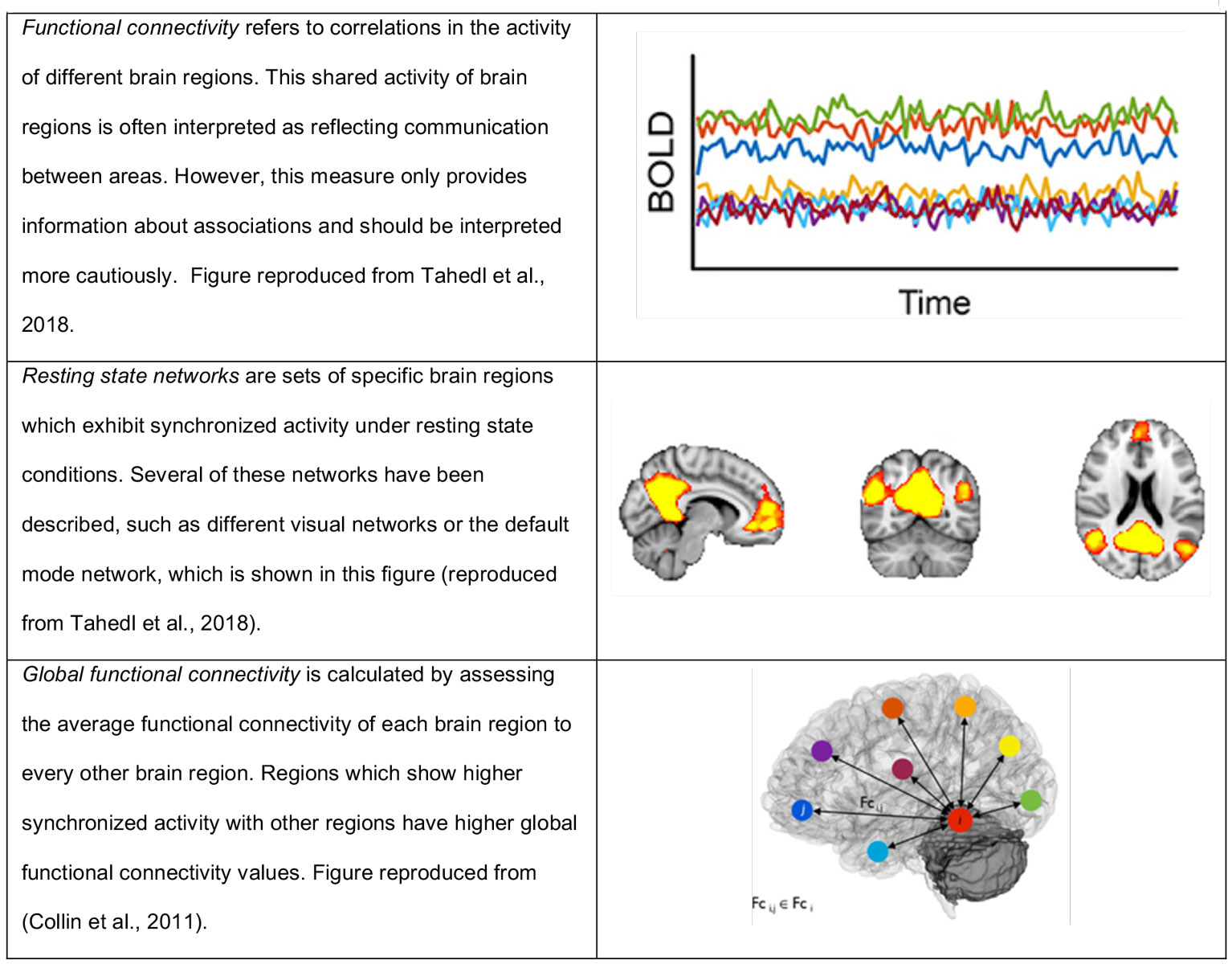
Figure 1 Key concepts of this article.
Figures reproduced from Tahedi et al., 2018 [12] and Collin et al. 2011 [13].
DOI: https://doi.org/10.4414/smw.2019.20124
Lysergic acid diethylamide (LSD) is a very potent hallucinogenic substance. Even small doses such as 100 µg can trigger profound changes in various mental domains. Among others, common effects are visual alterations, emotional and cognitive alterations, synaesthesia, derealisation and dissolution of the ego boundaries [1], all of which depend on LSD’s agonism at the serotonin2A-receptor [2].
After the discovery of LSD by the Swiss chemist Albert Hoffmann in 1943, the drug was commercialised by the Basel-based pharmaceutical company Sandoz for the use in psychiatry [3]. The indications mentioned covered two areas: firstly, administration of LSD as an adjunct to psychotherapy and, secondly, self-administration by the psychiatrist in order to gain insight into the world of patients with mental disorders, particularly psychosis. At first glance it seems contradictory that a drug should have such opposing effects. However, both areas – LSD as a treatment option for mental disorders and as a model for psychosis – were extensively investigated during the next decades. This research led – among others – to one of the first hypotheses that mental disorders might be caused by brain chemistry, when the similarity between LSD and serotonin was discovered [4]. During the 1950s and 1960s, more than 1000 reports on LSD were published, thousands of patients with various mental disorders were treated with LSD and results were often regarded as encouraging [4]. However, research stopped when LSD was prohibited in the 1970s.
In recent years, the research on hallucinogenic drugs has experienced a revival. In the UK and in Switzerland, the effects of LSD on healthy subjects were investigated by a number of researchers [1, 5, 6]. In 2015, a first study by the Swiss psychiatrist Peter Gasser investigated clinical effects of LSD as an anxiety treatment for patients with life-threatening diseases [7]. One of the most interesting questions in this field is how the typical effects of LSD are represented on a neuronal level. This question was recently addressed by three clinical trials, which used functional magnetic resonance imaging (fMRI) in healthy subjects. These studies were conducted by teams in London [5], Zurich [6] and by our research group in Basel [8, 9]. In the present paper we will summarise the results of these trials and provide an interpretation of how the observed neuronal effects might evoke the profound subjective effects related to this substance. We will focus on studies investigating resting state activities (participants did not engage in any task during the functional magnetic resonance imaging [fMRI] scan) of the brain and will not refer to task-related fMRI studies (e.g. [8–11]). In addition, because of these restrictions, this article focuses exclusively on functional connectivity, measurement of the correlation of brain activity between different regions. This concept and related terms used in this article are summarised in figure 1. The studies discussed in this review used moderate orally administered doses of 100 µg LSD [2, 8, 9] and 75 µg LSD intravenously [5]. In humans these moderate doses are expected to induce all typical effects associated with this drug [14]. All studies described below were conducted in healthy subjects and sample sizes were rather small (range 15–24 subjects). Details on the included studies are shown in table 1 below.

Figure 1 Key concepts of this article.
Figures reproduced from Tahedi et al., 2018 [12] and Collin et al. 2011 [13].
Table 1 Characteristic of the studies included in this review.
| Study authors | Centre | Sample size | Dosage | Sample characteristic | Placebo controlled | Functional connectivity measure |
|---|---|---|---|---|---|---|
| Carhart-Harris et al., 2016 [5] | London | 15 (healthy) | 75 µg LSD intravenously | Healthy, not hallucinogen-naïve | Yes | Within- and between-network functional connectivity |
| Tagliazucchi et al., 2016 [15] | Global functional connectivity | |||||
| Müller et al., 2018 [16] | Basel | 20 (healthy) | 100 µg orally | Healthy, mostly hallucinogen-naïve | Yes | Within- and between-network functional connectivity |
| Müller et al., 2017 [8, 9] | Global functional connectivity | |||||
| Preller et al., 2018 [2] | Zurich | 24 (healthy) | 100 µg orally | Healthy, mostly hallucinogen-naïve | Yes | Global functional connectivity |
The first published fMRI study on LSD focused on changes in functional connectivity within and between resting state networks [5]. Resting state networks are sets of specific brain regions which exhibit synchronised activity without further behavioural tasks or activity. Several brain connectivity networks have been described, such as the default mode network or different visual networks [17]. Carhart-Harris et al. investigated activity within several networks (connectivity between brain regions belonging to the same network) as well as connectivity between them [5]. They found decreased connectivity within several networks, but increased connectivity between the networks. In other words, these findings indicated that the synchronised activity normally seen within the respective network was less synchronised, but different networks were more synchronised with each other. This finding was interpreted as altered “integration and segregation”, that is, that LSD induces a state in which compartmentalisation between networks is blurred while the integrity of the individual networks is “breaking down”. It was concluded, that these brain states show typical drug effects induced by LSD. In line with this, the authors reported a significant correlation between decreased connectivity within the default mode network and a subjective drug effect “ego dissolution” (a state of temporary loss of the sense of a self) [5]. Details on the networks showing decreased connectivity after LSD are shown in table 2 and details on alterations in between-network connectivity are shown in figure 2.
Table 2 LSD-induced alterations in functional connectivity within resting state networks compared between studies.
| Resting state network | Carhart-Harris et al., 2016 [ 5 ] | Müller et al., 2018 [ 16 ] |
|---|---|---|
| Visual network 1 | ↓ | ↓ |
| Visual network 2 | ↓ | → |
| Visual network 3 | ↓ | ↓ |
| Auditory network | → | ↓ |
| Parietal cortex network | ↓ | Not investigated |
| Right frontopariatel network | ↓ | Not investigated |
| Default mode network | ↓ | ↓ |
| Sensorimotor network | ↓ | ↓ |
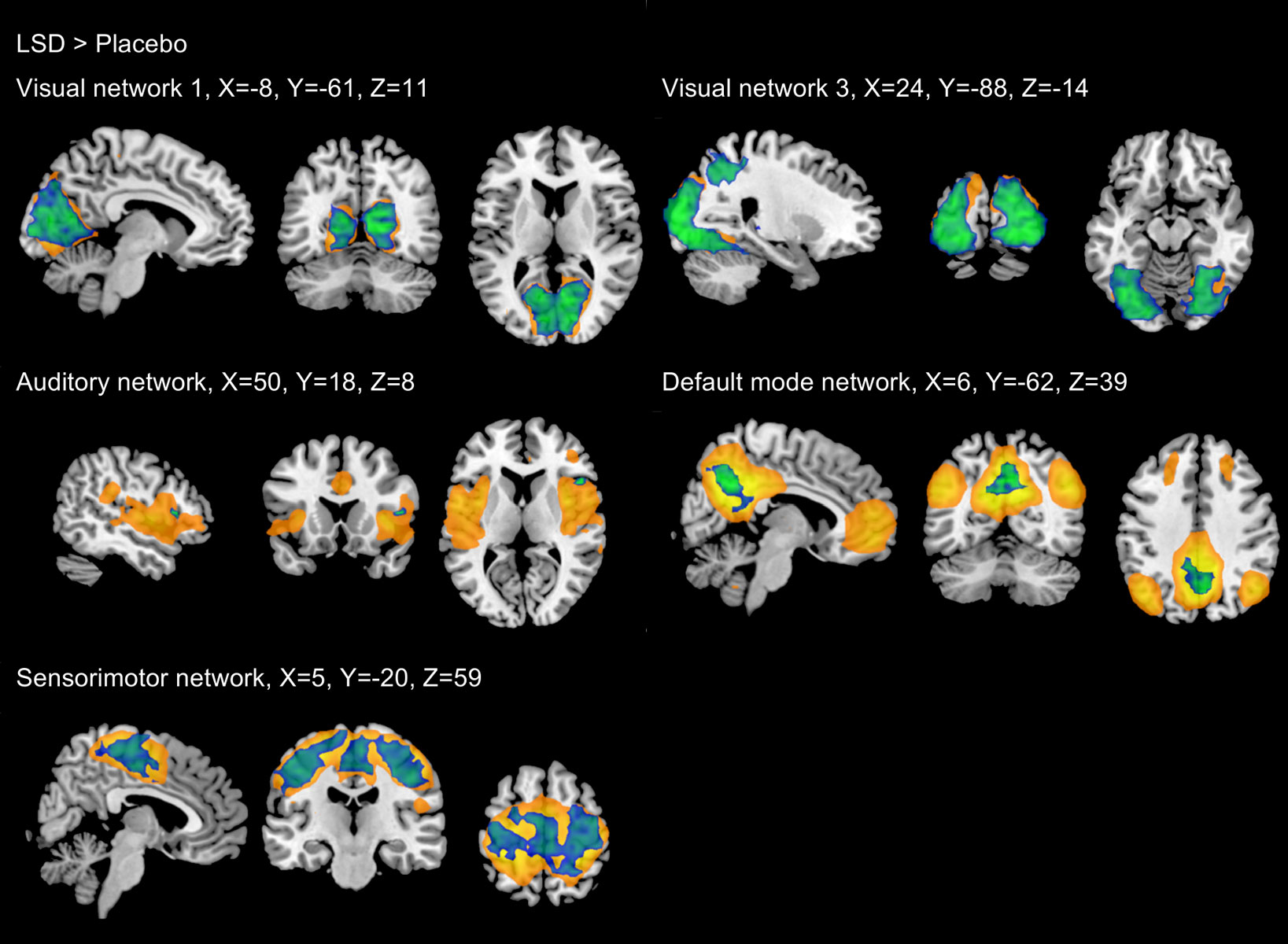
Figure 2 Significant increases in between-network functional connectivity after administration of LSD and psilocybin compared with placebo. Findings from Roseman et al., 2014 [18] are shown in green, findings from Carhart-Harris et al., 2016 [5] are shown in blue, Findings from Müller et al., 2018 [16] are shown in red. Black indicates networks which were not investigated by Carhart-Harris et al.
Figure reprinted from: Müller F, Dolder PC, Schmidt A et al. Altered network hub connectivity after acute LSD administration. NeuroImage Clin. 2018;18:694-701 [16], with permission from Elsevier.
Our team attempted to replicate these findings in a consecutive study [16]. We could confirm the findings regarding connectivity within networks and we also observed increased connectivity between networks (please see table 2, figs 2 and 3 for more details). However, consistency between studies was limited as connectivity between specific networks varied widely across studies. With regard to within-network connectivity, we noted that very similar alterations were observed in another study after the administration of a serotonin reuptake inhibitor [19]. The authors found that within-network connectivity within the default mode network, the visual networks 1–3, the sensorimotor network and the auditory network were decreased compared with placebo. As this drug is not associated with any subjective effects typical of a hallucinogenic drug, the observed alterations might not be specific to LSD or rather be an epiphenomenon of a nonspecific serotonergic stimulation. Moreover, we were not able to confirm associations between the observed neuronal alterations and the phenomenon of “ego dissolution” that were reported by the initial study and we did nor find any other associations [16].
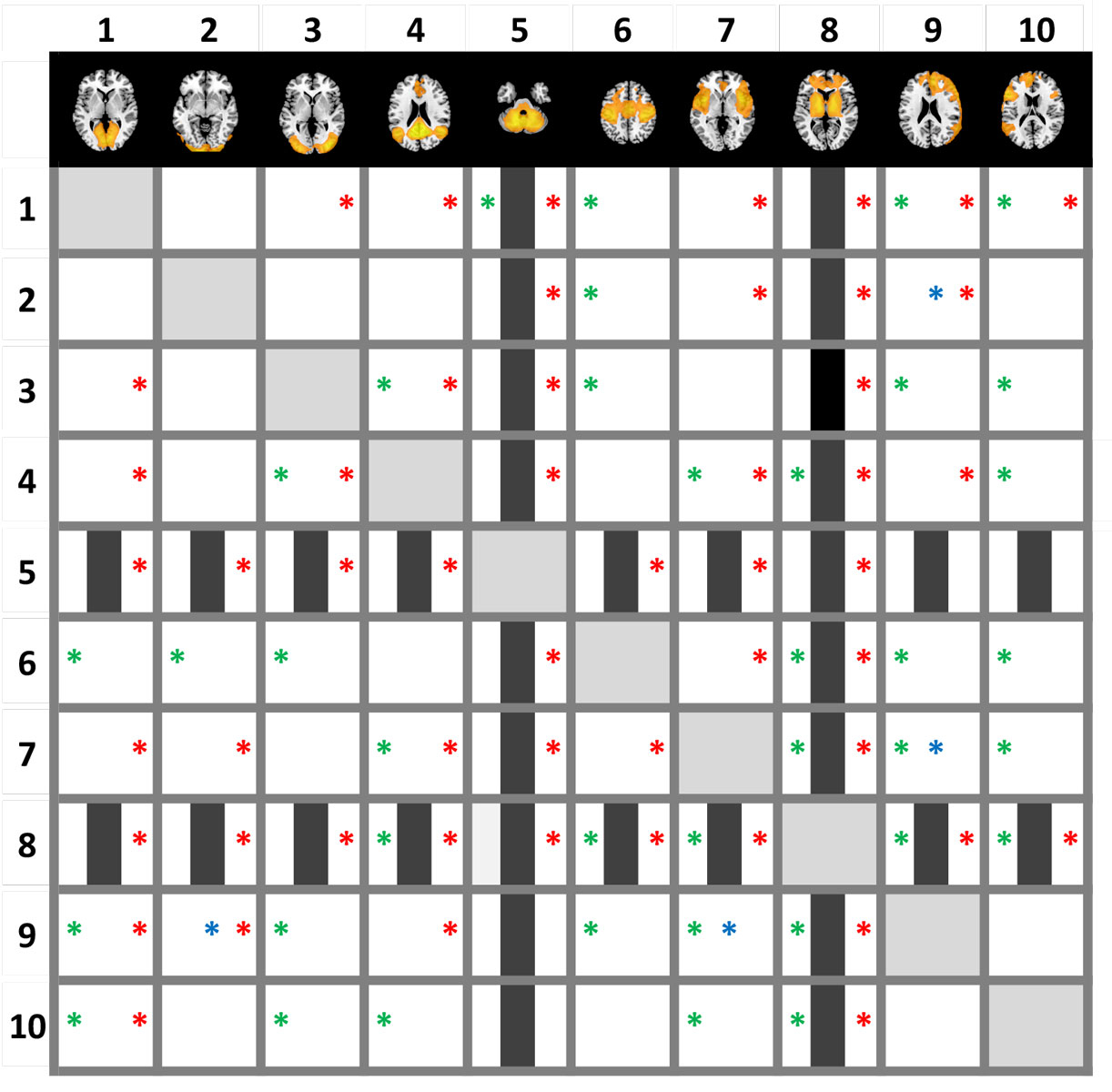
Figure 3 Decreased functional connectivity (shown in green-blue) within several resting state networks (shown in yellow) after LSD administration. Images are thresholded at p < 0.005 (two tailed), FWE (threshold free cluster enhancement). X, Y, and Z values indicate MNI coordinates. Right is the right side of the brain.
Figure reprinted from Figure reprinted from: Müller F, Dolder PC, Schmidt A et al. Altered network hub connectivity after acute LSD administration. NeuroImage Clin. 2018;18:694-701 [16], with permission from Elsevier.
Functional connectivity of the whole brain after LSD administration has been investigated by three studies so far [2, 8, 9, 15]). These studies used a measure called global functional connectivity, which is the mean correlation of a given region to all other regions. An advantage of this measure is that it is data-driven, meaning that no a priori hypotheses, such as which region or network might or might not be affected by LSD, are needed. Tagliazucchi et al. found relatively widespread increases in global connectivity in frontal, parietal, and temporal cortical regions [15]. In more detail, the authors described increased global connectivity in precuneus and thalamus, but no details were reported for other regions [15]. Compared with that, our group found increases in global connectivity that were limited to thalamic regions and part of the basal ganglia ([8, 9] please see fig. 4 for more details). The fact that alterations were spatially less widespread in our sample might be explained by slight methodological differences (global connectivity was calculated based on voxels compared to region of interest) resulting in a stricter statistical threshold.
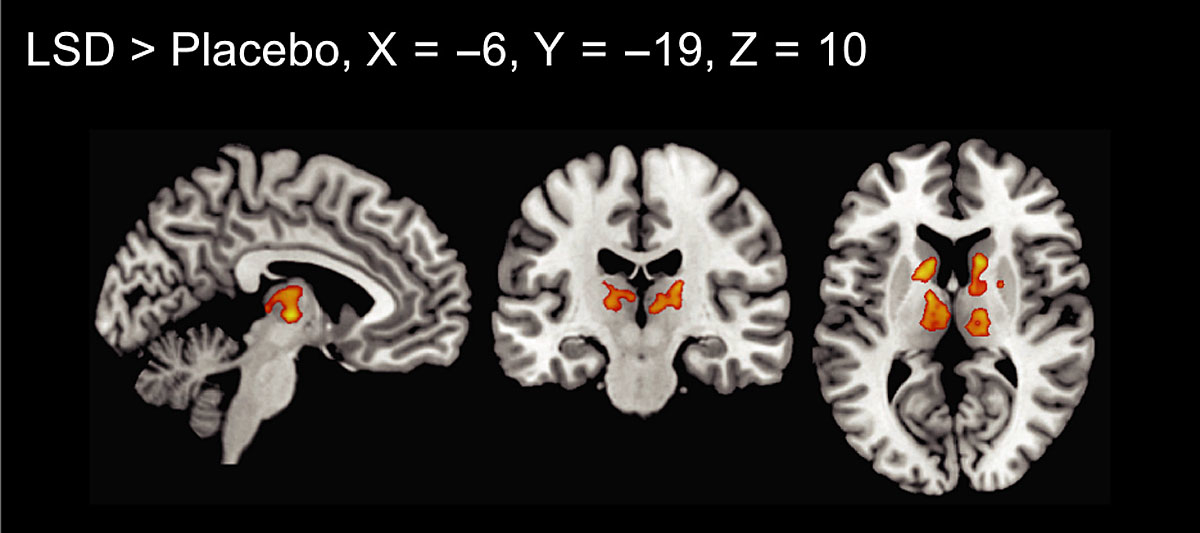
Figure 4 Global functional connectivity after LSD administration. Increased connectivity was seen in thalamic regions and the basal ganglia (p <0.05, cluster size FDR‐corrected on the basis of a voxel‐threshold of 0.05 FDR‐corrected). Right is right side of the brain. X, Y, and Z values indicate MNI coordinates.
Figure reprinted from Müller F, Lenz C, Dolder P et al. Increased thalamic resting-state connectivity as a core driver of LSD-induced hallucinations. Acta Psychiatr Scand. 2017;136:648-657 [8], with permission from Wiley.
Subsequently, Preller et al. used the same method for measuring whole brain connectivity [2] but additionally applied an approach called global signal regression [20]. Global signal regression is used to remove brain-wide variations of the signal, which are thought to reflect artefacts introduced by, for example, participants’ head motion or altered breathing patterns, which are common sources of bias in fMRI analyses [21]. Preller et al. found out that results differed markedly with and without global signal regression. With global signal regression, the authors reported increased global connectivity primarily in occipital and temporal regions and in the postcentral gyrus and precuneus, whereas other frontal, parietal and subcortical regions showed decreased connectivity. The authors also repeated their analysis without global signal regression. Findings changed significantly, including decreased connectivity in the right insula and increased connectivity in the cerebellum and parts of the basal ganglia and thalamus. Preller et al. speculated that the observed hyperconnectivity in somatosensory areas accompanied by hypoconnectivity in associative areas might lead to an imbalance, resulting in lacking integration of sensory integrations.
Psilocybin is a hallucinogenic drug naturally occurring in certain mushroom species. Like LSD, psilocybin primarily acts as a serotonin2A-receptor agonist [22]. Presumably, its subjective effects are very similar to those of LSD [23, 24]. It is therefore likely that psilocybin induces neural alterations very similar to those of LSD. However, reliable data have yet to be found. Compared with LSD, psilocybin has been examined by means of neuroimaging for some time, the first human study having been undertaken in the 1990s [25]. The group from London, who also conducted one of the LSD studies mentioned above, investigated comparable aspects of functional connectivity changes after psilocybin (2 mg intravenously) in 15 healthy subjects [15, 18]. The first study investigated connectivity between several resting state networks and reported widespread increases across several networks, in general accordance with findings obtained after LSD administration ([18]; please see fig. 2). However, as we have already pointed out elsewhere [16], agreements between these findings after psilocybin and alterations seen after LSD administration [5, 16] were quite limited. This raises the question about the specificity of the observed alterations for hallucinogenic drug effects. The second study investigated changes in global functional connectivity and found increased global connectivity in several regions, including the precuneus and the thalamus [15]. Therefore, these latter findings are in relatively good agreement with alterations seen after LSD administration [15, 16].
Several studies have indicated that substances such as LSD and psilocybin might have therapeutic effects in various mental disorders such as anxiety, depression and addiction [7, 26–28]. The question of how a single mechanism of action can exert positive effects in heterogeneous diseases is an interesting one. A recent model proposed that alterations in functional connectivity, as seen in the neuroimaging described above, might explain potential therapeutic effects of hallucinogenic drugs [29]. Nichols et al. hypothesised that this link can be found in altered hub connectivity induced by these drugs [29]. According to this model, pathological connectivity patterns associated with diverse mental diseases are acutely modified through destabilisation of hub functions with subsequent changes in functional connectivity between various brain regions. According to the authors, these events somehow give rise to the development of new connectivity patterns, which are stabilised after the acute effects have subsided, possibly through anti-inflammatory effects [29]. Others have followed a similar line of thought, stating that hallucinogens acutely induce a state of “disorder”, which opens the opportunity of reorganisation [30]. Some experimental fMRI findings pointed to lasting functional connectivity changes after the administration of a hallucinogenic drug [31], which might support these hypotheses.
The concept that LSD induces its subjective effects mainly via increases in functional connectivity between brain regions is tempting. For example, this concept provides a straightforward idea as to how LSD might induce effects like synaesthesia (e.g., the phenomenon of tasting music) or increased abilities for divergent thinking [32].
As described above, several findings suggest that LSD profoundly alters functional connectivity within the thalamocortical system. Our research specifically focused on this area and we will briefly summarise some theoretical aspects of these findings. It has been proposed that the thalamus is an important site of action for hallucinogenic drugs [4]. An important model proposed by Vollenweider and Geyer [33] suggested that hallucinogenic drugs work by disrupting thalamic gating of external and internal signals, leading to an increased information passage across the cortex. The thalamus shows widespread connections to other brain regions [34, 35] and is an important brain hub [36]. Together, cortex and thalamus form a complex system of extensive and reciprocal connections. These thalamocortical pathways are thought to serve integration and transfer of information between cortical regions, possibly via synchronisation [37]. Based on these findings, the thalamus has been called the “conductor” of the brain [38].
The concept that hallucinogens might act by affecting functioning of the thalamocortical system seems plausible, as the proposed neuronal model directly reflects core aspects of the subjective drug experience. Phenomenologically, the alterations induced by hallucinogenic drugs have often been described as a state of increased awareness, that is, conscious perception of aspects of the internal and external world that are normally suppressed. For example, after his self-experiment with mescaline (a hallucinogen very similar to LSD), Aldous Huxley speculated that this substance induces its effects by impairing these filter mechanisms [39]. A similar concept was formulated by the psychiatrist Stanislav Grof during his clinical studies with LSD in the 1950s. According to Grof, LSD acts as a nonspecific amplifier of brain function [40]. Ultimately, the neuronal basis of these empirical observations might be found in increased connectivity within the thalamocortical system, which facilitates increased conscious awareness of information flow.
It is intuitively appealing that the altered state of consciousness induced by LSD might be caused by widespread increases in functional connectivity. However, so far it has not been possible to reliably link these alterations to subjectively experienced drug effects. As noted above, profound changes in functional connectivity might appear without actual consequences for the subjective experience of individuals. On the other hand, it might be difficult to link very broad alterations in connectivity such as increased between-network connectivity to very specific subjective effects. In addition, all described studies were limited by relatively small sample sizes. Moreover, fMRI studies with such pharmacological substances face various problems that might induce biases. Functional connectivity measures are particularly sensitive to artefacts induced by participants’ head motion or modifications of physiological parameters [41, 42]. Participants’ motion might be attenuated after the administration of LSD, as participants might be restless during the scan. Moreover, LSD alters several physiological parameters such as heart rate, blood pressure and body temperature [1], all of which might affect neuroimaging data. Indeed, one of the presented studies reported significant differences in head motion between drug and placebo conditions [5, 15]. Lastly, hallucinogenic drugs might affect vascular tone or neurovascular coupling and hence neuroimaging measures, leading to alterations that do not actually reflect neuronal activity but unspecific effects [42, 43]. These latter pharmacological factors have so far remained largely unaddressed. In one of our investigations, we found no association between alterations in functional connectivity and heart rate, blood pressure and body temperature [16]. However, this analysis was based on measures taken only once just before the MRI scan and further continuous measurements taken during the scan might have been advantageous. Furthermore, results of Preller et al. might indicate that nonspecific effects such as LSD-induced alterations in vascular tone or neurovascular coupling might have a considerable impact on fMRI measurements [2]. Pharmacologically induced biases may be a main challenges for studies in this field, as it well known that such interventions can have wide-reaching consequences for fMRI measurements [41].
LSD is a controversial substance, believed to frequently cause “bad trips” and lasting impairments in mental health. However, LSD can be administered safely in research settings, given certain precautions such careful selection of participants and supervision during the study session [44]. Firstly, LSD use is not associated with any serious physical risks, so safety concerns primarily refer to possible mental side effects. During the acute phase of intoxication, LSD moderately increases heart rate and blood pressure [1, 14]. Therefore, participants with certain cardiovascular conditions should be excluded. LSD might also cause other side effects such as headache, nausea, tremor, dizziness, impaired psychomotor functions, exhaustion and others. Headache and exhaustion may outlast the acute drug effects by up to 72 hours in some cases [1, 14].
Secondly, with regard to mental side effects, one can differentiate between acute and long-lasting side effects. LSD might cause some acute mental side effects during the phase of intoxication. In laboratory studies using LSD and other psychoactive substances, mild or moderate anticipatory anxiety is relatively common at the beginning of the onset of the drug effects [1, 14]. Other acute effects include dysphoria, mood swings, depersonalisation and derealisation, mild paranoid thinking and others. However, negatively experienced acute drug effects are generally not predominant [1, 14].
Moreover, LSD is not considered to be a drug of dependence; that is, LSD does not cause compulsive drug seeking [4, 45]. Suspected prolonged severe mental side effects comprise hallucinogen persisting perception disorder (HPPD; “flashbacks”) and psychotic episodes. According to the Diagnostic and Statistical Manual of Mental Disorders (DSM), HPPD can be defined as a long-lasting syndrome characterised by a spontaneous recurrence of perceptual disturbances which are reminiscent of those generated while a subject was intoxicated with a hallucinogenic drug [46, 47]. The exact epidemiology of HPPD is unknown, but is considered to be a rare condition [48] and the course is typically limited [49]. The relationship between use of hallucinogenic drugs and psychotic episodes is still under debate. Recent epidemiological studies reported no association between use of hallucinogenic drugs and mental diseases, including psychosis [50, 51]. A survey on research with the hallucinogenic drugs LSD and mescaline during the 1950s found that one participant out of 1200 experienced a psychotic episode lasting longer than 48 hours [52]. Notably, this participant was an identical twin of patient suffering from schizophrenia. For safety reasons, present studies exclude subjects with (family) histories of psychotic disorders and also screen for risk factors associated with psychosis [2, 5, 8]). Notably, none of the modern LSD studies [2, 5, 8] reported severe adverse reactions, including psychotic episodes or HPPD. The same is true for psilocybin, which was also found be safe in research settings [53].
Previous neuroimaging studies on the acute effects of LSD in humans demonstrate widespread changes in functional connectivity after administering this drug. Several of these studies investigated specific aspects of these changes and await replication before findings could be regarded as conclusive. However, some findings have already been replicated and can therefore be considered as relatively reliable. These studies indicate that several resting state networks (two of three visual networks, the default mode network and the sensorimotor network) show decreased connectivity after LSD administration and that specific brain regions show marked increases in connectivity – the thalamic region being one of them. LSD might therefore induce its effects by compromising established patterns of brain connectivity while enhancing others. In this context, thalamic connectivity might be of special importance, given the importance of this region for data filtering and overall brain functioning. However, the observed alterations in connectivity have not yet been reliably linked to subjective drug effects. Furthermore, several sources of potential biases – such as LSD-induced alterations of physiological parameters – have not been addressed by previous studies. These factors should be addressed more consequently by future studies.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Schmid Y , Enzler F , Gasser P , Grouzmann E , Preller KH , Vollenweider FX , et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78(8):544–53. doi:.https://doi.org/10.1016/j.biopsych.2014.11.015
2 Preller KH , Burt JB , Ji JL , Schleifer CH , Adkinson BD , Stämpfli P , et al. Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. eLife. 2018;7:e35082. doi:.https://doi.org/10.7554/eLife.35082
3 Liechti ME . Modern Clinical Research on LSD. Neuropsychopharmacology. 2017;42(11):2114–27. doi:.https://doi.org/10.1038/npp.2017.86
4 Nichols DE . Psychedelics. Pharmacol Rev. 2016;68(2):264–355. doi:.https://doi.org/10.1124/pr.115.011478
5 Carhart-Harris RL , Muthukumaraswamy S , Roseman L , Kaelen M , Droog W , Murphy K , et al. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci USA. 2016;113(17):4853–8. doi:.https://doi.org/10.1073/pnas.1518377113
6 Preller KH , Razi A , Zeidman P , Stämpfli P , Friston KJ , Vollenweider FX . Effective connectivity changes in LSD-induced altered states of consciousness in humans. Proc Natl Acad Sci USA. 2019;116(7):2743–8. doi:.https://doi.org/10.1073/pnas.1815129116
7 Gasser P , Kirchner K , Passie T . LSD-assisted psychotherapy for anxiety associated with a life-threatening disease: a qualitative study of acute and sustained subjective effects. J Psychopharmacol. 2015;29(1):57–68. doi:.https://doi.org/10.1177/0269881114555249
8 Müller F , Lenz C , Dolder P , Lang U , Schmidt A , Liechti M , et al. Increased thalamic resting-state connectivity as a core driver of LSD-induced hallucinations. Acta Psychiatr Scand. 2017 a;136(6):648–57. doi:.https://doi.org/10.1111/acps.12818
9 Mueller F , Lenz C , Dolder PC , Harder S , Schmid Y , Lang UE , et al. Acute effects of LSD on amygdala activity during processing of fearful stimuli in healthy subjects. Transl Psychiatry. 2017 b;7(4):e1084. doi:.https://doi.org/10.1038/tp.2017.54
10 Preller KH , Herdener M , Pokorny T , Planzer A , Kraehenmann R , Stämpfli P , et al. The fabric of meaning and subjective effects in LSD-induced states depend on serotonin 2A receptor activation. Curr Biol. 2017;27(3):451–7. doi:.https://doi.org/10.1016/j.cub.2016.12.030
11 Schmidt A , Müller F , Lenz C , Dolder PC , Schmid Y , Zanchi D , et al. Acute LSD effects on response inhibition neural networks. Psychol Med. 2018;48(9):1464–73. doi:.https://doi.org/10.1017/S0033291717002914
12 Tahedl M , Levine SM , Greenlee MW , Weissert R , Schwarzbach JV . Functional Connectivity in Multiple Sclerosis: Recent Findings and Future Directions. Front Neurol. 2018;9:828. doi:.https://doi.org/10.3389/fneur.2018.00828
13 Collin G , Hulshoff Pol HE , Haijma SV , Cahn W , Kahn RS , van den Heuvel MP . Impaired cerebellar functional connectivity in schizophrenia patients and their healthy siblings. Front Psychiatry. 2011;2:73. doi:.https://doi.org/10.3389/fpsyt.2011.00073
14 Dolder PC , Schmid Y , Müller F , Borgwardt S , Liechti ME . LSD acutely impairs fear recognition and enhances emotional empathy and sociality. Neuropsychopharmacology. 2016;41(11):2638–46. doi:.https://doi.org/10.1038/npp.2016.82
15 Tagliazucchi E , Roseman L , Kaelen M , Orban C , Muthukumaraswamy SD , Murphy K , et al. Increased Global Functional Connectivity Correlates with LSD-Induced Ego Dissolution. Curr Biol. 2016;26(8):1043–50. doi:.https://doi.org/10.1016/j.cub.2016.02.010
16 Müller F , Dolder PCPC , Schmidt A , Liechti MEME , Borgwardt S . Altered network hub connectivity after acute LSD administration. Neuroimage Clin. 2018;18:694–701. doi:.https://doi.org/10.1016/j.nicl.2018.03.005
17 Smith SM , Fox PT , Miller KL , Glahn DC , Fox PM , Mackay CE , et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106(31):13040–5. doi:.https://doi.org/10.1073/pnas.0905267106
18 Roseman L , Leech R , Feilding A , Nutt DJ , Carhart-Harris RL . The effects of psilocybin and MDMA on between-network resting state functional connectivity in healthy volunteers. Front Hum Neurosci. 2014;8:204. doi:.https://doi.org/10.3389/fnhum.2014.00204
19 Klaassens BL , van Gorsel HC , Khalili-Mahani N , van der Grond J , Wyman BT , Whitcher B , et al. Single-dose serotonergic stimulation shows widespread effects on functional brain connectivity. Neuroimage. 2015;122:440–50. doi:.https://doi.org/10.1016/j.neuroimage.2015.08.012
20 Murphy K , Birn RM , Handwerker DA , Jones TB , Bandettini PA . The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi:.https://doi.org/10.1016/j.neuroimage.2008.09.036
21 Power JD , Mitra A , Laumann TO , Snyder AZ , Schlaggar BL , Petersen SE . Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–41. doi:.https://doi.org/10.1016/j.neuroimage.2013.08.048
22 Rickli A , Moning OD , Hoener MC , Liechti ME . Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur Neuropsychopharmacol. 2016;26(8):1327–37. doi:.https://doi.org/10.1016/j.euroneuro.2016.05.001
23 Hollister LE , Hartman AM . Mescaline, lysergic acid diethylamide and psilocybin comparison of clinical syndromes, effects on color perception and biochemical measures. Compr Psychiatry. 1962;3(4):235–41. doi:.https://doi.org/10.1016/S0010-440X(62)80024-8
24 Hollister LE , Sjoberg BM . Clinical syndromes and biochemical alterations following mescaline, lysergic acid diethylamide, psilocybin and combination of the three psychotomimetic drugs. Compr Psychiatry. 1964;5(3):170–8. doi:.https://doi.org/10.1016/S0010-440X(64)80030-4
25 Vollenweider FX , Leenders KL , Scharfetter C , Maguire P , Stadelmann O , Angst J . Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16(5):357–72. doi:.https://doi.org/10.1016/S0893-133X(96)00246-1
26 Bogenschutz MP , Forcehimes AA , Pommy JA , Wilcox CE , Barbosa PC , Strassman RJ . Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. 2015;29(3):289–99. doi:.https://doi.org/10.1177/0269881114565144
27Griffiths R. Overview of the Johns Hopkins psilocybin research project. Interdisciplinary Conference on Psychedelics Research. Amsterdam, June 3–5, 2016.
28 Johnson MW , Garcia-Romeu A , Cosimano MP , Griffiths RR . Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. 2014;28(11):983–92. doi:.https://doi.org/10.1177/0269881114548296
29 Nichols DE , Johnson MW , Nichols CD . Psychedelics as Medicines: An Emerging New Paradigm. Clin Pharmacol Ther. 2017;101(2):209–19. doi:.https://doi.org/10.1002/cpt.557
30 Froese T , Leenen I , Palenicek T . A role for enhanced functions of sleep in psychedelic therapy? Adapt Behav. 2018;26(3):129–35. doi:.https://doi.org/10.1177/1059712318762735
31 Sampedro F , de la Fuente Revenga M , Valle M , Roberto N , Domínguez-Clavé E , Elices M , et al. Assessing the psychedelic “after-glow” in ayahuasca users: Post-acute neurometabolic and functional connectivity changes are associated with enhanced mindfulness capacities. Int J Neuropsychopharmacol. 2017;20(9):698–711. doi:.https://doi.org/10.1093/ijnp/pyx036
32 Kuypers KPC . Out of the box: A psychedelic model to study the creative mind. Med Hypotheses. 2018;115:13–6. doi:.https://doi.org/10.1016/j.mehy.2018.03.010
33 Geyer MA , Vollenweider FX . Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci. 2008;29(9):445–53. doi:.https://doi.org/10.1016/j.tips.2008.06.006
34Jones E. The Thalamus, Second edition. New York: Cambridge University Press; 2007.
35 Parent A , Hazrati LN . Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20(1):91–127. doi:.https://doi.org/10.1016/0165-0173(94)00007-C
36 van den Heuvel MP , Sporns O . Rich-club organization of the human connectome. J Neurosci. 2011;31(44):15775–86. doi:.https://doi.org/10.1523/JNEUROSCI.3539-11.2011
37 Saalmann YB . Intralaminar and medial thalamic influence on cortical synchrony, information transmission and cognition. Front Syst Neurosci. 2014;8:83. doi:.https://doi.org/10.3389/fnsys.2014.00083
38 Malekmohammadi M , Elias WJ , Pouratian N . Human thalamus regulates cortical activity via spatially specific and structurally constrained phase-amplitude coupling. Cereb Cortex. 2015;25(6):1618–28. doi:.https://doi.org/10.1093/cercor/bht358
39Huxley A. The doors of perception. In: The Doors of Perception and Heaven and Hell. London: Chatto and Windus; 1954.
40Grof S. Realms of the Human Unconscious: Observations from LSD Research. New York: Viking Press; 1975.
41 Khalili-Mahani N , Chang C , van Osch MJ , Veer IM , van Buchem MA , Dahan A , et al. The impact of “physiological correction” on functional connectivity analysis of pharmacological resting state fMRI. Neuroimage. 2013;65:499–510. doi:.https://doi.org/10.1016/j.neuroimage.2012.09.044
42 Wise RG , Tracey I . The role of fMRI in drug discovery. J Magn Reson Imaging. 2006;23(6):862–76. doi:.https://doi.org/10.1002/jmri.20584
43 Tylš F , Páleníček T , Horáček J . Psilocybin--summary of knowledge and new perspectives. Eur Neuropsychopharmacol. 2014;24(3):342–56. doi:.https://doi.org/10.1016/j.euroneuro.2013.12.006
44 Johnson M , Richards W , Griffiths R . Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603–20. doi:.https://doi.org/10.1177/0269881108093587
45 Fantegrossi WE , Murnane KS , Reissig CJ . The behavioral pharmacology of hallucinogens. Biochem Pharmacol. 2008;75(1):17–33. doi:.https://doi.org/10.1016/j.bcp.2007.07.018
46 Cooper R . Diagnostic and Statistical Manual of Mental Disorders (DSM). Knowl Organ. 2018. doi:.https://doi.org/10.5771/0943-7444-2017-8-668
47 Orsolini L , Papanti GD , De Berardis D , Guirguis A , Corkery JM , Schifano F . The “Endless Trip” among the NPS users: Psychopathology and psychopharmacology in the Hallucinogen-persisting perception disorder. A systematic review. Front Psychiatry. 2017;8:240. doi:.https://doi.org/10.3389/fpsyt.2017.00240
48 Halpern JH , Pope HG, Jr . Hallucinogen persisting perception disorder: what do we know after 50 years? Drug Alcohol Depend. 2003;69(2):109–19. doi:.https://doi.org/10.1016/S0376-8716(02)00306-X
49 Strassman RJ . Adverse reactions to psychedelic drugs. A review of the literature. J Nerv Ment Dis. 1984;172(10):577–95. doi:.https://doi.org/10.1097/00005053-198410000-00001
50 Hendricks PS , Thorne CB , Clark CB , Coombs DW , Johnson MW . Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. J Psychopharmacol. 2015;29(3):280–8. doi:.https://doi.org/10.1177/0269881114565653
51 Johansen PO , Krebs TS . Psychedelics not linked to mental health problems or suicidal behavior: a population study. J Psychopharmacol. 2015;29(3):270–9. doi:.https://doi.org/10.1177/0269881114568039
52 Cohen S . Lysergic acid diethylamide: side effects and complications. J Nerv Ment Dis. 1960;130(1):30–40. doi:.https://doi.org/10.1097/00005053-196001000-00005
53 Studerus E , Kometer M , Hasler F , Vollenweider FX . Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. J Psychopharmacol. 2011;25(11):1434–52. doi:.https://doi.org/10.1177/0269881110382466
No financial support and no other potential conflict of interest relevant to this article was reported.