverter defibrillators for the prevention of sudden cardiac arrest: a health technology assessment and patient focus group study. Med Devices (Auckl). 2017;10:257–71. doi:.https://doi.org/10.2147/MDER.S144048
19 Feldman AM , Klein H , Tchou P , Murali S , Hall WJ , Mancini D , et al.; WEARIT investigators and coordinators; BIROAD investigators and coordinators. Use of a wearable defibrillator in terminating tachyarrhythmias in patients at high risk for sudden death: results of the WEARIT/BIROAD. Pacing Clin Electrophysiol. 2004;27(1):4–9. doi:.https://doi.org/10.1111/j.1540-8159.2004.00378.x
20 Lamichhane M , Gardiner JC , Bianco NR , Szymkiewicz SJ , Thakur RK . National experience with long-term use of the wearable cardioverter defibrillator in patients with cardiomyopathy. J Interv Card Electrophysiol. 2017;48(1):11–9. doi:.https://doi.org/10.1007/s10840-016-0194-6
21 Kao AC , Krause SW , Handa R , Karia D , Reyes G , Bianco NR , et al.; Wearable defibrillator use In heart Failure (WIF) Investigators. Wearable defibrillator use in heart failure (WIF): results of a prospective registry. BMC Cardiovasc Disord. 2012;12(1):123. doi:.https://doi.org/10.1186/1471-2261-12-123
22 Erath JW , Vamos M , Sirat AS , Hohnloser SH . The wearable cardioverter-defibrillator in a real-world clinical setting: experience in 102 consecutive patients. Clin Res Cardiol. 2017;106(4):300–6. doi:.https://doi.org/10.1007/s00392-016-1054-1
23 Basu-Ray I , Liu J , Jia X , Gold M , Ellenbogen K , DiNicolantonio J , et al. Subcutaneous Versus Transvenous Implantable Defibrillator Therapy: A Meta-Analysis of Case-Control Studies. JACC Clin Electrophysiol. 2017;3(13):1475–83. doi:.https://doi.org/10.1016/j.jacep.2017.07.017
24 Kadish A , Dyer A , Daubert JP , Quigg R , Estes NAM , Anderson KP , et al.; Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151–8. doi:.https://doi.org/10.1056/NEJMoa033088
25 Kutyifa V , Moss AJ , Klein HU , McNitt S , Zareba W , Goldenberg I . One-year follow-up of the prospective registry of patients using the wearable defibrillator (WEARIT-II Registry). Pacing Clin Electrophysiol. 2018;41(10):1307–13. doi:.https://doi.org/10.1111/pace.13448
No external funding was received for this study. None of the authors have any conflict of interests related to this study to declare.