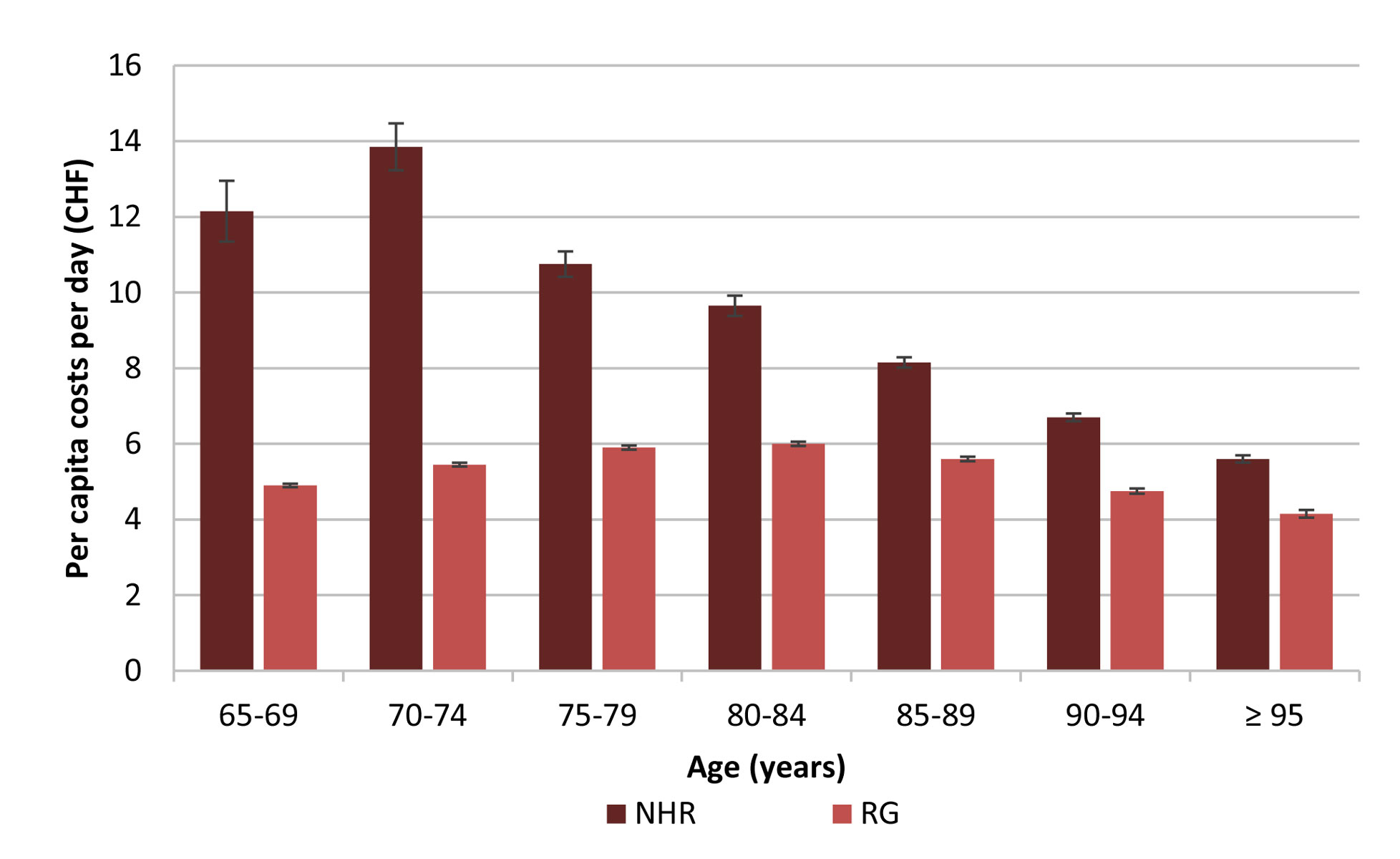
Figure 1 Average per capita drug costs per day in different age groups for NHR and the RG (individuals aged ≥65 years insured with Helsana, excluding NHR) with 95% confidence intervals, year 2016.
NHR = nursing home residents; RG = reference group
DOI: https://doi.org/10.4414/smw.2019.20126
In most high-income countries, the number of nursing home residents (NHR) is increasing. On average, 13% of Europeans aged over 65 years receive long-term care. Between-country variation is high, with the proportion of elderly long-term care receivers (not necessarily institutionalised) ranging from 2% in Portugal to more than 20% in Switzerland [1], where people aged 65 years or older accounted for 18.1% of the total population in 2016. It has been estimated that this proportion will further increase to 26% by 2045 [2]. Up to 91% of NHR regularly consume five or more medications [3]. Multimorbid individuals are the rule rather than the exception, which often leads to complex medication regimens and excessive polypharmacy [4]. The term polypharmacy refers to the concurrent use of multiple medications, especially if the medications are unnecessarily numerous or complex [5]. In a review by Morin et al., the total number of prescribed medications was reported to be the main driving factor for potentially inappropriate medication (PIM) use [6]. Medications are considered potentially inappropriate for use in the elderly if the risk of adverse events exceeds either the expected benefit, or when a better-tolerated or safer alternative is available [7]. PIM use has been found to be associated with an increased risk of poor health outcomes, including falls, confusion and mortality [8]. Furthermore, prescription of additional drugs to treat drug-induced symptoms can lead to prescribing cascades; this can occur when an adverse drug reaction is misinterpreted as a new medical problem [9]. Since the first related initiative of Beers et al. in 1991 in the USA [10], several tools to identify PIM have been developed. A recent review reported a prevalence of PIM in NHR between 18.5 and 82.6% in 17 studies on the basis of the Beers criteria [11]. Several countries established lists adapted to their local contexts; these include Canada [12], France [13], Germany (the PRISCUS list) [14] and seven EU countries in a combined approach (EU(7)-PIM list) [15].
Although polypharmacy and PIM are different concepts, they are strongly associated [16]. A recently published review found an increased risk of all-cause hospitalisation for NHR when exposed to polypharmacy and PIM [17]. Hospitalisations from nursing homes are associated with negative outcomes for residents and a substantial economic burden, with a relevant part thereof being preventable [17, 18]. Moreover, age-related physiological changes increase the risk of drug-related adverse events in the elderly [19].
Since drug prescribing plays a major role in nursing home settings, its optimisation has become a public-health issue worldwide [7]. Despite serious concerns about inappropriate drug prescribing and polypharmacy, only few studies have analysed medication utilisation in the elderly Swiss population [20–22]. To date, no comprehensive descriptive data on prescription patterns in Swiss nursing homes have been available. In the present study, we therefore aimed to analyse drug prescription patterns and to assess the prevalence of polypharmacy and use of PIM in Swiss NHR based on administrative claims data provided by the Swiss health insurance company Helsana.
We conducted a retrospective descriptive analysis of health insurance claims data covering the year 2016. The analysis was based on the electronic administrative claims database of the Swiss health insurance company Helsana.
All persons residing in Switzerland are required to purchase mandatory health insurance (with a very broad benefit package). Health insurers must in return offer mandatory insurance to everyone, irrespective of age or health status. Although insurance companies are private and there is no uniform national health insurance system, conditions and benefit packages are uniform across different providers. Federal authorities closely regulate the market. The Helsana group is one of the largest health insurers in Switzerland, providing mandatory health insurance to some 1.17 million individuals from all 26 cantons (year 2016). Helsana’s administrative claims data cover almost all healthcare invoices and are thus highly reliable. Recorded variables cover demographics, postal codes and claims sent to Helsana for reimbursement. The latter include drug prescriptions (including dose, galenic form and pack size), medical treatments and diagnostic tests. For the purpose of this analysis, one drug claim refers to the purchase of one single drug package; if several drugs were bought on a single occasion, these were treated as separate claims. Drugs were coded according to the Anatomical Therapeutic Chemical (ATC) classification system, which allows drug classes and individual active agents to be uniquely identified. However, data do not encompass insured persons’ personal characteristics such as smoking habits, alcohol consumption or weight / body mass index. Moreover, they do not cover symptoms, test results, outpatient diagnoses or medical resource use during acute hospitalisation. Thus, inpatient drug prescriptions are not available in the data, whereas outpatient drug prescriptions and most nursing home drug prescriptions (see below) are recorded.
We were granted access to anonymised datasets (from the database located at Helsana) covering the patient information and parameters relevant for this analysis.
In the Helsana database, we identified all NHR aged 65 years or older in 2016 who received at least one prescribed drug coded in the ATC classification system (99.8% of all NHR) and who had a minimum stay of 28 days in a nursing home (in order to focus on long-term institutionalised patients only). We then excluded all NHR whose invoices for nursing home care were based on lump sums, as in these cases drug utilisation was not detailed in the database (this affected, in particular, all NHR of the cantons Fribourg and Ticino).
To enable a comparison between NHR and the general elderly Swiss population, we identified a reference group (RG) consisting of all individuals aged 65 years or older insured with Helsana in 2016 who did not live in a nursing home and who had at least one claim of a prescribed drug coded in the ATC classification system.
Referring to existing literature [23], we defined polypharmacy as the concurrent use of ≥5 different prescribed drugs (according to their ATC code). Where a claim was present, we assumed it to indicate the use of the respective drug.
We used the 2015 Beers criteria and the PRISCUS list (2011) [8, 14] to identify PIM. Since the Beers criteria are of American origin and the PRISCUS list was adapted to the German market, our analyses considered only drugs licensed in Switzerland. We a priori defined long-term use of PIM as ≥3 claims of the same PIM in 2016 based on the assumption that one or a maximum of two claims of the same drug can be seen as short-term use for a defined time and diagnosis, whereas three or more separate claims of the same drug indicate regular use.
We assessed characteristics of the study population (such as sex and average age), the number of drug claims (overall and average per capita) including ATC codes and drug costs (overall and average per capita per day) in 2016 both in NHR and in the RG, and stratified our analyses by sex, age and canton of residence. We used means, standard deviations (SDs) and 95% confidence intervals (CIs) for descriptive statistics. Selected key parameters were standardised by 5-year age groups and sex to prevent confounding owing to different age or sex structures in NHR and the RG, using the Swiss population aged 65 and older as the standard population [24].
To estimate the number of different drugs taken concurrently and thus the prevalence of polypharmacy, we considered only drug claims in the second quarter of 2016. A 3-month time period was chosen to make a concurrent (rather than subsequent) use of the prescribed medications likely. A study based on Danish prescription data estimated that 80% of individuals who had purchased five or more drugs (excluding topical preparations and antineoplastic drugs) during 3 months were subject to an episode of polypharmacy (defined as the concurrent use of five or more drugs) at least once during the year [25]. We specifically chose the second quarter of the year as it is less susceptible to seasonal diseases (such as influenza). Exclusively in the polypharmacy analysis, we also included individuals who had no history of prescribed drugs in the respective period to achieve an overall estimate of the mean number of different concurrent drugs per person for the population of interest. In a sub-analysis, we excluded ATC codes representing topical drugs (e.g., galenic forms such as creams, ointments, gels, lotions, nasal sprays, aerosols or eye drops) to analyse systemic drugs only.
To identify explicit PIM in the dataset (independent of diagnoses or conditions), we created three separate lists of ATC codes (2015 Beers criteria; PRISCUS list; combination of both). We calculated prevalence of PIM use in NHR according to the number of claims (≥1 claim or ≥3 claims of the same PIM) and stratified the results by age, sex, canton of residence and set of criteria used.
To enable statements on a Swiss population level, we extrapolated all results according to age, sex and canton of residence using yearly published census data from the Swiss Federal Office for Statistics balancing out slight deviations of the structure of the Helsana population from that of the general Swiss population [26].
We used SAS 9.4 software (SAS Institute, Cary, NC) for the analyses.
We analysed claims data from a total of 91,166 NHR and 1,364,755 individuals in the RG (extrapolated). Table 1 summarises the characteristics of the study population, including drug claims and drug costs in 2016.
Table 1 Characteristics of Swiss NHR and the RG (individuals aged ≥65 years insured with Helsana, excluding NHR) including drug claims and drug costs, year 2016.
| NHR | RG | |
|---|---|---|
| Sex, n (%) | ||
| Total | 91,166 (100.0) | 1,364,755 (100.0) |
| Female | 65,514 (71.9) | 750,540 (55.0) |
| Male | 25,652 (28.1) | 614,215 (45.0) |
| Average age in years (SD) | ||
| Total | 85.7 (17.4) | 75.0 (17.7) |
| Female | 86.6 (16.3) | 75.5 (17.9) |
| Male | 83.6 (19.2) | 74.5 (17.5) |
| Number of overall drug claims in thousand (%) | ||
| Total | 5360.2 (100.0) | 42,100.0 (100.0) |
| Female | 3915.4 (73.0) | 24,142.9 (57.3) |
| Male | 1444.7 (27.0) | 17,957.1 (42.7) |
| Overall drug costs in million CHF (%) | ||
| Total | 210.4 (100.0) | 2711.9 (100.0) |
| Female | 149.0 (70.8) | 1406.9 (51.9) |
| Male | 61.4 (29.2) | 1305.0 (48.1) |
| Average per capita drug claims (SD) [standardised by age and sex*] | ||
| Total | 58.8 (48.2) [64.3 (48.6)] | 30.8 (29.1) [31.1 (29.1)] |
| Female | 59.8 (47.6) [66.2 (48.1)] | 32.2 (29.6) [32.4 (29.6)] |
| Male | 56.3 (49.8) [62.1 (50.3)] | 29.2 (28.3) [29.4 (28.3)] |
| Average per capita drug costs per day (CHF) (SD) [standardised by age and sex*] | ||
| Total | 8.55 (13.65) [11.45 (13.80)] | 5.45 (13.85) [5.45 (13.85)] |
| Female | 8.20 (13.00) [11.30 (13.25)] | 5.15 (12.75) [5.15 (12.75)] |
| Male | 9.45 (15.30) [11.60 (15.35)] | 5.80 (15.25) [5.85 (15.25)] |
NHR = nursing home residents; RG = reference group; SD = standard deviation * The overall results for NHR and the RG are standardised by age and sex, while the results for females and males are standardised by age
The average age was markedly higher in NHR than in the RG (NHR 85.7 years; RG 75.0 years). The same was true for the proportion of females (NHR 71.9%; RG 55.0%).
Over the 1-year observation period, irrespective of sex and across all age groups, NHR received on average nearly twice as many drug claims per capita as individuals in the RG (NHR 58.8; RG 30.8; NHR standardised by age and sex [sNHR] 64.6; RG standardised by age and sex [sRG] 31.1). Moreover, the average per capita drug costs per day of NHR were substantially higher compared with the RG (NHR CHF 8.55; RG CHF 5.45; sNHR CHF 11.45; sRG CHF 5.45).
In NHR, the average number of per capita drug claims was lower in men than in women, but men’s average per capita drug costs per day were higher (men CHF 9.45; women CHF 8.20). Standardised by age, this difference became relatively small (men CHF 11.60; women CHF 11.30). In the RG, we found the same pattern (table 1).
We observed a noticeable decrease of average per capita drug costs per day in NHR with advancing age (fig. 1). Costs decreased from over CHF 13.00 in 70- to 74-year-olds to below CHF 6.00 in residents aged 95 years and older. The RG, in contrast, showed maximum per capita costs per day of CHF 6.00 in individuals aged 80 to 84 years and minimum costs of CHF 4.15 in individuals aged 95 and older.

Figure 1 Average per capita drug costs per day in different age groups for NHR and the RG (individuals aged ≥65 years insured with Helsana, excluding NHR) with 95% confidence intervals, year 2016.
NHR = nursing home residents; RG = reference group
Figure 2 illustrates the relative deviation of the average per capita drug costs per day in nursing homes for each canton of Switzerland compared with the Swiss average value (CHF 8.55). The costs were highest in the canton Basel-Stadt at CHF 10.20 (+19.3%), and lowest in the canton Appenzell Ausserrhoden with CHF 5.85 (−31.6%). The observed cantonal pattern was similar in the RG (data not shown).
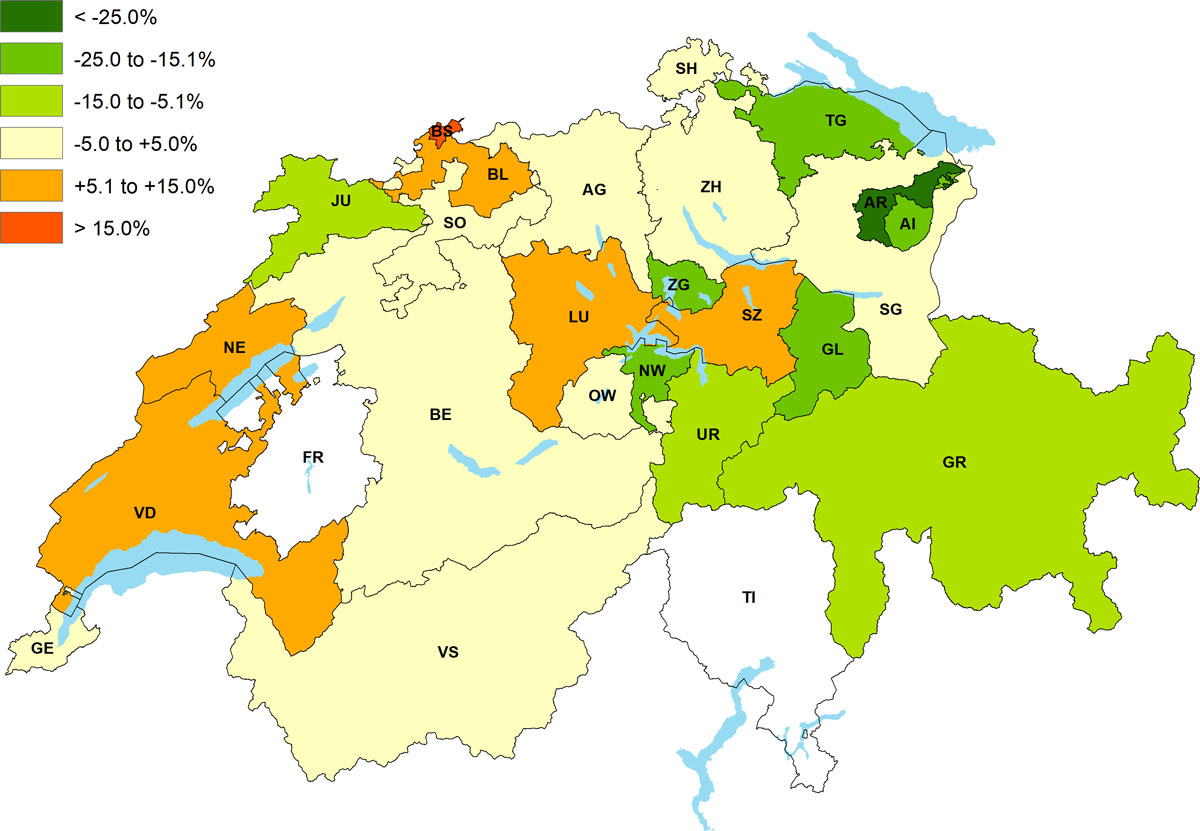
Figure 2 Relative deviation of per capita costs per day for drug claims in Swiss nursing homes compared with the Swiss average value of CHF 8.55, year 2016.
FR, TI: not applicable (invoices for nursing care based on lump sums)
AG = Aargau; AI = Appenzell Innerrhoden; AR = Appenzell Ausserrhoden; BE = Bern; BL = Basel-Landschaft; BS = Basel-Stadt; FR = Freiburg; GE = Genf; GL = Glarus; GR = Graubünden; JU = Jura; LU = Luzern; NE = Neuenburg; NW = Nidwalden; OW = Obwalden; SG = St. Gallen; SH = Schaffhausen; SO = Solothurn; SZ = Schwyz; TG = Thurgau; TI = Tessin; UR = Uri; VD = Waadt; VS = Wallis; ZG = Zug; ZH = Zürich
Table 2 characterises the prevalence of polypharmacy and the number of different concurrent drugs per person in NHR and the RG. Overall, polypharmacy was present in 85.5% of all NHR (sNHR 85.1%). In contrast, prevalence of polypharmacy was markedly lower in the RG (50.4%; sRG 50.5%). Whereas the prevalence of polypharmacy among NHR showed little variation across different age categories with minimum and maximum values of 81.4% (65- to 69-year-olds) and 87.0% (70- to 79-year-olds), respectively, it varied in the RG from a minimum of 39.8% among individuals aged 65 to 69 to a maximum of 63.5% among those aged 85 to 89 years.
Table 2 Prevalence of polypharmacy and number of different concurrent drugs per person in NHR and the RG (individuals aged ≥65 years insured with Helsana, excluding NHR), 2nd quarter of 2016.
| NHR | RG | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Total
(n = 74,290) |
Female
(n = 54,278) |
Male
(n = 20,012) |
Total
(n = 1,364,755) |
Female
(n = 750,540) |
Male
(n = 614,215) |
||||
| Prevalence of polypharmacy, % (95% CI) [standardised by age and sex*] | |||||||||
| 85.5 (84.9–86.1) | 85.8 (85.1–86.5) | 84.7 (83.5–86.0) | 50.4 (50.2–50.7) | 52.4 (52.1–52.7) | 48.1 (47.8–48.4) | ||||
| [85.1 (84.4–85.8)] | [85.9 (85.1–86.7)] | [84.0 (82.7–85.3)] | [50.5 (50.2–50.8)] | [52.3 (51.9–52.7)] | [48.2 (47.8–48.6)] | ||||
| Mean number of different concurrent drugs per individual, n (SD) [standardised by age and sex*] | |||||||||
| 9.4 (11.3) | 9.5 (11.1) | 9.3 (11.8) | 5.5 (11.6) | 5.8 (11.6) | 5.3 (11.6) | ||||
| [9.9 (13.1)] | [10.1 (13.0)] | [9.7 (9.7)] | [5.6 (11.5)] | [5.8 (11.5)] | [5.3 (11.5)] | ||||
| Number of individuals, categorised by the number of concurrent different drugs, n (%) | |||||||||
| 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 156,348 (11.5) | 79,264 (10.6) | 77,084 (12.6) | |||
| 1–3 | 6605 (8.9) | 4704 (8.7) | 1902 (9.5) | 392,873 (28.8) | 209,162 (27.9) | 183,711 (29.9) | |||
| 4–6 | 15,615 (21.0) | 11,247 (20.7) | 4368 (21.8) | 350,565 (25.7) | 191,447 (25.5) | 159,118 (25.9) | |||
| 7–9 | 19,227 (25.9) | 14,082 (25.9) | 5145 (25.7) | 223,114 (16.3) | 127,289 (17.0) | 95,825 (15.6) | |||
| 10–12 | 15,180 (20.4) | 11,294 (20.8) | 3886 (19.4) | 123,324 (9.0) | 72,409 (9.6) | 50,915 (8.3) | |||
| 13–15 | 9457 (12.7) | 7008 (12.9) | 2449 (12.2) | 61,847 (4.5) | 37,272 (5.0) | 24,575 (4.0) | |||
| >15 | 8207 (11.0) | 5943 (10.9) | 2263 (11.3) | 56,684 (4.2) | 33,697 (4.5) | 22,987 (3.7) | |||
CI = confidence interval; NHR = nursing home residents; RG = reference group; SD = standard deviation * The overall results for NHR and the RG are standardised by age and sex, while the results for females and males are standardised by age
In NHR and the RG, the average number of different drugs per person taken concurrently amounted to 9.4 (sNHR 9.9) and 5.5 (sRG 5.6), respectively (second quarter of 2016). In both groups, women received on average slightly more concurrent drugs than men (table 2). In NHR, we observed 9.6 drugs in 65- to 69-year-olds, a maximum of 10.5 drugs in 70- to 74-year-olds, and 8.5 drugs in the over 95-year-olds. In the RG, a minimum number of 4.6 and a maximum number of 6.7 different concurrently used drugs was found in 65- to 69-year-olds and 85- to 89-year-olds, respectively. In individuals aged 95 years and older, the average number of drugs amounted to 6.0 (fig. 3).

Figure 3 Average number of different concurrent drugs per person with 95% confidence intervals across different age groups in NHR and the RG (individuals aged ≥65 years insured with Helsana, excluding NHR), 2nd quarter of 2016.
NHR = nursing home residents; RG = reference group
In the subanalysis including systemic drugs only, the overall prevalence of polypharmacy remained high at 78.7 and 43.0% in NHR and the RG, respectively (sNHR 80.0 [80.0%]; sRG 43.0 [43.0%]). Accordingly, the average number of different concurrent drugs per person did not decrease substantially (NHR 8.0; RG 4.6; sNHR 8.5; sRG 4.6), indicating a predominant use of systemic drugs both in NHR and in the RG.
Table 3 shows the 15 most frequent active agents in nursing homes in 2016, by decreasing number of claims. Of these 15 agents, the majority were analgesics (ATC code starting with N02) and psycholeptics (ATC code starting with N05). Each of the 15 drugs was prescribed to a higher proportion of NHR than persons in the RG; 10 out of 15 drugs were prescribed to more than a quarter of all NHR. In particular, every second NHR received at least one prescription of paracetamol. A total of 22.4 and 11.2% of NHR received at least one prescription of quetiapine and fentanyl, respectively, whereas the same applied to only 1.7 and 1.1% of the RG. The corresponding analysis for the RG is shown in supplementary table S1 in appendix 1.
Table 3 The 15 most frequent drugs in NHR compared with the RG (individuals aged ≥65 years insured with Helsana, excluding NHR) and the prevalence of their use, ranked by number of drug claims, year 2016.
| Active agent | ATC code | Number of drug claims in NHR | Rank | Number of drug claims in RG | Rank | Prevalence of use in NHR (%) | Prevalence of use in the RG (%) |
|---|---|---|---|---|---|---|---|
| Paracetamol | N02BE01 | 260,211 | 1 | 1,202,864 | 2 | 54.4 | 34.6 |
| Metamizole | N02BB02 | 161,887 | 2 | 506,310 | 13 | 32.0 | 14.0 |
| Torasemide | C03CA04 | 143,192 | 3 | 511,161 | 12 | 38.3 | 11.7 |
| Quetiapine | N05AH04 | 136,026 | 4 | 106,810 | 85 | 22.4 | 1.7 |
| Pantoprazole | A02BC02 | 130,073 | 5 | 963,136 | 4 | 35.6 | 26.3 |
| Macrogol | A06AD65 | 129,455 | 6 | 318,776 | 22 | 32.2 | 12.6 |
| Cholecalciferol | A11CC05 | 128,905 | 7 | 640,780 | 7 | 32.0 | 17.8 |
| Fentanyl | N02AB03 | 110,441 | 8 | 105,330 | 88 | 11.2 | 1.1 |
| Artificial tear fluid | S01XA20 | 106,754 | 9 | 932,207 | 5 | 22.7 | 22.1 |
| Diclofenac (topical) | M02AA15 | 94,657 | 10 | 552,445 | 10 | 30.0 | 16.7 |
| Acetylsalicylic acid | B01AC06 | 93,777 | 11 | 1,220,562 | 1 | 32.1 | 29.4 |
| Calcium + vitamin D | A12AX | 84,489 | 12 | 614,066 | 8 | 25.7 | 17.6 |
| Urea | D02AE01 | 74,255 | 13 | 232,686 | 34 | 25.3 | 7.5 |
| Lorazepam | N05BA06 | 72,715 | 14 | 427,467 | 15 | 19.9 | 7.9 |
| Zolpidem | N05CF02 | 72,538 | 15 | 588,007 | 9 | 13.0 | 8.4 |
ATC = Anatomical Therapeutic Chemical classification system; NHR = nursing home residents; RG = reference group
Overall, 79.1% of NHR received at least one PIM when considering both the PRISCUS list and the 2015 updated Beers criteria (59.5% according to the PRISCUS list alone and 74.0% according to Beers criteria alone). Based on the PRISCUS list and Beers criteria combined, 56.2% of all NHR were long-term users (≥3 claims) of at least one PIM (40.9% according to the PRISCUS list alone and 51.3% according to Beers criteria alone). Prevalence of long-term PIM prescription was highest in NHR aged 70 to 74 years and decreased thereafter, irrespective of the tool used to identify PIM (supplementary fig. S1 in appendix 1).
Table 4 displays the 15 most prevalent PIMs among NHR. Psycholeptics were the most frequently prescribed PIM group including quetiapine, lorazepam, zolpidem, risperidone, pipamperone, haloperidol, oxazepam and hydroxyzine.
Table 4 The 15 most frequent PIM and the prevalence of their use in NHR according to the 2015 Beers criteria and PRISCUS list [8, 14], ranked by total number of recipients, year 2016.
| Rank | PIM active agent | ATC code | List: Beers (B) or PRISCUS (P) | No of NHR with claim | Prevalence of use in NHR (%) | No of NHR with long-term use (≥3 claims of same PIM) | Prevalence of long-term use in NHR (≥3 claims of same PIM) (%) |
|---|---|---|---|---|---|---|---|
| 1 | Quetiapine | N05AH04 | B | 20,384 | 22.4 | 13,888 | 15.2 |
| 2 | Lorazepam | N05BA06 | B/P | 18,378 | 20.2 | 8091 | 8.9 |
| 3 | Zolpidem | N05CF02 | B/P | 11,807 | 13.0 | 7794 | 8.5 |
| 4 | Paraffin oil | A06AA01 | P | 8760 | 9.6 | 5457 | 6.0 |
| 5 | Risperidone | N05AX08 | B | 8749 | 9.6 | 6220 | 6.8 |
| 6 | Pipamperone | N05AD05 | B | 7810 | 8.6 | 4563 | 5.0 |
| 7 | Haloperidol | N05AD01 | B/P | 7213 | 7.9 | 2620 | 2.9 |
| 8 | Ibuprofen | M01AE01 | B | 6780 | 7.4 | 2205 | 2.4 |
| 9 | Oxazepam | N05BA04 | B/P | 5948 | 6.5 | 3844 | 4.2 |
| 10 | Metoclopramide | A03FA01 | B | 5397 | 5.9 | 1602 | 1.8 |
| 11 | Belladonna alkaloids* | A03B | B | 4613 | 5.1 | 585 | 0.6 |
| 12 | Diclofenac p.o. | M01AB05 | B | 3965 | 4.3 | 1275 | 1.4 |
| 13 | Nitrofurantoin | J01XE01 | B/P | 3731 | 4.1 | 614 | 0.7 |
| 14 | Estriol | G03CA04 | B | 2807 | 3.1 | 1156 | 1.3 |
| 15 | Hydroxyzine | N05BB01 | B/P | 2784 | 3.0 | 1981 | 2.2 |
| Total PIM Beers and PRISCUS | B/P | 72,106 | 79.1 | 51,232 | 56.2 | ||
| Total PIM Beers | B | 67,490 | 74.0 | 46,798 | 51.3 | ||
| Total PIM PRISCUS | P | 54,253 | 59.5 | 37,268 | 40.9 | ||
ATC = Anatomical Therapeutic Chemical classification system; NHR = nursing home residents; No = number; PIM = potentially inappropriate medication; p.o. = peroral * Including derivatives such as butylscopolamine (antispasmodic agent)
Overall prevalence of PIM use was higher among female NHR than male NHR (women: 80.2%; men: 76.3%) (table S2 in appendix 1). Whereas a higher proportion of female NHR had one or more claims of benzodiazepines and related drugs (e.g., lorazepam, oxazepam and zolpidem), considerably more male NHR were prescribed paraffin oil (a laxative) or belladonna alkaloids and derivatives (antispasmodic agents).
Stratified by canton, we did not observe a clear pattern regarding prevalence of PIM (table S3 in appendix 1). Whereas the canton of Nidwalden showed a maximum value of 87.6%, only 67.2% of NHR in the neighbouring canton of Obwalden (both rather rural areas) had at least one claim of a PIM (PRISCUS list and Beers criteria combined). In total, 15 and 21 out of 24 analysed cantons deviated less than 5 and 10%, respectively, from the average PIM prevalence.
To our knowledge, the present study is the first systematic descriptive analysis of drug utilisation in Swiss nursing homes. The study is based on claims data of a large provider of Swiss mandatory health insurance; it includes elderly patients from all parts of the country who were either NHR or living in the community. Our results suggest that per capita drug claims, average per capita drug costs and the prevalence of polypharmacy were substantially higher in NHR than in the RG. Standardisation by age and sex did not materially alter these observations. Moreover, we identified a considerable amount of PIM use in elderly Swiss NHR.
The great majority (85.5%) of NHR were prescribed five or more different drugs at the same time. A recent study among NHR in Germany reported a similarly high prevalence of polypharmacy (83.5%) [27]. According to another study based on Helsana claims data, 41.2% of the Swiss general population aged 65 years or older (not living in nursing homes) were exposed to polypharmacy between 2008 and 2010 [20]. The prevalence of polypharmacy of 50.4% in our RG thus may suggest a trend towards an increasing number of individuals receiving polypharmacy over time.
Consistent with the latter study [20], we observed a decrease in the number of different drugs taken concurrently with increasing age. The reasons for this might be a de-prescribing of unnecessary medications (medications aiming mainly at a prolonged life expectancy rather than an improved quality of life), especially in very frail and old persons. In elderly patients, drug therapy in accordance with guidelines is often neither effective nor feasible due to different treatment approaches, possible drug-drug interactions and necessary dose adjustments [28]. To curb polypharmacy, clarifying and prioritising the diagnoses that do (not) require therapy is thus essential [29]. Cooper et al. reported that polypharmacy and associated adverse drug events could be minimised by reducing the use of nonessential (and potentially inappropriate) medications and by reinforcing adherence of NHR [30].
Eight out of ten NHR in our study population received at least one PIM when both the PRISCUS list and 2015 updated Beers criteria were taken into account. Furthermore, despite being considered PIMs, the psycholeptics quetiapine, lorazepam and zolpidem were found among the 15 most frequently prescribed drugs in nursing homes. Psycholeptics increase the risk of cerebrovascular events as well as the rate of cognitive decline and mortality in persons with dementia [8].
One in ten NHR received paraffin oil, making it the fourth most frequently used PIM among NHR. Viscous paraffin is usually indicated for the short-term treatment of constipation. In elderly people at risk of aspiration, side effects such as lipid pneumonia occur more frequently [8].
Two systematic reviews also emphasised the substantial occurrence of PIM in NHR: Storms et al. delineated a PIM prevalence in nursing homes between 18.5 and 82.6% according to Beers criteria [11]; Morin et al. described point prevalences of PIM use (varying criteria to identify PIM) ranging from 5.4 to 95%, with higher estimates reported in Europe than in other continents, and in studies conducted after 2005 compared with studies conducted between 1990 and 1999 [6].
Several limitations need to be considered when interpreting the results of the present study. Our analyses relied on healthcare invoices submitted to Helsana for reimbursement by mandatory health insurance. Consequently, we may have missed a number of drugs, in particular (1) non-prescription drugs purchased over-the-counter, (2) drugs paid for out-of-pocket by individuals whose healthcare expenses principally eligible for reimbursement by the mandatory health insurance did not exceed the annual deductible, (3) drugs not listed for reimbursement in the mandatory health insurance package, and (4) inpatient drug prescriptions. According to internal analyses by Swiss health insurers, the proportion of healthcare expenses below the annual deductible amounts to 2 to 3% of invoices [21]. Therefore, the drug utilisation reflected in our claims data may be a slight underestimation, whereas a potential selection bias is likely to be minor. Medications listed for reimbursement in the mandatory health insurance package account for 80% of the total medication costs in Switzerland [21]. Prevalence of polypharmacy might thus be underestimated to some extent. However, since all PIMs licensed in Switzerland are listed for reimbursement, it is very unlikely that we markedly underestimated their prevalence. Based on the lack of a universally used definition of “long-term use”, we defined long-term PIM use as three or more drug claims of the same PIM, assuming this definition to indicate a regular use. Therefore, a limited degree of misclassification may be expected. Furthermore, we had no information on whether the patients had actually taken the dispensed drugs, as adherence is not guaranteed solely by the purchase of a drug.
The present study was based on the 2015 updated Beers criteria and the PRISCUS list, both of which are widely accepted and evidence-based tools to identify PIM. Undoubtedly, these lists are not meant to override either the clinical judgment of the physician or an individual’s preference, values and needs [8]. Therefore, drugs identified as PIMs may be appropriate in individual clinical situations, under special monitoring and at an appropriate age-adjusted dose. Additionally, evidence of a significant association between PIM defined by Beers criteria and patient outcomes, such as adverse drug events or quality of life, is inconsistent [31, 32]. In contrast, PIMs defined by the START/STOPP criteria, another tool to identify PIM, have been shown to be significantly associated with adverse drug events and hospitalisation [33, 34]. However, the inappropriateness of drugs defined by STOPP is usually linked to exceeding a certain dosage or duration of use, a diagnosis, a co-medication, a duplication of drug classes or lack of an indication. Since required additional information such as outpatient diagnoses, kidney function or cumulative dosage was not captured in our data, we could not analyse implicit PIM (drugs being inappropriate in individual, specific clinical situations, or above a certain single/daily dose). The present study cannot, therefore, allow inferences about the appropriateness of prescriptions and the extent of overuse, underuse or misuse of medications on an individual level. Nevertheless, the prevalence of PIM, as observed in NHR, appears to be rather high and is presumably not solely explicable by individual clinical situations. The data further show that the average number of drug prescriptions per patient in Swiss nursing homes is rather high. However, we need to interpret these data carefully, as dermatological products for dry skin, artificial tears, calcium supplements or Vitamin D3 are also part of this assessment. Thus, whether the average number of drugs applied to NHR is indeed problematic or not needs to be interpreted in the context of the clinical situation. However, these findings are nevertheless in line with a growing body of evidence indicating the need to focus on drug safety, to improve prescribing practices and to prescribe as few drugs as absolutely necessary in the elderly [17, 20–22, 35].
Future national policies might aim at promoting rational drug use in geriatric patients. One possible approach is based on defining a responsible clinical pharmacist for every nursing home and on close interdisciplinary collaboration between healthcare providers in the nursing home setting. This approach has already been successfully tested at a cantonal level [36] and might be a promising approach for future interventions aimed to increase efficacy and quality of drug prescriptions in NHR.
To ensure transparent prescription practice, it is essential for all health professionals involved in the treatment of NHR to share information. Pharmacists have the opportunity to identify PIM or drugs with interaction potential before they reach the patient; physicians may gain an overview of the drugs used by consulting PIM criteria and have the opportunity to inform NHR about benefits and risks of a therapy; nursing professionals who personally know the NHR and administer drugs to them on a daily basis can make an important contribution by providing feedback to the other professional groups. In addition, the patients’ and relatives’ problem-awareness should be sharpened to develop an understanding that reducing the number of drugs is not necessarily associated with a reduction in performance, but may lead to a reduction of risks.
In conclusion, this study provides a comprehensive evaluation of drug utilisation in Swiss nursing homes. The results show that polypharmacy and use of PIM are frequent in NHR. Even though the absolute number of drugs is per se not a meaningful indicator of high or poor prescribing quality, we need to keep in mind that both polypharmacy and PIM are potentially problematic and may be associated with poor health outcomes in elderly people. The data further show that innovative high-cost drugs introduced in oncology, immunology and infectiology are rarely used in the nursing home setting. This results in average drug costs of only around CHF 8 per day for NHR, so that drug costs constitute a minor part of the total healthcare costs of patients in nursing homes. The present study quantifies drug utilisation of NHR and may indicate a need for effective interventions to optimise drug prescribing in Swiss nursing homes.
The appendix is available as a separate file for downloading at https://smw.ch/en/article/doi/smw.2019.20126/
The project was funded by Helsana Group, Zurich, Switzerland. The data have been partially reported in a report on drug use in Switzerland in German language (Helsana Arzneimittelreport 2017). The funder engaged neither in the development of the study design nor in the data analysis.
The authors have no relevant personal or financial conflicts of interests to declare.
1OECD. Recipients of long-term care. Health at a Glance 2017: OECD Indicators. OECD Publishing. 2017. pp. 206. Available at: https://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-2017_health_glance-2017-en. Last accessed on 09 Oct. 2018.
2Bundesamt für Statistik. Taschenstatistik der Schweiz 2018. Bevölkerung. Bundesamt für Statistik, Sektion Diffusion und Amtspublikationen. 2018. pp. 5. Available at: https://www.bfs.admin.ch/bfs/de/home/statistiken/kataloge-datenbanken/publikationen.assetdetail.4522206.html. Last accessed on 11 Oct. 2018.
3 Jokanovic N , Tan EC , Dooley MJ , Kirkpatrick CM , Bell JS . Prevalence and factors associated with polypharmacy in long-term care facilities: a systematic review. J Am Med Dir Assoc. 2015;16(6):535.e1–12. doi:.https://doi.org/10.1016/j.jamda.2015.03.003
4 Moore KL , Boscardin WJ , Steinman MA , Schwartz JB . Patterns of chronic co-morbid medical conditions in older residents of U.S. nursing homes: differences between the sexes and across the agespan. J Nutr Health Aging. 2014;18(4):429–36. doi:.https://doi.org/10.1007/s12603-014-0001-y
5 Fulton MM , Allen ER . Polypharmacy in the elderly: a literature review. J Am Acad Nurse Pract. 2005;17(4):123–32. doi:.https://doi.org/10.1111/j.1041-2972.2005.0020.x
6 Morin L , Laroche ML , Texier G , Johnell K . Prevalence of Potentially Inappropriate Medication Use in Older Adults Living in Nursing Homes: A Systematic Review. J Am Med Dir Assoc. 2016;17(9):862.e1–9. doi:.https://doi.org/10.1016/j.jamda.2016.06.011
7 Spinewine A , Schmader KE , Barber N , Hughes C , Lapane KL , Swine C , et al. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet. 2007;370(9582):173–84. doi:.https://doi.org/10.1016/S0140-6736(07)61091-5
8 By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227–46. doi:.https://doi.org/10.1111/jgs.13702
9 Rochon PA , Gurwitz JH . Optimising drug treatment for elderly people: the prescribing cascade. BMJ. 1997;315(7115):1096–9. doi:.https://doi.org/10.1136/bmj.315.7115.1096
10 Beers MH , Ouslander JG , Rollingher I , Reuben DB , Brooks J , Beck JC ; UCLA Division of Geriatric Medicine. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med. 1991;151(9):1825–32. doi:.https://doi.org/10.1001/archinte.1991.00400090107019
11 Storms H , Marquet K , Aertgeerts B , Claes N . Prevalence of inappropriate medication use in residential long-term care facilities for the elderly: A systematic review. Eur J Gen Pract. 2017;23(1):69–77. doi:.https://doi.org/10.1080/13814788.2017.1288211
12 McLeod PJ , Huang AR , Tamblyn RM , Gayton DC . Defining inappropriate practices in prescribing for elderly people: a national consensus panel. CMAJ. 1997;156(3):385–91.
13 Laroche ML , Charmes JP , Merle L . Potentially inappropriate medications in the elderly: a French consensus panel list. Eur J Clin Pharmacol. 2007;63(8):725–31. doi:.https://doi.org/10.1007/s00228-007-0324-2
14Holt S, Schmiedl S, Thürmann P. PRISCUS-Liste potenziell inadäquater Medikation für ältere Menschen. Berlin: Bundesministerium für Bildung und Forschung BMBF; 2011. Available at: http://priscus.net/download/PRISCUS-Liste_PRISCUS-TP3_2011.pdf
15 Renom-Guiteras A , Meyer G , Thürmann PA . The EU(7)-PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol. 2015;71(7):861–75. doi:.https://doi.org/10.1007/s00228-015-1860-9
16 Cahir C , Fahey T , Teeling M , Teljeur C , Feely J , Bennett K . Potentially inappropriate prescribing and cost outcomes for older people: a national population study. Br J Clin Pharmacol. 2010;69(5):543–52. doi:.https://doi.org/10.1111/j.1365-2125.2010.03628.x
17 Wang KN , Bell JS , Chen EYH , Gilmartin-Thomas JFM , Ilomäki J . Medications and Prescribing Patterns as Factors Associated with Hospitalizations from Long-Term Care Facilities: A Systematic Review. Drugs Aging. 2018;35(5):423–57. doi:.https://doi.org/10.1007/s40266-018-0537-3
18 Chiatti C , Bustacchini S , Furneri G , Mantovani L , Cristiani M , Misuraca C , et al. The economic burden of inappropriate drug prescribing, lack of adherence and compliance, adverse drug events in older people: a systematic review. Drug Saf. 2012;35(S1, Suppl 1):73–87. doi:.https://doi.org/10.1007/BF03319105
19 Mallet L , Spinewine A , Huang A . The challenge of managing drug interactions in elderly people. Lancet. 2007;370(9582):185–91. doi:.https://doi.org/10.1016/S0140-6736(07)61092-7
20 Blozik E , Rapold R , von Overbeck J , Reich O . Polypharmacy and potentially inappropriate medication in the adult, community-dwelling population in Switzerland. Drugs Aging. 2013;30(7):561–8. doi:.https://doi.org/10.1007/s40266-013-0073-0
21 Blozik E , Rapold R , Reich O . Prescription of potentially inappropriate medication in older persons in Switzerland: does the dispensing channel make a difference? Risk Manag Healthc Policy. 2015;8:73–80. doi:.https://doi.org/10.2147/RMHP.S78179
22 Reich O , Rosemann T , Rapold R , Blozik E , Senn O . Potentially inappropriate medication use in older patients in Swiss managed care plans: prevalence, determinants and association with hospitalization. PLoS One. 2014;9(8):e105425. doi:.https://doi.org/10.1371/journal.pone.0105425
23 Masnoon N , Shakib S , Kalisch-Ellett L , Caughey GE . What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. doi:.https://doi.org/10.1186/s12877-017-0621-2
24Bundesamt für Statistik. Alterung der Bevölkerung. March 2017. Available at: https://www.bfs.admin.ch/bfs/de/home/statistiken/bevoelkerung/alterung.html.Last accessed on 16 Apr. 2019]).
25 Bjerrum L , Rosholm JU , Hallas J , Kragstrup J . Methods for estimating the occurrence of polypharmacy by means of a prescription database. Eur J Clin Pharmacol. 1997;53(1):7–11. doi:.https://doi.org/10.1007/s002280050329
26Bundesamt für Statistik. Bevölkerung. April 2019. Available at: https://www.bfs.admin.ch/bfs/de/home/statistiken/bevoelkerung.html. Last accessed on 09 Apr. 2019.
27 Hoffmann F , Schmiemann G , Dörks M . Untersuchungen zu Polypharmazie: Eine Frage der Definition und der verwendeten Daten [Assessment of polypharmacy: A question of definition and underlying data]. Z Evid Fortbild Qual Gesundhwes. 2016;113:27–35. German. doi:.https://doi.org/10.1016/j.zefq.2016.03.004
28 Boyd CM , Darer J , Boult C , Fried LP , Boult L , Wu AW . Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–24. doi:.https://doi.org/10.1001/jama.294.6.716
29 Farmer C , Fenu E , O’Flynn N , Guthrie B . Clinical assessment and management of multimorbidity: summary of NICE guidance. BMJ. 2016;354:i4843. doi:.https://doi.org/10.1136/bmj.i4843
30 Cooper JA , Cadogan CA , Patterson SM , Kerse N , Bradley MC , Ryan C , et al. Interventions to improve the appropriate use of polypharmacy in older people: a Cochrane systematic review. BMJ Open. 2015;5(12):e009235. doi:.https://doi.org/10.1136/bmjopen-2015-009235
31 Onder G , Landi F , Liperoti R , Fialova D , Gambassi G , Bernabei R . Impact of inappropriate drug use among hospitalized older adults. Eur J Clin Pharmacol. 2005;61(5-6):453–9. doi:.https://doi.org/10.1007/s00228-005-0928-3
32 Laroche ML , Charmes JP , Nouaille Y , Picard N , Merle L . Is inappropriate medication use a major cause of adverse drug reactions in the elderly? Br J Clin Pharmacol. 2007;63(2):177–86. doi:.https://doi.org/10.1111/j.1365-2125.2006.02831.x
33 Pérez T , Moriarty F , Wallace E , McDowell R , Redmond P , Fahey T . Prevalence of potentially inappropriate prescribing in older people in primary care and its association with hospital admission: longitudinal study. BMJ. 2018;363:k4524. doi:.https://doi.org/10.1136/bmj.k4524
34 O’Mahony D , O’Sullivan D , Byrne S , O’Connor MN , Ryan C , Gallagher P . STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–8. doi:.https://doi.org/10.1093/ageing/afu145
35 Hovstadius B , Hovstadius K , Astrand B , Petersson G . Increasing polypharmacy - an individual-based study of the Swedish population 2005-2008. BMC Clin Pharmacol. 2010;10(1):16. doi:.https://doi.org/10.1186/1472-6904-10-16
36 Locca JF , Ruggli M , Buchmann M , Huguenin J , Bugnon O . Development of pharmaceutical care services in nursing homes: practice and research in a Swiss canton. Pharm World Sci. 2009;31(2):165–73. doi:.https://doi.org/10.1007/s11096-008-9273-9
The project was funded by Helsana Group, Zurich, Switzerland. The data have been partially reported in a report on drug use in Switzerland in German language (Helsana Arzneimittelreport 2017). The funder engaged neither in the development of the study design nor in the data analysis.
The authors have no relevant personal or financial conflicts of interests to declare.