Indications and associated factors for prescribing intravenous iron supplementation in Swiss general practice: a retrospective observational study
DOI: https://doi.org/10.4414/smw.2019.20127
Rahel
Meier, Ellen
Keizer, Thomas
Rosemann, Stefan
Markun
Institute of Primary Care, University of Zurich and University Hospital Zurich, Switzerland
Summary
BACKGROUND
Intravenous iron supplementation (IVIS) is increasing in Swiss general practice. This is controversial because of higher costs and risks compared with oral iron supplementation (OIS). In this study, we aimed to investigate indications for OIS and IVIS in Swiss general practice and test which factors are associated with prescribing IVIS instead of OIS.
METHODS
This was a retrospective observational study using data from electronic medical records of 279 Swiss general practitioners (GPs), including all their patients at their first OIS and IVIS administration who were at least 14 years old and with available pretreatment serum ferritin and haemoglobin measurements. We also collected data about the following comorbidities: chronic kidney disease (CKD) stage 3b or higher, inflammatory bowel disease (IBD), chronic heart failure and restless legs syndrome. Outcomes were proportions of cases treated with IVIS or OIS by assumed indication (anaemic or non-anaemic iron deficiency [NAID]) or the abovementioned comorbidities. We evaluated associations with IVIS prescription using regression models adjusting for patient and GP variables.
RESULTS
We assessed 1282 IVIS and 3003 OIS cases (median age 44 and 40 years, female in 86.7 and 88.6%, respectively). The most important indication for both IVIS and OIS was NAID (59.4 and 74.7%, respectively). Significant associations with prescribing IVIS instead of OIS were the severity of iron deficiency (reflected by serum ferritin levels and the presence of anaemia), the presence of advanced CKD, IBD and male sex of the GP. There was a time trend towards increasing serum ferritin levels associated with initiation of IVIS in NAID.
CONCLUSION
When preferring IVIS to OIS, Swiss GPs seem to consider severity of iron deficiency and comorbid conditions. However, pretreatment serum ferritin levels in IVIS for NAID are increasing, suggesting an ongoing indication extension and potential overuse.
Introduction
Iron deficiency is the leading cause of anaemia in central European countries, the most common cause being menstrual blood loss in women [1]. Iron deficiency can be diagnosed on the basis of serum ferritin levels before anaemia occurs [2]. However, target values for serum ferritin vary, as patient populations have been identified that benefit from significantly increased iron storage, namely patients with chronic kidney disease (CKD) [3, 4], chronic heart failure (CHF) [5] and restless legs syndrome (RLS) [6]. Evidence suggests that non-anaemic iron deficiency (NAID) may play a role in otherwise unexplained fatigue, further extending the spectrum of potential indications for iron supplementation beyond iron deficiency anaemia [7–9].
Iron supplementation may be administered orally or intravenously. Oral iron supplementation (OIS) is the recommended first-line treatment because of lower direct costs and fewer safety concerns, and intravenous iron supplementation (IVIS) is recommended as second-line treatment after OIS has proven ineffective [10, 11]. In specific patient populations, IVIS is a recognised first-line treatment, namely when intestinal iron absorption is impaired as in inflammatory bowel disease (IBD) [12] or when serum ferritin concentrations above 100 ng/ml are targeted, such as in CKD [13], CHF [14] or RLS [6].
In recent years, IVIS prescriptions have increased in Switzerland: Analysing data from insurance claims, Giger et al. found that IVIS prescription increased by 250% between 2006 and 2010 [15]. Also according to claims data, Bietry et al. found that IVIS prescription rates are a staggering thousand times higher in Switzerland than in the United Kingdom [16]. So far, it is unclear what causes this increase in IVIS prescriptions and several reasons seem plausible. One could be the increasing recognition of NAID [8, 9], based on the extension of the serum ferritin threshold defining iron deficiency above the value of 15 ng/ml proposed by the World Health Organization (WHO) [17, 18]. However, it is also possible that patients receiving IVIS are diagnosed with specific conditions proven to benefit from IVIS. Unfortunately, previous studies describing the increase in IVIS were based on claims data only and thus they were unable to investigate questions about the clinical circumstances under which iron supplementation was initiated.
Therefore, the aim of this study was to investigate indications for OIS and IVIS in Swiss general practice and test which factors are associated with prescribing IVIS instead of OIS.
Methods
Study design and setting
This study was a retrospective observational database analysis of Swiss general practice using data from the FIRE (Family Medicine ICPC-Research using Electronic Medical Records) project [19]. The FIRE project is a network of Swiss general practitioners (GPs) who export anonymised routine data from their electronic medical records (EMR); participation is free of charge and voluntary. The GPs contribute to a central database the following data: administrative data, laboratory values, medication data, vital signs and, if available, International Classification of Primary Care (ICPC) diagnoses. Since the project started in 2009, 441 GPs (8.6% of GPs working in the German-speaking region of Switzerland [20]) have joined the network, bringing with them over 518,000 patients and more than four million consultations. We assessed all data from the observation period included in the FIRE database (search range from January 2009 to August 2018).
Participants
All GPs in the FIRE network using EMR with interfaces exporting serum ferritin laboratory analyses (observation starting from the first exported serum ferritin value) were eligible.
Patient inclusion criterion was receiving any pure iron supplementation (OIS or IVIS, anatomical therapeutic chemical classification [ATC] codes [21]: B03AA, B03AB and B03AC) during the full observation period. If several iron supplements of the same kind (multiple treatment cycles) were issued to the same patient, only the first of each kind (OIS or IVIS) was included in order to prevent over-representation of individual patients. Patient were excluded if: (a) the initiation date of iron supplementation coincided with the date of first record in the FIRE database (because iron supplementation may have been ongoing in some of these patients and the encounter in question just be a follow-up, and not the true initiation of iron supplementation); (b) treatment was before age of 14 years. In cases meeting the inclusion criterion, we assessed whether serum ferritin and haemoglobin measurements were available to allow determination of anaemia and iron status. Cases with available measurements were included for further analysis. The GP, patient and case selection process is visualised in the study flowchart (see fig. 1 below).
Database query, variables and diagnostic criteria
From the included cases, we retrieved: (a) demographic data (age, sex and body mass index (BMI)); (b) treatment data (prescription date, treatment modality OIS or IVIS); (c) laboratory data (results of serum ferritin and haemoglobin measurements within 90 days preceding iron prescription, coding anaemia in female cases with haemoglobin <12 g/dl and male cases <13 g/dl) [22]; and (d) presence of concurrent conditions relevant to iron prescribing. We identified CKD stage 3b or higher, IBD, CHF and RLS by either considering diagnostic ICPC coding, prescribed drugs with high disease specificity or laboratory values with high specificity (CKD stage 3b or higher: at least two previous creatinine measures indicating an impaired glomerular filtration rate of <90 ml/min and at least one measurement <45 ml/min; IBD: ICPC code D94 or ATC codes A07EA06, A07EC01, A07EC02, A07FA; CHF: ICPC code K77; RLS: ICPC code N04). Cases without concurrent conditions were classified as iron deficiency anaemia (IDA) if anaemic and NAID if not anaemic.
Outcomes
Outcomes of the study were as follows:
- Proportions of cases by assumed indication for iron supplementation (IDA, NAID or presence of at least one specific comorbidity of either CKD, CHF or RLS). Cases with NAID were further subdivided into proportions of cases falling within predefined serum ferritin bands of <15 ng/ml, 15–29 ng/ml or ≥30ng/ml. Cases with IBD were included in the IDA or NAID group because the primary indication for these patients is not the IBD itself but the iron deficiency.
- Associations of patient and GP characteristics with treatment modality (IVIS or OIS).
- Time trends in serum ferritin levels associated with iron supplementation in NAID.
Ethical approval
According to the Local Ethics Committee of the Canton of Zurich, the project does not fall under the scope of the law on human research and therefore no ethical consent is necessary (BASEC-Nr. Req-2017-00797).
Data analysis
We described categorical data as counts and proportions (n, %) and numerical data as mean and standard deviation (SD) or median and interquartile range (IQR), as appropriate. For group comparisons, we used the Wilcoxon rank sum test, Welch two-sample t-test, χ2-test or Fisher’s exact test, as appropriate. To assess associations with treatment modality (IVIS or OIS), we used a generalised mixed model: the dependent variable was treatment modality; independent variables were patient characteristics (sex and age), year of treatment, comorbidities (IBD, CKD, CHF), severity of iron deficiency (anaemia and serum ferritin) and GP variables (sex and age), adjusting for GP identity as nested variable within the GP practice. We reported odds ratios (ORs) and 95% confidence intervals (CIs) for each factor included in the model.
To assess whether pretreatment serum ferritin levels in NAID changed over time we used graphical representation and a linear mixed model with serum ferritin as dependent variable. The independent variable was treatment modality. We adjusted for patient characteristics (sex and age), GP variables (sex and age) and GP identity as nested variable within the GP practice. We reported change over time as percentage increase per year. The p-value was set at ≤ 0.05. R (Version 3.5.0) was used for statistical analysis [23].
Results
Population
Data interfaces from 116 practices in the FIRE network exported serum ferritin measurements and were therefore eligible for this study. GPs (n = 279) in the dataset had a median age of 51 years (IQR 43–60); 61.4% were male and 83.0% were working in group practices. GPs’ median time under observation was 487 days (IQR 240–1017). A total of 10,912 patients met the inclusion criterion of starting OIS or IVIS; however, 2512 of these met at least one of the exclusion criteria at the patient level. On the level of cases, 4285 (OIS 3003 and IVIS 1282) met the inclusion criterion of available haemoglobin and serum ferritin measurements within 90 days preceding initiation of iron supplementation and thus composed the final dataset for subsequent analyses (fig. 1). Per individual GP, there was a median of 6 OIS cases (IQR 2–13, range 0–137) in the dataset and a median of 3 IVIS cases (IQR 1–9, range 0–57).
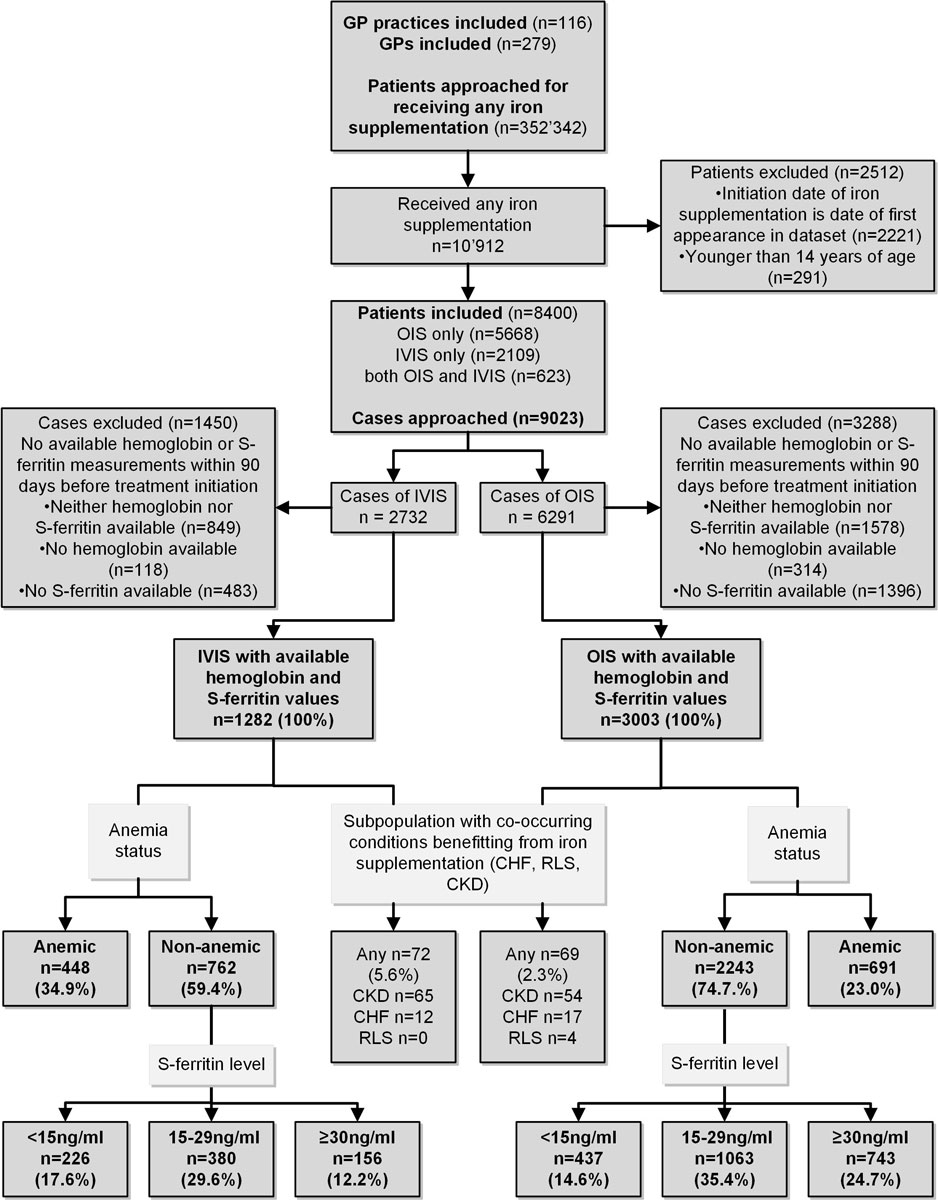
Figure 1 Study design and inclusion/exclusion criteria including numbers of patients and cases. GP = general practitioner; OIS = oral iron supplementation; IVIS = intravenous iron supplementation; S-ferritin = serum ferritin; CHF = chronic heart failure; RLS = restless legs syndrome; CKD = chronic kidney disease stage 3b or higher
Compared with OIS cases, IVIS cases were associated with significantly older age, lower serum ferritin and lower haemoglobin levels (table 1). Higher proportions of IBD and CKD stage 3b or higher were associated with IVIS. Of the 1282 IVIS cases, 240 (18.7%) had previously received OIS (median interval between OIS and IVIS 102 days, IQR 31–212).
Table 1 Description of cases (total n = 4285).
|
Variable
|
Intravenous iron
n = 1282
|
Oral iron
n = 3003
|
p-value
|
|
Median, mean or n
|
IQR
(SD) or %
|
Median, mean or n
|
IQR,
(SD) or %
|
| Age at iron supplementation (years) |
44 |
32–66 |
40 |
26–53 |
<0.001*
|
| Sex (female) |
1111 |
86.7% |
2662 |
88.6% |
0.0668†
|
| Body mass index (kg/m2) |
26.5 |
(6.5) |
25.7 |
(5.8) |
0.051‡
|
| Haemoglobin <3months pretreatment (g/dl) |
12.2 |
(1.7) |
12.8 |
(1.4) |
<0.001‡
|
| Serum ferritin <3months pretreatment (ng/ml) |
17 |
10–27 |
23 |
14–34 |
<0.001*
|
| Inflammatory bowel disease |
134 |
10.4% |
244 |
8.1% |
0.0139‡
|
| Chronic kidney disease stage 3b or higher |
65 |
5.1% |
54 |
1.8% |
<0.001*
|
| Chronic heart failure |
12 |
0.9% |
17 |
0.6% |
0.1762*
|
| Restless legs syndrome |
0 |
0% |
4 |
0.1% |
0.3241§
|
Indications for OIS and IVIS
The majority of cases for both OIS and IVIS were treated without having anaemia, CKD, CHF or RLS and thus fell into the NAID indication group. Indications differed substantially between OIS and IVIS: NAID 74.7 vs 59.4%; IDA 23.0 vs 34.9% and either CKD, CHF or IBD 2.3 vs 5.6%, respectively (see fig. 1 for comparative classification flow). Most NAID cases fell into the category of serum ferritin between 15 and 29 ng/ml, regardless of treatment modality.
Associations with treatment modality
We found that the strongest association for initiating IVIS instead of OIS was serum ferritin <15 ng/ml (OR 3.7, 95% CI 2.6–5.0, ref. serum ferritin ≥30 ng/ml). Other strong associations with IVIS initiation were serum ferritin 15 to 29 ng/ml (OR 2.2, 95% CI 1.7–2.7, ref. serum ferritin ≥30 ng/ml), chronic kidney disease stage 3b or higher (OR 2.0, 95% CI 1.2–3.4), anaemia (OR 2.0, 95% CI 1.6–2.5) and the GP being male (OR 1.8, 95% CI 1.1–2.9). We found a weak association for IBD (OR 1.4, 95% CI 1–1.9). See figure 2 for visualisation of associations with prescription of IVIS instead of OIS.
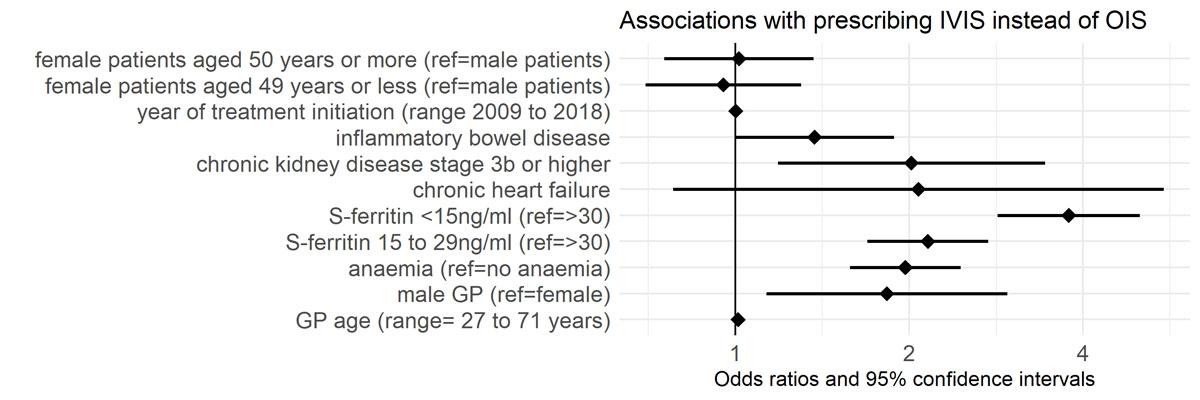
Figure 2 Odds ratios and 95% confidence intervals of variables’ associations with prescribing IVIS instead of OIS, n = 3484. OIS = oral iron supplementation; IVIS = intravenous iron supplementation; S-ferritin = serum ferritin; GP = general practitioners
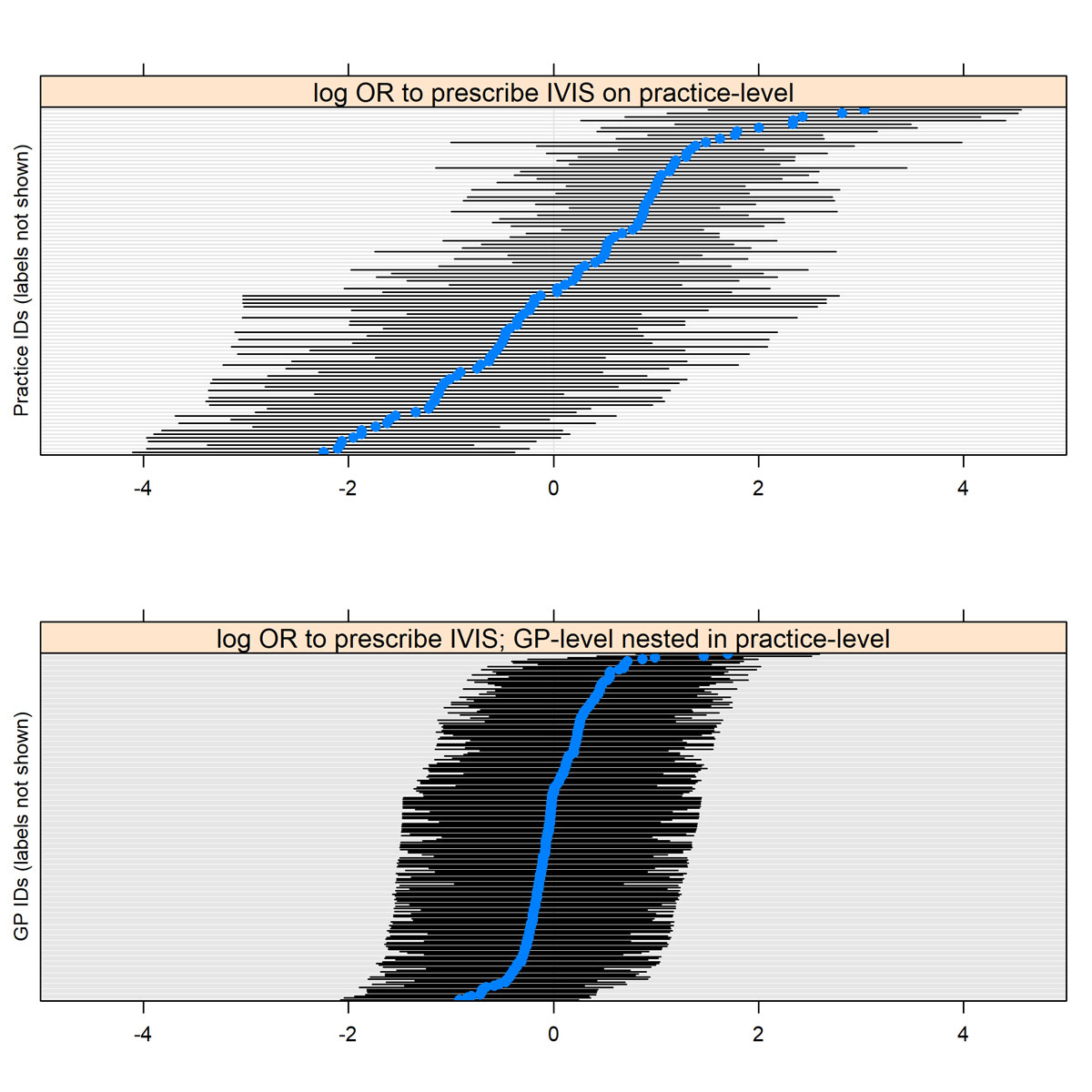
Multilevel adjustment for GPs nested in GP practices showed that there was a significant association on the level of GP practices with prescribing IVIS instead of OIS, but not on the level of individual GPs (see supplementary fig. S1 in appendix 1)
Time trend exploration
Graphical representation showed a trend towards increasing pretreatment serum ferritin thresholds with IVIS for NAID but not with OIS (fig. 3). The linear regression model confirmed the trend towards increase of serum ferritin levels; however, the trend was statistically not significant (yearly increase of serum ferritin in IVIS 3.0%; p = 0.096).
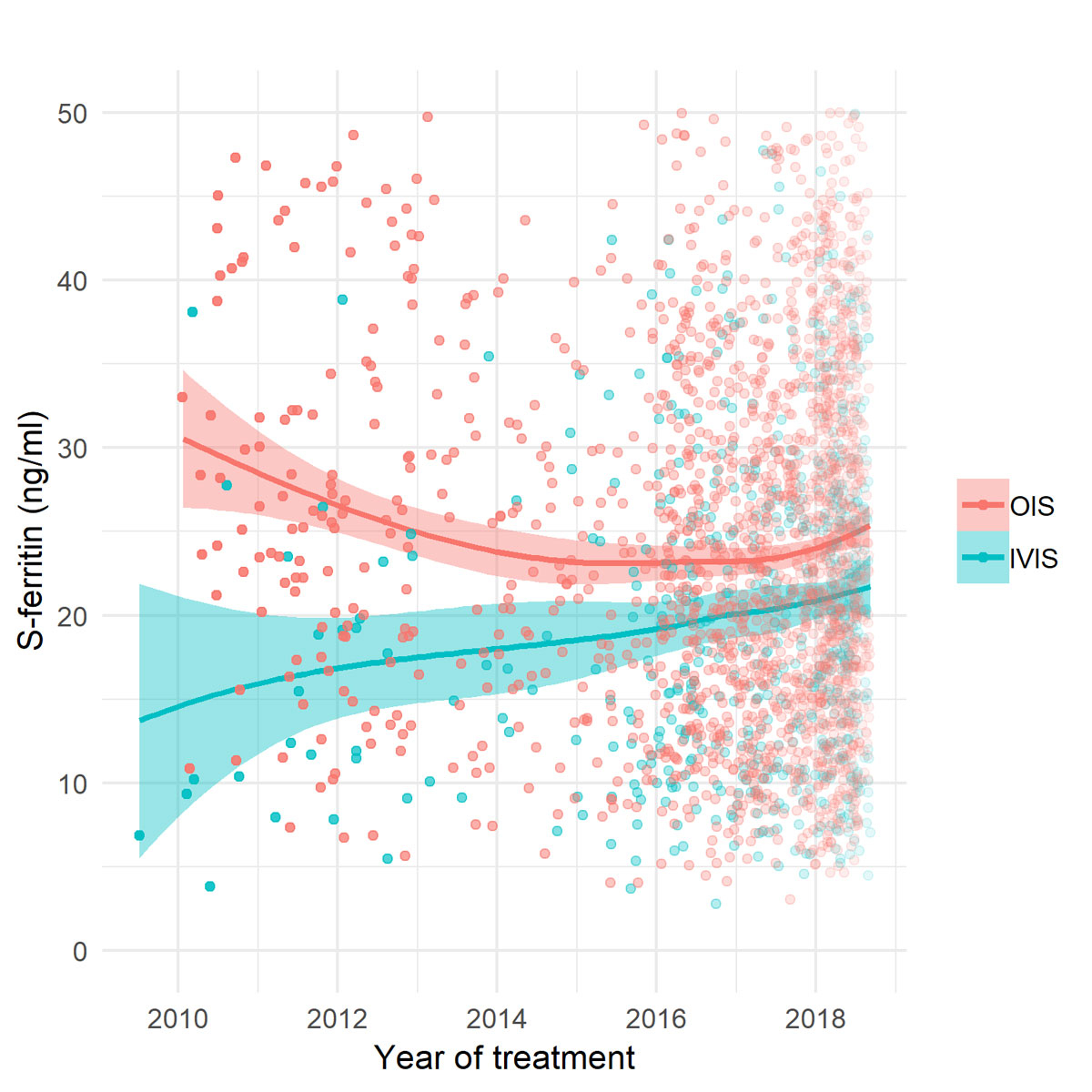
Figure 3 Pretreatment serum ferritin in patients with non-anaemic iron deficiency treated with OIS (red) and IVIS (blue). Curves are fitted with a least squares regression in localised subsets. OIS = oral iron supplementation; IVIS = intravenous iron supplementation; S-ferritin = serum ferritin
Discussion
This study showed that GPs used iron supplementation mainly in NAID and in most of these cases serum ferritin levels were above the threshold of 15 ng/ml recommended by the WHO [17] and renowned experts [11, 24]. This finding suggests an indication extension in Swiss general practice towards treatment for higher serum ferritin values and raises concerns about potential overtreatment. GPs’ decisions to use IVIS instead of OIS were associated with patient characteristics such as severity of iron deficiency and comorbidities, but also with GP characteristics, namely GPs’ sex and the GP practice. Serum ferritin levels prior to IVIS for NAID varied widely and seemed to rise over time, indicating a liberalisation of IVIS initiation.
Research in context
Previous studies have described prescription rates and costs of IVIS in Swiss outpatient care. Between 2006 and 2010, IVIS prescriptions increased by 244% and related costs increased by 340% (EUR 16.5 Mio) [15]. Between 2012 and 2014 prescription rates continued to increase and IVIS prescription rates became a thousand times higher in Switzerland than in the UK, according to Biétry et al. [16]. These studies, however, provided no information on indications and laboratory values associated with iron supplementation, which we investigated in our study. We found that one in 18 cases receiving IVIS belonged to a population with comorbidities primarily demanding intravenous treatment and serum ferritin targets of 100 ng/ml or higher (CHF, CKD). Only one in three cases of IVIS had haemoglobin values in the anaemic range. Moreover, at least every second case had neither a comorbid disease demanding IVIS nor anaemia, thus falling under the definition of NAID (fig. 1). Treatment of NAID with IVIS is most controversial because there is no evidence suggesting favourable effects on objective measures and only moderate evidence for fatigue, as a recent systematic review showed [25]. Fatigue is presumably among the most common reasons for treating NAID in general practice. Notably, the abovementioned systematic review found only two randomised controlled trials testing IVIS [26, 27]. One of these trials showed no effectiveness in the total study population, but the subgroup of individuals with serum ferritin levels <15 ng/ml seemed to benefit [27]. The other trial showed effectiveness, but had a serum ferritin level <15 ng/ml among its inclusion criteria [26]. Together these trials suggest that using this serum ferritin threshold of <15 ng/ml is reasonable in NAID, but for higher serum ferritin levels evidence is clearly lacking. Moreover, it has to be pointed out that OIS is similarly effective in fatigue in NAID, as two randomised controlled trials showed [28, 29]. In our study, we found that patients receiving OIS had more often NAID and serum ferritin above 15 ng/ml than patients receiving IVIS; however, potential overuse of OIS may be less relevant because of lower concerns about treatment risks and lower direct costs compared with IVIS.
Variability in healthcare and heterogeneity of recommendations
Patient factors associated with prescribing IVIS instead of OIS were the severity of iron deficiency and the presence of comorbid CKD and IBD. To consider CKD and IBD as a reason to favour IVIS is clearly supported by other evidence [12, 30]. The severity of iron deficiency itself, however, is not commonly specified by guidelines as a reason to forgo OIS and directly initiate IVIS. Swiss GPs may, however, follow the recommendations of the Health Technology Assessment published by the Swiss Medical Board in 2014, which explicitly recommends IVIS in severe iron deficiency because of faster restoration of iron stores and assumed superiority in quality-adjusted life years [31]. Another reason for choosing IVIS over OIS might be side effects previously experienced under OIS, which are expected to occur in 4 to 47% of cases, depending on the preparation [32]. In our study, we registered a previous attempt at treatment with OIS in one out of five IVIS cases.
GP-related factors associated with prescribing IVIS instead of OIS were sex and practice. The latter is not surprising, given that GPs working together in a practice share not only infrastructure but also are likely to follow similar guidelines and processes of care. The finding that male GPs were more likely than female GPs to prescribe IVIS suggests that gender-related factors contribute to treatment decisions. Such a provider-dependency in healthcare services, however, may be problematic because arguably indications primarily rest on patient factors.
In the literature there is substantial heterogeneity in the definition of iron deficiency, which may contribute to the heterogeneity of medical practice. An illustrative example for published but divergent expert opinions is Lopez et al., who recommended a serum ferritin threshold for iron deficiency of <15 ng/ml in The Lancet in 2016 and Camaschella, who recommended <30 ng/ml in The New England Journal of Medicine in 2015 [3, 24]. The implications of defining such margins for categorising a disease is shown by Schleiffenbaum et al., who determined that a surprising 7% of Swiss army draftees are iron deficient using a threshold of 30 ng/ml, or by Schueppbach et al. who found that 50% of healthy female Swiss hospital employees have serum ferritin below 22 ng/ml [33, 34].
Strengths and limitations
A major strength of this study is that it more precisely defines the setting where potential overuse of IVIS in Switzerland occurs. Thereby the study follows up on previous research that discovered a dramatic increase in IVIS in Switzerland over the last decade. A second major strength is the reach of the FIRE database, which covers 8% of GPs from the German-speaking area of Switzerland and their accrued real-life routine clinical data. However, GPs participating in the FIRE network might not be completely representative, as EMR users are younger and more often work in group practices [35]. Since GP age was not associated with our outcomes, we expect no relevant bias from this factor.
An important limitation of this study is information missing from EMR. Missing laboratory data (haemoglobin and ferritin) especially led to exclusion of a significant proportion of cases, which may convey a risk of selection bias. However, the proportion of cases excluded because of missing laboratory data did not differ between OIS and IVIS, which argues against an association with the main outcomes of our study. Interestingly, Giger et al. found very similar proportions of missing laboratory data in patients on iron supplementation [15]. This points to the possibility that physicians actually abstain from complete pretreatment testing. Nonetheless, technical reasons may also have contributed to missing laboratory data in the database. Patient histories preceding coverage in the FIRE project may have contained OIS that we missed in our study and therefore our rate of OIS attempts before IVIS is most likely an underestimation. Another risk of underestimation in our study concerns the recognition of comorbidities. Especially for CHF and RLS, we depended entirely on accurate ICPC coding, which might have been foregone in some cases by GPs. These comorbidities, however, are rare in the young population we identified and false negatives would hardly affect the main findings of our study. Ultimately, to interpret the study outcomes, it is important to understand that cases of iron supplementation were included only once at the initiation of the first treatment cycle in the database. Thus, the study outcomes represent situations at the start of iron supplementation, not recurrent treatment cycles and therefore not the entirety of iron supplementations.
Implications for further research
From a health-service research perspective, the evidence-performance gap reflected in overprescription of IVIS in patients with NAID needs to be better understood. The next steps in research of medical overuse should explore how clinical uncertainty and cognitive biases may lead to overuse [36]. Among possible drivers for overuse of IVIS are patient expectations, lack of knowledge, heterogeneity in the literature, financial incentives or cognitive biases from marketing campaigns deployed by pharmaceutical companies [37, 38].
Conclusion
When preferring IVIS to OIS, Swiss GPs seem to consider severity of iron deficiency and comorbid conditions. However, GPs use iron supplementation mostly in NAID and in most cases serum ferritin levels are above 15 ng/ml. Over the last decade, pretreatment serum ferritin levels in IVIS for NAID have increased, suggesting an ongoing indication extension and potential overuse.
Data sharing statement
The data are gathered within the ongoing FIRE project. Additional data that are regularly gathered are stored in the FIRE database. The FIRE database can be accessed at any time by the scientific team of the institute. For external requests, access has to be requested from the head of the institute.
Appendix 1 Supplementary figure
Acknowledgments
We thank the FIRE study group of general practitioners for contributing to the present study, Sima Djalali for fostering the FIRE database until the end of 2016, Fabio Valeri for statistical assistance and Isaac Gravestock for language editing.
Author contributions
RM, EK, TR and SM developed the study protocol. RM and SM extracted and analysed the data and wrote the final manuscript. All authors read, revised and approved the manuscript.
References
1
Kassebaum
NJ
,
Jasrasaria
R
,
Naghavi
M
,
Wulf
SK
,
Johns
N
,
Lozano
R
, et al.
A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615–24. doi:.https://doi.org/10.1182/blood-2013-06-508325
2
Mast
AE
,
Blinder
MA
,
Gronowski
AM
,
Chumley
C
,
Scott
MG
. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem. 1998;44(1):45–51.
3
Camaschella
C
. Iron-deficiency anemia. N Engl J Med. 2015;372(19):1832–43. doi:.https://doi.org/10.1056/NEJMra1401038
4
Kwack
C
,
Balakrishnan
VS
. Managing erythropoietin hyporesponsiveness. Semin Dial. 2006;19(2):146–51. doi:.https://doi.org/10.1111/j.1525-139X.2006.00141.x
5
Qian
C
,
Wei
B
,
Ding
J
,
Wu
H
,
Wang
Y
. The Efficacy and Safety of Iron Supplementation in Patients With Heart Failure and Iron Deficiency: A Systematic Review and Meta-analysis. Can J Cardiol. 2016;32(2):151–9. doi:.https://doi.org/10.1016/j.cjca.2015.06.009
6
Allen
RP
,
Picchietti
DL
,
Auerbach
M
,
Cho
YW
,
Connor
JR
,
Earley
CJ
, et al.; International Restless Legs Syndrome Study Group (IRLSSG). Evidence-based and consensus clinical practice guidelines for the iron treatment of restless legs syndrome/Willis-Ekbom disease in adults and children: an IRLSSG task force report. Sleep Med. 2018;41:27–44. doi:.https://doi.org/10.1016/j.sleep.2017.11.1126
7
Yokoi
K
,
Konomi
A
. Iron deficiency without anaemia is a potential cause of fatigue: meta-analyses of randomised controlled trials and cross-sectional studies. Br J Nutr. 2017;117(10):1422–31. doi:.https://doi.org/10.1017/S0007114517001349
8
Clénin
GE
. The treatment of iron deficiency without anaemia (in otherwise healthy persons). Swiss Med Wkly. 2017;147:w14434. doi:.https://doi.org/10.4414/smw.2017.14434
9
Pratt
JJ
,
Khan
KS
. Non-anaemic iron deficiency - a disease looking for recognition of diagnosis: a systematic review. Eur J Haematol. 2016;96(6):618–28. doi:.https://doi.org/10.1111/ejh.12645
10
Muñoz
M
,
Gómez-Ramírez
S
,
Bhandari
S
. The safety of available treatment options for iron-deficiency anemia. Expert Opin Drug Saf. 2018;17(2):149–59. doi:.https://doi.org/10.1080/14740338.2018.1400009
11
Goddard
AF
,
James
MW
,
McIntyre
AS
,
Scott
BB
; British Society of Gastroenterology. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60(10):1309–16. doi:.https://doi.org/10.1136/gut.2010.228874
12
Bonovas
S
,
Fiorino
G
,
Allocca
M
,
Lytras
T
,
Tsantes
A
,
Peyrin-Biroulet
L
, et al.
Intravenous Versus Oral Iron for the Treatment of Anemia in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore). 2016;95(2):e2308. doi:.https://doi.org/10.1097/MD.0000000000002308
13
Shepshelovich
D
,
Rozen-Zvi
B
,
Avni
T
,
Gafter
U
,
Gafter-Gvili
A
. Intravenous Versus Oral Iron Supplementation for the Treatment of Anemia in CKD: An Updated Systematic Review and Meta-analysis. Am J Kidney Dis. 2016;68(5):677–90. doi:.https://doi.org/10.1053/j.ajkd.2016.04.018
14
Ponikowski
P
,
Voors
AA
,
Anker
SD
,
Bueno
H
,
Cleland
JGF
,
Coats
AJS
, et al.; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. doi:.https://doi.org/10.1093/eurheartj/ehw128
15
Giger
M
,
Achermann
R
. Ambulante Eisensubstitution in der Schweiz – Kostensteigerung infolge venöser Applikation [Iron substitution in outpatients in Switzerland: Increase of costs associated with intravenous administration]. Z Evid Fortbild Qual Gesundhwes. 2013;107(4-5):320–6. doi:.https://doi.org/10.1016/j.zefq.2012.12.023
16
Biétry
FA
,
Hug
B
,
Reich
O
,
Susan
JS
,
Meier
CR
. Iron supplementation in Switzerland - A bi-national, descriptive and observational study. Swiss Med Wkly. 2017;147:w14444. doi:.https://doi.org/10.4414/smw.2017.14444
17World Health Organization, Centers for Disease Control and Prevention. Assessing the iron status of populations; Second edition, including Literature Reviews. 2007. Geneva: World Health Organization: 2007. Available from: https://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/9789241596107/en/
18
Seiler
C
. Healthy persons at risk for iron substitution. Swiss Med Wkly. 2017;147:w14452. doi:.https://doi.org/10.4414/smw.2017.14452
19
Chmiel
C
,
Bhend
H
,
Senn
O
,
Zoller
M
,
Rosemann
T
; FIRE study-group. The FIRE project: a milestone for research in primary care in Switzerland. Swiss Med Wkly. 2011;140:w13142. doi:.https://doi.org/10.4414/smw.2011.13142
20Swiss Medical Association (FMH). Aerztestatistik 2017. Updated 27 Mar. 2018 14 Dec. 2018. Available from: http://aerztestatistik.myfmh2.fmh.ch/.
21WHO Collaborating Centre for Drug Statistics Methodology. ATC-Structure and principles. Oslo, Norway: World Health Organization; 2018 Accessed 09 July 2019. Available from: https://www.whocc.no/atc/structure_and_principles/.
22World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System (WHO/NMH/NHD/MNM/111). Geneva: World Health Organization; 2011. Available from: http://www.who.int/vmnis/indicators/haemoglobin.pdf.
23R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. Available from: https://www.R-project.org/.
24
Lopez
A
,
Cacoub
P
,
Macdougall
IC
,
Peyrin-Biroulet
L
. Iron deficiency anaemia. Lancet. 2016;387(10021):907–16. doi:.https://doi.org/10.1016/S0140-6736(15)60865-0
25
Houston
BL
,
Hurrie
D
,
Graham
J
,
Perija
B
,
Rimmer
E
,
Rabbani
R
, et al.
Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: a systematic review of randomised controlled trials. BMJ Open. 2018;8(4):e019240. doi:.https://doi.org/10.1136/bmjopen-2017-019240
26
Favrat
B
,
Balck
K
,
Breymann
C
,
Hedenus
M
,
Keller
T
,
Mezzacasa
A
, et al.
Evaluation of a single dose of ferric carboxymaltose in fatigued, iron-deficient women--PREFER a randomized, placebo-controlled study. PLoS One. 2014;9(4):e94217. Published online April 23, 2014. doi:.https://doi.org/10.1371/journal.pone.0094217
27
Krayenbuehl
PA
,
Battegay
E
,
Breymann
C
,
Furrer
J
,
Schulthess
G
. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011;118(12):3222–7. doi:.https://doi.org/10.1182/blood-2011-04-346304
28
Vaucher
P
,
Druais
PL
,
Waldvogel
S
,
Favrat
B
. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. CMAJ. 2012;184(11):1247–54. Published online July 11, 2012. doi:.https://doi.org/10.1503/cmaj.110950
29
Verdon
F
,
Burnand
B
,
Stubi
CL
,
Bonard
C
,
Graff
M
,
Michaud
A
, et al.
Iron supplementation for unexplained fatigue in non-anaemic women: double blind randomised placebo controlled trial. BMJ. 2003;326(7399):1124. doi:.https://doi.org/10.1136/bmj.326.7399.1124
30
Albaramki
J
,
Hodson
EM
,
Craig
JC
,
Webster
AC
. Parenteral versus oral iron therapy for adults and children with chronic kidney disease. Cochrane Database Syst Rev. 2012;1:CD007857. doi:.https://doi.org/10.1002/14651858.CD007857.pub2
31Fachgremium Swiss Medical Board. Orale oder parenterale Behandlung des Eisenmangels. Bern: Swiss Medical Board; 2014. Available from: https://www.swissmedicalboard.ch/fileadmin/public/news/2014/bericht_smb_eisenmangel_lang_2014.pdf.
32
Cancelo-Hidalgo
MJ
,
Castelo-Branco
C
,
Palacios
S
,
Haya-Palazuelos
J
,
Ciria-Recasens
M
,
Manasanch
J
, et al.
Tolerability of different oral iron supplements: a systematic review. Curr Med Res Opin. 2013;29(4):291–303. doi:.https://doi.org/10.1185/03007995.2012.761599
33
Schuepbach
RA
,
Bestmann
L
,
Béchir
M
,
Fehr
J
,
Bachli
EB
. High Prevalence of Iron Deficiency among Educated Hospital Employees in Switzerland. Int J Biomed Sci. 2011;7(2):150–7.
34
Schleiffenbaum
BE
,
Schaer
DJ
,
Burki
D
,
Viollier
AF
,
Viollier
E
,
Stettler
ER
, et al.
Unexpected high prevalence of metabolic disorders and chronic disease among young male draftees--the Swiss Army XXI experience. Swiss Med Wkly. 2006;136(11-12):175–84.
35
Djalali
S
. Wer eHealth sucht, findet einen Haufen Papier. Schweiz Arzteztg. 2015;96(43):1575–8. doi: https://doi.org/10.4414/saez.2015.03985
36
Morgan
DJ
,
Brownlee
S
,
Leppin
AL
,
Kressin
N
,
Dhruva
SS
,
Levin
L
, et al.
Setting a research agenda for medical overuse. BMJ. 2015;351:h4534. doi:.https://doi.org/10.1136/bmj.h4534
37
Kale
MS
,
Korenstein
D
. Overdiagnosis in primary care: framing the problem and finding solutions. BMJ. 2018;362:k2820. doi:.https://doi.org/10.1136/bmj.k2820
38
Keyhani
S
,
Siu
AL
. The underuse of overuse research. Health Serv Res. 2008;43(6):1923–30. doi:.https://doi.org/10.1111/j.1475-6773.2008.00920.x