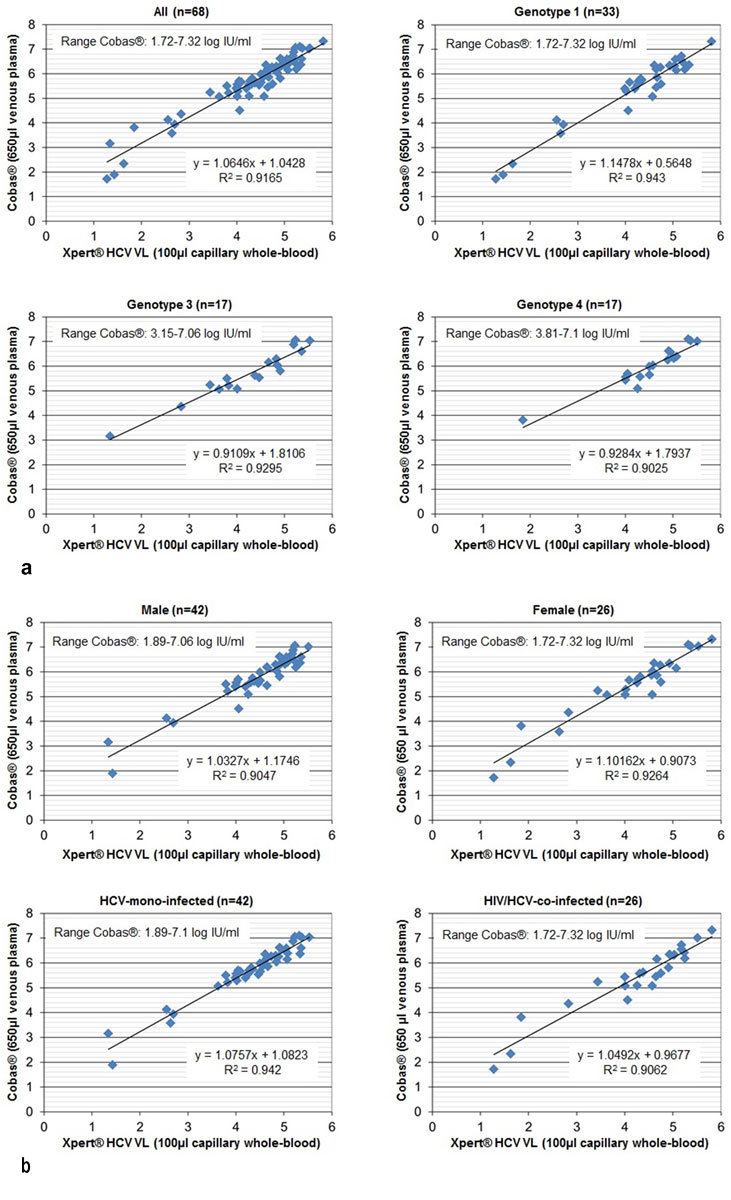
Figure 1 Quantitative performance of the Xpert® HCV Viral Load (VL) test: correlation of quantifiable HCV RNA results (log IU/ml) with the reference test Cobas®: overall, according to genotype, sex and HIV status.
DOI: https://doi.org/10.4414/smw.2019.20137
In order to reach the World Health Organization (WHO) goal “Hepatitis C elimination by 2030”, 80% treatment uptake is required [1]. With the latest well-tolerated pangenotypic direct acting antivirals (DAAs) (sofosbuvir/velpatasvir or glecaprevir/pibrentasvir) [2, 3], treatment duration decreased to 8–12 weeks, treatment success increased to 95–100% and genotyping became dispensable [4]. As a consequence, hepatitis C virus (HCV) treatment uptake in the Swiss Association for the Medical Management in Substance Users (SAMMSU) cohort increased from 55 to 65% between 1 May 2017 and 1 May 2018 [5]. However, only those diagnosed by HCV antibody screening, further evaluated with HCV RNA polymerase chain-reaction (PCR) testing and linked to care will eventually receive treatment. Diagnostic gaps in the HCV cascade have been described all over the world [6–9], particularly for the high-prevalence population of people who inject drugs with often difficult venous access.
Capillary tests are well accepted and potentially increase screening uptake [10]. Also, point-of-care tests (POCTs) providing results within minutes have been shown to improve linkage to care [11, 12]. However, they can only be recommended if sufficiently reliable. Although reliable rapid HCV antibody tests using capillary whole blood or saliva are already available [13], there is still need for optimising viraemia detection [14]. Dried blood spots (DBSs) were successfully used for HCV genotyping and HCV RNA quantification [15]. However, at 150–250 IU/ml, the detection threshold was significantly higher than in plasma [16] and turn-around times do not allow immediate decision making.
The Xpert® HCV Viral Load (VL) test is a POCT approved for 1 ml venous plasma or serum (limit of detection [LOD] 4.0 IU/ml, 95% confidence interval [CI] 2.8–5.2) and 6.1 IU/ml, 95% CI 4.2–7.9, respectively). It provides quantitative results within 105 min [17–19]. So far, only one study in 150 participants evaluated the performance of this test for finger-stick capillary whole blood. Compared with the Abbott® RealTime HCV Viral Load assay with 500 µl venous plasma, sensitivity was 95.5% (95% CI 84.5–99.4%) and specificity 98.1% (95% CI 93.4–99.8%) [20]. In September 2018, the Xpert® HCV Viral Load Finger-Stick (VL FS) test, specifically developed for 100 µl capillary whole-blood (LOD 40 IU/ml; 60 min), was approved. Compared with the Abbott® RealTime HCV Viral Load assay with 500 µl venous plasma, sensitivity was 98.3% (95% CI 91.1–100%) and specificity 100% (95% CI 96.6–100%) (167 participants) [21]. Both studies, conducted by The Kirby Institute in Australia, excluded patients under DAA treatment [20, 21].
The aim of our prospective study was to evaluate the diagnostic accuracy of rapid point-of-care HCV RNA quantification by GeneXpert® in 100 µl capillary whole blood overall and in different settings (according to HCV genotype, sex and human immunodeficiency virus [HIV] serostatus, with and without correction for haematocrit, on and off HCV treatment) using our laboratory-based standard quantitative HCV polymerase chain reaction (PCR) test (Roche Cobas® Ampliprep/Taqman) with 650 µl venous EDTA plasma as the reference test. Until October 2018, we used the Xpert® HCV VL test (105 min; developed for 1 ml plasma or serum). Thereafter, the Xpert® HCV VL FS test (60 min; specifically developed for 100 µl capillary whole blood) was evaluated.
The study was approved by the cantonal ethics committee (AG/SO 2012/091; PB_2016-02058). All participants gave written informed consent.
In a prospective study conducted between November 2016 and May 2019 in the Infectious Diseases Outpatient Clinic of the Cantonal Hospital Aarau, a tertiary care hospital, all adults with an indication for HCV RNA quantification and written informed consent provided both venous and capillary blood for parallel testing. To evaluate suitability of the capillary HCV RNA test for HCV treatment monitoring, specimens were collected during treatment, at the end of treatment (EOT) and 12 weeks thereafter (sustained virological response [SVR]).
The Xpert® HCV VL test was evaluated between November 2016 and October 2018 (194 paired samples from 88 patients) and the Xpert® HCV VL FS test between November 2018 and May 2019 (32 paired samples from 23 patients).
Xpert® HCV VL test: 100 µl capillary whole blood collected after a finger stick (MiniCollect Safety Lancet; Greiner Bio-One, Frickenhausen, Germany) into an EDTA minivette (Minivette® POCT 100 µl EDTA; Sarstedt, Nümbrecht, Germany) were immediately transferred into an Xpert® HCV VL cartridge (GXHCV-VL-CE-10; Cepheid, Sunnyvale, CA, USA), approved for 1 ml plasma (limit of quantification [LOQ] 10 IU/ml, LOD 4.0 IU/ml, 95% CI 2.8–5.2) or serum; results are available within 105 min. As described by Grebely et al. [20], 1 ml Cepheid buffer (Cepheid, Sunnyvale, CA, USA) was added with the 1 ml transfer pipette included in the kit. Based on the assumption of an average haematocrit of 45%, 100 µl whole blood contains ~55µl plasma, which is ~18-fold less than the 1 ml for which the cartridge is approved. Thus, capillary measurements were expected to be 1.3 log IU/ml lower than measurements in 1 ml plasma. Xpert® HCV VL testing of capillary whole blood was done on a GeneXpert® with four modules (GXIV-4-L System; Cepheid Sunnyvale, CA, USA).
Xpert® HCV VL FS test: 100 µl capillary whole-blood collected after a finger stick (see above) were immediately transferred into an Xpert® HCV VL FS cartridge (GXHCV-FS-CE-10; Cepheid, Sunnyvale, CA, USA) (approved for 100 µl capillary whole blood (LOQ 100 IU/ml, LOD 40 IU/ml; result within 60 min) and measured with a GeneXpert®.
Both index tests were used as POCT. The GeneXpert® containing four modules is connected to a laptop and transportable in a passenger car. For the duration of the study, the GeneXpert® was situated in the room of the Infectious Diseases Outpatient Clinic where blood sampling is performed. After instruction by the study team, the clinical nurses ran the Xpert® tests independently. Prior to the capillary blood draw, the GeneXpert® instrument and the software were started, patient data entered and the Xpert® cartridge scanned. Immediately after the capillary blood draw, the sample was transferred into the cartridge, which was directly loaded to the prepared GeneXpert®. Thus, the “puncture-to-test-start time” was less than 1 minute. Since the four modules operate independently of each other, the next test can be loaded while the first is still running. After the Xpert® test had been started, venepuncture was performed to collect venous plasma for the reference test and the third assay (see below). In the event of an error message from the GeneXpert® (“insufficient volume errors” occur after 3.5 minutes.), the patient was still available for another capillary blood draw. With the Xpert® HCV VL test running with 100 µl capillary whole blood and 1 ml buffer solution, we did not observe “insufficient volume errors”, whereas complete filling of the 100 µl EDTA minivette is crucial for the Xpert® HCV VL FS test, which uses only 100 µl capillary whole blood. Therefore, a lancet providing sufficient blood flow should be used. In the case of slow blood flow or air bubbles in the capillary, the blood draw should be repeated in order not to waste a cartridge.
HCV RNA in 650 µl venous EDTA plasma was quantified with real-time PCR using the Cobas® AmpliPrep / Cobas® TaqMan® HCV Test CAP/CTM version 2.0 (Roche®, Ref. Nr. 05532264) (LOQ 15 IU/ml), which is routinely run once a week in our laboratory. Samples are centrifuged within 6 hours of collection and plasma stored at −20°C until testing. Specimen preparation is automated using the Cobas® AmpliPrep instrument with amplification and detection automated using the Cobas® TaqMan® 48 analyser. The Cobas® AmpliPrep / Cobas® TaqMan® HCV test is based on three major processes: (1) specimen preparation to isolate HCV RNA; (2) reverse transcription of the target RNA to generate complementary DNA (cDNA), and (3) simultaneous PCR amplification of target cDNA and detection with a cleaved dual-labelled oligonucleotide detection probe specific to the target. Primers define a sequence within the highly conserved region of the 5'-untranslated region of the HCV genome. The nucleotide sequence of the primers has been optimized to yield comparable amplification of all HCV genotypes.
In the case of discordance between the index and the reference test, as well as discrepant quantifiable results (>2.3 log IU/ml or <0.3 log IU/ml difference between the Xpert® HCV VL test with 100 µl capillary whole-blood and the Cobas® assay with 650 µl venous plasma), an additional 1 ml venous plasma from the same blood draw was tested with the Xpert® HCV VL test according to the manufacturers’ recommendations to differentiate between material- (capillary whole blood vs venous plasma) and test- (Xpert® vs Cobas®) specific causes.
HCV genotypes were analysed by means of the VERSANT® HCV Genotype 2.0 Assay (LiPA) (Siemens, Zurich, Switzerland), which detects 6 genotypes and 19 subtypes.
Data regarding the Xpert® HCV VL and the Xpert® HCV VL FS test were analysed separately.
The following measures of accuracy were assessed: sensitivity (proportion of HCV RNA positives correctly identified by the capillary test), specificity (proportion of HCV RNA negatives correctly identified by the capillary test), positive predictive value (PPV; proportion of individuals with a positive capillary test result actually being HCV RNA positive), negative predictive value (NPV; proportion of individuals with a negative capillary test result actually being HCV RNA negative) and the proportion with concordant qualitative results. For the sensitivity/specificity analysis, we only considered qualitative results (detectable or not). “Detectable below 10 IU/ml” (Xpert® HCV VL test), “detectable below 100 IU/ml” (Xpert® HCV VL FS test) and “detectable below 15 IU/ml” (Cobas® assay) counted as positive results. We also determined diagnostic accuracy according to the following indications: pretreatment baseline, on-treatment follow-up, EOT, SVR, screening for reinfection, differentiation between spontaneous clearance and chronic hepatitis C, screening for acute infection.
Paired samples with quantifiable results in both tests underwent correlation analysis. A squared correlation coefficient (R2) and a fitted regression line were calculated.
Primers used by the Xpert® and the Cobas® platform might not recognise HCV RNA from different HCV genotypes equally well. Women have a lower haematocrit than men. Thus, on average, the plasma volume contained in 100 µl capillary whole blood is higher in women than in men. In human immunodeficiency virus (HIV) / HCV co-infected patients, there is interaction between the two viruses, resulting in enhanced HCV RNA replication and more rapid progression of HCV-related liver disease in co-infected patients [22, 23]. Accordingly, we investigated the correlation between Xpert® HCV VL and Cobas® results stratified by HCV genotype, sex and HIV serostatus and corrected [+log10(1000/(100−haematocrit(%))] versus uncorrected for the same-day haematocrit.
For paired quantifiable results, Bland-Altman plots were produced. We plotted the difference between the Cobas® and the respective Xpert® results against their average. Correction for the haematocrit was done only for the Xpert® HCV VL test.
Statistical analyses were performed with Stata Version 12.0 and OpenEpi (www.openepi.com).
Between November 2016 and October 2018, 194 Xpert® HCV VL tests were performed in 88 patients. Patient and sample characteristics are described in table 1. About 70% of the patients were male, 80% had ever used intravenous drugs, about half were on opioid agonist therapy, all but three were HCV antibody positive and 39% HIV/HCV co-infected. The median age was 48 years. More than half of the patients had more than one test (up to six tests per patient). HCV genotype 1 was the most frequent, followed by genotypes 3 and 4. On average, the same-day haematocrit was 3.3% higher in men than in women.
Table 1 Patient/sample characteristics.
|
Xpert® HCV VL test
(Nov. 2016 to Oct. 2018) |
Xpert® HCV VL FS test
(Nov. 2018 to May 2019) |
||
|---|---|---|---|
| Number of patients | 88 | 23 | |
| Male (%) | 69.3 (61) | 73.9 (17) | |
| Median age (years) (IQR) | 47.7 (40.4-52.8) | 45 (38.6-55.7) | |
| Ever intravenous drug use (%) | 79.5 (70) | 73.9 (17) | |
| On opioid agonist therapy (%) | 47.7 (42) | 52.2 (12) | |
| HCV antibody positive (%) | 96.6 (85) | 95.7 (22) | |
| HIV/HCV co-infected (%)* | 38.6 (34) | 13.0 (3) | |
| HIV mono-infected (%)* | 3.4 (3) | 0 (0) | |
| One test (%) | 45.5 (40) | 69.6 (16) | |
| Two tests (%) | 14.8 (13) | 17.4 (4) | |
| Three tests (%) | 22.7 (20) | 13.0 (3) | |
| Four tests (%) | 9.1 (8) | 0 (0) | |
| Five tests (%) | 6.8 (6) | 0 (0) | |
| Six tests (%) | 1.1 (1) | 0 (0) | |
| Number of samples | 194 | 33 | |
| HCV RNA positive with Cobas® (%) | 51.0 (99) | 45.5 (15) | |
| HCV genotype distribution among those HCV RNA positive with Cobas (%) | HCV genotype 1 | 42.4 (42) | 40 (6) |
| HCV genotype 2 | 0 (0) | 13.3 (2) | |
| HCV genotype 3 | 28.3 (28) | 40 (6) | |
| HCV genotype 4 | 27.3 (27) | 6.7 (1) | |
| HCV genotype 5 | 0 (0) | 0 (0) | |
| HCV genotype 6 | 2.0 (2) | 0 (0) | |
| Mean same-day haematocrit (%) (±SD) | All | 41.8 ± 3.9 (n = 156) | – |
| Men | 42.9 ± 3.5 (n = 104) | – | |
| Women | 39.6 ± 3.8 (n = 52) | – | |
| Mean difference (men – women) | 3.3 (95% CI 2.1–4.5) | – | |
HCV = hepatitis C virus; VL = viral load; FS = finger-stick; IQR = interquartile range; HIV = human immunodeficiency virus; SD = standard deviation; CI = confidence interval * HIV-infected people were almost exclusively under antiretroviral treatment and virologically suppressed
With the Cobas® assay in venous plasma (reference test), 51.0% (95% confidence interval [CI] 44.1–58.0%) samples were HCV RNA positive. Compared with this reference test, sensitivity of the Xpert® HCV VL assay using capillary blood was 97.0% (95% CI 91.5–99.0%) and specificity 94.7% (95% CI 88.3–97.7%) (positive predictive value 95.0%, 95% CI 88.9–97.9%; negative predictive value 96.8%, 95% CI 90.9–98.9%). The proportion with concordant qualitative results was 95.9% (95% CI 92.1–97.9%) (tables 2 and 3 ).
Table 2 Qualitative performance / diagnostic accuracy of the Xpert® HCV Viral Load (VL) and the Xpert® HCV Viral Load Finger-Stick (VL FS) tests.
|
Cobas® (reference test)
(650 µl venous plasma) |
||||
|---|---|---|---|---|
| Detected | Not detected | Total | ||
| Xpert® HCV VL (100 µl capillary whole blood) |
Detected | 96 | 5* | 101 |
| Not detected | 3* | 90 | 93 | |
| Total | 99 | 95 | 194 | |
|
Cobas® (reference test)
(650 µl venous plasma) |
||||
| Detected | Not detected | Total | ||
| Xpert® HCV VL FS (100 µl capillary whole-blood) |
Detected | 15 | 2† | 17 |
| Not detected | 0 | 16 | 16 | |
| Total | 15 | 18 | 33 | |
Xpert® HCV Viral Load test: limit of detection for 1 ml plasma 4 IU/ml Xpert® HCV Viral Load Finger-Stick test: limit of detection for 100 µl whole blood 40 IU/ml Cobas®: limit of detection for 650 µl plasma 15 IU/ml * See table 4 for detailed description of discordant results † Patient 1 (HCV genotype 1b, DAA treatment week 4): Xpert® HCV VL FS (100 µl capillary whole blood): detected <100 IU/ml, Cobas® (650 µl venous plasma): not detected, Xpert® HCV VL (1 ml venous plasma): not detected. Patient 2 (HCV genotype 4, DAA treatment week 3): Xpert® HCV VL FS (100 µl capillary whole blood): detected <100 IU/ml, Cobas® (650 µl venous plasma): not detected; Xpert® HCV VL (1 ml venous plasma): detected <100 IU/ml)
Table 3 Performance according to indication – Xpert® HCV Viral Load (VL) and Xpert® HCV Viral Load Finger-Stick (VL FS) tests.
| Indication | % of tests (n) | Prevalence (%) | Concordance (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| Xpert® HCV VL (100 µl capillary whole blood; n = 194) | |||||||
| Pretreatment baseline | 31.4 (61) | 100 (61/61) | 100 (61/61) | 100 (61/61) | – | 100 (61/61) | – |
| On treatment follow-up (weeks 1–8) |
22.2 (43) | 67.4 (29/43) | 83.7 (36/43) | 89.7 (26/29) | 71.4 (10/14) | 86.7 (26/30) | 76.9 (10/13) |
| EOT | 13.4 (26) | 3.8 (1/26) | 96.2 (25/26) | 100 (1/1) | 96.0 (24/25) | 50 (1/2) | 100 (24/24) |
| SVR | 19.6 (38) | 0 (0/38) | 100 (38/38) | – | 100 (38/38) | – | 100 (38/38) |
| Reinfection? | 5.7 (11) | 27.3 (3/11) | 100 (11/11) | 100 (3/3) | 100 (8/8) | 100 (3/3) | 100 (8/8) |
| SC or chronic hepatitis C? | 6.2 (12) | 41.7 (5/12) | 100 (12/12) | 100 (5/5) | 100 (7/7) | 100 (5/5) | 100 (7/7) |
| Acute infection? | 1.5 (3) | 0 (0/3) | 100 (3/3) | – | 100 (3/3) | – | 100 (3/3) |
| All | 100 (194) | 51.0 (99/194) | 95.9 (186/194) | 97.0 (96/99) | 94.7 (90/95) | 95.0 (96/101) | 96.8 (90/93) |
| Xpert® HCV VL FS (100 µl capillary whole blood; n = 33) | |||||||
| Pretreatment baseline | 27.2 (9) | 100 (9/9) | 100 (9/9) | 100 (9/9) | – | 100 (9/9) | – |
| On treatment follow-up (week 2–7) |
27.2 (9) | 11.1 (1/9) | 77.8 (7/9) | 100 (1/1) | 75 (6/8) | 33.3 (1/3) | 100 (6/6) |
| EOT | 12.1 (4) | 0 (0/4) | 100 (4/4) | – | 100 (4/4) | – | 100 (4/4) |
| SVR | 6.1 (2) | 0 (0/2) | 100 (2/2) | – | 100 (2/2) | – | 100 (2/2) |
| Reinfection? | 3.0 (1) | 0 (0/1) | 100 (1/1) | – | 100 (1/1) | – | 100 (1/1) |
| SC or chronic hepatitis C? | 21.2 (7) | 71.4 (5/7) | 100 (7/7) | 100 (5/5) | 100 (2/2) | 100 (5/5) | 100 (2/2) |
| Acute infection? | 3.0 (1) | 0 (0/1) | 100 (1/1) | – | 100 (1/1) | – | 100 (1/1) |
| All | 100 (33) | 45.5 (15/33) | 93.9 (31/33) | 100 (15/15) | 88.9 (16/18) | 88.2 (15/17) | 100 (16/16) |
PPV = positive predictive value; NPV = negative predictive value; EOT = end-of-treatment; SVR = sustained virological response; SC = spontaneous clearance
Among the eight discordant results (three false negative, five false positive), seven were 1 to 4 weeks after initiation of DAA treatment and one at EOT (table 4). The highest HCV RNA value missed by the capillary test was 68 IU/ml. For the other two false negative results, the Cobas®-result was “<15 IU/ml, detectable below the LOQ”. The three positive Cobas® results were confirmed by additional Xpert® HCV VL testing of 1 ml venous plasma. For all five false positive results, the capillary Xpert® HCV VL result was “HCV detected <10 IU/ml”. Additional Xpert® HCV VL testing of 1 ml venous plasma confirmed the capillary measurements and not the negative Cobas® results.
Table 4 Xpert® HCV Viral Load (VL) test: characteristics of the eight discordant and the two discrepant quantifiable results.
| Sex | HIV | HCV Genotype | Weeks after DAA start |
Hct (%)
(same day) |
(IU/ml) | (log IU/ml) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Cobas®
(650 µl venous plasma) |
Xpert® HCV VL |
Cobas®
(650 µl venous plasma) |
Xpert® HCV VL | |||||||
|
(100 µl capillary
whole blood; ~55 µl plasma) |
(1 ml venous plasma) |
(100 µl capillary
whole blood; ~55 µl plasma) |
(1 ml venous plasma) | |||||||
| Discordant results | ||||||||||
| M | Pos. | 4 | 1 | 42.8 | 68 | Neg. | 21 | 1.83 | Neg. | 1.32 |
| M | Pos. | 4 | 1 | 43.3 | Pos. <15 | Neg. | 22 | Pos. <1.20 | Neg. | 1.34 |
| F | Pos. | 3 | 1 | 30.3 | Pos. <15 | Neg. | Pos. <10 | Pos. <1.20 | Neg. | Pos. <1.00 |
| F | Neg. | 1 | 2 | 39.8 | Neg. | Pos. <10 | Pos. <10 | Neg. | Pos. <1.00 | Pos. <1.00 |
| M | Neg. | 3 | 4 | 45.7 | Neg. | Pos. <10 | Pos. <10 | Neg. | Pos. <1.00 | Pos. <1.00 |
| M | Neg. | 4 | 4 | 38.0 | Neg. | Pos. <10 | Pos. <10 | Neg. | Pos. <1.00 | Pos. <1.00 |
| F | Pos. | 1 | 4 | 41.2 | Neg. | Pos. <10 | Pos. <10 | Neg. | Pos. <1.00 | Pos. <1.00 |
| F | Neg. | 4 | 12 (EOT) | - | Neg. | Pos. <10 | 18 | Neg. | Pos. <1.00 | 1.26 |
| Discrepant quantifiable results* | ||||||||||
| M | Neg. | 3 | - | 45.0 | 643,810 | 33# | 947,000 | 5.81 | 1.52† | 5.98 |
| M | Pos. | 4 | - | 41.5 | 47,349 | 37,600 | 860,000 | 4.68 | 4.58 | 5.93 |
M = male, F = female, Pos. = positive, Neg. = negative, DAA = direct-acting antiviral, EOT = end-of-treatment, Hct = haematocrit * Since a ~18-fold smaller plasma volume is used in the capillary test (~55 µl instead of 1000 µl plasma), Xpert® results with capillary whole blood are expected to be ~1.3 log IU/ml lower than Cobas® results with venous plasma. Differences >2.3 log IU/ml or <0.3 log IU/ml were considered as discrepant quantifiable results. † Difficulties with capillary blood draw (potentially, the blood volume used was smaller than the intended 100 µl. The patient was retested 1 month later: Xpert® HCV VL (capillary whole blood) 71,000 IU/ml (4.85 log IU/ml); Cobas® (venous plasma) 1,088,010 IU/ml (6.04 log IU/ml); difference 1.19 log IU/ml, which is due to the ~18-fold smaller plasma volume used ~55 versus 1000 µl.)
After two pairs of tests were excluded because of problems with capillary blood draw and the reference test, respectively (table 4), 68 samples – 48.5% (33) genotype 1; 25% (17) genotype 3; 25% (17) genotype 4; 1.5% (1) genotype 6 – with quantifiable results in both tests remained for the correlation analysis (HCV RNA range with Cobas® 52–21,038,740 IU/ml = 1.72–7.32 log IU/ml; HCV range with the Xpert® HCV VL test: 19 – 652,000 IU/ml = 1.28–5.81 log IU/ml).
Cobas® and Xpert® HCV VL test values were highly correlated (R2 = 0.9165) irrespective of HCV genotype, HIV status and sex (~5% higher haematocrit in men results in ~5µl less plasma in 100µl whole blood.) (fig. 1). The equation for the fitted regression line was:

Figure 1 Quantitative performance of the Xpert® HCV Viral Load (VL) test: correlation of quantifiable HCV RNA results (log IU/ml) with the reference test Cobas®: overall, according to genotype, sex and HIV status.
Cobas® (log IU/ml) = 1.0646 × Xpert® (log IU/ml) + 1.0428
On average, capillary Xpert® HCV VL results were 1.32 (±0.34) log IU/ml lower (range 0.44-1.96) (Bland-Altman plot, supplementary fig. S1 in appendix 1). This corresponds to the ~18-fold smaller plasma volume used when using 100 µl whole blood with a haematocrit of 45% (55µl plasma) instead of 1 ml plasma (55/1000 = 0.055 = 1/18; log10(18) = 1.26).
For 57 (83.8%) of the 68 samples with paired quantifiable results, same-day haematocrit was available (range 31.5–50%; mean 41.8 ± 4.1%; mean in 23 women 39.1 ± 4.0%; mean in 34 men 43.6 ± 3.1%). Correction for the individual haematocrit (+log10(1000/(100−haematocrit(%)); range +log10(14.6) = 1.16 to +log10(20) = 1.30), reduced the mean difference between Cobas®- and capillary Xpert® HCV VL results from 1.34 ± 0.33 (range +0.44 to +1.96) to 0.11 ± 0.33 (range −0.83 to +0.77) (Bland-Altman plot, fig. S2), but did not improve correlation (R2 = 0.8906 vs R2 = 0.8895).
Eight Cobas®-results were “detectable below the LOQ” (15 IU/ml, 1.2 log IU/ml). Two of them were undetectable with the capillary Xpert® HCV VL test (table 4) and six “detectable below the LOQ” (10 IU/ml, 1.0 log IU/ml). In contrast, 31 capillary Xpert® HCV VL tests were “detectable below the LOQ” (fig. 2). The median corresponding Cobas® result was 23 IU/ml (interquartile range [IQR] 7.5–44) and 1.36 log IU/ml (IQR 0.88–1.64), respectively.
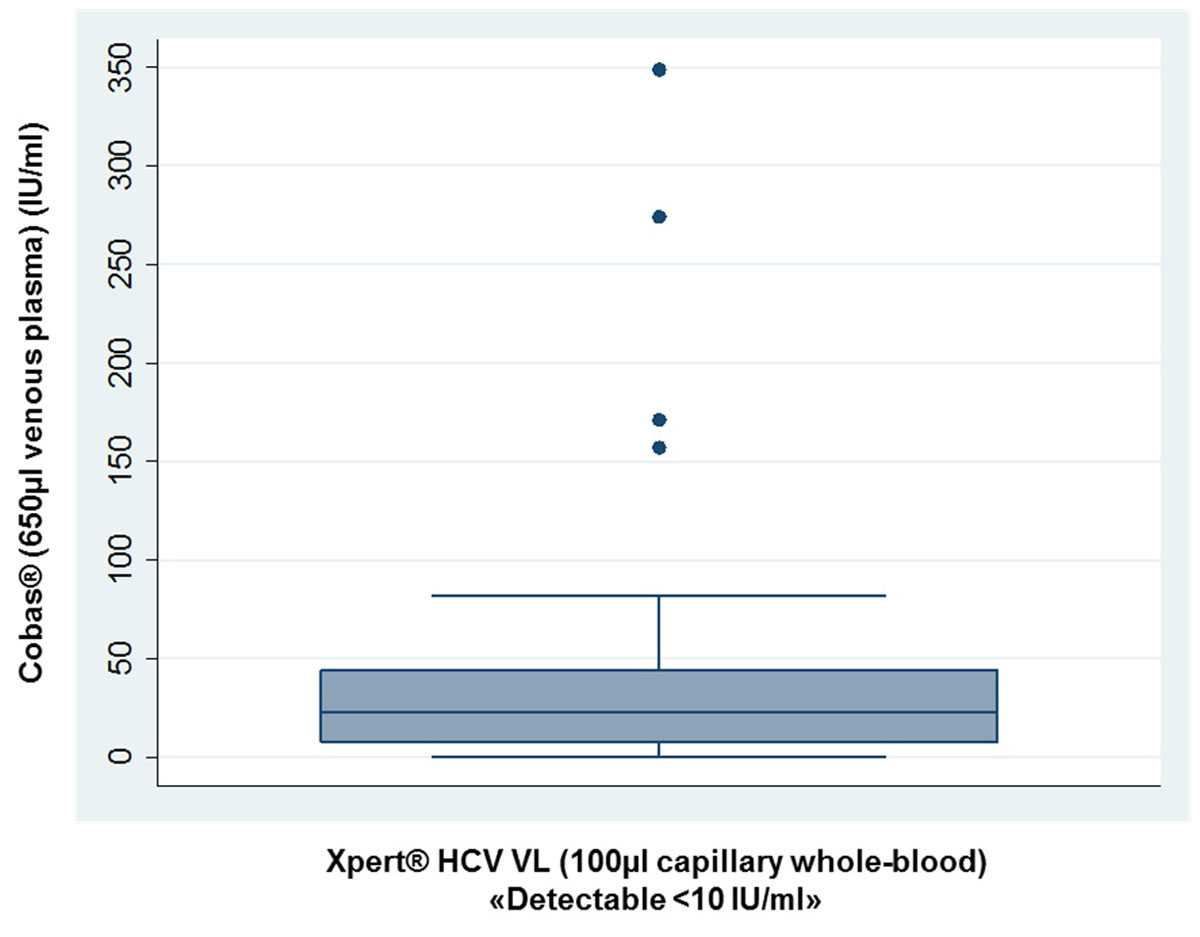
Figure 2 Xpert® HCV Viral Load (VL) test: Cobas® results (venous plasma) for the 31 capillary HCV RNA measurements below the limit of quantification of 10 IU/ml (1.0 log IU/ml).
For unquantifiable positive Cobas® results (“detectable below the limit of quantification of 15 IU/ml [1.2 log IU/ml]”), the mid-point between zero and the lower limit of quantification (15 IU/ml, 1.2 log IU/ml) was used (7.5 IU/ml, 0.88 log IU/ml).
Table 3 shows test performance according to HCV RNA indication. About two thirds of all tests were off treatment (pretreatment baseline, SVR, screening for acute infection / reinfection, differentiation between spontaneous clearance (SC) and chronic hepatitis C). Off treatment, no discrepancies were observed between Cobas® (650 µl venous plasma) and Xpert® HCV VL test (100 µl capillary blood). Sensitivity, specificity, PPV and NPV were 100%.
About one third of all tests were on treatment (on treatment follow-up [weeks 1–8] or EOT). Owing to three false negative Xpert® HCV VL tests (week 1), sensitivity and NPV were reduced to 90.0% (27/30) and 91.9% (34/37), respectively. Owing to five false positive Xpert® HCV VL tests (weeks 2, 4 and 12 [EOT]), specificity and PPV declined to 87.2% (34/39) and 84.4% (27/32), respectively.
Figure 3 shows the HCV RNA course under DAA treatment for eight patients (four genotype 1, two genotype 3, two genotype 4) with at least three paired HCV RNA measurements (prior to, during [weeks 1–6 or EOT] and after treatment [SVR]). Irrespective of genotype and DAA regimen used, there was a sharp HCV RNA decline during the first 2 weeks of treatment.
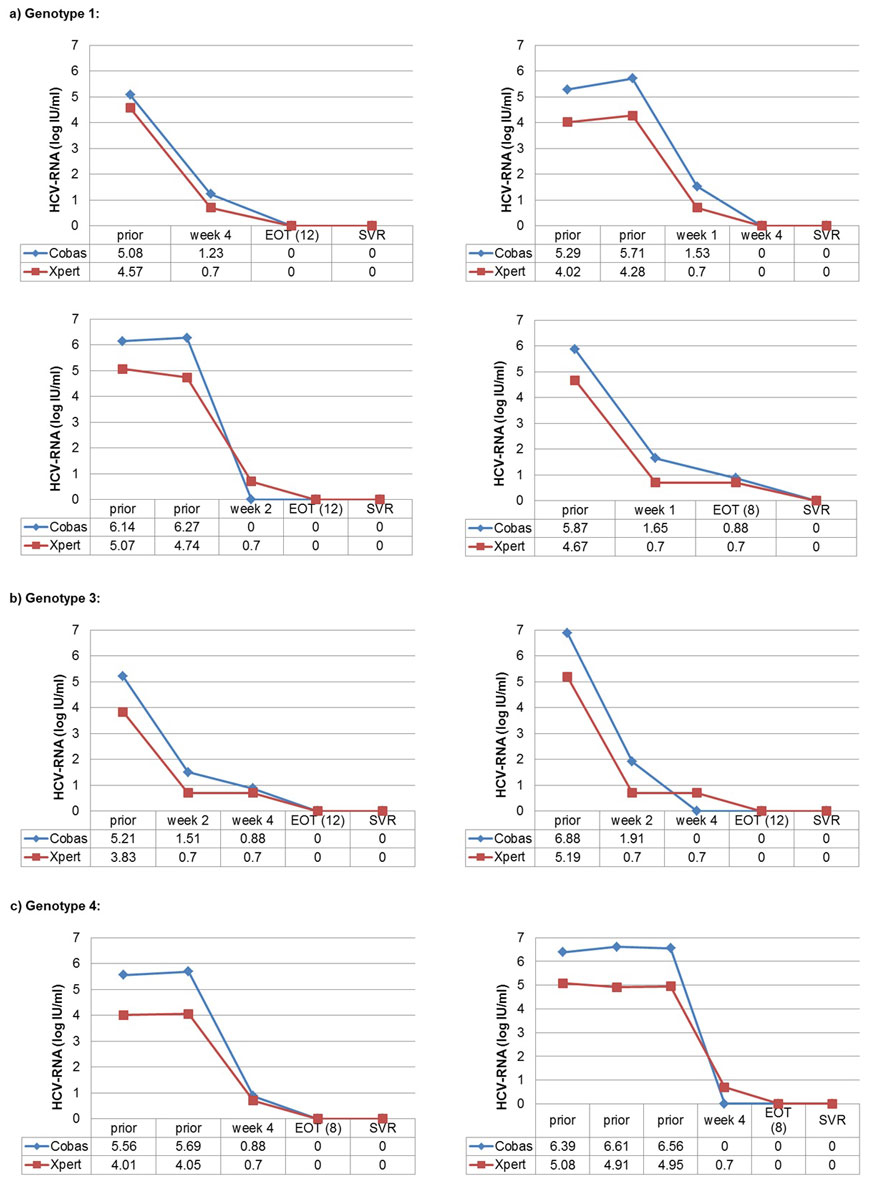
Figure 3 Xpert® HCV Viral Load (VL) test: HCV RNA course for 10 patients with parallel values prior to, during and after HCV-treatment.
Cobas = Cobas® assay with 650 µl venous plasma (limit of quantification 15 IU/ml = 1.2 log IU/ml); Xpert = Xpert® HCV Viral Load test with 100 µl finger-stick capillary whole blood (~55 µl plasma); prior = prior to HCV treatment; week 1, week 2, week 4, week 6 = respective weeks under HCV treatment; EOT (8) = end of treatment (week 8); EOT (12) = end of treatment (week 12); SVR = sustained virological response; 0 = HCV RNA not detectable.
For unquantifiable positive Cobas® results (“detectable below the limit of quantification of 15 IU/ml [1.2 log IU/ml]”), the mid-point between zero and the lower limit of quantification was used (7.5 IU/ml, 0.88 log IU/ml). For unquantifiable positive Xpert® HCV Viral Load test results (“detectable below the limit of quantification of 10 IU/ml [1.0 log IU/ml]”), the mid-point between zero and the lower limit of quantification was used (5 IU/ml, 0.70 log IU/ml).
One week after DAA start, all (12/12) HCV RNA measurements were still positive using Cobas®, all but two with an HCV RNA <100 IU/ml (2.0 log IU/ml). With the capillary Xpert® HCV VL test, 75.0% (9/12) were still positive, with three quantifiable results (19, 27 and 43 IU/ml) and six “detectable <10 IU/ml”. After 2 weeks, 73.3% (11/15) were positive with Cobas® (all but one HCV RNA <100 IU/ml) and 80% (12/15) with the capillary Xpert® HCV VL test (no quantifiable results). After 4 weeks, the proportions were 38.5% (5/13) (all HCV RNA <30 IU/ml) and 61.5% (8/13), respectively. After 6 and 8 weeks (one patient each), no HCV RNA was detectable. At EOT (week 8), one of 11 patients remained HCV RNA positive in both tests (both below the LOQ; SVR confirmed by both tests). At EOT (week 12), all 15 patients were HCV RNA negative with Cobas®, whereas one patient (6.7%) remained positive with the capillary Xpert® HCV VL test (SVR confirmed by both tests).
Between November 2018 and May 2019, 33 Xpert® HCV VL FS tests were performed in 23 patients. Patient and sample characteristics are described in table 1. The proportion with HIV/HCV co-infection was lower (13 vs 39%), and 70% of the patients had only one test (up to three tests per patient). Otherwise, patients were very similar to those tested with the Xpert® HCV VL assay. Again, about half of the samples were HCV RNA positive with Cobas® and 40% each were HCV genotype 1 and 3.
With the Cobas® assay in venous plasma (reference test), 45.5% (95% CI 29.8–62.0%) samples were HCV RNA positive. Compared with this reference test, sensitivity of the Xpert® HCV VL FS assay using capillary blood was 100% (95% CI 79.6–100%) and specificity 88.9% (95% CI 67.2–96.9%) (positive predictive value 88.2%, 95% CI 65.7–96.7; negative predictive value 100%, 95% CI 80.6–100). The proportion with concordant qualitative results was 93.9% (95% CI 80.4–98.3) (tables 2 and3).
The two discordant results (both false positive) were under DAA treatment (week 3, genotype 4 and week 4, genotype 1), when HCV RNA was near the LOQ. In both cases, the capillary Xpert® HCV VL FS result was “HCV detected <100 IU/ml”. Additional Xpert® HCV VL testing of 1 ml venous plasma confirmed the capillary measurement in the first patient (week 3, genotype 4; test result “HCV detected <10 IU/ml”), but not in the second patient.
Fourteen samples (six genotype 1; one genotype 2; six genotype 3; one genotype 4) with quantifiable results in both tests were available for the correlation analysis (HCV RNA range with Cobas® 678–3,558,156 IU/ml = 2.83–6.55 log IU/ml; HCV range with the Xpert® HCV VL FS test 247–3,100,000 IU/ml = 2.39–6.49 log IU/ml).
Cobas® and Xpert® HCV VL FS test values were highly correlated (R2 = 0.9899) (fig. 4). The equation for the fitted regression line was:
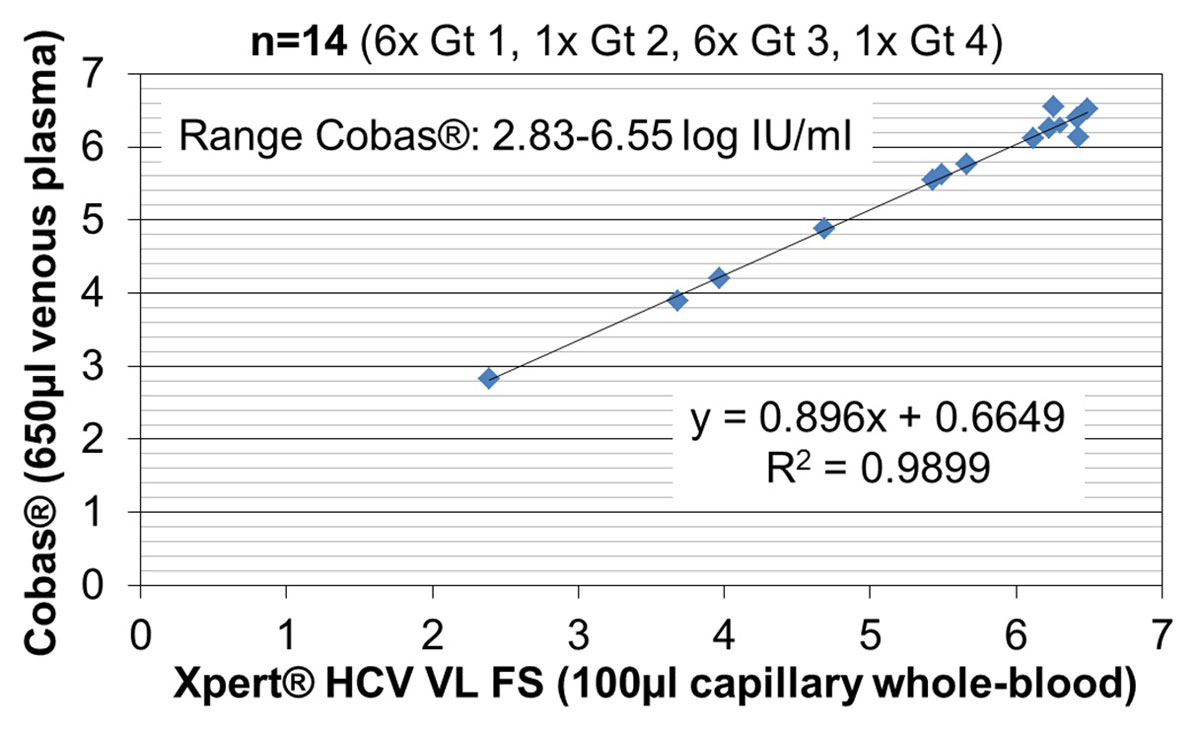
Figure 4 Quantitative performance of the Xpert® HCV Viral Load Finger-Stick (VL FS) test: correlation of quantifiable HCV RNA results (log IU/ml) with the reference test.
Gt = Genotype
Cobas® (log IU/ml) = 0.896 × Xpert® (log IU/ml) + 0.6649
On average, Xpert® HCV VL FS results were 0.10 (±0.17) log IU/ml lower (range −0.29 to +0.44) (Bland-Altman plot, supplementary fig. S3 in appendix 1).
There were no Cobas® results “detectable below the LOQ” (15 IU/ml, 1.2 log IU/ml), but three capillary Xpert® HCV VL FS tests were “detectable below the LOQ” (100 IU/ml, 2.0 log IU/ml). Two of them were undetectable with the Cobas® assay, and for one patient (DAA treatment week 2, genotype 2), the Cobas® result was 61 U/ml (1.79 log U/ml).
Table 3 shows test performance according to HCV RNA indication. About 60% of all tests were off treatment (pretreatment baseline, SVR, screening for acute infection/reinfection, differentiation between SC and chronic hepatitis C). Off treatment, no discrepancies were observed between the Cobas® (650 µl venous plasma) and Xpert® HCV VL FS test (100 µl capillary blood). Sensitivity, specificity, PPV and NPV were 100%.
About 40% of all tests were on treatment (on treatment follow-up [weeks 2–7] or EOT). Owing to two false positive Xpert® HCV VL FS tests (week 3 and 4), specificity and PPV were reduced to 83.3% (10/12) and 33.3% (1/3), respectively. With 11.1% versus 67.4%, HCV RNA-prevalence was markedly lower for the on treatment follow-up measurements in the Xpert® HCV VL FS test evaluation period. This is because HCV RNA was measured at later time-points (≤week 2 in 11.1% [1/9] vs 62.8% [27/43]).
HCV RNA quantification in 100 µl capillary whole blood with a commercial point-of-care rapid test (Xpert® HCV VL; 105 min; developed for 1 ml plasma or serum) proved highly reliable compared with the laboratory-based reference test (Cobas®) with venous plasma. Capillary Xpert® HCV VL results were 1.32 log IU/ml lower, corresponding to the smaller plasma volume used. All discordant results (three false negative, five false positive) were under DAA treatment (weeks 1–4 or EOT) with HCV RNA near the LOQ. Quantifiable Cobas®- and capillary Xpert® HCV VL results were strongly correlated irrespective of HCV genotype, HIV status, sex and correction for haematocrit.
Performance of the Xpert® HCV VL FS test (60 min; specifically developed for 100 µl capillary whole blood; approved in September 2018) was comparable, but convenience higher (no addition of buffer, more rapid results, no need for adjustment for the smaller plasma volume used).
Grebely et al. [20], the only previous publication evaluating the performance of HCV RNA quantification from finger-stick capillary whole blood with the Xpert® HCV VL test, excluded patients on DAA. In 150 patients, Xpert® HCV VL tests using finger-stick capillary whole blood and venous plasma were compared with Abbott® RealTime HCV Viral Load assay with venous plasma (LOQ 12 IU/ml). Both sensitivity and specificity of the capillary Xpert® HCV VL assay were comparable to those in our study (95.5% [42/44] vs 97.0% [96/99] and 98.1% [104/106] vs 94.7% [90/95], respectively). Similar to our results, the two false negative results had low viral load with the Abbott® assay (<12 IU/ml and 38 IU/ml). Remarkably, the capillary Xpert® HCV VL test performed equally well in our setting despite one third of HCV RNA measurements being collected under DAA treatment with viral loads near the LOD, a higher HCV RNA prevalence in our analysis (51.0 vs 29.3%) and different reference tests for venous plasma (Cobas® vs Abbott).
With 38 IU/ml [20] and 68 IU/ml, the highest HCV RNA values not detected by the capillary Xpert® test were lower than the 150–250 IU/ml described as the detection threshold for the DBS method [16]. Given the at least tenfold smaller volume used compared with analyses in venous plasma or serum, decreased sensitivity of capillary methods is not surprising. However, considering that most untreated patients have HCV viral loads >1000 IU/ml [24], capillary blood analysis is an easily applicable and reliable screening method for active hepatitis C.
Cell-associated HCV RNA in B lymphocytes present in whole blood but not in plasma might explain detectable viraemia in the capillary Xpert® test or DBS analysis, but not in parallel plasma measurements [15, 25]. Consistent with this hypothesis, Stapleton et al. found that whole blood-based HCV RNA detection was more sensitive than plasma-based methods [15, 26]. In our study, false positive results only occurred 2–4 and 12 weeks after DAA initiation. Longer persistence of cell-associated HCV RNA long after spontaneous clearance or successful treatment has been reported earlier [15, 27]. In any case, additional Xpert® HCV VL testing of 1 ml venous plasma (LOD 4 IU/ml) confirmed that our five false positive capillary Xpert® HCV VL tests were actually positive due to a higher sensitivity than the reference test. However, these discrepancies are probably not clinically relevant, because detectable virus under DAA treatment (including at EOT) does not predict subsequent treatment failure and/or relapse [12, 28].
Compared to the Cobas® assay with 650 µl venous plasma, the sensitivity of the Xpert® HCV VL FS test with 100 µl capillary whole blood was 100% (15/15), which is comparable to the 98.3% (59/60) observed in the study of Lamoury et al. with the Abbott® assay as reference test [21]. Specificity was somewhat lower in our setting (88.9% [16/18] vs 100% [107/107]). However, one of the two false positive results was confirmed to be positive by a third assay (Xpert® HCV VL test with 1 ml venous plasma). Different primers used in the Cobas® and Xpert® assays might be an additional possible explanation for the observed discordant results.
Among 565 participants in an Australian cohort study of people who inject drugs, 65% preferred finger-stick over venepuncture, with 61% of these preferring to receive results in 60 min. The most common reasons for preferring finger-stick testing were rapid result (62%) and venous access difficulties (21%) [29]. Feasibility of the Xpert® HCV VL FS assay was also demonstrated in 202 homeless people in Sydney, Australia [30].
Another recently published study with 170 participants showed that the Xpert® HCV VL test is able to accurately detect and quantify HCV RNA regardless of the HCV genotype in 50 µl venous whole blood collected in an EDTA tube and spotted onto a filter paper card (DBS technique) [31]. Sensitivity was 100% (119/119, 95% CI 96.9–100%) and specificity 90% (45/50, 95% CI 78.6–95.6%). On average, HCV RNA levels in whole blood were 1.93 ± 0.38 log IU/ml lower than in serum, which can be explained by the lower volume used (50 µl whole-blood eluted from DBS versus 500–1000 µl serum). There were no false negative, but five false positive results (all with HCV RNA below the LOQ of <10 IU/ml), supporting the hypothesis that whole blood-based HCV RNA detection is more sensitive than plasma-/serum-based methods.
Initially, we were concerned about an 8% error rate of the Xpert® HCV VL FS test in the study of Lamoury et al. [12, 21]. However, all errors we observed were “insufficient volume errors” occurring after 3.5 minutes. Thus, we asked the patients to wait until this critical time-point was passed and repeated the test if necessary, which was always successful. Since the Minivette POCT 100 µl EDTA tube must be completely filled, a lancet should be used that provides enough blood volume. In the case of slow blood flow, it is advisable to repeat the capillary blood draw. The time from finger-stick to test start should be minimised to further reduce the risk of clotting of the blood sample.
Further concerns regarding the use as POCT are the availability of the GeneXpert®-platform on site and its costs. Small GeneXpert® platforms with one, two or four modules can be transported in a passenger car. Thus, expensive but mobile equipment can be shared by several institutions.
Our study has several strengths. Rather than being excluded, patients under DAA treatment were deliberately monitored longitudinally throughout their HCV treatment up to the SVR check. With the Xpert® HCV VL test, a high proportion of patients (39%) was HIV co-infected. Correlation between quantifiable Xpert® HCV VL and Cobas® results was analysed stratified by HCV genotype, HIV status and sex, and took into account individual haematocrit values defining the plasma volume. Finally, the practicability of the capillary blood test in clinical routine is underlined by the fact that all the nurses working in our Infectious Diseases Outpatient Clinic successfully ran the tests without any support by a trained laboratory technician.
In view of the high correlation between Cobas® in venous plasma and Xpert® in capillary whole-blood, we additionally performed a parallel Xpert® HCV VL test with venous plasma only for the eight plus two discordant and the two discrepant quantifiable results.
As for any single-centre study with a rather small sample size, further confirmation by an independent study is desirable, especially for the evaluation of the Xpert® HCV VL FS test.
Point-of-care quantitative HCV RNA measurement in finger-stick capillary whole blood is a convenient, rapid and reliable method to diagnose active HCV infection, monitor treatment response and detect reinfection. Discordant results were rare and probably not clinically relevant, as they only occurred when HCV RNA was near the LOQ. For patients with difficult venous access after long-term intravenous drug use, capillary HCV RNA measurement removes a crucial barrier to HCV treatment and reinfection monitoring. Same-day results might improve linkage to care and allow a test-and-treat approach. The GeneXpert® IV system connected to a laptop is easily transportable by car, which is why we use it in peripheral drug substitution centres and addiction clinics, diminishing another barrier to treatment.
Whether the commercial availability of rapid point-of-care HCV RNA quantification in capillary whole blood translates into increased HCV treatment uptake remains to be determined. Reliable, rapid POCTs using capillary whole blood for anti-HAV [32, 33], anti-HBs, HBs-Ag and anti-HBc detection [34–37] remain an unmet need to identify patients requiring vaccination.
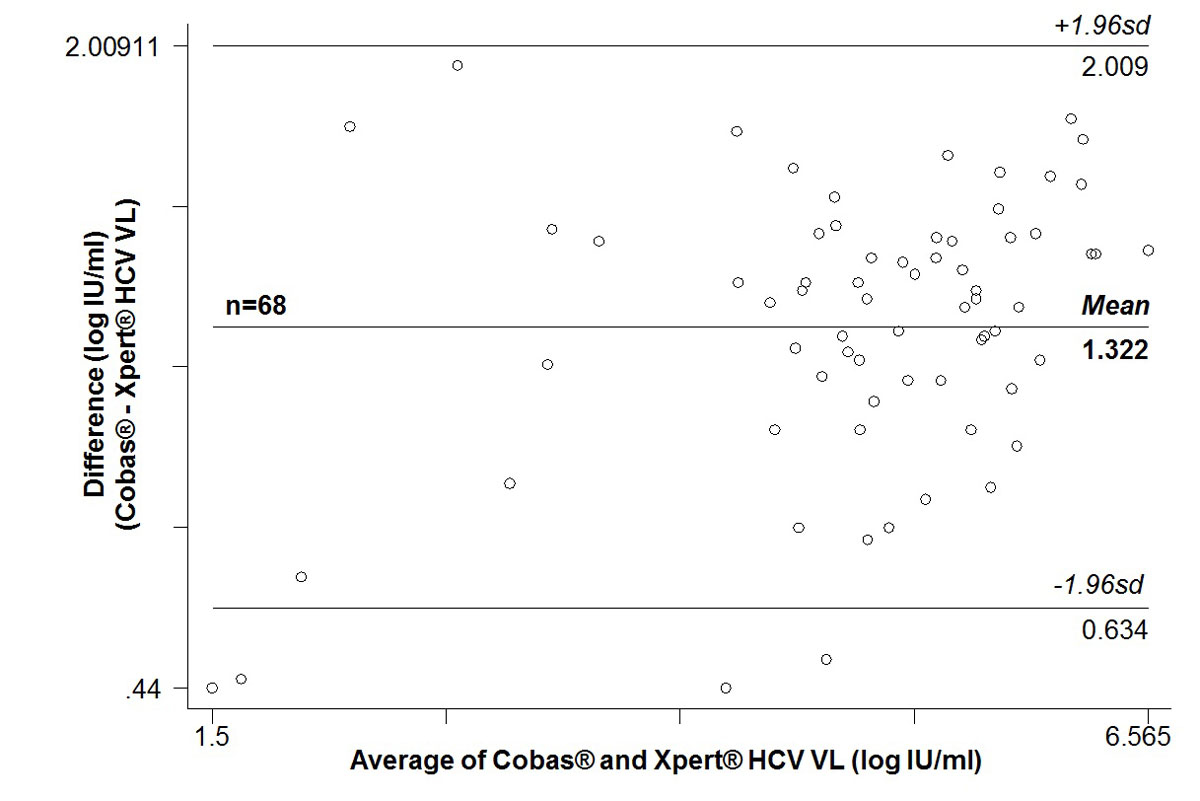
Figure S1 Bland-Altman plot of 68 paired quantifiable HCV RNA measurements: Cobas® in 650 µl venous plasma versus Xpert® HCV Viral Load (VL) test in 100 µl capillary whole blood
sd = standard deviation
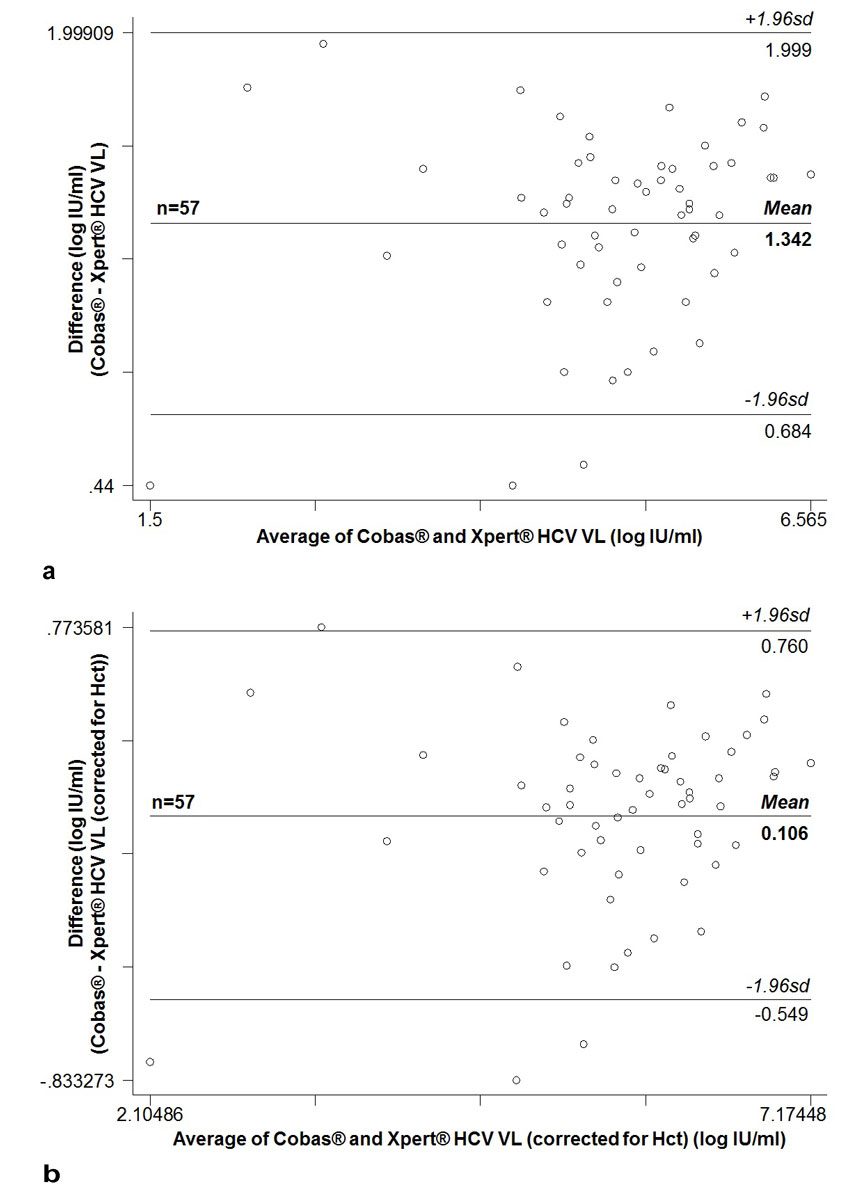
Figure S2 Bland-Altman plot of 57 paired quantifiable HCV RNA measurements for which same-day haematocrit was available: Cobas® in 650 µl venous plasma versus Xpert® HCV Viral Load (VL) test in 100 µl capillary whole blood. (a) Uncorrected; (b) Xpert® HCV Viral Load (VL) test results corrected for same-day haematocrit [+log10(1000/(100–haematocrit(%))]
sd = standard deviation, Hct = haematocrit
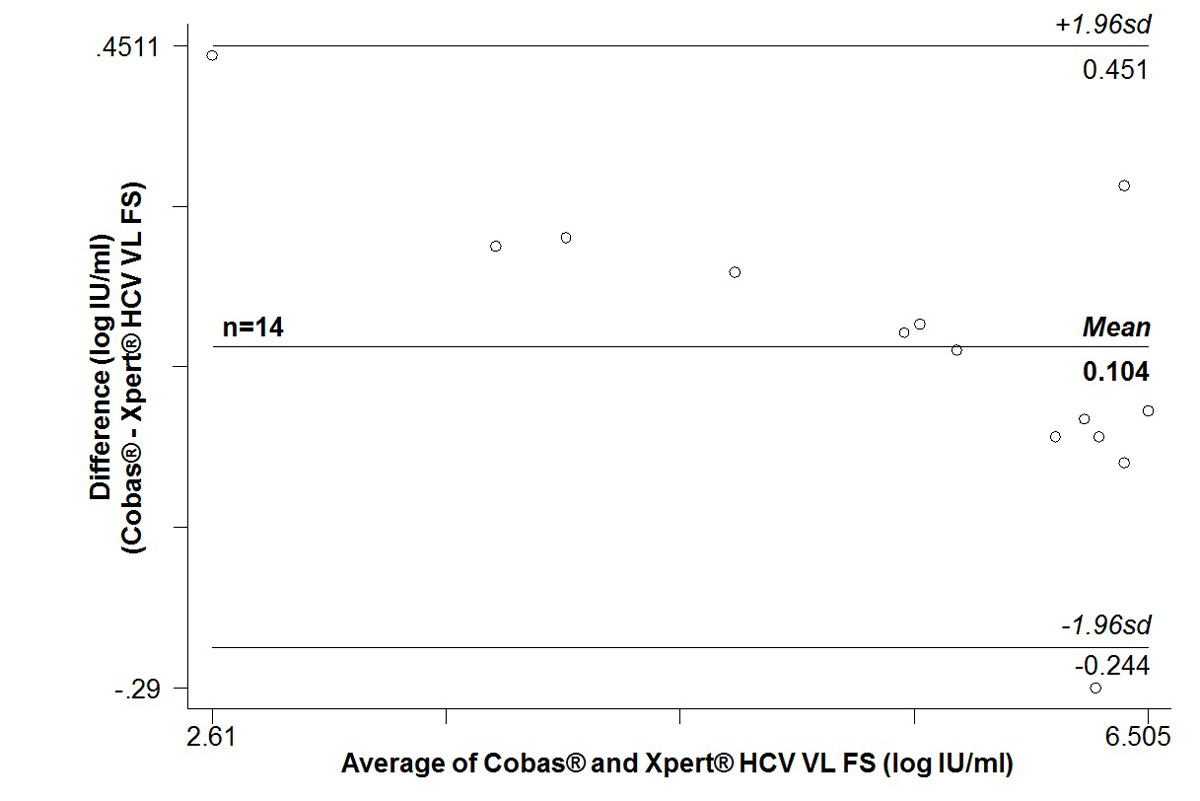
Figure S3 Bland-Altman plot of 14 paired quantifiable HCV RNA measurements: Cobas® in 650 µl venous plasma versus Xpert® HCV Viral Load Finger-Stick (VL FS) test in 100 µl capillary whole blood.
sd = standard deviation
We thank the Kirby Institute, Australia (Francois Lamoury, Tanya Applegate and Gregory Dore) for providing us with the protocol for the Xpert® HCV Viral Load test with capillary blood.
Preliminary results were presented as a poster at the Annual Meeting of the Swiss Society for Infectious Diseases in Basel, 30 August to 1 September 2017.
Axonlab® loaned us a GeneXpert® IV free of charge for one year. Afterwards, we could purchase one, thanks to financial support by the Hugo und Elsa Isler-Fonds. Cepheid® provided us with free lancets, EDTA minivettes and dilution buffer. Xpert® HCV Viral Load tests were sponsored by Gilead®. Additionally, the study was supported by the Swisslos-Fonds.
There is no conflict of interest for any of the authors.
1WHO. 05/2016, Combating hepatitis B and C to reach elimination by 2030 - Advocacy brief. http://apps.who.int/iris/bitstream/10665/206453/1/WHO_HIV_2016.04_eng.pdf (Accessed: 16 Jan. 2018)
2 Chahine EB , Sucher AJ , Hemstreet BA . Sofosbuvir/Velpatasvir: The First Pangenotypic Direct-Acting Antiviral Combination for Hepatitis C. Ann Pharmacother. 2017;51(1):44–53. doi:.https://doi.org/10.1177/1060028016668897
3 Puoti M , Foster GR , Wang S , Mutimer D , Gane E , Moreno C , et al. High SVR12 with 8-week and 12-week glecaprevir/pibrentasvir therapy: An integrated analysis of HCV genotype 1-6 patients without cirrhosis. J Hepatol. 2018;69(2):293–300. doi:.https://doi.org/10.1016/j.jhep.2018.03.007
4 Aghemo A , Colombo M . Glecaprevir/Pibrentasvir: The Final Piece in the Hepatitis C Virus Treatment Puzzle? Gastroenterology. 2018;154(4):1195–6. doi:.https://doi.org/10.1053/j.gastro.2018.01.035
5 Bregenzer A , Bruggmann P , Castro E , Moriggia A , Rothen M , Thurnheer MC , et al. Schweizer OAT-Programme auf ihrem Weg zur HCV-Elimination – die SAMMSU-Kohorte. Suchtmed. 2019;21(2):75–90.
6 Bregenzer A , Conen A , Knuchel J , Friedl A , Eigenmann F , Näf M , et al. Management of hepatitis C in decentralised versus centralised drug substitution programmes and minimally invasive point-of-care tests to close gaps in the HCV cascade. Swiss Med Wkly. 2017;147:w14544.
7 Janjua NZ , Kuo M , Yu A , Alvarez M , Wong S , Cook D , et al. The Population Level Cascade of Care for Hepatitis C in British Columbia, Canada: The BC Hepatitis Testers Cohort (BC-HTC). EBioMedicine. 2016;12:189–95. doi:.https://doi.org/10.1016/j.ebiom.2016.08.035
8 Yehia BR , Schranz AJ , Umscheid CA , Lo Re V, 3rd . The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One. 2014;9(7):e101554. doi:.https://doi.org/10.1371/journal.pone.0101554
9 Hajarizadeh B , Grebely J , McManus H , Estes C , Razavi H , Gray RT , et al. Chronic hepatitis C burden and care cascade in Australia in the era of interferon-based treatment. J Gastroenterol Hepatol. 2017;32(1):229–36. doi:.https://doi.org/10.1111/jgh.13453
10 Hayes B , Briceno A , Asher A , Yu M , Evans JL , Hahn JA , et al. Preference, acceptability and implications of the rapid hepatitis C screening test among high-risk young people who inject drugs. BMC Public Health. 2014;14(1):645. doi:.https://doi.org/10.1186/1471-2458-14-645
11 Bottero J , Boyd A , Gozlan J , Carrat F , Nau J , Pauti MD , et al. Simultaneous Human Immunodeficiency Virus-Hepatitis B-Hepatitis C Point-of-Care Tests Improve Outcomes in Linkage-to-Care: Results of a Randomized Control Trial in Persons Without Healthcare Coverage. Open Forum Infect Dis. 2015;2(4):ofv162. doi:.https://doi.org/10.1093/ofid/ofv162
12 Cooper C . Rapid HCV RNA testing: removing the final obstacle to elimination. Lancet Gastroenterol Hepatol. 2017;2(7):468–9. doi:.https://doi.org/10.1016/S2468-1253(17)30086-9
13 Tang W , Chen W , Amini A , Boeras D , Falconer J , Kelly H , et al. Diagnostic accuracy of tests to detect Hepatitis C antibody: a meta-analysis and review of the literature. BMC Infect Dis. 2017;17(S1, Suppl 1):695. doi:.https://doi.org/10.1186/s12879-017-2773-2
14 Ivanova Reipold E , Easterbrook P , Trianni A , Panneer N , Krakower D , Ongarello S , et al. Optimising diagnosis of viraemic hepatitis C infection: the development of a target product profile. BMC Infect Dis. 2017;17(S1, Suppl 1):707. doi:.https://doi.org/10.1186/s12879-017-2770-5
15 Greenman J , Roberts T , Cohn J , Messac L . Dried blood spot in the genotyping, quantification and storage of HCV RNA: a systematic literature review. J Viral Hepat. 2015;22(4):353–61. doi:.https://doi.org/10.1111/jvh.12345
16 Bennett S , Gunson RN , McAllister GE , Hutchinson SJ , Goldberg DJ , Cameron SO , et al. Detection of hepatitis C virus RNA in dried blood spots. J Clin Virol. 2012;54(2):106–9. doi:.https://doi.org/10.1016/j.jcv.2012.02.004
17 McHugh MP , Wu AHB , Chevaliez S , Pawlotsky JM , Hallin M , Templeton KE . Multicenter Evaluation of the Cepheid Xpert Hepatitis C Virus Viral Load Assay. J Clin Microbiol. 2017;55(5):1550–6. doi:.https://doi.org/10.1128/JCM.02460-16
18 Gupta E , Agarwala P , Kumar G , Maiwall R , Sarin SK . Point -of -care testing (POCT) in molecular diagnostics: Performance evaluation of GeneXpert HCV RNA test in diagnosing and monitoring of HCV infection. J Clin Virol. 2017;88:46–51. doi:.https://doi.org/10.1016/j.jcv.2017.01.006
19Cepheid®, Home → Cepheid Solutions → CE-IVD Tests → Virology → Xpert HCV Viral Load → Xpert HCV Viral Load Brochure - CE-IVD, http://www.cepheid.com/administrator/components/com_productcatalog/library-files/611eae732b51634d8a7e64878d8222a1-615085cee2245c0c5e547ebff5052119-Xpert-HCV-Viral-Load-Brochure-CEIVD-3043-02.pdf (Accessed: 16 Jan. 2018)
20 Grebely J , Lamoury FMJ , Hajarizadeh B , Mowat Y , Marshall AD , Bajis S , et al.; LiveRLife Study Group. Evaluation of the Xpert HCV Viral Load point-of-care assay from venepuncture-collected and finger-stick capillary whole-blood samples: a cohort study. Lancet Gastroenterol Hepatol. 2017;2(7):514–20. doi:.https://doi.org/10.1016/S2468-1253(17)30075-4
21 Lamoury FMJ , Bajis S , Hajarizadeh B , Marshall AD , Martinello M , Ivanova E , et al.; LiveRLife Study Group. Evaluation of the Xpert HCV Viral Load Finger-Stick Point-of-Care Assay. J Infect Dis. 2018;217(12):1889–96. doi:.https://doi.org/10.1093/infdis/jiy114
22 Yan Y , Huang F , Yuan T , Sun B , Yang R . HIV-1 Vpr increases HCV replication through VprBP in cell culture. Virus Res. 2016;223:153–60. doi:.https://doi.org/10.1016/j.virusres.2016.07.007
23 Lara J , Teka MA , Sims S , Xia GL , Ramachandran S , Khudyakov Y . HCV adaptation to HIV coinfection. Infect Genet Evol. 2018;65:216–25. doi:.https://doi.org/10.1016/j.meegid.2018.07.039
24 Tuaillon E , Mondain AM , Meroueh F , Ottomani L , Picot MC , Nagot N , et al. Dried blood spot for hepatitis C virus serology and molecular testing. Hepatology. 2010;51(3):752–8.
25 Santos C , Reis A , Dos Santos CV , Damas C , Silva MH , Viana MV , et al. The use of real-time PCR to detect hepatitis C virus RNA in dried blood spots from Brazilian patients infected chronically. J Virol Methods. 2012;179(1):17–20. doi:.https://doi.org/10.1016/j.jviromet.2011.06.012
26 Stapleton JT , Klinzman D , Schmidt WN , Pfaller MA , Wu P , LaBrecque DR , et al. Prospective comparison of whole-blood- and plasma-based hepatitis C virus RNA detection systems: improved detection using whole blood as the source of viral RNA. J Clin Microbiol. 1999;37(3):484–9.
27 Pham TN , MacParland SA , Mulrooney PM , Cooksley H , Naoumov NV , Michalak TI . Hepatitis C virus persistence after spontaneous or treatment-induced resolution of hepatitis C. J Virol. 2004;78(11):5867–74. doi:.https://doi.org/10.1128/JVI.78.11.5867-5874.2004
28 Fourati S , Guedj J , Chevaliez S , Nguyen THT , Roudot-Thoraval F , Ruiz I , et al. Viral kinetics analysis and virological characterization of treatment failures in patients with chronic hepatitis C treated with sofosbuvir and an NS5A inhibitor. Aliment Pharmacol Ther. 2018;47(5):665–73. doi:.https://doi.org/10.1111/apt.14478
29 Bajis S , Maher L , Treloar C , Hajarizadeh B , Lamoury FMJ , Mowat Y , et al.; LiveRLife Study Group. Acceptability and preferences of point-of-care finger-stick whole-blood and venepuncture hepatitis C virus testing among people who inject drugs in Australia. Int J Drug Policy. 2018;61:23–30. doi:.https://doi.org/10.1016/j.drugpo.2018.08.011
30 Bajis S , Grebely J , Cooper L , Smith J , Owen G , Chudleigh A , et al. Hepatitis C virus testing, liver disease assessment and direct-acting antiviral treatment uptake and outcomes in a service for people who are homeless in Sydney, Australia: The LiveRLife homelessness study. J Viral Hepat. 2019;26(8):969–79. doi:.https://doi.org/10.1111/jvh.13112
31 Wlassow M , Poiteau L , Roudot-Thoraval F , Rosa I , Soulier A , Hézode C , et al. The new Xpert HCV viral load real-time PCR assay accurately quantifies hepatitis C virus RNA in serum and whole-blood specimens. J Clin Virol. 2019;117:80–4. doi:.https://doi.org/10.1016/j.jcv.2019.06.007
32 Ahmed M , Munshi SU , Andalib S , Tabassum S , Islam MN . Testing Hepatitis A virus antibody in oral fluid among the prospective vaccinees foster the need of new oral HAV rapid test. Indian J Med Microbiol. 2011;29(1):72–3. doi:.https://doi.org/10.4103/0255-0857.76532
33 de Almeida LM , Azevedo RS , Marandino Guimarães AA , da Silva Freire Coutinho E , Struchiner CJ , Massad E . Detection of antibodies against hepatitis A virus in eluates of blood spotted on filter-paper: a pilot study in Rio de Janeiro, Brazil. Trans R Soc Trop Med Hyg. 1999;93(4):401–4. doi:.https://doi.org/10.1016/S0035-9203(99)90133-5
34 Shivkumar S , Peeling R , Jafari Y , Joseph L , Pai NP . Rapid point-of-care first-line screening tests for hepatitis B infection: a meta-analysis of diagnostic accuracy (1980-2010). Am J Gastroenterol. 2012;107(9):1306–13. doi:.https://doi.org/10.1038/ajg.2012.141
35 El-Ghitany EM , Farghaly AG . Evaluation of commercialized rapid diagnostic testing for some Hepatitis B biomarkers in an area of intermediate endemicity. J Virol Methods. 2013;194(1-2):190–3. doi:.https://doi.org/10.1016/j.jviromet.2013.08.026
36 Wu FY , Liao YW , Wu JF , Chen HL , Hsu HY , Chang MH , et al. A simple and rapid test-card method to detect hepatitis B surface antigen and antibody: potential application in young children and infants. Pediatr Neonatol. 2016;57(3):219–24. doi:.https://doi.org/10.1016/j.pedneo.2015.07.003
37 Poiteau L , Soulier A , Roudot-Thoraval F , Hézode C , Challine D , Pawlotsky JM , et al. Performance of rapid diagnostic tests for the detection of anti-HBs in various patient populations. J Clin Virol. 2017;96:64–6. doi:.https://doi.org/10.1016/j.jcv.2017.09.012
Axonlab® loaned us a GeneXpert® IV free of charge for one year. Afterwards, we could purchase one, thanks to financial support by the Hugo und Elsa Isler-Fonds. Cepheid® provided us with free lancets, EDTA minivettes and dilution buffer. Xpert® HCV Viral Load tests were sponsored by Gilead®. Additionally, the study was supported by the Swisslos-Fonds.
There is no conflict of interest for any of the authors.