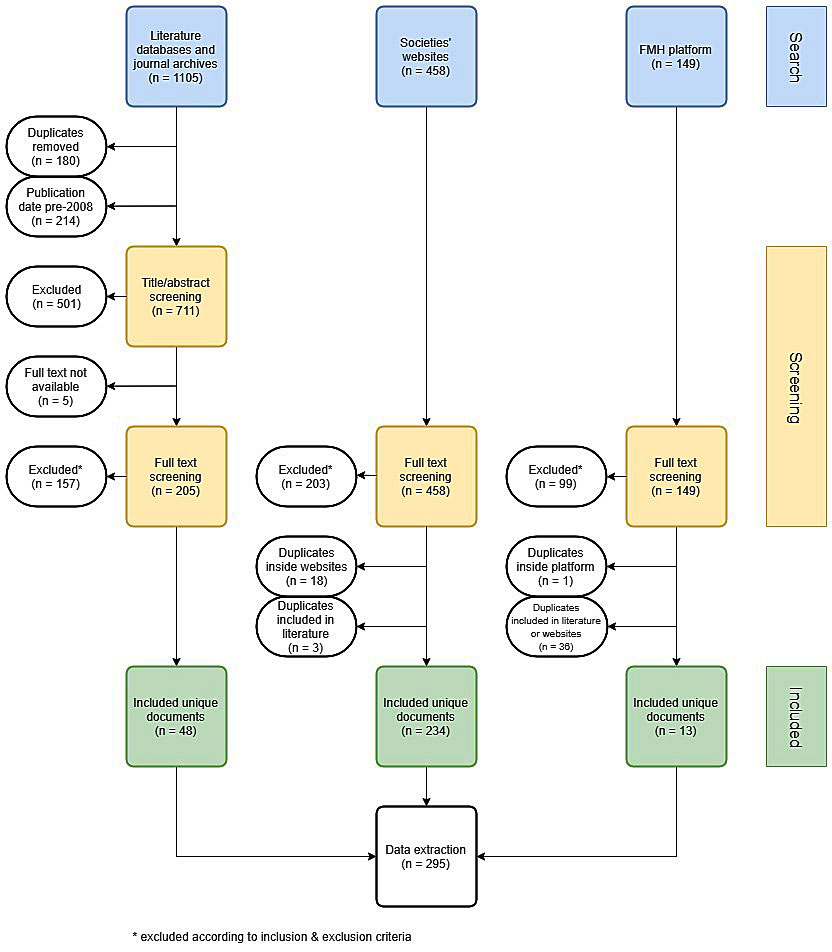
Figure 1 PRISMA flowchart of guideline search, screening and inclusion.
DOI: https://doi.org/10.4414/smw.2019.20134
Clinical practice guidelines (CPGs) provide systematically developed recommendations for medical doctors with the aim of optimising patient care [1, 2]. Their development in Switzerland is promoted by the Swiss Medical Association (FMH), as well as by the Swiss Academy of Arts and Sciences [3, 4]. Both organisations recognise medical societies as key players in CPG adaptation, adoption and development.
In contrast to some neighbouring countries, Switzerland has no single central institution to coordinate national CPG development [5]. Therefore, there is currently no comprehensive source of all national guidelines. Apart from a survey in 2001 [6], the overall CPG development efforts of Swiss medical societies have rarely been studied. With the aim to improve transparency and to interconnect CPG developers, FMH launched a platform for documenting Swiss CPGs in 2017 [7]. The submission of CPGs to the platform is voluntary, and it is unknown what fraction of all relevant CPGs is collected. A systematic effort to review all national guidelines has been undertaken in a few other countries [8], often limiting the scope to high quality [9] or single specialty [10] CPGs. No such systematic review of similar extent exists for Switzerland.
The aim of this study was to collect and review all current clinical practice guidelines developed or endorsed in Switzerland by medical societies. To achieve this, we systematically searched the scientific literature, Swiss medical societies’ websites and the FMH platform for guidelines. To capture the full range of guidelines, we used a broad definition of clinical practice guidelines and extracted indicators of their development, transparency and reporting methods.
The study was completed in two stages. In the first part of the study, we completed a systematic search for Swiss CPGs. Secondly, we extracted development and reporting data from the retrieved CPGs, and analysed the results. The study used principles of a systematic review and adhered to the reporting standards proposed in the PRISMA statement [11].
A broad search was performed to obtain a comprehensive overview of all CPGs developed or endorsed by Swiss medical societies. It included four sources: two literature search engines (PubMed and OVID), the archives of two major non-indexed Swiss medical journals (Schweizerische Ärztezeitung and Swiss Medical Forum), medical societies’ websites and the FMH guidelines platform at guidelines.fmh.ch. A list of synonyms of the word “guideline” in English, German, French and Italian was created. This CPG keyword list was used in the literature and journal archives search. Both searches were performed in June 2018. In PubMed and OVID, results were limited to publication date from 2008. From the journal archives, all articles containing a CPG keyword in the title were retrieved. A primary list of medical societies was compiled from the societies listed on the FMH website [12]. On the websites of these societies, a hand search was performed to identify additional sub- or inter-speciality societies. All collected websites were then searched for potential CPGs by a protocol-assisted hand search in September 2018. If a potential CPG was available in multiple languages, only one version was retrieved. Priority was given to English documents, followed by German, French and Italian. All guidelines submitted to the FMH platform were retrieved in November 2018. Documents that were not openly accessible were ignored. Items for which no full text document was found via the respective literature database are reported in figure 1. The search query for PubMed, strategy for the websites and the list of websites searched are given in appendix 1.
The selection criteria were formulated by all three authors (LB, AU, HD) and are analogous to the ones used in an Australian study [8]. A guideline was considered as endorsed if listed on the website of a society or submitted to the FMH platform by a medical society.
For inclusion, documents had to: (1) include recommendations intended to optimise patient care provided by medical doctors, (2) address specific clinical circumstances and (3) be produced, adapted or endorsed by a public Swiss national medical speciality society (“Fachgesellschaft”). Documents were excluded if they (1) had a publication date before 1 January 2008, or were (2) publications on dental medicine, (3) dietary recommendations for the general public or not meant primarily for advice given to patients by medical doctors, (4) public health recommendations aimed to optimise non-individualised prevention measures, the healthcare system or population health or (5) general advice on physician services and conduct, without specified clinical circumstances or patient population.
The screening was conducted by two authors (AU, LB) separately, disagreements were settled bilaterally or, if necessary, by a decision of the third author (HD). Search results from literature databases and journal archives were first screened by title and abstract, then a full text screening for all sources was performed.
The search and screening process is depicted in figure 1.
The extracted variables were based on adapted “Key Components of High-Quality and Trustworthy Guidelines”, proposed by the Guidelines International Network [13], and were predefined before data extraction. The variables correspond broadly to guideline development (e.g., publication and expiry dates), transparency (e.g., declaration of conflicts of interest and financial support) and methods (e.g., reporting of references and rating of evidence or recommendations). We also extracted guideline-signalling keywords from the title, and the health area reflected by the guideline (based on categories used in a similar review [8]).
The following categorical variables were extracted: health area reflected by the guideline, keyword in title, country(ies) of origin of the authoring societies and financial support for guideline development. Furthermore, the following binary (yes/no) variables were extracted: reporting of the publication and expiry dates, authors, their conflicts of interest, any methods of guideline development, decision making process, references, review of evidence, and rating of evidence and recommendations. Data were entered in a custom-made Excel document as a selection from predefined options for each variable. Data extraction was tested by reviewers in duplicate on a set of ten and subsequently 30 guidelines, to clarify the definitions of variables.
Data were extracted by two reviewers in duplicate (AU and LB) for sets of 30 guidelines sequentially. Inter-rater agreement of data entries was calculated, and the discordant data entries were resolved by discussion among the authors. The process was repeated until a pre-specified inter-rater agreement above 95% before the discussion was reached. After the second set of 30 guidelines, 95.5% agreement was reached, and the remaining guidelines were randomly assigned for data extraction to a single reviewer (AU or LB).
A single source of the finally selected set of guidelines (literature search, societies’ websites or FMH guideline database) was recorded in the review.
Extracted data were analysed using Microsoft Excel and R [14].
The PRISMA flowchart of the guideline selection is shown in figure 1. We identified 1105, 458 and 149 documents in the literature, websites and FMH platform, respectively. After removal of duplicates and screening for the inclusion and exclusion criteria, 295 documents were included in the final analysis. From these, 199 were found only on the societies’ websites, 44 only in the literature and 13 only in the FMH platform. Thirty-nine guidelines were found on at least two of these sources. The distribution of guidelines by their source is shown in figure 2. A list of the included guidelines is provided in appendix 2.

Figure 1 PRISMA flowchart of guideline search, screening and inclusion.
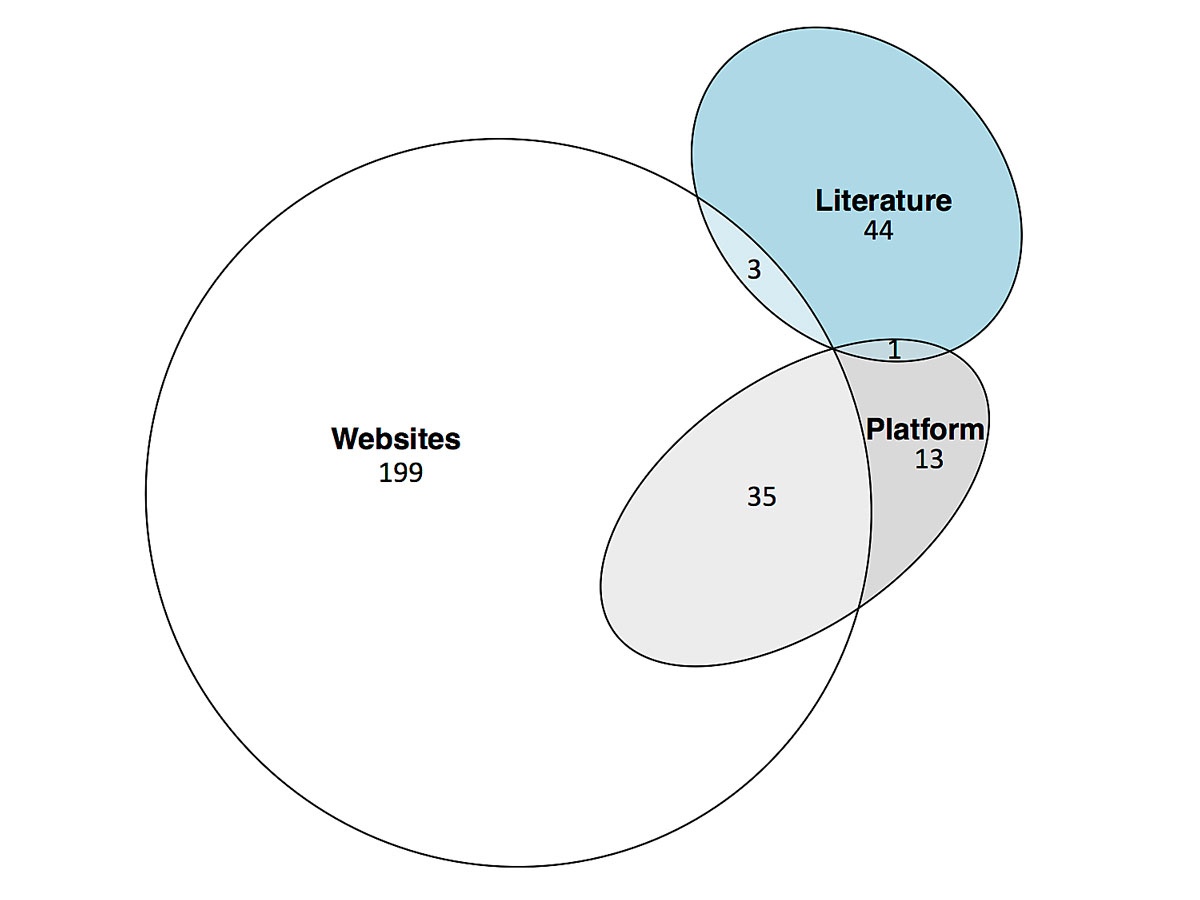
Figure 2 Sources of the guidelines included in the review.
Literature – scientific literature databases and archives of two major non-indexed Swiss medical journals, Platform – FMH guidelines platform guidelines.fmh.ch, Websites – Swiss medical societies’ websites.
In total, 159 (54%) of the included CPGs had at least one predefined keyword in the title. “Empfehlung” and “guideline” were the most frequent keywords, mentioned 63 (21%) and 46 (16%) times, respectively. Health areas with the most numerous corresponding CPGs were cardiovascular and infectious diseases, 55 (19%) and 52 (18%) of CPGs, respectively (table 1). Seventy-six CPGs were initially assigned to the category “other”, and upon reviewing included rheumatology (22), neurology (8), anaesthesiology and reanimatology (8), gynaecology (7), allergology (6), dermatology (5), palliative care (5), and other specialties (15) CPGs. Overall, 212 (72%) of included CPGs were developed by Swiss medical societies or authors affiliated with Swiss medical institutions, 6 (2%) with cooperation of German physician societies only, 23 (8%) with cooperation of German and Austrian physician societies, and 54 (18%) were developed by European, American or other physician societies, with or without explicit contribution from Switzerland.
Table 1 Health area and country(ies) of origin.
| Health area | Developed in | Total | |||
|---|---|---|---|---|---|
| Switzerland | Cooperation with German society | Cooperation with German and Austrian society |
Europe, USA
or other |
||
| Cancer | 10 (56%) | 0 (0%) | 2 (11%) | 6 (33%) | 18 |
| Cardiovascular disease | 16 (29%) | 0 (0%) | 1 (2%) | 38 (69%) | 55 |
| Diabetes | 19 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 19 |
| Drugs and alcohol | 2 (50%) | 0 (0%) | 1 (25%) | 1 (25%) | 4 |
| Infectious diseases | 49 (94%) | 0 (0%) | 2 (4%) | 1 (2%) | 52 |
| Injury | 3 (75%) | 0 (0%) | 0 (0%) | 1 (25%) | 4 |
| Mental health | 19 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 19 |
| Musculoskeletal disease | 6 (86%) | 0 (0%) | 1 (14%) | 0 (0%) | 7 |
| Non-infectious pulmonary disease | 12 (80%) | 1 (7%) | 1 (7%) | 1 (7%) | 15 |
| Obesity | 3 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 |
| Pregnancy and childbirth | 13 (62%) | 0 (0%) | 6 (29%) | 2 (10%) | 21 |
| Renal disease | 2 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 |
| Other | 58 (76%) | 5 (7%) | 9 (12%) | 4 (5%) | 76 |
| Total | 212 (72%) | 6 (2%) | 23 (8%) | 54 (18%) | 295 |
Guidelines reflecting different health areas had differing distributions by country of origin (table 1). Cardiovascular diseases guidelines were developed by international societies or in cooperation with other countries frequently (69% of cardiovascular guidelines), whereas pregnancy and childbirth guidelines were developed in cooperation with Austrian and German physician societies frequently (29% of the guidelines).
Reporting of guideline development was incomplete in many of the guidelines (table 2). At least one guideline author (member of the guideline developing team) was reported in 246 (83%), and the publication date in 276 (94%) of the CPGs. However, an expiry date was provided in only 48 (16%). A conflict of interest statement was provided directly or via a reference in 139 (44%), and a declaration of financial support for the guideline development in 86 (29%) of the included guidelines. Of these 86 guidelines, 19 (22%) reported no financial support, 10 (12%) support from industry and 57 (66%) support from non-industry related sources.
Table 2 Quality and transparency indicators.
| Total number of CPGs = 295 | ||
|---|---|---|
| Yes | No | |
| One or more members of guideline development group listed | 246 (83%) | 49 (17%) |
| Conflict of interest statement | 129 (44%) | 166 (56%) |
| Publication date | 276 (94%) | 19 (6%) |
| Expiration date | 48 (16%) | 247 (84%) |
| Declaration of funding/other support | 86 (29%) | 209 (71%) |
| References | 239 (81%) | 56 (19%) |
| Systematic review of evidence | 29 (10%) | 266 (90%) |
| Decision-making process described | 36 (12%) | 259 (88%) |
| Any method described | 165 (56%) | 130 (44%) |
| Rating of evidence | 68 (23%) | 227 (77%) |
| Rating of recommendation | 60 (20%) | 235 (80%) |
Any method of guideline development was reported in 165 (56%) guidelines (table 2). A total of 239 (81%) guidelines provided at least some references to the evidence and evidence syntheses used for the guideline development, but only 29 (10%) claimed in the methods to have used a systematic review of evidence. Thirty-six (12%) of CPGs described some procedure of decision making to reach a consensus in the guideline recommendation statements (13 Nominal group, 5 Delphi, 18 modified, combined or other methods). A system of evidence quality rating was defined and used in 68 (23%), and a system of recommendation strength rating in 60 (20%) CPGs.
Keywords in the title were associated with different frequencies of using the methodology of a systematic review (fig. 3). A systematic review was performed in CPGs titled “Leitlinie” most frequently (58% of “Leitlinie”). Country of origin was also associated with the type of evidence review (fig. 4). Guidelines developed in Switzerland (without international cooperation) reported a systematic review of evidence least frequently (2%), and it was more prevalent with an international cooperation between Switzerland, Germany and Austria or other countries (fig. 4).
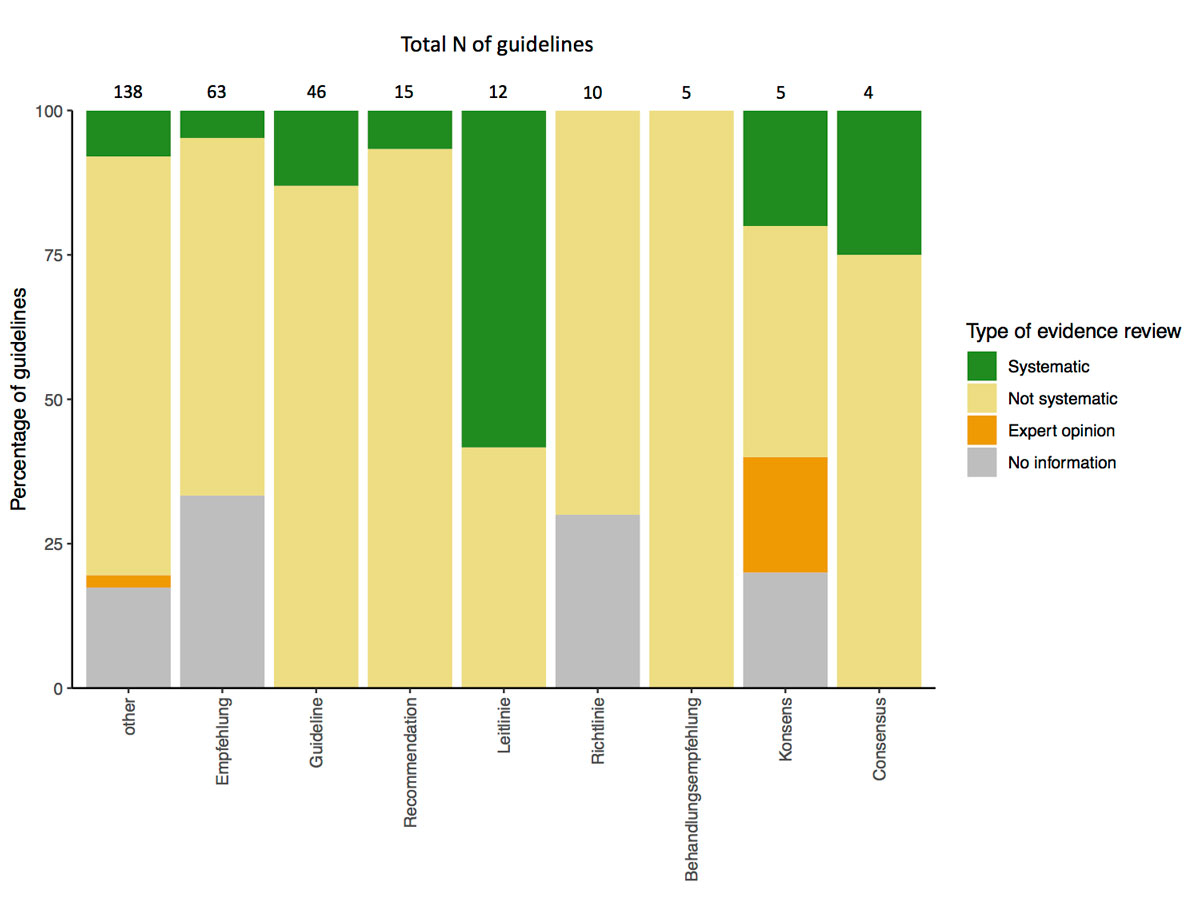
Figure 3 Keyword in title of guideline and review of evidence
Only keywords with more than two corresponding guidelines included are shown (“guida” and “racommandation” had a single guideline each). Three CPGs had two keywords in the title and were assigned for both categories in the table.
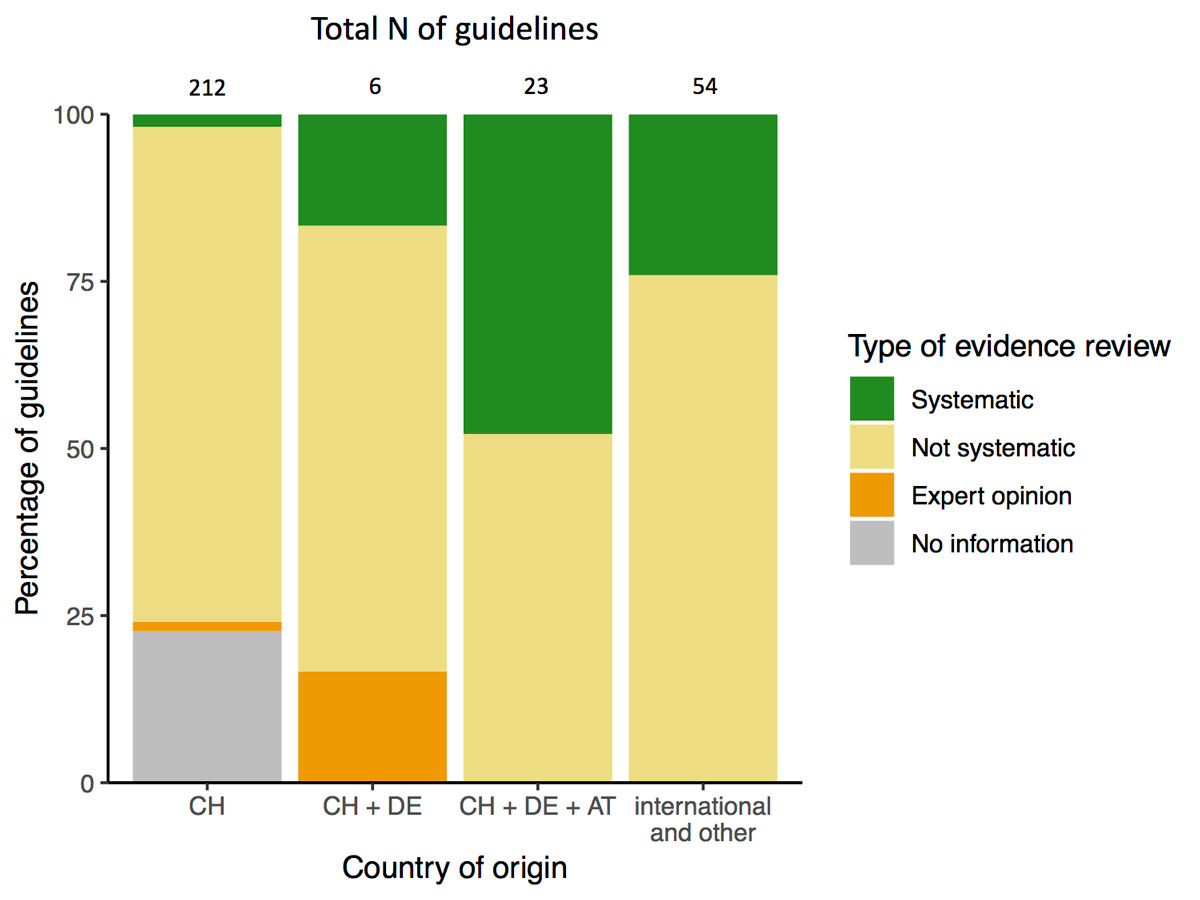
Figure 4 Country of origin and review of evidence.
We identified 295 clinical practice guidelines (CPGs) developed or endorsed by Swiss medical societies and published after 2008. The majority of CPGs reported basic elements of development (at least one author, publication date and at least some references). However, many other key components of high-quality CPGs [13], such as reporting of conflict of interests of authors and financial support for the guideline, a systematic review of evidence, the decision-making process, and rating of evidence or recommendations, were present only in a minority of the analysed CPGs.
Because of different study methodologies, the total number of CPGs found is difficult to compare with those in other studies. In 2001, a survey of 46 Swiss medical societies reported 84 guidelines produced or endorsed in Switzerland [6]. In Australia, 313 CPGs were identified [8], including regional CPGs and guidelines produced by government agencies. A study in Argentina identified 144 CPGs when guidelines with no development method described were excluded [9]. The number of CPGs identified depends also on the time span analysed (2008–2018 in our study, 1974–1999 in [6], 2003–2007 in [8] and 1994–2004 in [9]).
For inclusion in our study, documents did not have to fully meet the often cited American Institute of Medicine definition of a CPG [1], which implies a systematic review of evidence and an assessment of benefit and harms of alternative care options. Applying such a strict definition might only give a limited view on guidelines in the broader sense – documents intended to optimise patient care provided by physicians. However, we did restrict the analysis to CPGs developed or endorsed by national medical societies, excluding guidelines authored by physician networks, cantonal bodies or hospitals. Similarly, “Choosing Wisely” lists were not the focus of our study. Still, six of these lists of recommendations are included in this review. Admittedly, such guidelines could have a significant local impact on medical practice. The analysis of these CPGs, as well as the uptake and applicability of the different types of guidelines in Switzerland, will have to be explored in future studies.
Most CPGs were found on websites only (see fig. 2), potentially rendering them difficult to find for researchers, practitioners and policy makers. During the search process in this study, we observed that CPGs on societies’ websites are not always easily found (e.g., archived in various and sometimes non-intuitive subsections of the website). Consequently, the website searches relied heavily on hand search, which might be less reproducible than a search in a literature database. Medical societies currently submit only a fraction of their guidelines uploaded on their websites to the FMH platform (fig. 2). The reason for the low submission rate might be the novelty of the platform and the effort required to prepare and send a CPG to the platform. Furthermore, medical societies’ websites did not contain 44 CPGs that were published in the literature only. According to a survey at a Swiss university hospital, although physicians thought guidelines are useful as a source of advice, their use was limited by low awareness of existing guidelines, among other factors [15]. Making guidelines easier to find and more accessible might improve their uptake in Switzerland.
A range of terms are used to title CPGs in Switzerland. Whereas more than half of the identified guidelines used one of the predefined terms in their title (see fig. 3), only a relatively small number were called a “guideline”. Even though (only) the term “guideline” has an official definition in the Swiss CPG context [2], CPGs titled “guideline” were based on a systematic review of evidence no more often than CPGs with other or no keywords in the title (fig. 3). In contrast, the German translation of the term, “Leitlinie”, was associated with a systematic review more frequently. Some of the CPGs identified in this study were documents with recommendations that potentially do not intend to be a CPG, even if they could be interpreted and used as such. This might point to a need for finer categorisation of recommendations for clinical practice, defining some of these documents not as a guideline, but rather as a less formal tool of communicating recommendations. Additionally, a more rigid definition of “guidelines” could be developed in consensus among Swiss developers and other stakeholders. For example, in the German AWMF framework the term “Leitlinie” is used in the context of CPG development uniformly [16].
The development and endorsement process of CPGs seems generally quite heterogeneous. In the health areas “cardiovascular disease” and “pregnancy and childbirth”, many CPGs were either endorsed international guidelines or developed in cooperation with German or Austrian societies (see table 1). In other health areas, no such pattern was recognisable. CPGs produced in cooperation with other countries were more frequently based on a systematic review of evidence. Although our study cannot provide a definitive conclusion on the quality of these CPGs, a systematic review is internationally considered an essential and resource-intensive criterion for a high-quality CPG. Only 10% of CPGs were based on a systematic review of evidence in our study. Systematic review was similarly uncommon in a study of Croatian CPGs (11.5%) and the corresponding European CPGs (12.5%) [17]. Thus, international cooperation could be a possible strategy for providing CPGs in accordance with the international standard. As a guideline’s development can cost up to USD 200,000 [18], it seems reasonable to look for synergies among developers.
Reporting quality of some aspects of the analysed Swiss CPGs seemed rather low. Some of the shortcomings might be related to the diverse reporting formats of some CPGs, especially those published on the societies’ websites. For example, the dissemination format of a leaflet or other type of short guideline might omit references, even though they have been used in the document’s development. If there were no financial support for a guideline’s development, the authors might not feel a need to report the fact and might omit the information instead. However, the omission of such information precludes evaluation of a guideline’s quality by its users. Universal use of some reporting standard such as the RIGHT statement [19] would greatly help to assess the trustworthiness of CPGs. Currently, only single elements of CPG’s reporting quality can be compared internationally; for example, conflict of interest was declared in 44% of CPGs in our study, in 8% of Croatian and 92% of corresponding European CPGs [17], and 21% of Australian CPGs [8].
Our study is currently the most comprehensive review of CPGs in Switzerland. In comparison with the other mentioned national studies, we included a much broader range of guidelines and sources in an effort to capture the real-life situation. We employed a predefined systematic search and data-extraction strategy, aiming for reproducible methodology. The variables extracted were based on the essential criteria for trustworthy CPGs, listed by GIN [13]. Our broad definition of CPGs meant that the majority of CPGs did not contain information required for evaluation with the AGREE-II [20] or RIGHT tools, commonly used for guideline reporting assessment. Still, many corresponding elements of these tools were represented in our extracted variables.
Our study is also subject to some limitations. First, a few CPGs might have been missed in the search, such as CPGs published in other non-indexed Swiss speciality journals. Second, we only extracted the absolute number of CGPs per health area, which might not represent the extent of guideline development activity in the respective area as well as, for example, the total number of individual recommendations. Some CPGs also cover overlapping health areas, which was not reflected well in our categorisation. Third, we only analysed the quality of reporting, not the comprehensive quality of the guideline itself. For example, mentioning having used a systematic review might not equal a high-quality systematic review being conducted and interpreted appropriately. No formal conclusion regarding the overall quality of CPGs can be made as we did not use validated tools, such as AGREE-II or the RIGHT-checklist. Further, some imprecision could have happened as both original and modified versions of the same guideline might have been included owing to the long timespan (10 years) of publication dates included. Similarly, we did not discriminate between the different dissemination formats of the same guideline, such as long and short versions as in the German AWMF framework. Additional documents describing the methodology of a CPG might not always have been found if they were provided in the society’s website but not mentioned within the guideline itself. In those cases, we could underestimate the reporting quality. We also noticed that it is difficult to differentiate between guideline modification (adaptation) and de novo development with another guideline used only as a source of information. Therefore, any modification was categorised as development of a (new) guideline. Finally, 136 CPGs did not have one of the predefined keywords in the title (fig. 3). Because of the heterogeneous formatting of the included CPGs, we decided to extract keywords only from the title, as it is identifiable in almost any kind of document. However, the keyword in some cases might have been present elsewhere – in the section heading in a journal, in the subtitle or abstract. This may have caused some misclassification within this variable.
In conclusion, there is relevant CPG activity in Switzerland: the number of guidelines present is substantial, but the quality of their development, transparency and methods reporting is widely varying. CPGs could potentially be improved by using common definitions and standards on development and reporting. In order to be able to compare the quality of Swiss CPGs with that of other countries, standard tools should be used for CPG reporting. We hope that this study will facilitate discussions on the need of a guideline framework in Switzerland by providing a first general overview on Swiss CPGs developed by medical societies.
Search strategy for clinical practice guidelines in PubMed, OVID, non-indexed Swiss medical journals and Swiss medical societies’ websites.
The appendices are available in a separate file for downloading at https://smw.ch/en/article/doi/smw.2019.20134/
We would like to thank Stefanie Hostettler (FMH) for her valuable input concerning the FMH platform guidelines.fmh.ch and the planning of the study. We also would like to thank the main applicant of NRP 74 Project 26, Prof. Dr. Matthias Schwenkglenks, and co-applicant PD Dr. Viktor von Wyl, for their support.
The contribution of AU was supported by the Swiss National Science Foundation (SNSF) National Research Programme "Smarter Health Care" (NRP 74), Project 26 “How do guidelines and recommendations influence medical treatment?” (grant number 407440_167349).
The authors report no conflict of interest.
1IOM (Institute of Medicine). Clinical Practice Guidelines We Can Trust [Internet]. Graham R, Mancher M, Miller Wolman D, Greenfield S, Steinberg E, editors. Clinical Practice Guidelines We Can Trust. Washington (DC): National Academies Press; 2011. 266 p. Available from: http://www.nap.edu/catalog/13058
2 Eicher E . Guideline für Guidelines. Schweiz Arzteztg. 1999;80(10):581–4.
3Akademien der Wissenschaften der Schweiz. Ein nachhaltiges Gesundheitssystem für die Schweiz. Roadmap der Akademien der Wissenschaften Schweiz. 2014; Available from: https://www.samw.ch/dam/jcr:56db98e2-3bc2-4741-a168-6fc44ab5aca8/roadmap_samw_nachhaltiges_gesundheitssystem_schweiz.pdf
4 FMH. Medizinische Guidelines: Voraussetzungen und Anwendung. Schweiz Arzteztg. 2014;95(03):52–3. doi:.https://doi.org/10.4414/saez.2014.02166
5 Legido-Quigley H , Panteli D , Brusamento S , Knai C , Saliba V , Turk E , et al. Clinical guidelines in the European Union: mapping the regulatory basis, development, quality control, implementation and evaluation across member states. Health Policy. 2012;107(2-3):146–56. doi:.https://doi.org/10.1016/j.healthpol.2012.08.004
6 Obrist R . Guidelines in den schweizerischen medizinischen Fachgesellschaften. Eine Bestandesaufnahme. Schweiz Arzteztg. 2001;82(24):1282–5. doi:.https://doi.org/10.4414/saez.2001.08236
7 Hostettler S , Kraft E , Bosshard C . Neue Online-Plattform «Guidelines Schweiz». Schweiz Arzteztg. 2017;98(2627):862. doi:.https://doi.org/10.4414/saez.2017.05747
8 Buchan HA , Currie KC , Lourey EJ , Duggan GR . Australian clinical practice guidelines--a national study. Med J Aust. 2010;192(9):490–4. doi:.https://doi.org/10.5694/j.1326-5377.2010.tb03604.x
9 Esandi ME , Ortiz Z , Chapman E , Dieguez MG , Mejía R , Bernztein R . Production and quality of clinical practice guidelines in Argentina (1994-2004): a cross-sectional study. Implement Sci. 2008;3(1):43. doi:.https://doi.org/10.1186/1748-5908-3-43
10 Venkatesh AK , Savage D , Sandefur B , Bernard KR , Rothenberg C , Schuur JD . Systematic review of emergency medicine clinical practice guidelines: Implications for research and policy. PLoS One. 2017;12(6):e0178456. doi:.https://doi.org/10.1371/journal.pone.0178456
11 Moher D , Liberati A , Tetzlaff J , Altman DG , Group TP . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement (Reprinted from Annals of Internal Medicine). Phys Ther. 2009;89(9):873–80. Available at: http://dx.plos.org/10.1371/journal.pmed.1000097.
12FMH. Fachgesellschaften [Internet]. Available from: https://www.fmh.ch/ueber_fmh/aerzteorganisationen/fachgesellschaften.html
13 Qaseem A , Forland F , Macbeth F , Ollenschläger G , Phillips S , van der Wees P ; Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156(7):525–31. Available at: http://annals.org/article.aspx?doi=10.7326/0003-4819-156-7-201204030-00009. doi:.https://doi.org/10.7326/0003-4819-156-7-201204030-00009
14R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2018.
15 Birrenbach T , Kraehenmann S , Perrig M , Berendonk C , Huwendiek S . Physicians’ attitudes toward, use of, and perceived barriers to clinical guidelines: a survey among Swiss physicians. Adv Med Educ Pract. 2016;7:673–80. doi:.https://doi.org/10.2147/AMEP.S115149
16AWMF. Leitlinien [Internet]. Available from: https://www.awmf.org/leitlinien.html.
17 Tokalić R , Viđak M , Buljan I , Marušić A . Reporting quality of European and Croatian health practice guidelines according to the RIGHT reporting checklist. Implement Sci. 2018;13(1):135. doi:.https://doi.org/10.1186/s13012-018-0828-4
18 Burgers JS , Grol R , Klazinga NS , Mäkelä M , Zaat J ; AGREE Collaboration. Towards evidence-based clinical practice: an international survey of 18 clinical guideline programs. Int J Qual Health Care. 2003;15(1):31–45. doi:.https://doi.org/10.1093/intqhc/15.1.31
19 Chen Y , Yang K , Marušic A , Qaseem A , Meerpohl JJ , Flottorp S , et al.; RIGHT (Reporting Items for Practice Guidelines in Healthcare) Working Group. A Reporting Tool for Practice Guidelines in Health Care: The RIGHT Statement. Ann Intern Med. 2017;166(2):128–32. doi:.https://doi.org/10.7326/M16-1565
20 Brouwers MC , Kho ME , Browman GP , Burgers JS , Cluzeau F , Feder G , et al.; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting, and evaluation in health care. Prev Med. 2010;51(5):421–4. doi:.https://doi.org/10.1016/j.ypmed.2010.08.005
Shared first authorship
The contribution of AU was supported by the Swiss National Science Foundation (SNSF) National Research Programme "Smarter Health Care" (NRP 74), Project 26 “How do guidelines and recommendations influence medical treatment?” (grant number 407440_167349).
The authors report no conflict of interest.