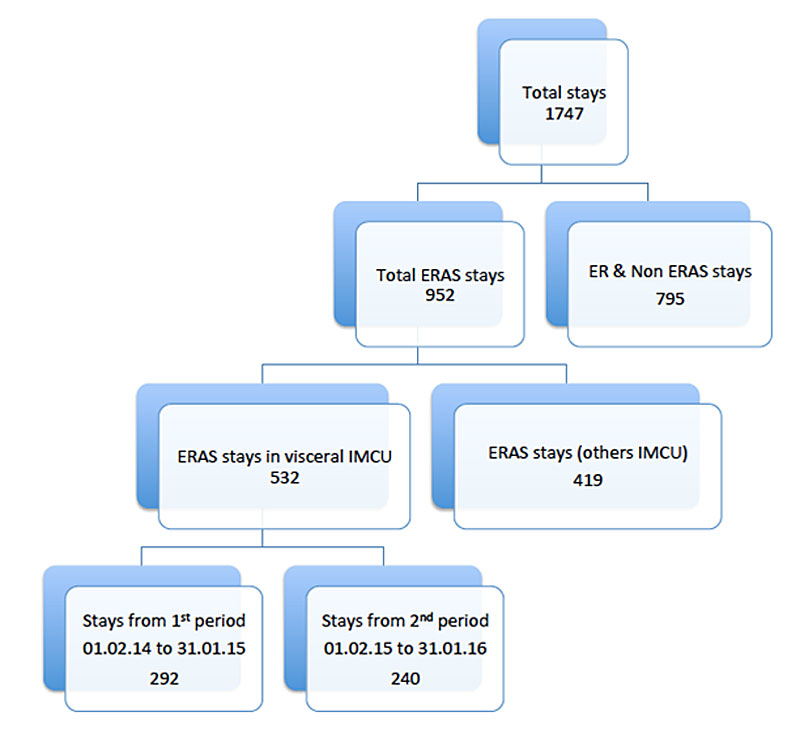
Figure 1 Selection of the analysed population.
DOI: https://doi.org/10.4414/smw.2019.20117
Modern gastrointestinal and hepatopancreaticobiliary (HPB) surgery is characterised by complex surgical procedures performed in an aging patient population with multiple co-morbidities [1, 2]. Subsequently, specialisation of the advanced healthcare professionals involved has become mandatory. This has gained more importance, as increasing healthcare costs are nowadays a major issue worldwide and cost containment in Western countries requires a careful use of healthcare resources.
Since the 1980s, staffing intensive care units (ICUs) with critical care specialists (CCSs) has been shown to improve short- and long-term survival [3, 4]. A systematic review demonstrated that ICU management by CCSs reduced overall hospital mortality, ICU mortality, total length of stay (LOS), and intermediate care unit (IMCU) and ICU LOS. Implementation of CCSs optimises utilisation of resources [5] such as central venous lines and arterial catheters for invasive monitoring, as well as organisation of general ICU functioning [6, 7].
More recently, IMCUs have become important in many institutions, because ICUs were chronically overbooked and also occupied by some patients actually not requiring ICU level of care. Implementation of minimally invasive surgical approaches and enhanced recovery protocols such as Enhanced Recovery After Surgery (ERAS) further promoted development of IMCUs. Of note, IMCUs were traditionally integrated into the respective surgical speciality and surgeons were in charge of the patients. The main limitations of this concept were the lack of surgeons trained in critical care and the time the surgical staff dedicated to managing these patients [8]. To overcome these limitations in our department, a CCS was recruited and subsequently a semi-closed model implemented in the IMCU [4]. This model included interdisciplinary work, which meant that surgeons remained in charge of the surgical management whereas the CCS was responsible for medical care.
The aim of the present study was to assess the short-term impact of a semi-closed IMCU system on patient outcome, using parameters of efficiency (total, ICU and IMCU LOS, readmission and preventable operation rates) to confirm a feeling shared by the healthcare providers that management of acute visceral surgery patients outside the ICU was better.
The methodological framework was based on Donabedian’s model [9], which collects information based on three different domains (structure, process, outcome) to estimate quality of care. In our case, the modification of part of the structure by recruitment of a CCS could have a potential effect on the process (diagnosis, treatments, etc.) as well as on the outcome (effects of healthcare on patients). The change in the structure was the sharing of responsibility for the 11-bed surgical IMCU by transferring medical management from surgeons to a CCS. Two consecutive 12-month periods were compared. During the first period (1 February 2014 to 31 January 2015), surgeons were in charge of the entire patient management in the IMCU. The second period (1 February 2015 to 31 January 2016) covered the shared responsibility model, where surgeons and a CCS were in charge. Of note, the CCS was present on weekdays, with the surgical team on call for weekends and night shifts.
This change, from an open IMCU where patients are admitted under the care of the attending physician, with the CCS being available to provide expertise via elective consultation [10], to a semi-closed IMCU where a CCS provides direct patient care in collaboration with other involved physicians, is in common with many surgical and cardiothoracic ICUs using this model [11].
The following outcomes were analysed: total LOS, duration of ICU or IMCU LOS. Because of the heterogeneous types of intervention, durations of stay were analysed by sub-speciality, even where samples were small.
In addition, rates of readmission (from ward to IMCU or ICU and from IMCU to ICU), the rate of potentially preventable readmission (considered non-predictable at discharge and occurring within 30 days) [12] and with at least one potentially preventable re-intervention (operating theatre, interventional radiology) were assessed.
Some of the patients were prospectively included in ERAS programmes, and thus well documented in institutional databases. All patients who underwent elective or emergency upper gastrointestinal, liver, pancreas or colorectal surgery were included. This made a total of 1747 hospital stays; all of them included at least one stay in the IMCU. Of all IMCU stays, 952 were ERAS stays and 795 were non-ERAS stays (e.g., multiple organ failure, acute pancreatitis, abdominal abscess). Of the 952 ERAS stays, 532 were in the visceral surgery IMCU and the other 419 stays were admitted to other IMCUs where daily supervision by a CCS was not available. Of the 532 ERAS visceral surgery IMCU stays, there were 292 stays in the first period (before CCS implementation), and 240 stays in the second period (after CCS implementation). Figure 1 summarises the selection of the analysed population.

Figure 1 Selection of the analysed population.
Of note, 25% of all IMCU stays (438 out of 1747) were transfers from the ICU.
ASA score, mean age and gender were recorded to describe the patients’ characteristics (table 1). The patients were divided into two groups of the basis of their ASA score: ASA 1–2 (healthy or with mild systemic disease) and ASA 3–4 (severe or life-threatening disease). The groups were identical, except for oesophageal surgery with significantly more ASA 3–4 patients in the second period (p = 0.04).
Table 1 ASA scores of the patients.
| Number of patients |
ASA 1-2
n (%) |
ASA 3-4
n (%) |
p-value
(<0.05) |
Mean age
(years) |
Female patients
% |
||
|---|---|---|---|---|---|---|---|
| Colorectal surgery | Total | 193 | 100 (52%) | 93 (48%) | 0.97 | ||
| 1st period | 118 | 61 (52%) | 57 (48%) | 66 | 40 | ||
| 2nd period | 75 | 39 (52%) | 36 (48%) | 68 | 41 | ||
| Pancreatic surgery | Total | 95 | 59 (62%) | 36 (38%) | 0.76 | ||
| 1st period | 43 | 26 (61%) | 17 (40%) | 67 | 56 | ||
| 2nd period | 52 | 33 (64%) | 19 (37%) | 65 | 54 | ||
| Hepatic surgery | Total | 86 | 67 (78%) | 19 (22%) | 0.53 | ||
| 1st period | 66 | 51 (77%) | 15 (23%) | 59 | 39 | ||
| 2nd period | 20 | 16 (80%) | 4 (20%) | 58 | 46 | ||
| Oesophageal surgery | Total | 71 | 38 (54%) | 33 (47%) | 0.04 | ||
| 1st period | 38 | 20 (59%) | 18 (49%) | 61 | 24 | ||
| 2nd period | 33 | 14 (41%) | 19 (51%) | 62 | 26 | ||
| Gastric bypass surgery | Total | 54 | 33 (61%) | 21 (39%) | 0.29 | ||
| 1st period | 28 | 19 (68%) | 9 (32%) | 44 | 82 | ||
| 2nd period | 26 | 14 (54%) | 12 (46%) | 42 | 77 | ||
| Missing | 3 | 30 | |||||
Definition of ASA score (expresses the preoperative health of a patient): 1 = healthy patient; 2 = patient with a minimal systemic disease; 3 = patient with a severe disease; 4 = patient with a life-threatening disease
During the two periods of observation, surgeons in charge or surgical techniques and strategies included ERAS protocols did not change in the five sub-specialties analysed.
Because of the skewed distribution of LOS data, non-parametric methods were used. Bivariate analyses were conducted on each of the outcomes chosen (Pearson chi [2] or Fisher’s exact test for categorical variables and the Wilcoxon rank sum test or Kruskall-Wallis test with Bonferroni correction for continuous variables). Because of small sample sizes no multivariate analysis was performed.
The 532 visceral surgery IMCU stays were divided into five subspecialties, most patients being colorectal (n = 193) (table 1). From the 532 stays, the majority were single stays (n = 505; table 2). One patient after oesophageal surgery had two stays. In the colorectal group, 168 patients accounted for the 193 stays. Eleven patients had two stays and one patient had three stays. For hepatic, pancreatic and gastric bypass surgery, all patients had one stay. Details of patients and stays are summarised in tables 1 and 2 .
Table 2 Details of the 532 hospital stays.
|
Patients with 1 hospital stay
n = 505 |
Patients with 2 hospital stays
n = 12 |
Patients with 3 hospital stays
n = 1 |
|
|---|---|---|---|
| Colorectal surgery (n = 193 stays) |
168 | 11 | 1 |
| Pancreatic surgery (n = 95) |
95 | 0 | 0 |
| Hepatic surgery (n = 117 stays) |
117 | 0 | 0 |
| Oesophageal surgery (n = 73) |
71 | 1 | 0 |
| Gastric bypass surgery (n = 54) |
54 | 0 | 0 |
Table 3 Details of the ICU, IMCU LOS, readmissions in ICU or IMCU and potentially preventable readmission and operation.
| Sub-specialties | Total period |
1st period
(1 Feb. 2014 – 31 Jan. 2015) |
2nd period
(1 Feb. 2015 – 31 Jan. 2016) |
p-value (p = 0.05) |
|
|---|---|---|---|---|---|
| Colorectal surgery | Mean total LOS (±SD) | 17 (± 15) | 17 (±13) | 17 (±18) | 0.33 |
| Mean ICU total LOS (±SD) | 7 (± 8) | 7 (± 10) | 6 (± 5) | 0.85 | |
| Mean IMCU total LOS (±SD) | 4 (± 6) | 3 (± 3) | 5 (± 8) | 0.47 | |
| Readmission in ICU | 12 (6%) | 7 (6%) | 5 (7%) | 0.86 | |
| Readmission in IMCU | 21 (11%) | 10 (9%) | 11 (15%) | 0.19 | |
| Potentially preventable readmission | 20 (10%) | 15 (13%) | 5 (7%) | 0.17 | |
| Potentially preventable operation | 48 (25%) | 28 (25%) | 20 (26%) | 0.79 | |
| Pancreatic surgery | Mean total LOS (±SD) | 28 (± 22) | 27 (± 21) | 27 (± 23) | 0.77 |
| Mean ICU total LOS (±SD) | 6 (± 4) | 4 (± 3) | 7 (± 5) | 0.12 | |
| Mean IMCU total LOS (±SD) | 7 (± 8) | 7 (± 7) | 7 (± 9) | 0.36 | |
| Readmission in ICU | 15 (16%) | 6 (14%) | 9 (17%) | 0.66 | |
| Readmission in IMCU | 13 (14%) | 8 (19%) | 5 (10%) | 0.20 | |
| Potentially preventable readmission | 10 (11%) | 4 (9%) | 6 (12%) | 0.72 | |
| Potentially preventable operation | 23 (25%) | 13 (32%) | 10 (20%) | 0.78 | |
| Hepatic surgery | Mean total LOS (±SD) | 14 (± 13) | 14 (± 10) | 15 (± 15) | 0.33 |
| Mean ICU total LOS (±SD) | 3 (± 2) | 2 (± 2) | 4 (± 4) | 0.14 | |
| Mean IMCU total LOS (±SD) | 4 (± 3) | 4 (± 3) | 3 (± 3) | 0.71 | |
| Readmission in ICU | 8 (7%) | 6 (9%) | 2 (4%) | 0.34 | |
| Readmission in IMCU | 6 (5%) | 6 (9%) | 0 (0%) | 0.04 | |
| Potentially preventable readmission | 6 (5%) | 5 (7%) | 1 (2%) | 0.21 | |
| Potentially preventable operation | 7 (6%) | 4 (± 6%) | 3 (± 6%) | 0.98 | |
| Oesophageal surgery | Mean total LOS (±SD) | 32 (± 29) | 36 (± 32) | 29 (± 27) | 0.18 |
| Mean ICU total LOS (±SD) | 10 (± 17) | 10 (± 12) | 10 (± 21) | 0.005 | |
| Mean IMCU total LOS (±SD) | 9 (± 10) | 10 (± 12) | 8 (± 8) | 0.19 | |
| Readmission in ICU | 12 (16%) | 6 (18%) | 6 (15%) | 0.80 | |
| Readmission in IMCU | 17 (23%) | 7 (21%) | 10 (26%) | 0.61 | |
| Potentially preventable readmission | 3 (4%) | 2 (6%) | 1 (3%) | 0.48 | |
| Potentially preventable operation | 20 (28%) | 13 (39%) | 7 (18%) | 0.04 | |
| Gastric bypass surgery | Mean total LOS (±SD) | 6 (± 9) | 7 (± 10) | 6 (± 8) | 0.77 |
| Mean ICU total LOS (±SD) | No data | ||||
| Mean IMCU total LOS (±SD) | 1 (± 3) | 2 (± 3) | 1 (± 1) | 0.60 | |
| Readmission in ICU | No data | ||||
| Readmission in IMCU | 1 (2%) | 1 (4%) | 0 (0%) | 0.33 | |
| Potentially preventable readmission | 2 (4%) | 2 (8%) | 1 (4%) | 0.95 | |
ICU = intensive care unit; IMCU = intermediate care unit; LOS = length of stay; SD = standard deviation
The mean total hospital LOS in the five sub-specialties was not different with or without the CCS (colorectal surgery p = 0.33, pancreatic surgery p = 0.77, hepatic surgery p = 0.33, oesophageal surgery p = 0.18, gastric surgery p = 0.77).
The mean total ICU LOS was significantly reduced after oesophageal surgery (p = 0.005), but not after colorectal (p = 0.85), pancreatic (p = 0.12) or hepatic (p = 0.14) surgery. There were no ICU stays after gastric surgery.
The mean total IMCU LOS was not different with or without a CCS (colorectal surgery p = 0.47, pancreatic surgery p = 0.36, hepatic surgery p = 0.71, oesophageal surgery p = 0.19, gastric surgery p = 0.60).
Readmission to the ICU was not different with or without a CCS (colorectal surgery p = 0.86, pancreatic surgery p = 0.66, hepatic surgery p = 0.34, oesophageal surgery p = 0.80).
There was a significant reduction in IMCU readmissions from the ward after hepatic surgery (p = 0.04) after CCS implementation. No difference was observed in the other subspecialties (colorectal surgery p = 0.19, pancreatic surgery p = 0.20, oesophageal surgery p = 0.61, gastric surgery p = 0.33).
There was a significant reduction in the rate of potentially preventable operations for oesophageal surgery (p = 0.04). No difference was observed in the other subspecialties (colorectal surgery p = 0.79, pancreatic surgery p = 0.78, hepatic surgery p = 0.98, gastric surgery p = 0.97).
The aim of the present study was to assess the impact of a CCS on the LOS in patients undergoing elective/urgent gastrointestinal, hepatobiliary and pancreatic surgery.
The main observation was a statistically significant reduction in ICU LOS after oesophageal surgery, and a reduction, although nonsignificant, after pancreatic and hepatic surgery. After implementing a CCS, only patients with major organ dysfunction were transferred to the ICU postoperatively. Patients undergoing oesophageal surgery were usually directly managed in the IMCU, only patients presenting severe cardiorespiratory comorbidities were admitted to the ICU.
We also observed a difference in the numbers of patients admitted to the IMCU after hepatic (66 vs 20) and colorectal (118 vs 75) surgery between the first and the second periods. There could be two explanations for this. Firstly, more restrictive patient selection through implementation of IMCU monitoring criteria (no IMCU admission for asymptomatic patients with epidural catheters, or after minor hepatic and colorectal procedures). Secondly, prioritisation of the management of the most fragile patients in the visceral IMCU and not in other surgical units. To confirm those elements, the number of hepatic and colorectal patients was similar between the two analysed periods: in the first period (1 February 2014 to 31 January 2015) there were 73 hepatic patients and 215 colorectal patients; in the second period (1 February 2015 to 31 January 2016) there were 63 hepatic patients and 198 colorectal patients.
The presence of a CCS could theoretically increase the total hospital and IMCU LOS because of more complex and invasive management. However, this study showed that changing from an open to a semi-closed IMCU system by adding a daily available CCS did not affect the patients’ overall LOS.
For colorectal surgery, results showed no significant differences. The reduced number of patients transiting through the IMCU between the first and the second period (from 118 to 75) likely reflects more restrictive IMCU patient selection by the CCS and therefore a more appropriate use of resources [5]. The colorectal subgroup was the oldest (mean age 67 years), and thus avoiding an IMCU stay may have a positive impact on cognitive complications, such as acute confusional states and delirium, which is known to increase LOS and in-hospital mortality (4–17%) [13, 14]. This result is in line with those of Nathens et al., who showed in a study from 2006 that “the risk of death in the elderly is reduced by almost 50% with the involvement of an intensivist. This effect might relate to a greater understanding of the impact and care of coexisting diseases in this population” [15].
A significant reduction in readmissions to the IMCU after hepatic surgery and a non-significant reduction of ICU LOS after both pancreatic (p = 0.12) and hepatic (p = 0.14) surgery suggests that for fragile patients, the presence of a CCS enables complications to s to be anticipated and their impact reduced. This indicator (ICU readmission) has been proven to be a reliable qualitative parameter [16].
For oesophageal surgery, the benefit of a CCS seemed important, probably because this population is extremely fragile and comorbid, and the procedures complex. The main observation was the reduction in ICU stays, probably by only admitting patients presenting severe cardiorespiratory comorbidities to the ICU, all other patients being admitted directly to the IMCU. IMCU LOS was reduced as well but not significantly, probably because of the small sample size. Early identification of complications associated with an active patient management may in part explain these findings. The result is particularly interesting as there are more ASA 3–4 patients during the second period (51 vs 41% in the first period). Even in such fragile and comorbid patients, the IMCU management proved to be efficient and sufficient.
The longer stays after gastric surgery can be attributed to bias as one single patient transferred from an outside hospital, spent 41 days in acute units (30 days in ICU, 11 days in the IMCU) because of respiratory and major surgical complications. Normally, gastric bypass patients returned directly to the ward or spent 12–24 hours in the IMCU for respiratory monitoring in the case of untreated obstructive sleep apnoea syndrome.
In relation to the general functioning of the department of visceral surgery, reductions in ICU and IMCU LOS and reduction in the number of patient transfers might have a positive impact on administrative workload, reduce the risks of medical errors and increase the time dedicated to patient care. The implementation of a CCS implied a more active management of the patients and consequently a more important workload for nursing staff.
Some limitations of the present study need to be mentioned. First, this was a retrospective study and some important items, such as aspects of cost-effectiveness, were not assessed. Second, the small sample size of the subgroups limited the interpretation. Furthermore, there was no time gap between the “before CCS” and the “with CCS” periods. There might have been a more profound impact on the outcomes if the data collection had begun once the CCS was fully established and the system adapted.
Restriction of the analysis to an ERAS population meant that almost half of the patients (n = 795) were dropped. The ERAS population mainly consisted of patients undergoing elective surgery. In our ERAS population, about 18% were emergencies, in three subspecialties (colorectal, hepatic and pancreatic surgery). The emergencies excluded (peritonitis with multiple organ dysfunction, pancreatitis) might have had a major impact on outcomes, as morbidity and mortality of emergency/urgent patients is known to be higher [17]. The second drop out comes from patients not admitted to the 11-bed surgical IMCU (n = 419). These patients were daily managed by surgeons and nursing teams unused to visceral pathologies; the CCS was called only for emergencies. An anticipated management of specific situation was therefore not possible (fig. 1).
Reorganisation from an open to a semi-closed IMCU by involving a CCS improves daily management of acute surgical patients. It offers the complementarity of close surgical follow up associated with specific medical competences of a CCS with benefit for the patients and the surgical staff. This interdisciplinary approach probably represents the future, given the increasing age of the patient population in a complex medical environment.
No financial support and no potential conflict of interest related to this study was reported.
1 Etzioni DA , Liu JH , Maggard MA , Ko CY . The aging population and its impact on the surgery workforce. Ann Surg. 2003;238(2):170–7. doi:.https://doi.org/10.1097/01.SLA.0000081085.98792.3d
2 Joliat GR , Hahnloser D , Demartines N , Schäfer M . Future development of gastrointestinal cancer incidence and mortality rates in Switzerland: a tumour registry- and population-based projection up to 2030. Swiss Med Wkly. 2015;145:w14188. doi:.https://doi.org/10.4414/smw.2015.14188
3 Brown JJ , Sullivan G . Effect on ICU mortality of a full-time critical care specialist. Chest. 1989;96(1):127–9. doi:.https://doi.org/10.1378/chest.96.1.127
4 Tai DY , Goh SK , Eng PC , Wang YT . Impact on quality of patient care and procedure use in the medical intensive care unit (MICU) following reorganisation. Ann Acad Med Singapore. 1998;27(3):309–13.
5 Pollack MM , Katz RW , Ruttimann UE , Getson PR . Improving the outcome and efficiency of intensive care: the impact of an intensivist. Crit Care Med. 1988;16(1):11–7. doi:.https://doi.org/10.1097/00003246-198801000-00003
6 Li TC , Phillips MC , Shaw L , Cook EF , Natanson C , Goldman L . On-site physician staffing in a community hospital intensive care unit. Impact on test and procedure use and on patient outcome. JAMA. 1984;252(15):2023–7. doi:.https://doi.org/10.1001/jama.1984.03350150023014
7 Pronovost PJ , Angus DC , Dorman T , Robinson KA , Dremsizov TT , Young TL . Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288(17):2151–62. doi:.https://doi.org/10.1001/jama.288.17.2151
8 Juneja D , Nasa P , Singh O . Physician staffing pattern in intensive care units: Have we cracked the code? World J Crit Care Med. 2012;1(1):10–4. doi:.https://doi.org/10.5492/wjccm.v1.i1.10
9 Donabedian A , Wheeler JR , Wyszewianski L . Quality, cost, and health: an integrative model. Med Care. 1982;20(10):975–92. doi:.https://doi.org/10.1097/00005650-198210000-00001
10Rothschild J. Closed intensive care units and other models of care for critically ill patients. In: Shojania KG, Duncan BW, McDonald KM, et al., editors. Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Evidence Report/Technology Assessment No. 43. Rockville, MD: Agency for Healthcare Research and Quality; 2001. pp 413–22.
11 Tinti MS , Haut ER , Horan AD , Sonnad S , Reilly PM , Schwab CW , et al. Transition to a semiclosed surgical intensive care unit (SICU) leads to improved resident job satisfaction: a prospective, longitudinal analysis. J Surg Educ. 2009;66(1):25–30. doi:.https://doi.org/10.1016/j.jsurg.2008.09.005
12 Halfon P , Eggli Y , van Melle G , Chevalier J , Wasserfallen JB , Burnand B . Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55(6):573–87. doi:.https://doi.org/10.1016/S0895-4356(01)00521-2
13 Rudolph JL , Jones RN , Rasmussen LS , Silverstein JH , Inouye SK , Marcantonio ER . Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. 2007;120(9):807–13. doi:.https://doi.org/10.1016/j.amjmed.2007.02.026
14 Norkiene I , Ringaitiene D , Misiuriene I , Samalavicius R , Bubulis R , Baublys A , et al. Incidence and precipitating factors of delirium after coronary artery bypass grafting. Scand Cardiovasc J. 2007;41(3):180–5. doi:.https://doi.org/10.1080/14017430701302490
15 Nathens AB , Rivara FP , MacKenzie EJ , Maier RV , Wang J , Egleston B , et al. The impact of an intensivist-model ICU on trauma-related mortality. Ann Surg. 2006;244(4):545–54.
16 Plate JDJ , Peelen LM , Leenen LPH , Houwert RM , Hietbrink F . A proposal for an intermediate care unit-quality measurement framework. Crit Care Res Pract. 2018;2018:4560718. doi:.https://doi.org/10.1155/2018/4560718
17 Pearse RM , Harrison DA , James P , Watson D , Hinds C , Rhodes A , et al. Identification and characterisation of the high-risk surgical population in the United Kingdom. Crit Care. 2006;10(3):R81. doi:.https://doi.org/10.1186/cc4928
No financial support and no potential conflict of interest related to this study was reported.