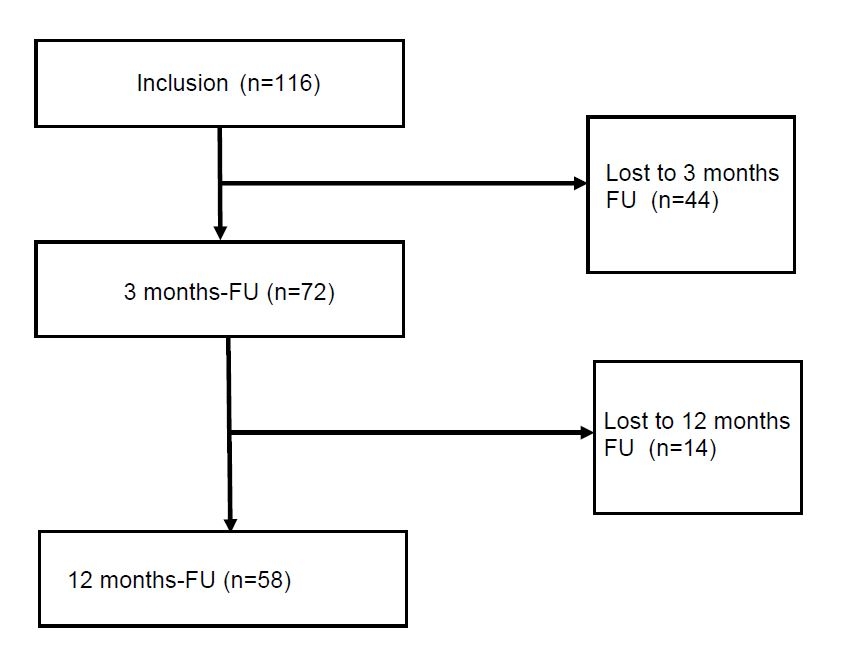
Figure 1 Flowchart of the prospective gout cohort at the University Hospital Basel.
DOI: https://doi.org/10.4414/smw.2019.20121
Gout is the most common inflammatory joint disease in adults and is caused by the deposition of monosodium urate crystals in the joints or soft tissue [1]. Gout flares are extremely painful and have an acute onset. The typical attack lasts for several days and is followed by asymptomatic intervals. If left untreated, chronic gout with nephrolithiasis [2] and the formation of tophi in various internal organs can also occur [3–5].
According to the American College of Rheumatology (ACR) / European League Against Rheumatism (EULAR) gout classification criteria 2015 [6], arthritis is classified as gout in the presence of a typical presentation, laboratory findings or imaging, even without synovial fluid analysis.
The prevalence of gout ranges from 1 to 3% in Europe [7] and is 3.9% in the US [8], but is increased in patients with impaired renal function and those having a reduced uric acid excretion [9, 10]. Incidence rates increase exponentially with higher serum uric acid levels [11]. The annual incidence rate in patients with uric acid levels above 9 mg/dl (535 μmol/l) is as high as 4.9% [11].
The disease can be debilitating and impacts on function and quality of life [12]. Gout is associated with increased all-cause disease mortality [13]. The total disability-adjusted life years because of gout has increased globally between 2007 and 2017 by 30% [14]. Moreover, hyperuricaemia, and hence gout, is a cardiovascular risk factor [15] and an independent risk factor for hypertension [16] and diabetes [17]. Comorbidities are thus prevalent [18]. In multimorbid patients, flares of gout are notably frequent, because of diuretic treatment or renal function impairment [19, 20]. Gout can be cured if the serum urate level is permanently lowered below a proposed threshold of 360 μmol/l [21]. Despite the huge impact of gout on health and the establishment of treat-to-target (T2T) strategies [22], treatment remains unsatisfactory to date. In a French cohort, the serum uric acid (SUA) target (<360 μmol/l) was achieved in about a quarter of the patients in the first year of therapy [23]. New register data from the US suggest that only one third of the patients with gout are under urate lowering treatment [8]. In another register study from the Netherlands, only 51% of the patients had ULT medication coverage of at least 80% of the study days [24].
Most of these data stem from cross-sectional register studies, which precludes detailed analysis of the factors associated with failure of reaching the SUA target.
Subsets of patients (e.g., hospitalised patients) are probably even more difficult to treat, owing to existing comorbidities (e.g., reduced kidney function) and to polypharmacy [25].
We therefore aimed to study treatment adherence and outcome, and to analyse potential factors for treatment failure in a population of gout patients seen in a university hospital.
We used existing reporting guidelines for observational studies such as Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [26, 27] for this longitudinal cohort study. Patients seen by a rheumatologist because of a gout flare at the University Hospital Basel, either in the inpatient department or the outpatient clinic were asked to participate in our single-centre gout cohort. The study was approved by the ethics committee (EKNZ 75/2012), and all subjects gave written informed consent. The inclusion period was between January 2013 and April 2017. At presentation, patients were invited for follow-up visits at 3 and 12 months after inclusion; patients under the age of 18 years were excluded. A rheumatologist performed the 3- and 12-month follow-up assessments at the rheumatology clinic. In between the follow-up visits, the patients were not restricted in their choice of caring physician.
Gout was diagnosed either by detection of urate crystals in joint aspirates or by fulfilment of the American College of Rheumatology classification criteria for gout [28]. Dual energy computed tomography (DECT) with characteristic findings for gout was used to confirm crystal deposition when a synovial analysis was not available [29]. Chronic gout was defined as a disease lasting more than 12 months. At each visit, the following were assessed: (i) clinical manifestation of gout (acute flares, arthritis, tophi and number and localisation of involved joints) including number of gout flares, disease duration; (ii) laboratory parameters (SUA, creatinine, C-reactive protein [CRP]); and (iii) comorbidities (history of diabetes, hypertension, renal insufficiency [30], myocardial infarction, stroke, dyslipidaemia, autoimmune disease, organ transplantation, nephrolithiasis and body mass index [BMI in kg/m2]) and current medication. The patient interviews and assessments were standardised and defined in the study protocol. The data were collected in an electronic database (Secu Trial®) managed by the hospital’s clinical trial unit.
Patients who missed the 3- or 12-month visits were contacted by telephone and asked for their reasons for not attending. These data were collected retrospectively. SUA levels from patients without follow-up data were retrieved from general practitioner (GP) or hospital files in 12 patients.
Patients who achieved the SUA target of 360 μmol/l (according to the EULAR guidelines 2016 [31]), at the 3- and/or 12-month follow-up were compared with patients who did not achieve the SUA target with respect to clinical characteristics, gout presentation and laboratory data. Patients who suffered from acute gout attacks by the 3- or 12-month follow-up were excluded from the SUA outcome analysis [32, 33]. We also compared patients with or without flares during the follow-up period (between 3- and 12-month follow-up or at 12-month follow-up presentation).
Categorical variables were reported as frequencies and compared using Pearson’s χ2-tests or Fisher’s exact-tests. Continuous variables showing normal distribution were reported as means and standard deviations and compared using the Mann-Whitney U-test. Continuous data showing non-normal distribution were reported as medians and interquartile ranges (IQRs). All analyses were conducted using Graph Pad Prism Version 8 (GraphPad Software, Inc., La Jolla, CA USA). A significance level of α = 0.05 was maintained for all analyses.
Between January 2013 and April 2017, 116 patients were included (2013 n = 38, 2014 n = 41, 2015 n = 26, 2016 n = 8, 2017 n = 3). Fifty-two percent of the patients were hospitalised at the time of inclusion. The vast majority of patients had either crystal- (98 patients, 84.5%) or DECT-proven gout (7 patients). All patients fulfilled the ACR 1977 criteria for gout [28]. At baseline, 74% of patients presented with a flare. Fifty-seven (49.1%) patients had chronic disease, including 14 patients with longstanding disease of over 10 years. In one patient, spinal gout was detected. At baseline, 41 patients were on ULT, either allopurinol (n = 36) or febuxostat (n = 5). Allopurinol daily doses were 50 mg in 1 patient, 100 mg in 19, 150 mg in 1, 200 mg in 5, 300 mg in 8 and 400 mg in 2. Febuxostat daily doses were 60 mg in one and 80 mg in four patients. The mean daily dose of allopurinol was 135 mg (range 50–400, SD 115). Thirty patients received prophylactic treatment: colchicine (26 patients), prednisone (2 patients), or nonsteroidal anti-inflammatory drugs (NSAIDs; 2 patients). Twenty-eight patients received flare treatment with colchicine (9), prednisone (8) or nonsteroidal analgesics (11).
The most prevalent comorbidity was hypertension, followed by chronic kidney disease and obesity. Detailed patient characteristics and laboratory data at the time of inclusion are given in table 1.
Table 1 Patient characteristics at baseline (n = 116).
| Gender male | 98 (84.5%) |
| Mean age (years) | 66.9 (15.1) Range: 23–94 |
| Mean serum uric acid level (μmol/l) | 472.1 (149.9) |
| Mean GFR (ml/min/1.73 m2) | 67.4 (33.3) |
| Median CRP (mg/l) | 17.5 (84.2) |
| Hypertension | 78/108 (72.2%) |
| Dyslipidaemia | 34/108 (31.5%) |
| Chronic kidney disease | 59/108 (54.6%) |
| Myocardial infarction | 24/108 (22.2%) |
| Stroke | 9/108 (8.3%) |
| Obesity (BMI >30 kg/m2) | 35/99 (35.4%) |
| Nephrolithiasis | 9/108 (8.3%) |
| Diabetes | 8/108 (7.4%) |
| Autoimmune disease | 7/108 (6.5%) |
| Transplantation | 2/108 (1.9%) |
| Mean BMI (kg/m2) | 29.1 (5.7) |
| First diagnosis of gout at inclusion | 22.4% |
| Median disease duration (months) | 12.0 (51.3) |
| Oligo- or polyarticular gout | 52.2% |
| Tophaceous gout | 25.9% |
BMI = body mass index (assessed in 99 patients); CRP = C-reactive protein; GFR = glomerular filtration rate estimated with MDRD short formula; MDRD = modification of diet in renal disease Continuous variables are represented as mean and standard deviation (SD) if normally distributed and as median, interquartile range (IQR) if not normally distributed, and categorical variables as frequency (%).
Seventy-two of 116 patients (62.1%) attended the 3-month follow-up and 58 (50%) the 12-month follow-up (fig. 1). Six patients presented at the 12-month but not at the 3-month follow-up. Twelve patients died during the follow-up period. Causes of deaths were malignant diseases (n = 5), cardiac failure (n = 2), sepsis (n = 1) and unknown (n = 4).

Figure 1 Flowchart of the prospective gout cohort at the University Hospital Basel.
Three patients were hospitalised at the time of the 3-month follow-up and four at the 12-month follow-up. Additionally, two patients were immobilised, and two suffered from dementia and were thus unable to attend the follow-up visits. Four patients indicated GP visits as the reason for their absence from follow-up visits. Twenty-five patients withdrew consent, and six patients were lost to follow up for unknown reasons. Thirty-five percent (n = 40) of the included patients were treated in the rheumatology outpatient clinic.
At the 3-month follow-up, three patients reported one new comorbidity (obesity, nephrolithiasis and diabetes); at the 12-month follow-up one patient reported de novo chronic kidney disease (CKD) and two de novo diabetes. None of these patients achieved the SUA target level at 3- or 12-month follow-up.
Sixteen of 72 patients (22%) achieved the SUA level below 360 μmol/l at the 3-month follow-up visit and 33/58 patients (57%) at the 12-month follow-up visit. Patients who did not reach the SUA target at 12 months more often reported flares after 3 months of follow-up (44 vs 30%, p = 0.41). Seventeen patients were on de novo allopurinol treatment at the 3-month follow-up. In 15 patients, the allopurinol dosage had been increased since inclusion. The mean daily dose of allopurinol at the 3-month follow-up was 171 mg (SD 96, range 50–400). At the 3-month follow-up five patients were treated with febuxostat, two of these de novo (one patient with 40 mg and the other with 80 mg per day) and one switched to allopurinol between baseline and 3-month follow-up. In one patient, the dosage was increased from 80 to 120 mg per day. The two other patients who were on febuxostat since baseline had a stable dosage of 80 mg/d.
At the 12-month follow-up, two patients had started febuxostat, both with 80 mg/d. One patient had the dosage reduced from 120 to 80 mg per day. In 18 patients, the daily allopurinol dose had been increased since the 3-month follow-up, and eight patients received de novo ULT at the 12-month follow-up. The mean allopurinol daily dose (n = 46) was 212 mg (SD 111, range 50–600 mg), and the median febuxostat daily dose (n = 8) was 80 mg (IQR 0). Twenty-two patients took a daily dose of 300 mg allopurinol; the maximum febuxostat dose was 80 mg at that time. Allopurinol doses during follow-up were lower in patients with reduced kidney function (fig. 2).

Figure 2 Renal function, SUA levels and allopurinol dosage at baseline and follow-up. Violin plots (red lines = medians) of (A) Glomerular filtration rate (GFR in ml/min/1.73 m2) and corresponding SUA (serum uric acid) levels in μmol/l, (B) GFR and corresponding allopurinol dosages (mg/d), (C) Allopurinol dosages and corresponding SUA levels for the 3 time-points of the observation period.
At the 3- and 12-month follow-up visits, 52 and 47%, respectively, of patients reported at least one flare since inclusion. Two thirds had active arthritis at the 3-month follow-up and a quarter at the 12-month follow-up. Patients with flares at the 12-month follow-up had a lower median glomerular filtration rate at that time (53.2 ml/min/m2, IQR 35.5) than those without flares (66.6 ml/min/m2, IQR 50; p = 0.25). Patients with flares between 3-month follow-up and 12-month follow-up had a shorter median duration of allopurinol medication (9 months, IQR 10.9 vs 12 months, IQR 4.4; p = 0.016).
At 3- and 12-month follow-up, 30 and 36% of patients, respectively, had received flare prophylaxis. Most often, colchicine was given, followed by prednisone and interleukin-1 antagonists. Only one patient received NSAIDs at 12-month follow-up. Eleven patients experienced flares during the preceding 9 months before the 12 month follow-up visit, despite flare prophylaxis.
To identify risk factors for not achieving the SUA target, we compared patients achieving the target of SUA level <360 μmol/l at either the 3- or the 12-month follow-up (group 1 “achievers” n = 42) with those who did not (group 2 “non-achievers” n = 36). Achievers had significantly lower median SUA (403 vs 473 μmol/l, p = 0.02) and CRP (5.5 vs 23 mg/l, p = 0.04) levels at baseline. The mean glomerular filtration rate in the non-achiever subset was lower than in the achievers (65.1 vs 53.2 ml/min/m2, p = 0.06). The median allopurinol dose at 12-month follow-up was higher in the group of the achievers. (table 2, fig. 2).
Table 2 Comparison of serum uric acid target level achieved or not (and no concomitant flare at follow-up).
| Parameter |
Group 1
(3m or 12m FU SUA target achieved) |
Group 2
SUA target not achieved (at either FU time-point) |
p-value |
|---|---|---|---|
| Number of patients | 42 | 36 | |
| Median age (years) | 68 (16.3) | 70 (21.5) | 0.77* |
| Gender male | 35 (83.3%) | 34 (94.4%) | 0.17† |
| Median GFR (ml/min/1.73 m2) at BL | 65.1 (44.5) | 53.2 (43.4) | 0.06‡ |
| Median SUA (μmol/l) at BL | 403 (222.0) | 473 (153.3) | 0.02* |
| Median CRP (mg/l) at BL | 5.5 (16.7) | 23.0 (82.9) | 0.04* |
| Chronic gout | 40.5% | 61.1% | 0.11† |
| Median allopurinol dose at 12m FU | 300 mg/d (200) | 100 mg/d (200) | 0.03* |
| BL = baseline; CRP = C-reactive protein, FU = follow-up; GFR = glomerular filtration rate estimated using the MDRD short formula; MDRD = modification of diet in renal disease; SUA = serum uric acid level Continuous variables are represented as mean and standard deviation (SD) if normally distributed and as median and interquartile range (IQR) if not normally distributed, and categorical variables as frequency (%). Seventy-two patients attended the 3-month FU visits, 6 patients attended the 12-month FU but not the 3-month FU, thus FU data are available from 78 patients. * Mann Whitney Test, † Fisher’s exact test, ‡ unpaired t-test |
|||
Patients who were regularly followed up more than once (also outside of the study visits) at the rheumatology outpatient clinic (53% of patients) were more likely to achieve the SUA target at 12-month follow-up: of the 33 patients who achieved the SUA target, 22 (66.6%) were followed up in the rheumatology outpatient department; of the 25 patients who did not achieve the SUA target only 9 (36%) were followed up in the rheumatology outpatient department (p = 0.033).
In 24 patients without follow-up at 3 months (n = 8) and 12 months (n = 16), SUA serum levels could be obtained from hospital files and laboratory results sent by the caring physicians. Thus, we retrieved 16 additional SUA results, in total from 74 patients at the 12-month follow-up.
Patients attending follow-up and patients not attending follow-up visits did not differ regarding age or prevalence of comorbidities. There was a trend for a higher risk of loss to follow-up of women (n = 11, 61.1% without follow-up vs men, n = 26, 26.5%, p = 0.006). Pre-existing ULT was significantly more frequent in patients who attended follow-up visits (41.8%) than in those who did not (21.6%, p = 0.039). Mean SUA in the 16 patients without follow-up at 3 months was 367.3 versus 417.2 μmol/l in those attending follow-up (p = 0.30) and median SUA at 12-month follow-up was 345 vs 360 μmol/l, p = 0.390, respectively. Data on concomitant flares at the time of SUA measurement were not available in the patients without follow-up.
The longitudinal analysis of the patients included in our local prospective gout cohort at the University Hospital Basel showed a poor outcome of gout patients even in the era of T2T strategies [22]. Only half of the patients eventually reached the SUA target of 360 μmol/l at 12 months, and 50% of patients did not attend the follow-up visits. Data from the Mexican GRESGO cohort that also prospectively followed up gout patients showed that even a lower proportion of patients achieved the SUA target and GRESGO had a loss to follow-up of 50% [34]. Compared with ours, the GRESGO cohort included younger patients with a more often preserved kidney function. The epidemiological register studies from the UK [35] and the Netherlands [24] revealed that less than 50% of patients in general practice receive ULT. In Taiwan, with a high prevalence of gout, the proportion of patients with gout receiving ULT was even lower, at 23%, in 2015 [36]. The population of included patients in these register studies was most likely broader and therefore differed from our patients, and individual risk factors for failing to reach the SUA target, dosing of treatment as well as comorbidities were not assessed in these studies. Moreover, most of these register studies were cross-sectional.
When rheumatologists are involved in the care of gout patients, SUA targets are more often reached. Data from a Spanish transversal study suggest that the implementation of T2T guidelines eventually led to some progress in achieving the SUA target by rheumatologists (66% of patients compared with less than 50% of patients 5 years ago) [37]. A cohort study from Singapore, located at a rheumatology clinic, showed successful outcomes; 72% of the patients achieved the SUA target at 12 months [38]. This is in line with the observation that our patients followed up in the department of rheumatology more often reached the SUA target than those followed up elsewhere.
The high proportion of multimorbid elderly hospitalised patients in our local cohort highlights the increasing problem of decreased urate clearance due to renal impairment and the use of diuretics in an ever older population, which presents treatment obstacles [39]. Consequently, patients with a better renal function in our cohort had a trend to a higher likelihood of reaching the SUA target at follow-up.
Comorbidities were highly prevalent in our patients, and mortality during follow-up was high, with 12 deaths amongst 116 patients. Gout may be only one of several health problems, and polypharmacy may pose problems with adherence and medication interactions. Treatment is hampered by reduced kidney or liver function, or by real or feared medication interactions. The prescription of ULT such as allopurinol and the prescription of colchicine needs a good knowledge of medication side effects, limitations and interactions with other treatments. Moreover, colchicine is not licensed in Switzerland, and physicians may thus be reluctant to prescribe it. This may explain why patients regularly followed up at the department of rheumatology of the University Hospital Basel were more likely to achieve the SUA target than those who did not and were followed up elsewhere.
Although patients were invited to the follow-up visits, only half of them (n = 52) attended both follow-up visits. This is in line with the reported low treatment adherence of gout patients [40]. The proportion of loss to follow-up was higher in female than in male patients, and patients lost to follow-up were significantly less frequently receiving ULT at study inclusion. Patients already under ULT at inclusion may have received previous information from the prescriber and were hence potentially more sensitised to their disease. Successful gout management depends on the patient’s compliance and the physician’s expertise in gout management. To this end, educating patients about the disease and careful explanation of the management plan is essential. Many patients are insufficiently informed [41]. First studies with healthcare workers involved in the care of gout patients show that better information and repeated contact with the patients dramatically improve the outcome of gout [42].
The retrospectively collected SUA values from 32% of the patients who were lost to follow-up at 3 and 12 months did not differ significantly from those attending follow-up. However, we cannot exclude that the patients lost to follow-up and without available SUA measurements outside the study might have had a poorer adherence and hence a lower probability of achieving the SUA target. Moreover, SUA measurement during flares with falsely low SUA levels cannot be excluded.
Overall in the patients treated with allopurinol, the dose of ULT was increased reluctantly, presumably because of existing chronic kidney disease; dosages of allopurinol higher than 400 mg/d were prescribed for one patient only. The allopurinol dosage was adapted in less than a third of the patients between baseline and 3-month follow-up and less than half of them between 3- and 12-month follow-up. Flare prophylaxis was given to only one third of patients, and flares were frequent.
Insufficient dosing is still a problem, although treatment for patients with impaired renal function is available: for example, allopurinol and colchicine in adapted doses [43, 44] or febuxostat, a new xanthine oxidase inhibitor, which may be prescribed without dose adaption in patients with moderate renal insufficiency.
Continuous medical education about and adoption of a treat-to-target strategy (T2T) [22], as well as the development of new treatments, may help to more widely implement T2T strategies amongst general practitioners also.
There are limitations to this study. Only gout patients presenting at the rheumatology and internal medicine departments of a university hospital were included, probably leading to a sample of patients with severe gout. Since the majority of gout patients are treated by their general practitioner, future studies in primary care settings are also needed. Data relating to patient-reported outcomes, diet, and socioeconomics and other gout outcomes (e.g., number of flares), treatment costs, and drug safety were not assessed in this study. Studies have demonstrated considerable correlations between SUA and many of these. Thus, future prospective clinical studies should include such outcome domains to characterise potential differences between treatment strategies [31, 45, 46]. Statistical analysis of our data was limited because of the low number of patients compared with register studies. However, the detailed prospectively collected data within an observational study and the high number of patients with crystal-proven gout, in contrast to most of the existing data from cohort and register studies, allowed a detailed analysis of the factors associated with failure to reach therapeutic targets.
This study highlights the urgent need for an improvement in gout patient management. Solutions for the “gout problem” [47] are still needed.
We thank all the patients from the gout cohort and all involved physicians and study nurses from the Departments of Rheumatology, Internal Medicine, and the clinical trial unit at the University Hospital Basel.
The study was supported by a research grant from the Department of Medicine of the University Hospital Basel, the Hedwig Widmer Stiftung Zurich and the Freie Akademische Gesellschaft, Basel. CTB is supported by the Margot und Erich Goldschmidt & Peter René Jacobson-Stiftung.
The authors declare no conflict of interest.
1 Harris MD , Siegel LB , Alloway JA . Gout and hyperuricemia. Am Fam Physician. 1999;59(4):925–34.
2 Landgren AJ , Jacobsson LTH , Lindström U , Sandström TZS , Drivelegka P , Björkman L , et al. Incidence of and risk factors for nephrolithiasis in patients with gout and the general population, a cohort study. Arthritis Res Ther. 2017;19(1):173. doi:.https://doi.org/10.1186/s13075-017-1376-z
3 Ayoub I , Almaani S , Brodsky S , Nadasdy T , Prosek J , Hebert L , et al. Revisiting medullary tophi: a link between uric acid and progressive chronic kidney disease? Clin Nephrol. 2016;85(2):109–13. doi:.https://doi.org/10.5414/CN108663
4 Iacobellis G , Iacobellis G . A rare and asymptomatic case of mitral valve tophus associated with severe gouty tophaceous arthritis. J Endocrinol Invest. 2004;27(10):965–6. doi:.https://doi.org/10.1007/BF03347542
5 Khanna D , Tang S-J , Wallace WD , Roth BE , Hahn BH . Gouty tophi in a pancreatic pseudocyst. Arthritis Rheum. 2002;46(2):565–6. doi:.https://doi.org/10.1002/art.10067
6 Neogi T , Jansen TLTA , Dalbeth N , Fransen J , Schumacher HR , Berendsen D , et al. 2015 Gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2015;74(10):1789–98. doi:.https://doi.org/10.1136/annrheumdis-2015-208237
7 Kuo C-F , Grainge MJ , Zhang W , Doherty M . Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11(11):649–62. doi:.https://doi.org/10.1038/nrrheum.2015.91
8 Chen-Xu M , Yokose C , Rai SK , Pillinger MH , Choi HK . Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007-2016. Arthritis Rheumatol. 2019;71(6):991–9. doi:.https://doi.org/10.1002/art.40807
9 Krishnan E . Chronic kidney disease and the risk of incident gout among middle-aged men: a seven-year prospective observational study. Arthritis Rheum. 2013;65(12):3271–8. doi:.https://doi.org/10.1002/art.38171
10 Mohammed E , Browne LD , Kumar AUA , Adeeb F , Fraser AD , Stack AG . Prevalence and treatment of gout among patients with chronic kidney disease in the Irish health system: A national study. PLoS One. 2019;14(1):e0210487. doi:.https://doi.org/10.1371/journal.pone.0210487
11 Campion EW , Glynn RJ , DeLabry LO . Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82(3):421–6. doi:.https://doi.org/10.1016/0002-9343(87)90441-4
12 Scirè CA , Manara M , Cimmino MA , Govoni M , Salaffi F , Punzi L , et al.; KING Study Collaborators. Gout impacts on function and health-related quality of life beyond associated risk factors and medical conditions: results from the KING observational study of the Italian Society for Rheumatology (SIR). Arthritis Res Ther. 2013;15(5):R101. doi:.https://doi.org/10.1186/ar4281
13 Disveld IJM , Zoakman S , Jansen TLTA , Rongen GA , Kienhorst LBE , Janssens HJEM , et al. Crystal-proven gout patients have an increased mortality due to cardiovascular diseases, cancer, and infectious diseases especially when having tophi and/or high serum uric acid levels: a prospective cohort study. Clin Rheumatol. 2019;38(5):1385–91. doi:.https://doi.org/10.1007/s10067-019-04520-6
14 Kyu HH , Abate D , Abate KH , Abay SM , Abbafati C , Abbasi N , et al.; GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1859–922. doi:.https://doi.org/10.1016/S0140-6736(18)32335-3
15 Krishnan E , Baker JF , Furst DE , Schumacher HR . Gout and the risk of acute myocardial infarction. Arthritis Rheum. 2006;54(8):2688–96. doi:.https://doi.org/10.1002/art.22014
16 Grayson PC , Kim SY , LaValley M , Choi HK . Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2011;63(1):102–10. doi:.https://doi.org/10.1002/acr.20344
17 Rho YH , Lu N , Peloquin CE , Man A , Zhu Y , Zhang Y , et al. Independent impact of gout on the risk of diabetes mellitus among women and men: a population-based, BMI-matched cohort study. Ann Rheum Dis. 2016;75(1):91–5. doi:.https://doi.org/10.1136/annrheumdis-2014-205827
18 Kuo C-F , Grainge MJ , Mallen C , Zhang W , Doherty M . Comorbidities in patients with gout prior to and following diagnosis: case-control study. Ann Rheum Dis. 2016;75(1):210–7. doi:.10.1136/annrheumdis-2014-206410 https://doi.org/10.1136/annrheumdis-2014-206410
19 Hunter DJ , York M , Chaisson CE , Woods R , Niu J , Zhang Y . Recent diuretic use and the risk of recurrent gout attacks: the online case-crossover gout study. J Rheumatol. 2006;33(7):1341–5.
20 Rothenbacher D , Primatesta P , Ferreira A , Cea-Soriano L , Rodríguez LAG . Frequency and risk factors of gout flares in a large population-based cohort of incident gout. Rheumatology (Oxford). 2011;50(5):973–81. doi:.https://doi.org/10.1093/rheumatology/keq363
21 Perez-Ruiz F . Treating to target: a strategy to cure gout. Rheumatology (Oxford). 2009;48(Suppl 2):ii9–14. doi:.https://doi.org/10.1093/rheumatology/kep087
22 Kiltz U , Smolen J , Bardin T , Cohen Solal A , Dalbeth N , Doherty M , et al. Treat-to-target (T2T) recommendations for gout. Ann Rheum Dis. 2017;76(4):632–8. doi:.https://doi.org/10.1136/annrheumdis-2016-209467
23 Pascart T , Norberciak L , Ea H-K , Guggenbuhl P , Lioté F . GOSPEL 4 - Patients With Early-Onset Gout and Development of Earlier Severe Joint Involvement and Metabolic Comorbid Conditions: Results From a Cross-Sectional Epidemiologic Survey. Arthritis Care Res (Hoboken). 2019;71(7):986–92.
24 Janssen CA , Oude Voshaar MAH , Vonkeman HE , Krol M , van de Laar MAFJ . A retrospective analysis of medication prescription records for determining the levels of compliance and persistence to urate-lowering therapy for the treatment of gout and hyperuricemia in The Netherlands. Clin Rheumatol. 2018;37(8):2291–6. doi:.https://doi.org/10.1007/s10067-018-4127-x
25 Wright S , Chapman PT , Frampton C , O’Donnell JL , Raja R , Stamp LK . Management of Gout in a Hospital Setting: A Lost Opportunity. J Rheumatol. 2017;44(10):1493–8. doi:.https://doi.org/10.3899/jrheum.170387
26 von Elm E , Altman DG , Egger M , Pocock SJ , Gøtzsche PC , Vandenbroucke JP ; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi:.https://doi.org/10.1371/journal.pmed.0040296
27 Vandenbroucke JP , von Elm E , Altman DG , Gøtzsche PC , Mulrow CD , Pocock SJ , et al.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. doi:.https://doi.org/10.1371/journal.pmed.0040297
28 Wallace SL , Robinson H , Masi AT , Decker JL , McCarty DJ , Yü TF . Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20(3):895–900. doi:.https://doi.org/10.1002/art.1780200320
29 Choi HK , Burns LC , Shojania K , Koenig N , Reid G , Abufayyah M , et al. Dual energy CT in gout: a prospective validation study. Ann Rheum Dis. 2012;71(9):1466–71. doi:.https://doi.org/10.1136/annrheumdis-2011-200976
30 Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150.
31 Richette P , Doherty M , Pascual E , Barskova V , Becce F , Castañeda-Sanabria J , et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76(1):29–42. doi:.https://doi.org/10.1136/annrheumdis-2016-209707
32 Zhao T , Lv X , Cao L , Guo M , Zheng S , Xue Y , et al. Renal excretion is a cause of decreased serum uric acid during acute gout. Int J Rheum Dis. 2018;21(9):1723–7. doi:.https://doi.org/10.1111/1756-185X.13348
33 Urano W , Yamanaka H , Tsutani H , Nakajima H , Matsuda Y , Taniguchi A , et al. The inflammatory process in the mechanism of decreased serum uric acid concentrations during acute gouty arthritis. J Rheumatol. 2002;29(9):1950–3.
34 Alvarado-de la Barrera C , López-López CO , Álvarez-Hernández E , Peláez-Ballestas I , Gómez-Ruiz C , Burgos-Vargas R , et al. Are target urate and remission possible in severe gout? A five-year cohort study. J Rheumatol. 2019;181214. doi:.https://doi.org/10.3899/jrheum.181214
35 Kuo C-F , Grainge MJ , Mallen C , Zhang W , Doherty M . Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis. 2015;74(4):661–7. doi:.https://doi.org/10.1136/annrheumdis-2013-204463
36 Kuo C-F , Grainge MJ , See L-C , Yu K-H , Luo S-F , Zhang W , et al. Epidemiology and management of gout in Taiwan: a nationwide population study. Arthritis Res Ther. 2015;17(1):13. doi:.https://doi.org/10.1186/s13075-015-0522-8
37 Perez Ruiz F , Sanchez-Piedra CA , Sanchez-Costa JT , Andrés M , Diaz-Torne C , Jimenez-Palop M , et al. Improvement in Diagnosis and Treat-to-Target Management of Hyperuricemia in Gout: Results from the GEMA-2 Transversal Study on Practice. Rheumatol Ther. 2018;5(1):243–53. doi:.https://doi.org/10.1007/s40744-017-0091-1
38 Lim AY , Shen L , Tan CH , Lateef A , Lau TC , Teng GG . Achieving treat to target in gout: a clinical practice improvement project. Scand J Rheumatol. 2012;41(6):450–7. doi:.https://doi.org/10.3109/03009742.2012.689325
39 Abhishek A . Managing Gout Flares in the Elderly: Practical Considerations. Drugs Aging. 2017;34(12):873–80. doi:.https://doi.org/10.1007/s40266-017-0512-4
40 De Vera MA , Marcotte G , Rai S , Galo JS , Bhole V . Medication adherence in gout: a systematic review. Arthritis Care Res (Hoboken). 2014;66(10):1551–9. doi:.https://doi.org/10.1002/acr.22336
41 Coburn BW , Bendlin KA , Sayles H , Hentzen KS , Hrdy MM , Mikuls TR . Target Serum Urate: Do Gout Patients Know Their Goal? Arthritis Care Res (Hoboken). 2016;68(7):1028–35. doi:.https://doi.org/10.1002/acr.22785
42 Doherty M , Jenkins W , Richardson H , Sarmanova A , Abhishek A , Ashton D , et al. Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial. Lancet. 2018;392(10156):1403–12. doi:.https://doi.org/10.1016/S0140-6736(18)32158-5
43 Stamp LK , O’Donnell JL , Zhang M , James J , Frampton C , Barclay ML , et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412–21. doi:.https://doi.org/10.1002/art.30119
44 Wason S , Mount D , Faulkner R . Single-dose, open-label study of the differences in pharmacokinetics of colchicine in subjects with renal impairment, including end-stage renal disease. Clin Drug Investig. 2014;34(12):845–55. doi:.https://doi.org/10.1007/s40261-014-0238-6
45 Khanna D , Khanna PP , Fitzgerald JD , Singh MK , Bae S , Neogi T , et al.; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447–61. doi:.https://doi.org/10.1002/acr.21773
46 Qaseem A , Harris RP , Forciea MA ; Clinical Guidelines Committee of the American College of Physicians. Management of Acute and Recurrent Gout: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166(1):58–68. doi:.https://doi.org/10.7326/M16-0570
47 Rai SK , Choi HK , Choi SHJ , Townsend AF , Shojania K , De Vera MA . Key barriers to gout care: a systematic review and thematic synthesis of qualitative studies. Rheumatology (Oxford). 2018;57(7):1282–92. doi:.https://doi.org/10.1093/rheumatology/kex530
The study was supported by a research grant from the Department of Medicine of the University Hospital Basel, the Hedwig Widmer Stiftung Zurich and the Freie Akademische Gesellschaft, Basel. CTB is supported by the Margot und Erich Goldschmidt & Peter René Jacobson-Stiftung.
The authors declare no conflict of interest.