Biomarkers in cardiovascular medicine: towards precision medicine
DOI: https://doi.org/10.4414/smw.2019.20125
Danielle Menosi
Gualandroab, Raphael
Twerenbolda, Jasper
Boeddinghausa, Thomas
Nestelbergera, Christian
Puelachera, Christian
Muellera
aCardiovascular Research Institute Basel (CRIB) and Department of Cardiology, University Hospital Basel, University of Basel, Switzerland
bHeart Institute (InCor), University of Sao Paulo Medical School, Brazil
Summary
Biomarkers are noninvasive, inexpensive, highly reproducible tools that allow clinicians to quantify pathophysiological processes relevant to a specific disease. Although the concept of biomarker-guided precision medicine is still in its infancy once a specific cardiovascular diagnosis is established, biomarker guidance has become the standard of care in the early diagnosis of acute cardiovascular disease in patients presenting to the emergency department with common symptoms such as acute chest pain or acute dyspnoea. This review highlights recent advances and remaining uncertainties regarding the use of the most relevant cardiovascular biomarkers, namely high-sensitivity cardiac troponin and natriuretic peptides in established indications such as the early diagnosis of acute myocardial infarction and heart failure. In addition, we address emerging indications such as the screening for perioperative myocardial infarction.
Abbreviations:
- AMI
-
acute myocardial infarction
- BNP
-
B-type natriuretic peptide
- cTn
-
cardiac troponin
- ECG
-
electrocardiogram
- ESC
-
European Society of Cardiology
- hs-cTn
-
high-sensitivity cardiac troponin
- NSTEMI
-
non-ST-segment-elevation myocardial infarction
- NT-proBNP
-
N-terminal-pro-B-type natriuretic peptide
- PMI
-
perioperative myocardial infarction/injury
- sST2
-
soluble suppression of tumourigenicity 2
High-sensitivity cardiac troponin
High-sensitivity cardiac troponin plays a major role in the diagnostic work-up of patients presenting to the emergency department with symptoms suggestive of acute myocardial infarction (AMI) [1]. Clinical assessment and the 12-lead electrocardiogram (ECG) are important, but unfortunately do not have high enough accuracy to reliably diagnose or exclude non-ST-segment-elevation myocardial infarction (NSTEMI). Therefore, the addition of blood tests to quantify the extent of myocardial injury, a pathophysiological hallmark of AMI, using cardiac troponin (cTn) T or I, form one cornerstone for the early diagnosis of AMI.
Recent advances in the analytical sensitivity and precision of cTn-assays have led to the development of high-sensitivity cardiac troponin assays and thereby the clinical ability to detect and precisely quantify even small amounts of cardiomyocyte injury [2–35]. In comparison with the conventional cTn assay, these new assays increased the diagnostic accuracy for AMI at presentation, reducing the “troponin-blind” interval and allowing the development of novel strategies for the rapid rule-out or early rule-in of NSTEMI [2–34]. These improved assays are classified as “sensitive” when able to detect cTn in ~20–50% of healthy individuals and as “high-sensitivity” if they detect a cTn level in >50% of apparently healthy subjects, and if they have a coefficient of variation of <10% at the 99th percentile upper reference limit of the assay [8]. High-sensitivity assays have the ability to accurately detect cTn at lower levels than older generation assays, giving them higher sensitivity for the detection of AMI at presentation, which means that the time interval to the second measurement of high-sensitivity cTn (hs-cTn) can be significantly shortened to 3 hours or even 1 hour using the algorithms described in detail below [2–36]. Additionally, these strategies reduce the time to discharge from the emergency department, with substantial cost savings as hs-cTn assays are inexpensive tests. The cost of hs-cTn assays is identical to that of conventional cTn assays. It varies substantially from country to country worldwide ranging from about EUR 2–15.
Both cTnT and cTnI are structural proteins specific to the heart. However, they are not disease-specific markers. Hs-cTnT and hs-cTnI assays exactly quantify the amount of myocardial injury [3, 35, 36]. They must be interpreted as quantitative variables and not in a binary fashion (negative/positive) like a pregnancy test. From a diagnostic perspective, it is highly inappropriate to label a patient as “cTn-positive”, as this would put together patients with only mildly elevated cTn levels barely above the 99th percentile and an associated positive predictive value for NSTEMI of only about 40–50% with patients with markedly elevated cTn levels (e.g., about five times above the 99th percentile) and an associated positive predictive value of 90%. The higher the cTnT/I concentration, the higher the likelihood for the presence of AMI. To differentiate AMI from other causes of chest pain, absolute rather than relative hs-cTn changes are preferable [12, 19–21]. The larger the absolute cTnT/I change within 1, 2, or 3 hours, the higher the likelihood for the presence of AMI [12, 19–21]. Continuing medical education and training of physicians in these concepts is essential to avoid inappropriate interpretation as AMI of chronic mild elevations of cTnT/I associated with, for example, heart failure or other structural cardiac disorders.
“False-positive” cardiac troponin measurements, “troponinaemia” and “troponinitis”
In the absence of overt myocardial ischaemia, elevated cTnT/I concentrations are sometimes labelled as “false positive” cTnT/I results, “troponinaemia” or “troponinitis”. These terms should definitely be avoided, as the vast majority of these unexpected cTnT/I elevations, are “true positive” for myocardial injury (rather than AMI) and reflect previously undetected or underestimated myocardial disease including valvular heart disease, heart failure and chronic coronary artery disease, or even AMI with atypical symptoms. The clinical use of hs-cTn assays has taught us that multiple cardiac as well as primarily noncardiac disorders (such as septic shock) may lead to substantial amounts of myocardial injury and thereby hs-cTnT/I elevations (table 1) [3, 8]. It is important to note that patients with stable cTnT/I elevations (chronic myocardial injury) have a worse prognosis than similar patients without a cTnT/I elevation, irrespective of the underlying disease. This is true regardless of whether the patient has heart failure, renal dysfunction-associated cardiac disease, gastrointestinal bleeding, sepsis, respiratory disease, pulmonary embolism, subarachnoid haemorrhage or stroke, or whether the patient is asymptomatic without known cardiovascular disease [37]. Obviously, the medical consequences of myocardial injury as quantified by cTnT/I elevations will be highly individualised and different from that in patients with AMI.
Table 1 Conditions other than myocardial infarction associated with cardiac troponin elevations.
| Tachyarrhythmias |
| Heart failure |
| Hypertensive emergencies |
| Critical illness (e.g., shock, sepsis, burns) |
| Myocarditis |
| Tako-tsubo cardiomyopathy |
| Structural heart disease (e.g., aortic stenosis) |
| Aortic dissection |
| Pulmonary embolism, pulmonary hypertension |
| Renal dysfunction and associated cardiac disease |
| Coronary spasm |
| Acute neurological event (e.g., stroke or subarachnoid haemorrhage) |
| Cardiac contusion or cardiac procedures (e.g., CABG, PCI, ablation, pacing, cardioversion, or endomyocardial biopsy) |
| Hypo- and hyperthyroidism |
| Infiltrative diseases (e.g., amyloidosis, haemochromatosis, sarcoidosis, scleroderma) |
| Myocardial drug toxicity or poisoning (e.g., doxorubicin, 5-fluorouracil, trastuzumab, snake venoms) |
| Extreme endurance efforts |
| Rhabdomyolysis |
CABG = coronary artery bypass surgery; PCI = percutaneous coronary intervention
Adapted from Twerenbold et al. [4] with permission from the publisher. Twerenbold R, Boeddinghaus J, Nestelberger T, Wildi K, Rubini Gimenez M, Badertscher P, et al. Clinical Use of High-Sensitivity Cardiac Troponin in Patients With Suspected Myocardial Infarction. J Am Coll Cardiol. 2017;70(8):996–1012. |
There are two uncommon, but well-described exceptions to this rule. First, particularly hs-cTnI assays may have “true false-positive” values due to heterophilic antibodies. These heterophilic antibodies are endogenous human antibodies that bind to immunoglobulins of other species and can occasionally exist in blood samples. In high titres, they can bind to the antibody of the hs-cTnI immunoassay, giving a positive result in the absence of the antigen to be detected (cardiac troponin I) [38]. Second, particularly the hs-cTnT assay may have “true false-positive” values as a result of rare chronic skeletal muscle diseases such as Duchenne [39]. If such an analytical problem is expected, measurement of the other hs-cTn (hs-cTnT and I, respectively) and cardiac imaging including magnetic resonance imaging usually rapidly clarifies the case [38]. In case of “true elevations”, hs-cTnI and hs-cTnT are concordant and both elevated. In case of “false elevations”, hs-cTnI and hs-cTnT are discordant.
Hs-cTn in patients with suspected acute myocardial infarction
Rapid triage algorithms using high-sensitivity cardiac troponins
The most important clinical and economic advantage of hs-cTnT/I assays is their ability to substantially reduce the “troponin-blind” interval in the first hours of an AMI. This allowed the development of several troponin-based strategies aimed at early rule-out or rule-in of NSTEMI. Two of them, the 0/1h-algorithm and the 0/3h-algorithm, are currently recommended by the European Society of Cardiology (ESC) as a class I recommendation [3].
It is important to consider the following aspects when applying troponin-based strategies in clinical practice (fig. 1). First, these early hs-cTn-based algorithms should be used only in conjunction with full clinical assessment. Second, they should be considered as triage strategies rather than definite diagnostic strategies, and additional imaging tests such as invasive coronary angiography, stress testing, echocardiography or coronary computed tomography angiography may be necessary for a definite diagnosis. Third, the percentage of patients eligible for rule-out or rule-in varies widely from about 10 to 80% depending on the underlying algorithm, the hs-cTnT/I assay used and the clinical setting, including the prevalence of NSTEMI [20, 23]. Fourth, these strategies should only be applied after the initial ECG has excluded ST-segment-elevation myocardial infarction, since these high-risk patients need prompt identification based on the ECG and immediate reperfusion therapy without the need for hs-cTnT/I testing [2]. Fifth, like any other triage strategy, these novel hs-cTnT/I based strategies should be implemented only after extensive training of all involved clinicians and nurses, and embedded in the local standard operating procedures of the emergency department.
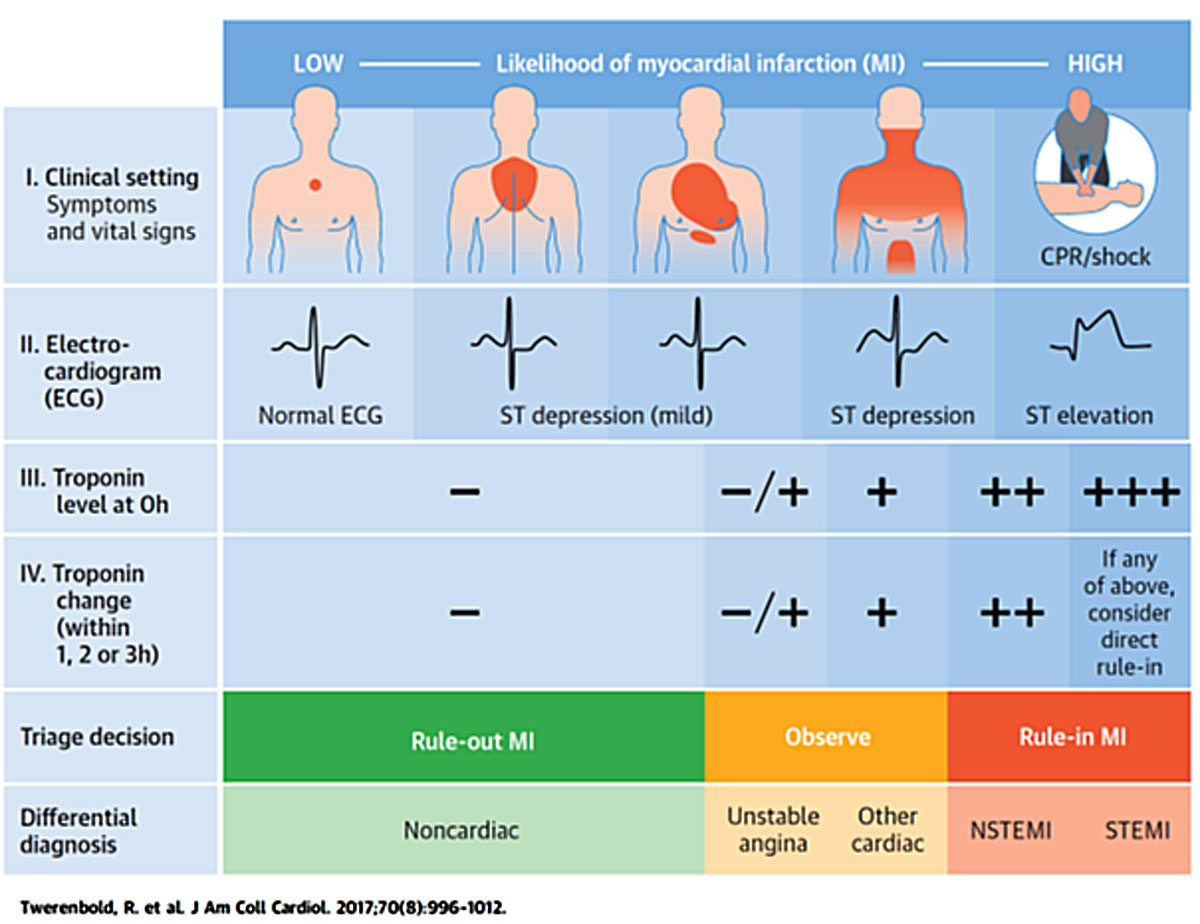
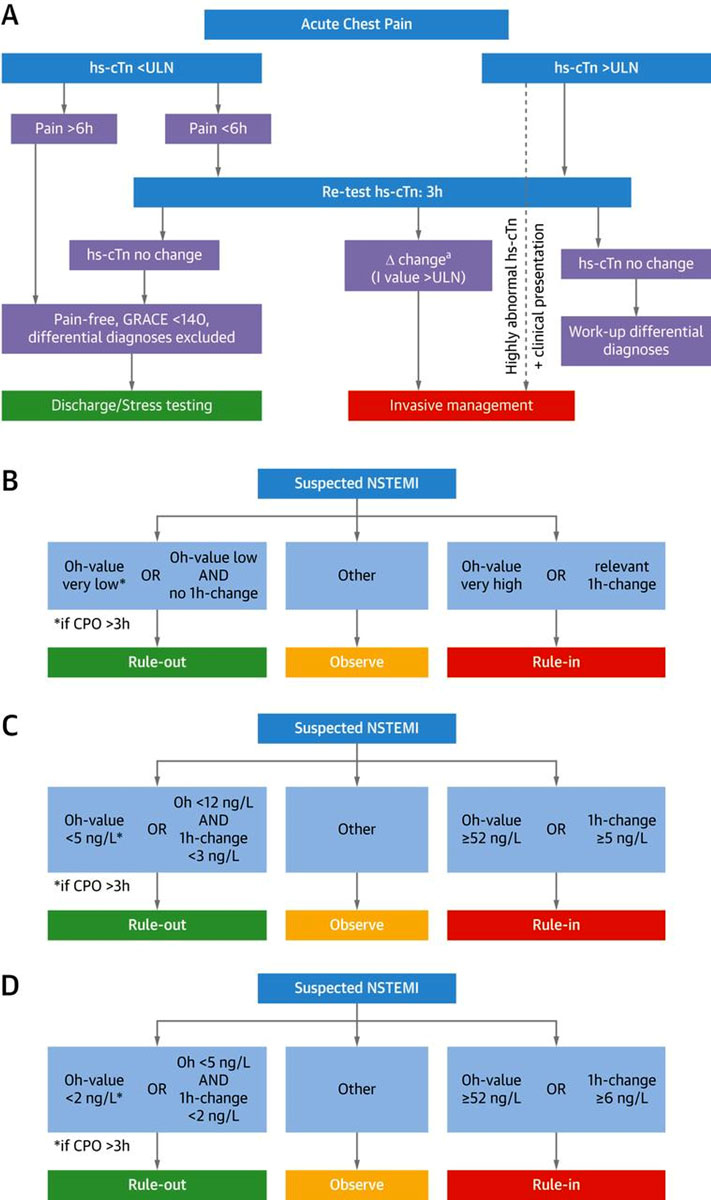
Figure 1
Clinical assessment of patients presenting with suspected acute coronary syndrome. The initial assessment is based on the integration of low likelihood and/or high likelihood features derived from clinical setting (symptoms, vital signs), 12-lead ECG, and cardiac troponin determined at presentation to the emergency department and serially thereafter. “Other cardiac” includes, among others, myocarditis, Tako-tsubo cardiomyopathy and congestive heart failure. “Noncardiac” refers to thoracic diseases such as pneumonia or pneumothorax. Cardiac troponin and its change during serial sampling should be interpreted as a quantitative marker: the higher the 0h-level or the absolute change during serial sampling, the higher the likelihood for the presence of myocardial infarction. In patients presenting with cardiac arrest or haemodynamic instability of presumed cardiovascular origin, echocardiography should be performed/interpreted by trained physicians immediately following a 12-lead ECG. If the initial evaluation suggests aortic dissection or pulmonary embolism, D-dimers and multi-detector computed tomography angiography are recommended according to dedicated algorithms.
CPR = cardiopulmonary resuscitation; ECG = electrocardiogram; MI = myocardial infarction; NSTEMI = non-ST-segment-elevation myocardial infarction; STEMI=ST-segment-elevation myocardial infarction
Adapted from Twerenbold et al. [4] with permission from the publisher. Twerenbold R, Boeddinghaus J, Nestelberger T, Wildi K, Rubini Gimenez M, Badertscher P, et al. Clinical Use of High-Sensitivity Cardiac Troponin in Patients With Suspected Myocardial Infarction. J Am Coll Cardiol. 2017;70(8):996–1012.
Recent studies have shown that the ESC 0/1h-algorithm provides even better performance characteristics than the ESC 0/3h-algorithm and should be the preferred triage algorithm (fig. 2), as it provides the most favourable balance between patient safety and triage efficacy [40–43].
The concept of the ESC 0/1h-algorithm is exclusively based on hs-cTn concentrations at the emergency department presentation and their absolute change after 1 hour, using assay-specific cutoffs. This concept is depicted in figure 2B; an example with assay-specific cutoffs for the hs-cTnT assay is in figure 2C. [3, 20, 29, 30, 44, 45]. In addition to serial sampling, the ESC 0/1h-algorithm allows direct rule-out of NSTEMI based on a single hs-cTnT/I measurement if hs-cTnT/I concentration at presentation is extremely low, but only in patients with a chest pain onset >3 hours prior to the emergency department presentation, as safety of this single measurement strategy was reduced in the very early presenters. [46] The ESC-0/1h-algorithm results in safe rule-out of NSTEMI (negative predicted value of 99–100%) and allows an accurate early triage in about 75% of patients: 60% towards rule-out and in 15% towards rule-in of NSTEMI [45]. The application of the ESC 0/1h-algorithm is also possible in institutions with a median assay turn-around time of more than 1 hour since the 1 hour only refers to the timing of the serial sample. In these institutions, the second blood draw simply needs to be performed while the results from the first blood draw are still awaited, because these values have anyway to be evaluated together in order to determine the hs-cTn delta value.
The ESC 0/1h-algorithm may also be applied in patients with renal dysfunction and the elderly without the need to adjust cut-off values, as excellent safety was confirmed in these high-risk patients also [47–49]. However, fewer patients will be triaged to the rule-out group given the higher pre-test probability for NSTEMI in these subgroups.
Additional helpful steps in the observe zone
The ESC 0/1h-algorithms [3, 20, 29, 30, 44, 45] are very helpful for either rapid rule-in or rule-out of NSTEMI in about 75% of patients, leaving about 25% in the observe zone [3, 18, 20, 29, 30, 44, 45, 50, 51]. These patients are typically older men with known coronary artery disease. Patients in the observe zone require detailed clinical assessment, additional an hs-cTn measurement at 3 hours and, usually, cardiac imaging including echocardiography to make an accurate diagnosis [52] The clinical interpretation of mildly abnormal hs-cTn levels is crucial for physicians in the emergency department since up to one third of patients triaged to the observe zone are finally diagnosed with NSTEMI or unstable angina. Therefore, further serial hs-cTn re-testing at 3 hours should be performed to better differentiate an acute cardiac disease (such as NSTEMI) associated with a dynamic hs-cTn course from a chronic cardiac disease reflected by stable hs-cTn course (chronic myocardial injury). Coronary angiography (in those with high likelihood for NSTEMI), echocardiography and functional stress imaging (in those with low likelihood for NSTEMI) seem to be the preferred tests in observe zone patients [52].
Coronary computed tomography angiography seems a suitable imaging modality in only a minority [53]. A randomised controlled trial recently showed no benefit of routine coronary computed tomography angiography over standard optimal care encompassing hs-cTnT in patients with suspected acute coronary syndrome, with regard to identification of significant coronary artery disease requiring revascularisation within 30 days, duration of hospital stay or direct discharge from the emergency department [54]. Functional instead of anatomical testing is mandatory to differentiate coronary lesions resulting in myocardial ischaemia and acute chest pain at rest from lesions that are innocent bystanders in relation to the acute chest pain episode leading to emergency department presentation [52].
Overruling a triage recommendation
Hs-cTn-based triage algorithms must always be used in conjunction with detailed clinical assessment and interpretation of the ECG. This analysis may result in overruling a “rule-out” recommendation provided by the hs-cTn-based algorithms in some patients perceived to be at high-risk of NSTEMI. Overruling should then lead to the identical process described for patients assigned the observe zone and should always include an additional hs-cTn measurement at 3 hours. For example, if a patient was classified as “rule-out” of AMI, but has chest pain characteristics typical for unstable angina, he/she would still possibly benefit from hospitalisation and intensification of medical therapy, and probably from coronary angiography or noninvasive imaging for coronary artery disease.
Rule-out of non-ST-segment-elevation myocardial infarction does not always equal outpatient management
The novel strategies were developed to safely rule-out NSTEMI, but not other disorders that still may require hospital admission such as unstable angina, pulmonary embolism, aortic dissection or severe sepsis from pneumonia. Accordingly, the percentage of patients who can possibly be managed in an out-patient clinic is smaller than the percentage of patients ruled-out for NSTEMI. Besides, standard operating procedures should be in place to ensure appropriate follow-up of patients rapidly discharged from the emergency department, which often will include outpatient functional cardiac stress testing in patients with intermediate to high-risk probability for coronary artery disease.
Emerging indication: patients undergoing noncardiac surgery
More than 300 million surgical procedures are performed annually worldwide [55]. In spite of improvements in surgical and anaesthetic techniques, the morbidity and mortality related to these procedures remain higher than expected [56]. Cardiac complications, including perioperative myocardial infarction/injury (PMI), are important contributors to mortality after non-cardiac surgery [57–59]. Therefore, it is paramount to identify which patients are at greater risk for postoperative complications, not only to provide the best medical care, but also to assess the risk-benefit ratio of surgical procedures. The currently available risk stratification tools to identify these patients, such as the Revised Cardiac Risk Index, have only moderate accuracy [60–62]. Biomarkers could improve the prediction of cardiac complications and mortality after noncardiac surgery. Additionally, biomarkers are essential for prompt diagnosis of postoperative complications, including PMI. Currently, the most solid scientific evidence available indicates that cardiac troponins and natriuretic peptides are the most useful biomarkers in the perioperative setting [58, 63].
Perioperative evaluation: perioperative myocardial Infarction/injury, the silent and neglected killer
Elevated preoperative hs-cTnT/I concentrations are independent predictors of mortality and major cardiac events after noncardiac surgery [64–67]. Current guidelines differ in their recommendation regarding the preoperative measurement of hs-cTnT/I concentrations, indicating ongoing controversy whether the improvement in risk stratification documented in recent studies is of clinical value [2, 68, 69]. Similarly, current guidelines also differ in their recommendation regarding perioperative screening for PMI in high-risk patients by measurement of hs-cTn after surgery [2, 68–70]. As a considerable number of patients undergoing surgery have chronic myocardial injury, it seems reasonable to obtain a preoperative sample from these patients in order to differentiate acute from chronic myocardial injury after surgery [68–70].
Observational studies have also addressed the prognostic role of postoperative hs-cTnT/I elevations [57, 58, 65, 67, 71, 72]. In the VISION study (the vascular events in noncardiac surgery patients cohort evaluation), the authors measured hs-cTnT on the first three days after noncardiac surgery, in a prospective cohort of 21,842 patients aged above 45 years. The peak postoperative hs-cTnT concentration was significantly associated with 30-day mortality and the higher the hs-cTnT concentration, the higher the mortality (even for patients with concentrations within the normal range, when compared with patients with undetectable values) [57]. These results corroborate the findings of previous studies that reported the adverse impact of hs-cTn values on prognosis of patients with several clinical conditions (diabetes, coronary artery disease, heart failure, renal failure) and also in the primary prevention setting [73–76]. In order to determine the extent and the true impact of surgery itself on prognosis, it is paramount to differentiate the acute myocardial injury precipitated by surgery from chronic myocardial injury.
In the Basel-PMI study, hs-cTnT concentrations were measured before and for 2 days after 2546 noncardiac surgeries in consecutive patients either older than 65 years or older than 45 years with documented coronary, cerebral or peripheral artery disease. In order to distinguish acute from chronic injury, PMI was defined as an absolute increase in hs-cTnT of 14 ng/l (the 99th percentile upper reference limit for the assay studied) above preoperative concentrations or between two postoperative concentrations. PMI occurred after 16% of the procedures, and only 6% of the patients had chest pain. This documents the major clinical problem that more than 90% of cases of PMI are asymptomatic and can therefore only be detected by routine hs-cTnT/I screening. After 30 days, nearly 10% of patients with PMI had died versus 2% of patients without PMI. Mortality was identical in patients with PMI in whom other criteria required for the definition of spontaneous AMI such as ECG changes and/or chest discomfort were also present versus those in whom no additional criteria were present. The occurrence of PMI was independently related to mortality [58].
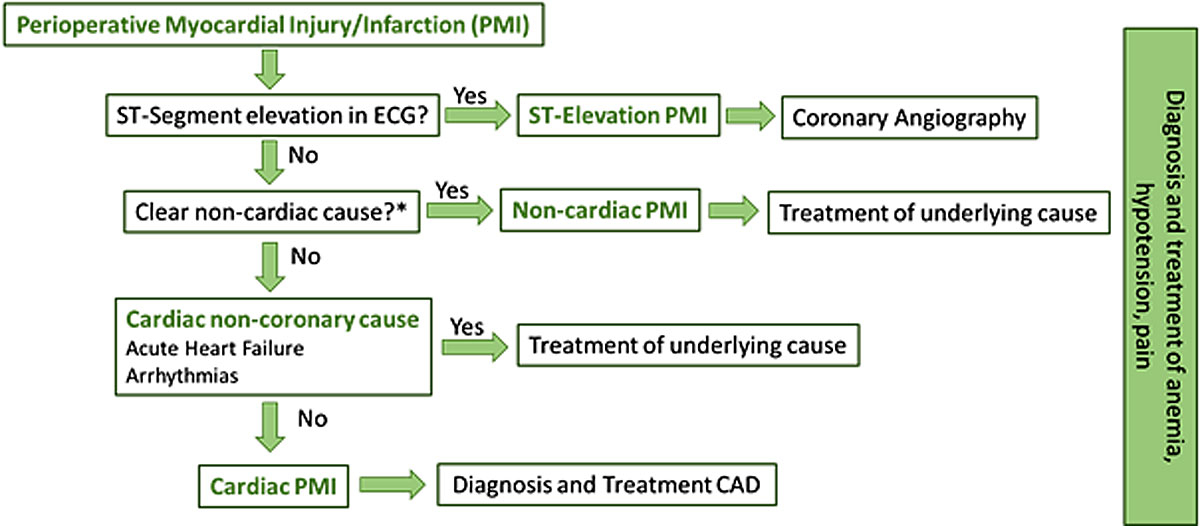
As with spontaneous myocardial infarction, different pathophysiological processes may underlie the development of PMI [59, 77]. Type 1 myocardial infarction (atherosclerotic plaque rupture and/or erosion) and type 2 myocardial infarction (supply-demand mismatch of oxygen) seem to be the most common, but several other cardiac and even primarily noncardiac conditions may contribute to myocardial injury and therefore to the differential diagnosis of PMI (table 1). Therefore, the approach to patients with PMI should be personalised, with a careful clinical evaluation, including an ECG, with the aim to identify and treat the most likely mechanism (fig. 3). In the presence of ST-segment-elevation myocardial infarction (a rare event in the perioperative period), this should be treated as usual with primary percutaneous coronary intervention. As these patients are at increased bleeding risk, dual antiplatelet therapy with clopidogrel rather than more potent antiplatelet agents should be preferred. The next step is to exclude a primarily noncardiac cause, such as severe sepsis, stroke, cardiac trauma or pulmonary embolism [78]. In the presence of a primarily noncardiac cause, the specific condition should be treated. Then a cardiac but non-coronary cause, such as acute heart failure or arrhythmias, should be identified and treated according to current guidelines [79]. Potential causes for type 2 myocardial infarction, such as anaemia, tachycardia due to pain and hypotension should always be assessed and treated accordingly. For the remaining patients, diagnosis and treatment of coronary artery disease should be performed. The recommendation and timing for invasive or noninvasive diagnosis depends on patient characteristics, clinical presentation and ECG findings, and should be individualised [68, 80]. The Basel-PMI study revealed that the vast majority of cases of PMI (86%) are cardiac and about 14% have a primarily noncardiac trigger [58].
Insights gained in VISION, Basel-PMI and other recent studies fully support the description of PMI as a silent (>90% are asymptomatic!) and neglected (these events are missed in most institutions worldwide!) killer with a 30-day mortality exceeding 10%. These findings fully support the recommendation within the universal definition of myocardial infarction for systematic screening using hs-cTnT/I measurements once before surgery and once daily in the first two postoperative days in high-risk patients such as those older than 65 years or older than 45 years with documented coronary, cerebral or peripheral artery disease if undergoing major noncardiac surgery [2, 68–70].
Natriuretic peptides
Natriuretic peptides – B-type natriuretic peptide (BNP) or N-terminal-pro-B-type natriuretic peptide (NT-proBNP) – are quantitative markers of haemodynamic cardiac stress and heart failure [81–83]. As the extent of heart failure, often unknown to patients and their physicians, is a powerful predictor of mortality and cardiac complications [84–86], preoperative concentrations of BNP and NT-proBNP have consistently been shown to be accurate and independent predictors of mortality and cardiac events after noncardiac surgery [63, 66, 87–90]. The higher the natriuretic peptide values, the higher the risk for cardiac complications or death. In an individual patient data meta-analysis, preoperative BNP concentrations above 100 pg/ml or NT-proBNP concentrations above 300 pg/ml worked well as cut-off values [63]. Preoperative measurements of BNP/NT-proBNP increased the accuracy of the Revised Cardiac Risk Index score for prediction of postoperative events [66, 89, 91]. Guidelines suggest measurement of BNP or NT-ProBNP in high-risk patients undergoing noncardiac surgery, such as patients older than 65 years, or patients older than 55 years with at least one cardiovascular risk factor [68–70]. Future studies need to define in more detail which cardiac work-up and/or therapy would allow mitigation of the increased risk identified by the elevated BNP/NT-proBNP concentrations. The postoperative measurement of BNP/NT-proBNP should be restricted to symptomatic patients with unclear dyspnoea for the diagnosis of acute heart failure [92].
Other cardiovascular biomarkers
Several pilot studies have evaluated the potential clinical utility of several other cardiovascular biomarkers in the perioperative setting. ST2 is an interleukin-1 family receptor, and its soluble isoform (sST2) is related to cardiac hypertrophy and fibrosis following cardiac overload. In patients with acute and chronic heart failure, high sST2 levels have been related to worse prognosis and might be used for risk stratification [93]. With regard to noncardiac surgery, in a small study including 175 patients sST2 concentrations were measured in the first 3 days after surgery. Peak sST2 concentration was independently related to 30-day major adverse cardiac events (death, nonfatal cardiac arrest, myocardial infarction and acute heart failure) [94]. Copeptin, the C-terminal fragment of the provasopressin peptide, was related to postoperative events, but did not provide added value on top of NT-proBNP and cTn [95]. Similarly, concentrations of midregional pro-adrenomedullin, leptin, heart-type fatty acid-binding protein and survivin have been shown to associated with adverse events after noncardiac surgery in small studies [96–99]. Further research is needed to determine the potential clinical utility of these biomarkers in patients undergoing noncardiac surgery.
Conclusion
Routine clinical use of cardiovascular biomarkers, specifically hs-cTnT/I represents a critical step towards precision medicine in the early diagnosis of acute myocardial infarction, and in the early detection of perioperative myocardial infarction/injury. In order to optimise its use, physicians need to be aware of three essential concepts:
- Hs-cTnT/I concentrations should always be interpreted as a quantitative variable, and not binary like a pregnancy test.
- In the case of unexpected hs-cTnT/I elevations, “true elevations” due to myocardial injury from unknown or underestimated cardiac disease such as heart failure are much more likely than “false elevations” due to analytical problems.
- Hs-cTnT/I concentrations should always be interpreted within the context of the clinical presentation of the patient using all other clinical information available and not in isolation.
Author contributions
Danielle Menosi Gualandro and Raphael Twerenbold contributed equally to this manuscript.
References:
1
Blomkalns
AL
,
Gibler
WB
. Chest pain unit concept: rationale and diagnostic strategies. Cardiol Clin. 2005;23(4):411–21, v. doi:.https://doi.org/10.1016/j.ccl.2005.08.011
2
Thygesen
K
,
Alpert
JS
,
Jaffe
AS
,
Chaitman
BR
,
Bax
JJ
,
Morrow
DA
, et al.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40(3):237–69. doi:.https://doi.org/10.1093/eurheartj/ehy462
3
Roffi
M
,
Patrono
C
,
Collet
JP
,
Mueller
C
,
Valgimigli
M
,
Andreotti
F
, et al.; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267–315. doi:.https://doi.org/10.1093/eurheartj/ehv320
4
Twerenbold
R
,
Boeddinghaus
J
,
Nestelberger
T
,
Wildi
K
,
Rubini Gimenez
M
,
Badertscher
P
, et al.
Clinical Use of High-Sensitivity Cardiac Troponin in Patients With Suspected Myocardial Infarction. J Am Coll Cardiol. 2017;70(8):996–1012. doi:.https://doi.org/10.1016/j.jacc.2017.07.718
5
Twerenbold
R
,
Boeddinghaus
J
,
Mueller
C
. Update on High-sensitivity cardiac troponin in patients with suspected myocardial infarction. Eur Heart J Suppl. 2018;20(suppl_G):G2–10. doi:.https://doi.org/10.1093/eurheartj/suy020
6
Twerenbold
R
,
Boeddinghaus
J
,
Nestelberger
T
,
Wildi
K
,
Rubini Gimenez
M
,
Badertscher
P
, et al.
How to best use high-sensitivity cardiac troponin in patients with suspected myocardial infarction. Clin Biochem. 2018;53:143–55. doi:.https://doi.org/10.1016/j.clinbiochem.2017.12.006
7
Thygesen
K
,
Mair
J
,
Katus
H
,
Plebani
M
,
Venge
P
,
Collinson
P
, et al.; Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J. 2010;31(18):2197–204. doi:.https://doi.org/10.1093/eurheartj/ehq251
8
Thygesen
K
,
Mair
J
,
Giannitsis
E
,
Mueller
C
,
Lindahl
B
,
Blankenberg
S
, et al.; Study Group on Biomarkers in Cardiology of ESC Working Group on Acute Cardiac Care. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J. 2012;33(18):2252–7. doi:.https://doi.org/10.1093/eurheartj/ehs154
9
Reichlin
T
,
Hochholzer
W
,
Bassetti
S
,
Steuer
S
,
Stelzig
C
,
Hartwiger
S
, et al.
Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361(9):858–67. doi:.https://doi.org/10.1056/NEJMoa0900428
10
Keller
T
,
Zeller
T
,
Peetz
D
,
Tzikas
S
,
Roth
A
,
Czyz
E
, et al.
Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361(9):868–77. doi:.https://doi.org/10.1056/NEJMoa0903515
11
Giannitsis
E
,
Kurz
K
,
Hallermayer
K
,
Jarausch
J
,
Jaffe
AS
,
Katus
HA
. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56(2):254–61. doi:.https://doi.org/10.1373/clinchem.2009.132654
12
Haaf
P
,
Drexler
B
,
Reichlin
T
,
Twerenbold
R
,
Reiter
M
,
Meissner
J
, et al.
High-sensitivity cardiac troponin in the distinction of acute myocardial infarction from acute cardiac noncoronary artery disease. Circulation. 2012;126(1):31–40. doi:.https://doi.org/10.1161/CIRCULATIONAHA.112.100867
13
Reiter
M
,
Twerenbold
R
,
Reichlin
T
,
Haaf
P
,
Peter
F
,
Meissner
J
, et al.
Early diagnosis of acute myocardial infarction in the elderly using more sensitive cardiac troponin assays. Eur Heart J. 2011;32(11):1379–89. doi:.https://doi.org/10.1093/eurheartj/ehr033
14
Reiter
M
,
Twerenbold
R
,
Reichlin
T
,
Benz
B
,
Haaf
P
,
Meissner
J
, et al.
Early diagnosis of acute myocardial infarction in patients with pre-existing coronary artery disease using more sensitive cardiac troponin assays. Eur Heart J. 2012;33(8):988–97. doi:.https://doi.org/10.1093/eurheartj/ehr376
15
Apple
FS
. A new season for cardiac troponin assays: it’s time to keep a scorecard. Clin Chem. 2009;55(7):1303–6. doi:.https://doi.org/10.1373/clinchem.2009.128363
16
Rubini Gimenez
M
,
Twerenbold
R
,
Reichlin
T
,
Wildi
K
,
Haaf
P
,
Schaefer
M
, et al.
Direct comparison of high-sensitivity-cardiac troponin I vs. T for the early diagnosis of acute myocardial infarction. Eur Heart J. 2014;35(34):2303–11. doi:.https://doi.org/10.1093/eurheartj/ehu188
17
Meune
C
,
Reichlin
T
,
Irfan
A
,
Schaub
N
,
Twerenbold
R
,
Meissner
J
, et al.
How safe is the outpatient management of patients with acute chest pain and mildly increased cardiac troponin concentrations?
Clin Chem. 2012;58(5):916–24. doi:.https://doi.org/10.1373/clinchem.2011.178053
18
Reichlin
T
,
Cullen
L
,
Parsonage
WA
,
Greenslade
J
,
Twerenbold
R
,
Moehring
B
, et al.
Two-hour algorithm for triage toward rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Am J Med. 2015;128(4):369–79.e4. doi:.https://doi.org/10.1016/j.amjmed.2014.10.032
19
Reichlin
T
,
Irfan
A
,
Twerenbold
R
,
Reiter
M
,
Hochholzer
W
,
Burkhalter
H
, et al.
Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation. 2011;124(2):136–45. doi:.https://doi.org/10.1161/CIRCULATIONAHA.111.023937
20
Reichlin
T
,
Schindler
C
,
Drexler
B
,
Twerenbold
R
,
Reiter
M
,
Zellweger
C
, et al.
One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172(16):1211–8. doi:.https://doi.org/10.1001/archinternmed.2012.3698
21
Mueller
M
,
Biener
M
,
Vafaie
M
,
Doerr
S
,
Keller
T
,
Blankenberg
S
, et al.
Absolute and relative kinetic changes of high-sensitivity cardiac troponin T in acute coronary syndrome and in patients with increased troponin in the absence of acute coronary syndrome. Clin Chem. 2012;58(1):209–18. doi:.https://doi.org/10.1373/clinchem.2011.171827
22
Mueller
C
. Biomarkers and acute coronary syndromes: an update. Eur Heart J. 2014;35(9):552–6. doi:.https://doi.org/10.1093/eurheartj/eht530
23
Than
M
,
Cullen
L
,
Reid
CM
,
Lim
SH
,
Aldous
S
,
Ardagh
MW
, et al.
A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): a prospective observational validation study. Lancet. 2011;377(9771):1077–84. doi:.https://doi.org/10.1016/S0140-6736(11)60310-3
24
Than
M
,
Cullen
L
,
Aldous
S
,
Parsonage
WA
,
Reid
CM
,
Greenslade
J
, et al.
2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol. 2012;59(23):2091–8. doi:.https://doi.org/10.1016/j.jacc.2012.02.035
25
Cullen
L
,
Mueller
C
,
Parsonage
WA
,
Wildi
K
,
Greenslade
JH
,
Twerenbold
R
, et al.
Validation of high-sensitivity troponin I in a 2-hour diagnostic strategy to assess 30-day outcomes in emergency department patients with possible acute coronary syndrome. J Am Coll Cardiol. 2013;62(14):1242–9. doi:.https://doi.org/10.1016/j.jacc.2013.02.078
26
Than
M
,
Aldous
S
,
Lord
SJ
,
Goodacre
S
,
Frampton
CM
,
Troughton
R
, et al.
A 2-hour diagnostic protocol for possible cardiac chest pain in the emergency department: a randomized clinical trial. JAMA Intern Med. 2014;174(1):51–8. doi:.https://doi.org/10.1001/jamainternmed.2013.11362
27
Meller
B
,
Cullen
L
,
Parsonage
WA
,
Greenslade
JH
,
Aldous
S
,
Reichlin
T
, et al.
Accelerated diagnostic protocol using high-sensitivity cardiac troponin T in acute chest pain patients. Int J Cardiol. 2015;184:208–15. doi:.https://doi.org/10.1016/j.ijcard.2015.02.006
28
Hammarsten
O
,
Fu
ML
,
Sigurjonsdottir
R
,
Petzold
M
,
Said
L
,
Landin-Wilhelmsen
K
, et al.
Troponin T percentiles from a random population sample, emergency room patients and patients with myocardial infarction. Clin Chem. 2012;58(3):628–37. doi:.https://doi.org/10.1373/clinchem.2011.171496
29
Reichlin
T
,
Twerenbold
R
,
Wildi
K
,
Gimenez
MR
,
Bergsma
N
,
Haaf
P
, et al.
Prospective validation of a 1-hour algorithm to rule-out and rule-in acute myocardial infarction using a high-sensitivity cardiac troponin T assay. CMAJ. 2015;187(8):E243–52. doi:.https://doi.org/10.1503/cmaj.141349
30
Rubini Gimenez
M
,
Twerenbold
R
,
Jaeger
C
,
Schindler
C
,
Puelacher
C
,
Wildi
K
, et al.
One-hour rule-in and rule-out of acute myocardial infarction using high-sensitivity cardiac troponin I. Am J Med. 2015;128(8):861–870.e4. doi:.https://doi.org/10.1016/j.amjmed.2015.01.046
31
Body
R
,
Carley
S
,
McDowell
G
,
Jaffe
AS
,
France
M
,
Cruickshank
K
, et al.
Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol. 2011;58(13):1332–9. doi:.https://doi.org/10.1016/j.jacc.2011.06.026
32
Rubini Giménez
M
,
Hoeller
R
,
Reichlin
T
,
Zellweger
C
,
Twerenbold
R
,
Reiter
M
, et al.
Rapid rule out of acute myocardial infarction using undetectable levels of high-sensitivity cardiac troponin. Int J Cardiol. 2013;168(4):3896–901. doi:.https://doi.org/10.1016/j.ijcard.2013.06.049
33
Hollander
JE
,
Than
M
,
Mueller
C
. State-of-the-Art Evaluation of Emergency Department Patients Presenting With Potential Acute Coronary Syndromes. Circulation. 2016;134(7):547–64. doi:.https://doi.org/10.1161/CIRCULATIONAHA.116.021886
34
Keller
T
,
Zeller
T
,
Ojeda
F
,
Tzikas
S
,
Lillpopp
L
,
Sinning
C
, et al.
Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011;306(24):2684–93. doi:.https://doi.org/10.1001/jama.2011.1896
35
Twerenbold
R
,
Rubini Gimenez
M
,
Nestelberger
T
,
Boeddinghaus
J
,
Wildi
K
,
Mueller
C
. Optimising the early rule-out and rule-in of myocardial infarction using biomarkers. Cardiovasc Med. 2019;22:w02010. doi:.https://doi.org/10.4414/cvm.2019.02010
36
Amsterdam
EA
,
Wenger
NK
,
Brindis
RG
,
Casey
DE, Jr
,
Ganiats
TG
,
Holmes
DR, Jr
, et al.
2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–228. doi:. Correction in: J Am Coll Cardiol. 2014;64(24):2713–4. doi:https://doi.org/10.1016/j.jacc.2014.09.017
37
Hollander
JE
. Managing Troponin Testing. Ann Emerg Med. 2016;68(6):690–4. doi:.https://doi.org/10.1016/j.annemergmed.2016.05.023
38
Mair
J
,
Lindahl
B
,
Müller
C
,
Giannitsis
E
,
Huber
K
,
Möckel
M
, et al.
What to do when you question cardiac troponin values. Eur Heart J Acute Cardiovasc Care. 2018;7(6):577–86. doi:.https://doi.org/10.1177/2048872617708973
39
Schmid
J
,
Liesinger
L
,
Birner-Gruenberger
R
,
Stojakovic
T
,
Scharnagl
H
,
Dieplinger
B
, et al.
Elevated Cardiac Troponin T in Patients With Skeletal Myopathies. J Am Coll Cardiol. 2018;71(14):1540–9. doi:.https://doi.org/10.1016/j.jacc.2018.01.070
40
Badertscher
P
,
Boeddinghaus
J
,
Twerenbold
R
,
Nestelberger
T
,
Wildi
K
,
Wussler
D
, et al.; APACE Investigators. Direct Comparison of the 0/1h and 0/3h Algorithms for Early Rule-Out of Acute Myocardial Infarction. Circulation. 2018;137(23):2536–8. doi:.https://doi.org/10.1161/CIRCULATIONAHA.118.034260
41
Chapman
AR
,
Anand
A
,
Boeddinghaus
J
,
Ferry
AV
,
Sandeman
D
,
Adamson
PD
, et al.
Comparison of the Efficacy and Safety of Early Rule-Out Pathways for Acute Myocardial Infarction. Circulation. 2017;135(17):1586–96. doi:.https://doi.org/10.1161/CIRCULATIONAHA.116.025021
42
Chapman
AR
,
Hesse
K
,
Andrews
J
,
Ken Lee
K
,
Anand
A
,
Shah
ASV
, et al.
High-Sensitivity Cardiac Troponin I and Clinical Risk Scores in Patients With Suspected Acute Coronary Syndrome. Circulation. 2018;138(16):1654–65. doi:.https://doi.org/10.1161/CIRCULATIONAHA.118.036426
43
Twerenbold
R
,
Costabel
JP
,
Nestelberger
T
,
Campos
R
,
Wussler
D
,
Arbucci
R
, et al.
Outcome of Applying the ESC 0/1-hour Algorithm in Patients With Suspected Myocardial Infarction. J Am Coll Cardiol. 2019;74(4):483–94. doi: https://doi.org/10.1016/j.jacc.2019.05.046
44
Mueller
C
,
Giannitsis
E
,
Christ
M
,
Ordóñez-Llanos
J
,
deFilippi
C
,
McCord
J
, et al.; TRAPID-AMI Investigators. Multicenter Evaluation of a 0-Hour/1-Hour Algorithm in the Diagnosis of Myocardial Infarction With High-Sensitivity Cardiac Troponin T. Ann Emerg Med. 2016;68(1):76–87.e4. doi:.https://doi.org/10.1016/j.annemergmed.2015.11.013
45
Twerenbold
R
,
Neumann
JT
,
Sörensen
NA
,
Ojeda
F
,
Karakas
M
,
Boeddinghaus
J
, et al.
Prospective Validation of the 0/1-h Algorithm for Early Diagnosis of Myocardial Infarction. J Am Coll Cardiol. 2018;72(6):620–32. doi:.https://doi.org/10.1016/j.jacc.2018.05.040
46
Boeddinghaus
J
,
Nestelberger
T
,
Twerenbold
R
,
Wildi
K
,
Badertscher
P
,
Cupa
J
, et al.
Direct Comparison of 4 Very Early Rule-Out Strategies for Acute Myocardial Infarction Using High-Sensitivity Cardiac Troponin I. Circulation. 2017;135(17):1597–611. doi:.https://doi.org/10.1161/CIRCULATIONAHA.116.025661
47
Twerenbold
R
,
Wildi
K
,
Jaeger
C
,
Gimenez
MR
,
Reiter
M
,
Reichlin
T
, et al.
Optimal Cutoff Levels of More Sensitive Cardiac Troponin Assays for the Early Diagnosis of Myocardial Infarction in Patients With Renal Dysfunction. Circulation. 2015;131(23):2041–50. doi:.https://doi.org/10.1161/CIRCULATIONAHA.114.014245
48
Twerenbold
R
,
Badertscher
P
,
Boeddinghaus
J
,
Nestelberger
T
,
Wildi
K
,
Puelacher
C
, et al.
0/1-Hour Triage Algorithm for Myocardial Infarction in Patients With Renal Dysfunction. Circulation. 2018;137(5):436–51. doi:.https://doi.org/10.1161/CIRCULATIONAHA.117.028901
49
Boeddinghaus
J
,
Nestelberger
T
,
Twerenbold
R
,
Neumann
JT
,
Lindahl
B
,
Giannitsis
E
, et al.; APACE, BACC, and TRAPID-AMI Investigators. Impact of age on the performance of the ESC 0/1h-algorithms for early diagnosis of myocardial infarction. Eur Heart J. 2018;39(42):3780–94. doi:.https://doi.org/10.1093/eurheartj/ehy514
50
Druey
S
,
Wildi
K
,
Twerenbold
R
,
Jaeger
C
,
Reichlin
T
,
Haaf
P
, et al.
Early rule-out and rule-in of myocardial infarction using sensitive cardiac Troponin I. Int J Cardiol. 2015;195:163–70. doi:.https://doi.org/10.1016/j.ijcard.2015.05.079
51
Boeddinghaus
J
,
Reichlin
T
,
Cullen
L
,
Greenslade
JH
,
Parsonage
WA
,
Hammett
C
, et al.
Two-Hour Algorithm for Triage toward Rule-Out and Rule-In of Acute Myocardial Infarction by Use of High-Sensitivity Cardiac Troponin I. Clin Chem. 2016;62(3):494–504. doi:.https://doi.org/10.1373/clinchem.2015.249508
52
Nestelberger
T
,
Wildi
K
,
Boeddinghaus
J
,
Twerenbold
R
,
Reichlin
T
,
Giménez
MR
, et al.
Characterization of the observe zone of the ESC 2015 high-sensitivity cardiac troponin 0h/1h-algorithm for the early diagnosis of acute myocardial infarction. Int J Cardiol. 2016;207:238–45. doi:.https://doi.org/10.1016/j.ijcard.2016.01.112
53
Schlett
CL
,
Hoffmann
U
,
Geisler
T
,
Nikolaou
K
,
Bamberg
F
. Cardiac computed tomography for the evaluation of the acute chest pain syndrome: state of the art. Radiol Clin North Am. 2015;53(2):297–305. doi:.https://doi.org/10.1016/j.rcl.2014.11.007
54
Dedic
A
,
Lubbers
MM
,
Schaap
J
,
Lammers
J
,
Lamfers
EJ
,
Rensing
BJ
, et al.
Coronary CT Angiography for Suspected ACS in the Era of High-Sensitivity Troponins: Randomized Multicenter Study. J Am Coll Cardiol. 2016;67(1):16–26. doi:.https://doi.org/10.1016/j.jacc.2015.10.045
55
Weiser
TG
,
Haynes
AB
,
Molina
G
,
Lipsitz
SR
,
Esquivel
MM
,
Uribe-Leitz
T
, et al.
Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet. 2015;385(Suppl 2):S11. doi:.https://doi.org/10.1016/S0140-6736(15)60806-6
56
Pearse
RM
,
Moreno
RP
,
Bauer
P
,
Pelosi
P
,
Metnitz
P
,
Spies
C
, et al.; European Surgical Outcomes Study (EuSOS) group for the Trials groups of the European Society of Intensive Care Medicine and the European Society of Anaesthesiology. Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380(9847):1059–65. doi:.https://doi.org/10.1016/S0140-6736(12)61148-9
57
Devereaux
PJ
,
Biccard
BM
,
Sigamani
A
,
Xavier
D
,
Chan
MTV
,
Srinathan
SK
, et al.; Writing Committee for the VISION Study Investigators. Association of Postoperative High-Sensitivity Troponin Levels With Myocardial Injury and 30-Day Mortality Among Patients Undergoing Noncardiac Surgery. JAMA. 2017;317(16):1642–51. doi:.https://doi.org/10.1001/jama.2017.4360
58
Puelacher
C
,
Lurati Buse
G
,
Seeberger
D
,
Sazgary
L
,
Marbot
S
,
Lampart
A
, et al.; BASEL-PMI Investigators. Perioperative Myocardial Injury After Noncardiac Surgery: Incidence, Mortality, and Characterization. Circulation. 2018;137(12):1221–32. doi:.https://doi.org/10.1161/CIRCULATIONAHA.117.030114
59
Puelacher
C
,
Lurati-Buse
G
,
Singeisen
H
,
Dang
M
,
Cuculi
F
,
Müller
C
. Perioperative myocardial infarction/injury after noncardiac surgery. Swiss Med Wkly. 2015;145:w14219. doi:.https://doi.org/10.4414/smw.2015.14219
60
Lee
TH
,
Marcantonio
ER
,
Mangione
CM
,
Thomas
EJ
,
Polanczyk
CA
,
Cook
EF
, et al.
Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–9. doi:.https://doi.org/10.1161/01.CIR.100.10.1043
61
Bilimoria
KY
,
Liu
Y
,
Paruch
JL
,
Zhou
L
,
Kmiecik
TE
,
Ko
CY
, et al.
Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–42.e3. doi:.https://doi.org/10.1016/j.jamcollsurg.2013.07.385
62
Gualandro
DM
,
Puelacher
C
,
LuratiBuse
G
,
Llobet
GB
,
Yu
PC
,
Cardozo
FA
, et al.; GREAT network. Prediction of major cardiac events after vascular surgery. J Vasc Surg. 2017;66(6):1826–1835.e1. doi:.https://doi.org/10.1016/j.jvs.2017.05.100
63
Rodseth
RN
,
Biccard
BM
,
Le Manach
Y
,
Sessler
DI
,
Lurati Buse
GA
,
Thabane
L
, et al.
The prognostic value of pre-operative and post-operative B-type natriuretic peptides in patients undergoing noncardiac surgery: B-type natriuretic peptide and N-terminal fragment of pro-B-type natriuretic peptide: a systematic review and individual patient data meta-analysis. J Am Coll Cardiol. 2014;63(2):170–80. doi:.https://doi.org/10.1016/j.jacc.2013.08.1630
64
Gualandro
DM
,
Puelacher
C
,
LuratiBuse
G
,
Lampart
A
,
Strunz
C
,
Cardozo
FA
, et al.; TropoVasc and BASEL-PMI Investigators. Comparison of high-sensitivity cardiac troponin I and T for the prediction of cardiac complications after non-cardiac surgery. Am Heart J. 2018;203:67–73. doi:.https://doi.org/10.1016/j.ahj.2018.06.012
65
Nagele
P
,
Brown
F
,
Gage
BF
,
Gibson
DW
,
Miller
JP
,
Jaffe
AS
, et al.
High-sensitivity cardiac troponin T in prediction and diagnosis of myocardial infarction and long-term mortality after noncardiac surgery. Am Heart J. 2013;166(2):325–332.e1. doi:.https://doi.org/10.1016/j.ahj.2013.04.018
66
Weber
M
,
Luchner
A
,
Seeberger
M
,
Mueller
C
,
Liebetrau
C
,
Schlitt
A
, et al.
Incremental value of high-sensitive troponin T in addition to the revised cardiac index for peri-operative risk stratification in non-cardiac surgery. Eur Heart J. 2013;34(11):853–62. doi:.https://doi.org/10.1093/eurheartj/ehs445
67
Gillmann
HJ
,
Meinders
A
,
Grohennig
A
,
Larmann
J
,
Bünte
C
,
Calmer
S
, et al.
Perioperative levels and changes of high-sensitivity troponin T are associated with cardiovascular events in vascular surgery patients. Crit Care Med. 2014;42(6):1498–506. doi:.https://doi.org/10.1097/CCM.0000000000000249
68
Gualandro
DM
,
Yu
PC
,
Caramelli
B
,
Marques
AC
,
Calderaro
D
,
Fornari
LS
, et al.
3rd Guideline for Perioperative Cardiovascular Evaluation of the Brazilian Society of Cardiology. Arq Bras Cardiol. 2017;109(3, Supl 1):1–104. doi:.https://doi.org/10.5935/abc.20170140
69
Kristensen
SD
,
Knuuti
J
,
Saraste
A
,
Anker
S
,
Bøtker
HE
,
Hert
SD
, et al., Authors/Task Force Members. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383–431. doi:.https://doi.org/10.1093/eurheartj/ehu282
70
Duceppe
E
,
Parlow
J
,
MacDonald
P
,
Lyons
K
,
McMullen
M
,
Srinathan
S
, et al.
Canadian Cardiovascular Society Guidelines on Perioperative Cardiac Risk Assessment and Management for Patients Who Undergo Noncardiac Surgery. Can J Cardiol. 2017;33(1):17–32. doi:.https://doi.org/10.1016/j.cjca.2016.09.008
71
Kavsak
PA
,
Walsh
M
,
Srinathan
S
,
Thorlacius
L
,
Buse
GL
,
Botto
F
, et al.
High sensitivity troponin T concentrations in patients undergoing noncardiac surgery: a prospective cohort study. Clin Biochem. 2011;44(12):1021–4. doi:.https://doi.org/10.1016/j.clinbiochem.2011.05.017
72
Noordzij
PG
,
van Geffen
O
,
Dijkstra
IM
,
Boerma
D
,
Meinders
AJ
,
Rettig
TC
, et al.
High-sensitive cardiac troponin T measurements in prediction of non-cardiac complications after major abdominal surgery. Br J Anaesth. 2015;114(6):909–18. doi:.https://doi.org/10.1093/bja/aev027
73
Omland
T
,
de Lemos
JA
,
Sabatine
MS
,
Christophi
CA
,
Rice
MM
,
Jablonski
KA
, et al.; Prevention of Events with Angiotensin Converting Enzyme Inhibition (PEACE) Trial Investigators. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361(26):2538–47. doi:.https://doi.org/10.1056/NEJMoa0805299
74
Jia
X
,
Sun
W
,
Hoogeveen
RC
,
Nambi
V
,
Matsushita
K
,
Folsom
AR
, et al.
High-Sensitivity Troponin I and Incident Coronary Events, Stroke, Heart Failure Hospitalization, and Mortality in the ARIC Study. Circulation. 2019;139(23):2642–53. doi:.https://doi.org/10.1161/CIRCULATIONAHA.118.038772
75
Felker
GM
,
Mentz
RJ
,
Teerlink
JR
,
Voors
AA
,
Pang
PS
,
Ponikowski
P
, et al.
Serial high sensitivity cardiac troponin T measurement in acute heart failure: insights from the RELAX-AHF study. Eur J Heart Fail. 2015;17(12):1262–70. doi:.https://doi.org/10.1002/ejhf.341
76
Cavender
MA
,
White
WB
,
Jarolim
P
,
Bakris
GL
,
Cushman
WC
,
Kupfer
S
, et al.
Serial Measurement of High-Sensitivity Troponin I and Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus in the EXAMINE Trial (Examination of Cardiovascular Outcomes With Alogliptin Versus Standard of Care). Circulation. 2017;135(20):1911–21. doi:.https://doi.org/10.1161/CIRCULATIONAHA.116.024632
77
Gualandro
DM
,
Campos
CA
,
Calderaro
D
,
Yu
PC
,
Marques
AC
,
Pastana
AF
, et al.
Coronary plaque rupture in patients with myocardial infarction after noncardiac surgery: frequent and dangerous. Atherosclerosis. 2012;222(1):191–5. doi:.https://doi.org/10.1016/j.atherosclerosis.2012.02.021
78
Gualandro
DM
,
Puelacher
C
,
Mueller
C
. High-sensitivity cardiac troponin in acute conditions. Curr Opin Crit Care. 2014;20(5):472–7. doi:.https://doi.org/10.1097/MCC.0000000000000132
79
Ponikowski
P
,
Voors
AA
,
Anker
SD
,
Bueno
H
,
Cleland
JGF
,
Coats
AJS
, et al.; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. doi:.https://doi.org/10.1093/eurheartj/ehw128
80
Amsterdam
EA
,
Wenger
NK
,
Brindis
RG
,
Casey
DE, Jr
,
Ganiats
TG
,
Holmes
DR, Jr
, et al.; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(25):e344–426.. Correction in: Circulation. 2014;130(25):e433–4.
81
Kozhuharov
N
,
Sabti
Z
,
Wussler
D
,
Nowak
A
,
Badertscher
P
,
Twerenbold
R
, et al.; BASEL V Investigators. Prospective validation of N-terminal pro B-type natriuretic peptide cut-off concentrations for the diagnosis of acute heart failure. Eur J Heart Fail. 2019;21(6):813–5. doi:.https://doi.org/10.1002/ejhf.1471
82
Seferović
PM
,
Polovina
M
,
Bauersachs
J
,
Arad
M
,
Gal
TB
,
Lund
LH
, et al.
Heart failure in cardiomyopathies: a position paper from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(5):553–76. doi:.https://doi.org/10.1002/ejhf.1461
83
Mueller
C
,
Buser
P
. B-type natriuretic peptide (BNP): can it improve our management of patients with congestive heart failure?
Swiss Med Wkly. 2002;132(43-44):618–22.
84
Hammill
BG
,
Curtis
LH
,
Bennett-Guerrero
E
,
O’Connor
CM
,
Jollis
JG
,
Schulman
KA
, et al.
Impact of heart failure on patients undergoing major noncardiac surgery. Anesthesiology. 2008;108(4):559–67. doi:.https://doi.org/10.1097/ALN.0b013e31816725ef
85
Healy
KO
,
Waksmonski
CA
,
Altman
RK
,
Stetson
PD
,
Reyentovich
A
,
Maurer
MS
. Perioperative outcome and long-term mortality for heart failure patients undergoing intermediate- and high-risk noncardiac surgery: impact of left ventricular ejection fraction. Congest Heart Fail. 2010;16(2):45–9. doi:.https://doi.org/10.1111/j.1751-7133.2009.00130.x
86
Fayad
A
,
Ansari
MT
,
Yang
H
,
Ruddy
T
,
Wells
GA
. Perioperative Diastolic Dysfunction in Patients Undergoing Noncardiac Surgery Is an Independent Risk Factor for Cardiovascular Events: A Systematic Review and Meta-analysis. Anesthesiology. 2016;125(1):72–91. doi:.https://doi.org/10.1097/ALN.0000000000001132
87
Karthikeyan
G
,
Moncur
RA
,
Levine
O
,
Heels-Ansdell
D
,
Chan
MT
,
Alonso-Coello
P
, et al.
Is a pre-operative brain natriuretic peptide or N-terminal pro-B-type natriuretic peptide measurement an independent predictor of adverse cardiovascular outcomes within 30 days of noncardiac surgery? A systematic review and meta-analysis of observational studies. J Am Coll Cardiol. 2009;54(17):1599–606. doi:.https://doi.org/10.1016/j.jacc.2009.06.028
88
Ryding
AD
,
Kumar
S
,
Worthington
AM
,
Burgess
D
. Prognostic value of brain natriuretic peptide in noncardiac surgery: a meta-analysis. Anesthesiology. 2009;111(2):311–9. doi:.https://doi.org/10.1097/ALN.0b013e3181aaeb11
89
Rodseth
RN
,
Lurati Buse
GA
,
Bolliger
D
,
Burkhart
CS
,
Cuthbertson
BH
,
Gibson
SC
, et al.
The predictive ability of pre-operative B-type natriuretic peptide in vascular patients for major adverse cardiac events: an individual patient data meta-analysis. J Am Coll Cardiol. 2011;58(5):522–9. doi:.https://doi.org/10.1016/j.jacc.2011.04.018
90
Kopec
M
,
Duma
A
,
Helwani
MA
,
Brown
J
,
Brown
F
,
Gage
BF
, et al.
Improving Prediction of Postoperative Myocardial Infarction With High-Sensitivity Cardiac Troponin T and NT-proBNP. Anesth Analg. 2017;124(2):398–405. doi:.https://doi.org/10.1213/ANE.0000000000001736
91
Biccard
BM
,
Naidoo
P
. The role of brain natriuretic peptide in prognostication and reclassification of risk in patients undergoing vascular surgery. Anaesthesia. 2011;66(5):379–85. doi:.https://doi.org/10.1111/j.1365-2044.2011.06694.x
92
Ponikowski
P
,
Voors
AA
,
Anker
SD
,
Bueno
H
,
Cleland
JGF
,
Coats
AJS
, et al.; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. doi:.https://doi.org/10.1093/eurheartj/ehw128
93
van Vark
LC
,
Lesman-Leegte
I
,
Baart
SJ
,
Postmus
D
,
Pinto
YM
,
Orsel
JG
, et al.; TRIUMPH Investigators. Prognostic Value of Serial ST2 Measurements in Patients With Acute Heart Failure. J Am Coll Cardiol. 2017;70(19):2378–88. doi:.https://doi.org/10.1016/j.jacc.2017.09.026
94
Yang
HS
,
Hur
M
,
Yi
A
,
Kim
H
,
Kim
J
. Prognostic Role of High-sensitivity Cardiac Troponin I and Soluble Suppression of Tumorigenicity-2 in Surgical Intensive Care Unit Patients Undergoing Non-cardiac Surgery. Ann Lab Med. 2018;38(3):204–11. doi:.https://doi.org/10.3343/alm.2018.38.3.204
95
Jarai
R
,
Mahla
E
,
Perkmann
T
,
Jarai
R
,
Archan
S
,
Tentzeris
I
, et al.
Usefulness of pre-operative copeptin concentrations to predict post-operative outcome after major vascular surgery. Am J Cardiol. 2011;108(8):1188–95. doi:.https://doi.org/10.1016/j.amjcard.2011.06.024
96
Gu
Z
,
Sun
C
,
Xiang
D
. Postoperative Adverse Cardiovascular Events Associated with Leptin and Adverse Age After Elective Major Non-Cardiac Surgery: An Asian Single-Center Study. Med Sci Monit. 2018;24:2119–25. doi:.https://doi.org/10.12659/MSM.906797
97
Marković
D
,
Jevtović-Stoimenov
T
,
Ćosić
V
,
Stošić
B
,
Dinić
V
,
Marković-Živković
B
, et al.
Clinical Utility of Survivin (BIRC5), Novel Cardiac Biomarker, as a Prognostic Tool Compared to High-sensitivity C-reactive Protein, Heart-type Fatty Acid Binding Protein and Revised Lee Score in Elderly Patients Scheduled for Major Non-cardiac Surgery: A Prospective Pilot Study. J Med Biochem. 2018;37(2):110–20. doi:.https://doi.org/10.1515/jomb-2017-0046
98
Golubović
M
,
Janković
R
,
Sokolović
D
,
Ćosić
V
,
Maravić-Stojkovic
V
,
Kostić
T
, et al.
Preoperative Midregional Pro-Adrenomedullin and High-Sensitivity Troponin T Predict Perioperative Cardiovascular Events in Noncardiac Surgery. Med Princ Pract. 2018;27(3):278–84. doi:.https://doi.org/10.1159/000488197
99
Gillmann
HJ
,
Meinders
A
,
Larmann
J
,
Sahlmann
B
,
Schrimpf
C
,
Aper
T
, et al.
Adrenomedullin Is Associated With Surgical Trauma and Impaired Renal Function in Vascular Surgery Patients. J Intensive Care Med. 2019;34(1):67–76. doi:.https://doi.org/10.1177/0885066616689554