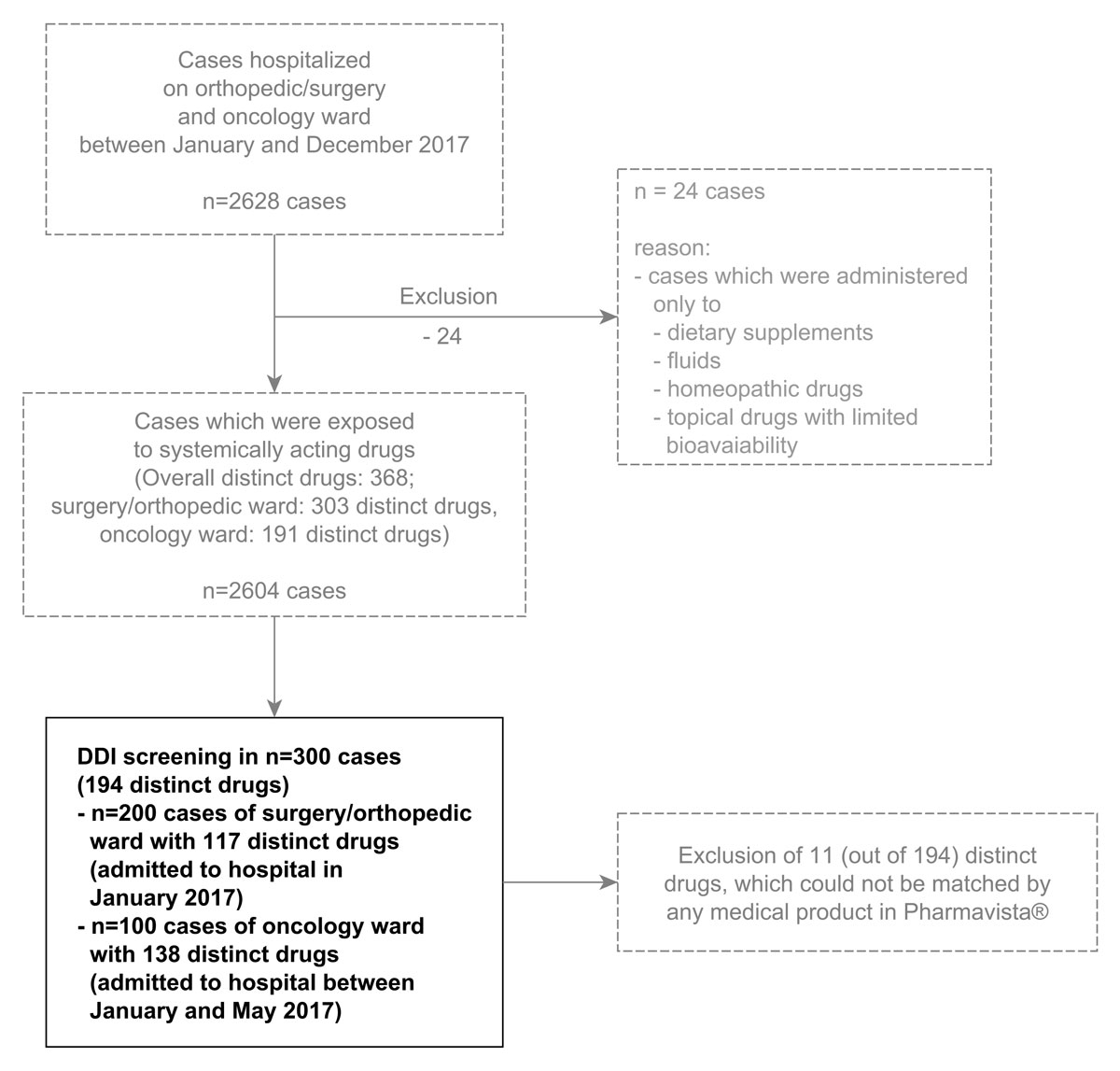
Figure 1 Selection of 300 patient cases for drug-drug interaction (DDI) screening. Cases were selected chronologically, starting from January 2017.
DOI: https://doi.org/10.4414/smw.2019.20103
Clinical decision support systems (CDSS) aim to increase the safety of a drug therapy [1, 2].They frequently contain an information module on co-medication with a drug-drug interaction (DDI) screening tool [1]. There are several DDI screening tools, with various severity ratings, specificities (exclusion of clinically irrelevant DDIs) and sensitivities (detection of clinically relevant DDIs that are associated with an increased risk of adverse drug reactions (ADRs)) on the market [2, 3]. Pharmavista® (HCI Solutions, AG, Bern, Switzerland) is a DDI screening tool that provides literature- and/or label-based monographs together with a classification of clinical relevance based on the ABDA interaction database (ABDATA Pharma-Daten-Service, Eschborn, Germany; http://abdata.de/datenangebot/abda-datenbank/interaktionen/). The tool performed well compared to similar tools with regard to the comprehensiveness of its monographs, as well as its specificity and sensitivity, on a test set of 60 drug pairs [3].
DDIs are considered an important risk factor for ADRs, and have been identified as the cause of 0.6% (range 0.1–3.7%) [4, 5] of hospital admissions. One percent (12 out of 1193) of paediatric cases with severe ADRs reported to a Canadian national spontaneous reporting system were caused by DDIs [6]. DDIs have also been associated with increases in the length [7, 8] and cost of hospitalisation [9], and have been found to be a frequent drug-related problem [10]. The major risk factor for DDIs appears to be polypharmacy (routine use of four or more drugs [11]), both in adults [12] and children [13, 14], along with genetic polymorphisms and the use of drugs with a narrow therapeutic index [15]. While polypharmacy increases with age, age alone seems to be an independent risk factor for DDI-induced ADRs [15]. This may be explained, at least in part, by age-related changes in pharmacokinetics (PK) and pharmacodynamics (PD) [15]. In children, developmental changes can also explain changes in the pharmacokinetics of DDIs with age [16].
DDIs are not always associated with ADRs, and different patients react differently to DDIs, which is why the term “potential DDI” is often used to describe them. “Potential DDIs” are DDIs which can – but do not always – cause ADRs. Potential DDIs have been estimated to occur in 3.8% of paediatric outpatients [17] and in 49–75% [18, 19] of paediatric inpatients (any grade). Potential DDIs with a “contraindicated” rating have been estimated to occur in 6% of paediatric patients in intensive care [18]. In adults, 4.6–31.6% [7, 20, 21] of hospitalised cases with potential DDIs have been associated with ADRs. To the best of our knowledge, the proportion of DDIs that have caused ADRs in children has not yet been studied.
Only some electronic interaction alerts are considered clinically relevant [10], depending on the applied screening tool [2] and the patient population [22]. For this reason, it has been recommended that institutions evaluate DDI screening tools for completeness (sensitivity), accuracy (specificity) and the risk of alert fatigue before their implementation [23]. The number of DDIs detected in children and their relevance may indeed differ from the number of DDIs observed in adults, as discussed above [15, 16]. Pharmavista® is a tool that may be being used increasingly in paediatric hospitals in Switzerland, but no data is currently available regarding its use in paediatrics.
The primary objective of this study was to evaluate the case-specific prevalence of high-risk DDIs in paediatric inpatients (drug combinations rated as “contraindicated” or “contraindicated by precaution” because of probable or possible severe consequences) according to the interaction tool Pharmavista®, since we believe that the prevalence of these alerts will be the primary factor driving alert fatigue. Further aims were to estimate the overall case-specific DDI prevalence (summarising DDIs of any grade) and the overall alert rate (prevalence per number of prescriptions), to assess the duration of the administration of drug combinations associated with a DDI warning, and to critically assess the appropriateness of high-risk DDI alerts in this setting.
This study was set up as a retrospective, single-centre study using routine electronic medical data from a cohort of paediatric patients hospitalised on the surgery/orthopaedic and oncology wards at the University of Basel Children’s Hospital (UKBB) in Basel, Switzerland between January and December 2017. The aim was to analyse 300 cases in total (two thirds from the surgery/orthopaedic ward and one third from the oncology ward, i.e. the first 200 and 100 cases of the year 2017). We hypothesised that the prevalence of high-risk DDIs would lie between 1% (surgery/orthopaedics) and 10% (oncology), and targeted a power of ≥91% (α = 0.05) to reject the null hypothesis (prevalence of high-risk DDIs = 5%) at the extremes of this range (two-sided one-sample test for proportion). Study approval was obtained from the local ethics committee of North-Western/Central Switzerland (EKNZ 2017-01729).
Data were obtained from electronic medical documentation (nursing documentation) in Phoenix (Version: 7.9.1-17, Compu Group Medical Schweiz AG). The following variables were extracted for each hospitalisation case: individual identifier number, date of hospitalisation, hospitalisation ward, medicines administered (medicinal product, dose, date, time and route of administration), and patient demographics, including gender, date of birth and weight. Active ingredients were added manually to medicinal products, which were recorded as free text (i.e. not standardised). Route of administration (also recorded as free text) was classified into systemic (including intravenous, intra-articular, intramuscular, intraperitoneal, intrathecal, jejunal, oral, via feeding tube, sublingual, rectal, subcutaneous, transdermal/cutaneous, vaginal, inhalation/nasal) and topical (including transdermal/cutaneous, local, ocular) administration. All patient cases with systemically administered drugs were included, while cases where only topically administered drugs with limited bioavailability, fluids, dietary supplements or homeopathic drugs were used were excluded (fig. 1).

Figure 1 Selection of 300 patient cases for drug-drug interaction (DDI) screening. Cases were selected chronologically, starting from January 2017.
Age, day and total length of hospitalisation, total daily drug dose and the number of distinct drugs given were calculated for each case from the extracted variables. Categorical variables were summarised by numbers (n) and percentages (%), continuous variables by their median, interquartile range (IQR) and overall range.
For each ward, the total number of prescribed drugs was calculated, and the frequencies of their administration were summarised.
DDI screening was performed in January and February 2018 using the free online version of Pharmavista® (available at www.compendium.ch). The product names of concomitantly (defined as the same day) administered drugs were entered for each case and day of hospital stay. Products which could not be identified by the screening tool were replaced, if possible, with another product name containing the same active ingredient. All DDIs detected were documented with their severity grading for each hospitalisation day per case, and were categorised into PK or PD interactions.
The prevalence of high-risk DDIs was calculated, with 95% confidence intervals (95%CI), as the number of cases with at least one DDI of the severity grade “contraindicated” or “contraindicated by precaution”. The overall DDI prevalence was calculated in a similar manner, including all six severity grades (“contraindicated”, “contraindicated by precaution”, “therapy monitoring/modification”, “therapy monitoring/modification in case of risk factors”, “therapy monitoring by precaution”, “no measures required”). The overall alert rate was calculated as
All variables were calculated for each ward separately and for the 300 cases in total.
A longitudinal summary of the DDIs detected during hospitalisation, the cumulative drug exposure and the cumulative DDI exposure was generated. For each drug combination associated with a DDI, the number of cases with this DDI and the duration of exposure were summarised.
In a post-hoc analysis, the correlation of the number of drugs given and the number of DDIs with the age and length of hospitalisation was investigated graphically, and summarised numerically by the non-parametric Spearman’s correlation coefficient (ρ) for each ward.
The amount of missing demographic data was summarised. The statistical analyses described above were performed using the free statistical software R (R Foundation for Statistical Computing, Vienna, Austria, www.R-project.org, version: 3.3.1 (2016-06-21)).
The clinical relevance of the high-risk DDIs detected was evaluated by a literature review on Medline (considering reviews, original research papers (clinical trials and observational/epidemiologic studies) and case reports, as well as pharmacovigilance analyses). In particular, we searched for estimates of the frequency and/or other quantifications of ADRs due to a particular DDI, as well as risk factors for ADRs due to that particular drug combination, with a focus on the patient’s age and the paediatric setting. The search terms used included drug names alone, drug names with or without the terms “interaction” and “drug-drug interaction”, and drug names with or without the specific expected ADR (all fields or MeSH terms). Paediatric investigations were searched by filtering for age (corresponding to searching for "infant"[MeSH Terms] OR "child"[MeSH Terms] OR "adolescent"[MeSH Terms]). References of relevant articles were also considered.
The 300 analysed cases included 200 from the surgery/orthopaedic ward (195 individuals, admitted to hospital in January 2017) and 100 from the oncology ward (43 individuals, admitted to hospital between January and May 2017). Overall, 23 cases were hospitalised repeatedly within this period (range: two to seven hospitalisations). Patient demographics are summarised in table 1. A total of 194 distinct drugs were prescribed and analysed (138 on the oncology ward, 117 on the surgery/orthopaedic ward, supplementary table S1 in appendix 1) out of 368 distinct drugs prescribed during the complete year 2017 (fig. 1). The median number of distinct drugs given per case was 5 (IQR 3–8) overall, with 7 (5–10) on the oncology ward and 4 (2–6) on surgery/orthopaedic ward. Figure 2A illustrates the number of drugs prescribed for each case and the hospitalisation day in a longitudinal manner.
Table 1 Patient demographics and summary of drug use and detected drug-drug interactions (DDI).
| Surgery/orthopaedic ward | Oncology | Both wards | |
|---|---|---|---|
| Analysed cases | 200 | 100 | 300 |
| Individuals | 195 | 43 | 238 |
| Female [number (%)] | 84 (43.1) | 18 (41.9) | 102 (42.9) |
| Male [number (%)] | 111 (56.9) | 25 (58.1) | 136 (57.1) |
| Age [years] median (IQR; range) |
9.5 (3.1–14.3; 0.0–26.7*) | 9.3 (3.5–14.3; 0.2–19.6*) | 9.5 (3.2–14.3; 0.0–26.7) |
| Weight [kg] median (IQR; range) |
26.0 (13.0–49.0; 3.0–109.0)† | 25.0 (15.5–35.0; 7.0–85.0)‡ | 25 (15–45; 3–109) |
| Length of hospitalisation [days] median (IQR; range) |
3 (2–6; 1–37) | 3 (2–8; 1–47) | 3 (2–6; 1–47) |
| Number of distinct drugs administered per case median (IQR; range) | 4 (2–6; 1–15) | 7 (5–10; 1–22) | 5 (3–8; 1–22) |
| Total number of prescribed drugs (active ingredients) | 117 | 138 | 194 |
| Drugs given in ≥10% of cases | Paracetamol, mefenamic acid, metamizole, midazolam, ketorolac, amoxicillin/clavulanic acid, nalbuphine, ibuprofen, enoxaparin, ondansetron, lidocaine/prilocaine |
Ondansetron, SMX/TMP, paracetamol, vincristine, mesna, metamizole, furosemide, cholecalciferol, esomeprazole, macrogol, methylprednisolone, prednisone, fluconazole, lidocaine/prilocaine, dexamethasone, ceftazidime, clemastine, sodium picosulfate | |
| Prevalence of high-risk DDIs [% (number of cases, 95% CI)] |
1.5 (3/200, 0.3–4.7%) |
2.0 (2/100, 0.3–7.7%) |
1.7 (5/300, 0.6–4.1%) |
| Overall prevalence of DDIs [% (number of cases, 95% CI)] | 11 (22/200, 7.2–16.4%) |
36 (36/100, 26.8–36.3%) |
15.7 (47/300, 11.8–20.4%) |
| Overall alert rate [% (number of drugs/number of DDIs, 95% CI)] | 2.9 (27/929, 2.0–4.3%) | 17.6 (134/761, 15.0–20.5%) | 9.5 (161/1690, 8.2–11.1%) |
| Total number of distinct DDIs | 21 | 71 |
CI = confidence interval; IQR = interquartile range; SMX/TMP = sulfamethoxazole + trimethoprim * Some patients who have reached adult age can exceptionally be treated in a paediatric hospital, depending on their medical condition. † 29 missing values, ‡ 6 missing values. Drugs printed in bold are classified as high-risk drugs which could harm patients with incorrect use (internal hospital guidelines).
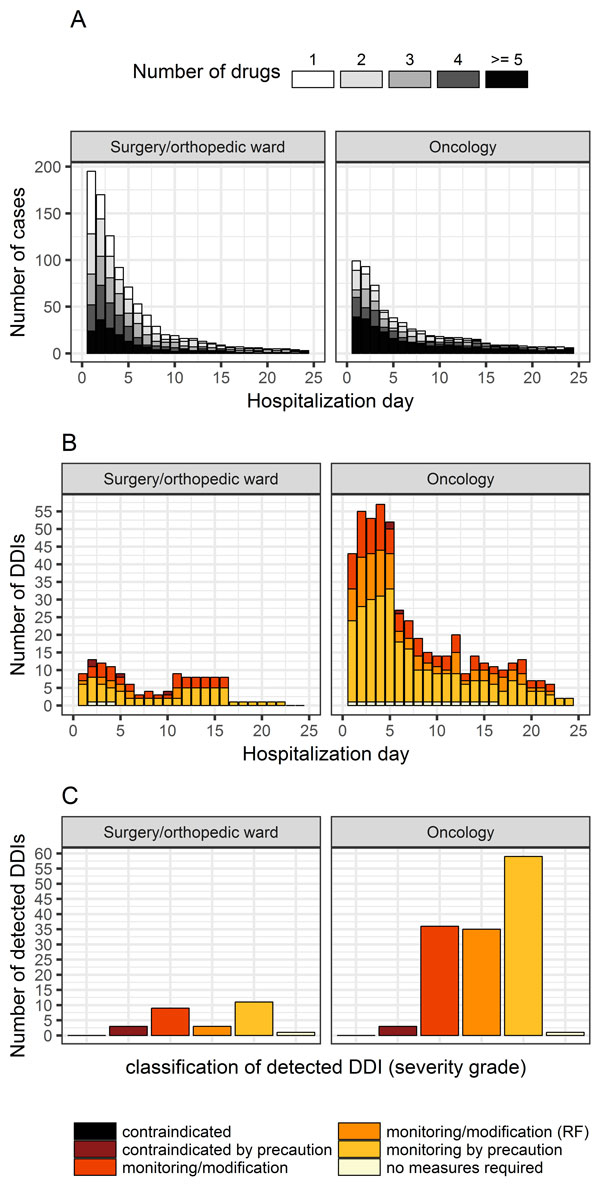
Figure 2 A: Daily drug exposure and B: Detected drug-drug interactions (DDIs) over the hospitalisation period. C: Distribution and classification of detected drug-drug interactions (DDIs) according to assigned severity grades. RF = therapy monitoring/modification in case of risk factors.
The drugs which were given to ≥10% of cases are summarised for each ward in table 1. Paracetamol, metamizole, and the combination of lidocaine + prilocaine can be found for both wards.
Thirty-one products could not be identified by Pharmavista®. Of these, 20 could be replaced with an alternative product containing the same active ingredient, while 11 products had to be excluded from the analysis (see table S2 in appendix 1).
Five out of the 300 cases analysed (2/100 from the oncology ward and 3/200 from the surgery/orthopaedic ward) were found to have ≥1 DDI (range 1–2) with a severity grading “contraindicated by precaution”, while no interaction rated as “contraindicated” was detected (estimated prevalence of high-risk DDIs 1.7%, 95% CI 0.6–4.1%; table 1). These drug combinations were tramadol/fentanyl/morphine with nalbuphine (n = 3 cases), droperidol + ondansetron (n = 1 case) and methotrexate + metamizole (n = 1 case). They were given for a duration of 1-2 days (table 2). Adverse drug events (ADEs) due to these three combinations (QT prolongation with the combination droperidol + ondansetron, reduced effect of opioid agonists with nalbuphine, and increased haematotoxicity with methotrexate-metamizole) were not documented in the patients’ medical records.
Table 2 Summary of the detected high-risk drug-drug interactions (DDIs, all pharmacodynamic interactions for drug combinations rated as “contraindicated by precaution”).
| Drug combination | Number of cases with DDI | Duration (days) | Effect of DDI | Ward |
|---|---|---|---|---|
| Ondansetron + droperidol | 1 | 2 | Increased risk of QT prolongation | Surgery /orthopaedic |
| Opioid agonist (tramadol, fentanyl, morphine) + nalbuphine | 3 | 1 | Reduced effect of the opioid-agonist / increased risk for withdrawal | Surgery / orthopaedic and oncology |
| Methotrexate + metamizole | 1 | 1 | Increased risk of haematoxicity | Oncology |
Drugs printed in bold are classified as high-risk drugs which could harm patients with incorrect use (internal hospital guidelines).
The overall prevalence of DDIs detected per patient-case (any grade) was 15.7% (11.8–20.4%), 36% (26.8–36.3%) on the oncology ward and 11% (7.2–16.4%) on the surgery/orthopaedic ward. The corresponding overall alert rates per number of prescriptions were 9.5% (8.2–11.1%) overall, 17.6% (134/761, 15.0–20.5%) on the oncology ward, and 2.9% (27/929, 2.0–4.3%) on the surgery/orthopaedic ward. Most of the detected DDIs were rated as “therapy monitoring by precaution”. Figures 2B and 2C illustrate the classification and distribution of the detected DDIs according to their severity grade for each ward. Figure 2B gives a longitudinal summary of the detected DDIs for each hospitalisation day. Most DDIs occurred on the second hospitalisation day on the surgery/orthopaedic ward, and on days 2–5 on the oncology ward.
A summary of all detected DDIs, with their explanation, is given in table 3 (pharmacokinetic interactions) and table 4 (pharmacodynamic interactions). The correlation of the number of drugs given and number of DDIs with age and the length of hospitalisation is illustrated in detail in supplementary figure S1 (appendix 1). Briefly, the number of drugs used was correlated with the length of hospitalisation (ρ = 0.61–0.71), but less correlated with age (ρ = 0.17–0.40). The number of DDIs was correlated with the number of drugs used (ρ = 0.35–0.63) and the length of hospitalisation (ρ = 0.27–0.55), but less correlated with age (ρ = 0.19–0.24).
Table 3 Summary of other pharmacokinetic drug-drug interactions (DDIs) issued (not rated as “contraindicated” or “contraindicated by precaution”).
|
Classification of DDI severity
Drug combination |
Duration
(days) |
Effect
(Mechanism) |
|---|---|---|
| Therapy monitoring/modification | ||
| Phenobarbital + lamotrigine | 8 | ↓ lamotrigine exposure (UGT induction by phenobarbital) |
| Rifampicin + metronidazole | 6 | ↓ metronidazole exposure (CYP3A4 induction by rifampicin) |
| Aprepitant + dexamethasone | 2-3 | ↑ dexamethasone exposure (CYP3A4 inhibition by aprepitant) |
| Fluconazole + vincristine, vindesine | 1–2 | ↑ vincristine/vindesine exposure (CYP3A4 inhibition by fluconazole) |
| Magaldrate + dexamethasone | 1 | ↓ dexamethasone exposure (reduced absorption) |
| Cholestyramine + ursodeoxycholic acid | 1 | ↓ ursodeoxycholic exposure (reduced absorption) |
| Methotrexate + amoxicillin | 1 | ↑ methotrexate exposure (competitive OAT binding) |
| Methotrexate + sulfamethoxazole/trimethoprim | 1 | ↑ methotrexate toxicity (reduced renal clearance + additive dihydrofolate inhibition) |
| Therapy monitoring/modification in case of risk factors | ||
| Paracetamol + carbamazepine, phenobarbital, rifampicin | 1–4 | ↑ paracetamol toxicity (increased toxic metabolite formation by CYP induction) |
| Fluconazole + budesonide/dexamethasone/ methylprednisolone/prednisone | 1–2 | ↑ steroid exposure (CYP3A4 inhibition by fluconazole) |
| Fluconazole + es-/omeprazole | 1–2 | ↑ es-/omeprazole exposure (CYP2C19 and -3A4 inhibition) |
| Miconazole + methylprednisolone | 1–3 | ↑ steroid exposure (CYP3A4 inhibition by azoles) |
| Therapy monitoring by precaution | ||
| Rifampicin + esomeprazole | 5 | ↓ esomeprazole exposure (induction of CYP450 enzymes) |
| Rifampicin + ondansetron | 5 | ↓ ondansetron exposure (CYP3A4 induction by rifampicin) |
| Oxcarbazepine + lamotrigine | 22 | ↓ lamotrigine exposure (UGT induction by oxcarbazepine) |
| Esomeprazole + diazepam | 11 | ↑ diazepam exposure (CYP2C19 inhibition by esomeprazole) |
| Furosemide + cephalosporins (cefepime, ceftazidime) | 1–4 | ↑ cephalosporin exposure (Probably reduced renal clearance) |
| Ciclosporin + steroids (budesonide, methylprednisolone, prednisone) | 7–10 | ↑ ciclosporin/steroid exposure ↑ risk of seizures |
| Methotrexate + esomeprazole | 1–2 | ↑ methotrexate exposure (competitive OAT binding) |
| Tacrolimus + steroids (methylprednisolone, prednisone) | 4–30 | ↑ or ↓ tacrolimus exposure |
| Tacrolimus + pantoprazole | 10–32 | ↑ tacrolimus exposure (possible CYP3A4 inhibition postulated) |
| Aprepitant + vincristine | 1 | ↑ vincristine exposure (CYP3A4 inhibition by aprepitrant) |
| No measures required | ||
| Ciclosporin + esomeprazole | 16 | Increased/decreased exposure of ciclosporin (unknown mechanism) |
OAT = organic ion transporter; UGT = UDP-glucuronosyltransferase Drugs printed in bold are classified as high-risk drugs which could harm patients with incorrect use (internal hospital guidelines).
Table 4 Summary of other pharmacodynamic drug-drug interactions (DDIs) issued (not rated as “contraindicated” or “contraindicated by precaution”).
|
Classification of DDI severity
Drug combination |
Duration
(days, range) |
Effect |
|---|---|---|
| Therapy monitoring/modification | ||
| Ibuprofen + steroids (hydrocortisone, betamethasone, methylprednisolone) | 1–4 | Increased risk of gastrointestinal bleeding |
| Opioids (morphine, fentanyl) + benzodiazepines (diazepam, midazolam) | 1 | Increased risk of sedation and respiratory depression |
| Ethanol + oxcarbazepine, Morphine, dimetinden | 1–2 | Increased sedation |
| ondansetron + clarithromycin, fluconazole, pentamidine diisethionate | 1–2 | Increased risk of QT prolongation/TdP |
| Tacrolimus + teicoplanin, vancomycin | 8 | Increased risk of nephrotoxicity |
| Amikacin + amphotericin B, furosemide | 1–4 | Increased risk of nephrotoxicity |
| Tacrolimus + enalapril maleate | 22 | Increased risk of hyperkalaemia |
| Ciclosporin + potassium chloride | 2 | Increased risk of hyperkalaemia |
| Lisinopril + trimethoprim | 1 | Increased risk of hyperkalaemia |
| Furosemide + oxcarbazepine | 1 | Increased risk of hyponatraemia |
| Cholecalciferol + hydrochlorothiazide | 21 | Increased risk of hypercalcaemia |
| Therapy monitoring/modification in case of risk factors | ||
| Amikacin + ceftazidime | 2–5 | Increased risk of nephrotoxicity |
| Diuretics (furosemide, hydrochlorothiazide) + proton pump inhibitor (omeprazole, esomeprazole, pantoprazole) | 1–21 | Increased risk of hypomagnesaemia |
| Furosemide + sodium picosulfate | 2 | Increased risk of hypokalaemia |
| Therapy monitoring by precaution | ||
| Ciclosporin + furosemide | 11 | Increased risk of nephrotoxicity and hyperuricaemia |
| Ciclosporin + trimethoprim | 9 | Increased risk of nephrotoxicity |
| Prednisone + vaccines (DTPP, M, P) | 1 | Increased risk of insufficient immunisation |
| Diuretics (hydrochlorothiazide, furosemide) + steroids (dexamethasone, methylprednisolone, prednisone) | 1–19 | Increased risk of hypokalaemia |
| Anticoagulants (heparin, enoxaparin) + cephalosporins (cefepime, cefazoline) | 3–7 | Increased bleeding risk |
| Tacrolimus + ondansetron | 2–4 | Increased risk of QT prolongation/TdP |
| Tacrolimus + pentamidine diisethionate | 1–2 | Increased risk of QT prolongation/TdP |
| Tramadol + ondansetron | 1 | Reduced analgesic effect of tramadol |
| No measures required | ||
| Ciprofloxacin + mefenamic acid | 3 | Increased seizure risk under quinolone treatment with NSAIDs |
DTPP = diphtheria-tetanus-pertussis-poliomyelitis vaccine; M = meningococcal C vaccine; NSAID = nonsteroidal anti-inflammatory drug; P = pneumococcal vaccine; TdP = torsades de pointes Drugs printed in bold are classified as high-risk drugs which could harm patients with incorrect use, drugs printed in italics are classified as high-risk drugs when used i.v. (potassium chloride) or at high concentrations (heparin >500 U/vial) (internal hospital guidelines).
The results of the literature review are presented as part of the discussion section.
In this study, we determined, for the first time in a paediatric setting, the prevalence of high-risk DDIs (drug combinations rated as “contraindicated” or “contraindicated by precaution”) according to the Swiss DDI tool Pharmavista®. The study population was patients hospitalised on a surgery/orthopaedic and an oncology ward of a Swiss children’s hospital. With our low prevalence, 1.7% of analysed patient cases (95% CI 0.6–4.1%, all “contraindicated by precaution”), we expect that over-alerting of contraindicated DDIs would not be a problem if Pharmavista® tool were to be implemented as a CDSS in a paediatric hospital. No severe ADEs were documented in the medical records of cases exposed to contraindicated DDIs. While the prevalence of contraindicated DDIs did not differ between the two wards analysed, the prevalence of DDIs of any grade was higher for the oncology ward (≥1 DDI in 36% of patient cases, 26.8–36.3%) than for the surgery/orthopaedic ward (≥1 DDI in 11% of cases, 7.2–16.4%). If active alerts were to be given for all DDIs independent of their severity grading, alerts would be expected to occur once for every 5–6 drugs prescribed on the oncology ward, but only once for every 33 drugs prescribed on the surgery/orthopaedic ward (alert rates 17.6% (15–21%) versus 2.9% (2.0–4.3%)). Therefore, the optimal severity level of active alerts may be chosen differently for different paediatric wards.
Critical evaluation of the severity grading of the detected contraindicated DDIs (all “by precaution”) suggests that some of these alerts may be downgraded in this setting. In fact, some of these drug combinations are routinely used in particular clinical situations, with favourable benefit-risk profiles reported.
According to Pharmavista®, the risk of cardiac arrhythmias (torsade de pointes, TdP) is increased when administering droperidol with other QT-prolonging agents such as ondansetron. The frequency of drug-induced TdP is estimated to be 1:100,000–1:1,000,000 for non-cardiac drugs, and is mainly dose dependent. Risk factors are advanced age, polypharmacy, female sex, electrolyte disorders (including hypokalaemia, hypocalcaemia and hypomagnesemia), existing heart diseases (including hypertension and tachyarrhythmia), inherited long QT syndrome and a history of QTc prolongation [24–27]. Both droperidol and ondansetron are rated as “drugs which prolong QT interval and/or can cause TdP”, and as “drugs which should be avoided in patients with existing congenital long QT syndrome” in CredibleMeds lists [28, 29].
Ondansetron plus low-dose droperidol is intentionally used, however, to prevent post-operative nausea and vomiting (PONV) after general anaesthesia. The risk of post-operative vomiting is approximately two-fold higher in children than in adults, and combination therapy for PONV is superior to single drug use [30, 31]. The QT interval prolongation of droperidol has been quantified as 37–59 ms under high-dose neuroleptic treatment (0.1–0.25 mg/kg for 75 kg bodyweight [BW]) [32], and 15–22 ms under low-dose treatment (0.008–0.017 mg/kg for 75 kg BW) in the context of PONV management [33]. The TdP risk for low-dose droperidol is controversial: no risk for ventricular tachycardia was found in studies where more than 20,000 patients treated for PONV management were analysed [34, 35], while a black box warning due to several cases with TdP and death, mostly at neuroleptic doses greater than 2.5 mg (0.33 mg/kg for 75 kg BW) was issued. In children, low droperidol doses (off-label) of 0.01-0.015 mg/kg as monotherapy and 0.015 mg/kg in combination with ondansetron 0.1 mg/kg (licensed dose for children >1 month) are recommended for PONV management [30]. For ondansetron, dose-dependent QT-prolongation has been quantified as 5.8–19.6 ms at doses of 8–32 mg (0.11–0.43 mg/kg for 75 kg BW) [36]. No or subadditive effects on the QTc interval (increase by 0–7 ms) have been described for droperidol combined with ondansetron in adult and paediatric studies [31, 37, 38], where QTc intervals did not exceed the normal limits and no cardiac arrhythmias occurred [31, 37]. Since increased age is a risk factor for QT prolongation (and thus TdP), children may be less vulnerable to the development of cardiac side effects under this drug combination compared to adults and elderly patients [25, 27]. No ECG to evaluate whether QT prolongation occurred was recorded in the medical record of our patient.
The administration of opioid mu-receptor antagonists, including the mixed opioid agonist and antagonist nalbuphine, has been shown to induce opioid withdrawal symptoms in methadone-dependent adults [39]. Opioid withdrawal can be highly uncomfortable, but is rarely life-threatening. Symptoms may include behavioural changes (anxiety, agitation, insomnia and tremor) and physiological signs (nausea, vomiting, diarrhoea, increased muscle tone, tachypnoea, fever, sweating, hypertension and tachycardia). Physical opioid dependence, and sometimes tolerance, can develop within 5–7 days of treatment. Risk factors for iatrogenic withdrawal symptoms are abrupt opioid cessation, rapid dose reduction, decreasing drug level (e.g. due to formulation or opioid change) and administration of an opioid antagonist [40].
In opioid naive patients however, opioid agonists and antagonists have been successfully combined to reduce gastrointestinal or dermal side effects (e.g. i.v. nalbuphine or naloxone), while nalbuphine did not reduce analgesic effects [41]. Also, randomised studies have shown that co-medication with nalbuphine can reduce the incidence of post-operative, morphine-induced pruritus in opioid-naive patients [42, 43], including children [44]. Neither reduced efficacy of opioid agonists nor withdrawal symptoms after combination with nalbuphine could be observed in several studies [41, 42, 45–47]. An additive analgesic effect was even noted in a randomised cohort trial when the drugs were given simultaneously, probably explained by the agonistic and hence additional analgesic effect of nalbuphine on the kappa receptor [45]. Neither withdrawal (including hypertension, sweating, nausea, vomiting, diarrhoea, fever [40]) nor reduced analgesic effects were documented in our patients, who were only exposed to nalbuphine in combination with an opioid agonist for one day. It is not clear whether the medications were combined intentionally.
The combination has been classified as “contraindicated by precaution” due to potential additive haematotoxic effects, especially in older adults. The incidence rate of metamizole-associated agranulocytosis has been estimated at 0.46–1.63 cases per million person-days of metamizole in Switzerland [48]. About 3.7–4% of case reports associated with metamizole-induced agranulocytosis were reported in patients less than 20 years old [48, 49]. Long-term use has been suggested as a risk factor [50]. The combination of methotrexate with metamizole was deemed related to fatal outcomes in four out of seven elderly patients [48]. Female gender, older age and triple blood cell line disorder were among the further risk factors for a fatal outcome [48]. Risk factors for methotrexate-associated haematotoxicity include high-dose treatment, poor renal function and omission of folic acid [51].
The combination methotrexate and metamizole has not been systematically evaluated for its potentially increased haematotoxic effects. Metamizole is not a first-line analgesic drug [52], but can be an interesting non-opioid analgesic with opioid-sparing effects [53], including in oncology [53], when paracetamol or NSAIDs show insufficient analgesic effects [52] or carry an additional risk of hepato-[54]/nephrotoxicity [55] and/or gastrointestinal bleeding [56]. Still, caution seems to be justified in paediatric patients co-treated with methotrexate, especially when duration of metamizole use is long [50].
Despite the low prevalence of contraindicated DDIs, we would not query the usefulness of a DDI screening tool when considering the large number of distinct drugs administered (table 1, fig. 1), the large number of drugs only occasionally prescribed for single cases, and the large number of potential DDIs of different severity grades (tables 1–3 ). While a reduction in the frequency and severity of ADEs after the implementation of an interaction screening tool could not be demonstrated in the ambulatory setting [20], the number of potential DDIs can be reduced significantly [57, 58], and awareness of clinically relevant DDIs can be improved by personal communication [59–61]. Bertsche et al. [60] also showed a reduction in ADEs in the intensive care setting under these circumstances. This suggests that the safety of drug therapy is increased when DDI tools are used as part of a multidisciplinary approach – not only or necessarily by reducing the number of DDIs, but also by appropriate dose modification and therapy monitoring.
Our prevalence of high-risk DDIs per case was close to the prevalences of 5% and 6% that were estimated in a large cohort of paediatric patients hospitalised on different medical wards [18, 19]. It appears to be smaller than the high-risk DDI prevalence in hospitalised adults when they were screened for DDIs by an earlier version of Pharmavista® [62]. Interestingly, the DDIs reported for adults using the same tool differ from those presented in tables 3 and 4 [62], emphasising the need for population-specific assessments. Our overall prevalence per case of both wards combined, 15.7% (11.8–20.4%), was low compared to other studies, which reported that 49-75% of paediatric admissions were associated with at least one DDI [18, 19].
Overall, more DDIs were identified on the oncology ward (36%, 26.8–36.3%) than the surgery/orthopaedic ward (11%, 7.2–16.4%), probably due to the higher number of different drugs administered per case and the generally low therapeutic index of most oncology drugs. Nevertheless, the overall prevalence of DDIs on the oncology ward of 36% may be considered surprisingly low. A prospective observational study in paediatric haemato-oncology patients <12 years old estimated a more than 1.5-fold higher prevalence of 60% [63]. This difference may be explained by differences in polypharmacy, because these children were exposed to a median total number of 13 drugs per treatment (compared to a median of only 7 in our case) [63]. Polypharmacy is indeed considered a main risk factor for the occurrence of DDIs and ADRs [13, 14]. This is also the case in cancer patients [64]. Furthermore, our study found a correlation between the number of drugs prescribed and the number of potential DDIs in a post-hoc analysis. The number of potential DDIs was also correlated with the length of hospitalisation and, to a degree, with age (fig. S1), possibly because these factors were associated with a higher number of prescribed drugs. In adults, age has been reported as associated with the number of DDIs and ADEs among older patients, probably due to age-dependent comorbidities and polypharmacy, and the reduced elimination capacity for many drugs in elderly patients [15].
The longitudinal summary of the DDIs detected over the hospitalisation period showed that most DDIs occurred on the second hospitalisation day on the surgery/orthopaedic ward, which is also the day on which the most medications were prescribed (figs 2A and 2B). The second day of hospitalisation was also the day of surgery. On the oncology ward, however, the number of DDIs rose during the first five days of hospitalisation, by which point the total number of prescribed drugs was already decreasing. This again suggests that not only the presence of polypharmacy, but also the interaction potential and the therapeutic index of drugs can be responsible for the occurrence of DDIs.
There are factors that may have led to an over- or underestimation of the frequency of DDIs. Dietary supplements could have interacted with other drugs, e.g. by reducing absorption due to complexation, but these were excluded from the analysis. Since we considered all drugs that were given on the same day to be prescribed concomitantly, drugs that were switched may have been falsely considered as combined. Also, interactions with enzyme inducers or inhibitors with a long half-life may have been missed. Given that the database of the Swiss screening tool Pharmavista® was not able to recognise unlicensed products, DDIs associated with such drugs could not be evaluated, which may be a limitation for its use, especially in a paediatric setting, where extemporaneous preparations are regularly used. Drug-excipient interactions may have been both falsely recorded and missed because we replaced unavailable drug formulations with alternative products containing the same active ingredient. Only pairwise DDIs are considered by the tool, and the severity grading of DDIs in which more than two drugs were involved may be underestimated. The generalisability of the results may be limited by the single centre nature of the study, and the fact that only two specific wards investigated.
The sensitivity of the DDI tool could not be assessed in this retrospective study. Taegtmeyer et al. found that 6 out of 153 (4%) clinically relevant DDIs were missed by Pharmavista® when compared to personal, clinical pharmacology assessments [10], suggesting that some relevant interactions may have been missed. Also, the tool was not compared with other DDI tools. Other authors have advocated the commercial software Drug Interaction Checker (Micromedex®) for the prevention of DDI-related ADRs [5].
In summary, the low prevalence of contraindicated DDIs suggests that Pharmavista® has a low risk of over-alerting when used in a Swiss paediatric hospital. Still, literature suggests that the severity ratings of DDIs rated as “contraindicated by precaution” could be partially downgraded in this setting, and that a few relevant interactions may be missing. This suggests the need for a patient/population-specific evaluation of DDI alerts, ideally using a multidisciplinary approach. Further investigations of the appropriateness of DDI alerts in a paediatric setting are needed, including the critical assessment of lower-level DDI alerts. Ideally, this would be done in a prospective manner to assess the effect of implementing such a CDSS on patient safety and outcomes.
Table S1A: Drugs administered on surgery/orthopaedic ward.
Table S1B: Drugs administered on the oncology ward.
Table S2: List of drugs which could not be identified by Pharmavista® and that could not be replaced by their active ingredient.
Figure S1: Correlation between age, number of prescribed drugs and number of DDIs and correlation between the length of hospitalization, number of prescribed drugs and number of DDIs.
The appendix is available as a separate file for downloading at: https://smw.ch/en/article/doi/smw.2019.20103/
The authors would like to thank Karin Meier and Mehmet Tümen for IT assistance with data extraction.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Carli-Ghabarou D , Seidling HM , Bonnabry P , Lovis C . A survey-based inventory of clinical decision support systems in computerised provider order entry in Swiss hospitals. Swiss Med Wkly. 2013;143:w13894 .https://doi.org/10.4414/smw.2013.13894
2 Vitry AI . Comparative assessment of four drug interaction compendia. Br J Clin Pharmacol. 2007;63(6):709–14 .https://doi.org/10.1111/j.1365-2125.2006.02809.x
3 Vonbach P , Dubied A , Krähenbühl S , Beer JH . Evaluation of frequently used drug interaction screening programs. Pharm World Sci. 2008;30(4):367–74 .https://doi.org/10.1007/s11096-008-9191-x
4 Becker ML , Kallewaard M , Caspers PW , Visser LE , Leufkens HG , Stricker BH . Hospitalisations and emergency department visits due to drug-drug interactions: a literature review. Pharmacoepidemiol Drug Saf. 2007;16(6):641–51 .https://doi.org/10.1002/pds.1351
5 Muhič N , Mrhar A , Brvar M . Comparative analysis of three drug-drug interaction screening systems against probable clinically relevant drug-drug interactions: a prospective cohort study. Eur J Clin Pharmacol. 2017;73(7):875–82 .https://doi.org/10.1007/s00228-017-2232-4
6 Carleton BC , Smith MA , Gelin MN , Heathcote SC . Paediatric adverse drug reaction reporting: understanding and future directions. Can J Clin Pharmacol. 2007;14(1):e45–57.
7 Bucşa C , Farcaş A , Cazacu I , Leucuta D , Achimas-Cadariu A , Mogosan C , et al. How many potential drug-drug interactions cause adverse drug reactions in hospitalized patients? Eur J Intern Med. 2013;24(1):27–33 .https://doi.org/10.1016/j.ejim.2012.09.011
8 Moura C , Prado N , Acurcio F . Potential drug-drug interactions associated with prolonged stays in the intensive care unit: a retrospective cohort study. Clin Drug Investig. 2011;31(5):309–16 .https://doi.org/10.1007/BF03256929
9 Moura CS , Acurcio FA , Belo NO . Drug-drug interactions associated with length of stay and cost of hospitalization. J Pharm Pharm Sci. 2009;12(3):266–72 .https://doi.org/10.18433/J35C7Z
10 Taegtmeyer AB , Kullak-Ublick GA , Widmer N , Falk V , Jetter A . Clinical usefulness of electronic drug-drug interaction checking in the care of cardiovascular surgery inpatients. Cardiology. 2012;123(4):219–22 .https://doi.org/10.1159/000343272
11World Health Organization. WHO Global Patient Safety Challenge 2017 [Internet]. 2017. Available from: http://apps.who.int/iris/bitstream/handle/10665/255263/WHO-HIS-SDS-2017.6-eng.pdf;jsessionid=C98EADFD3A4F92A60419136EFAAFB119?sequence=1
12 Johnell K , Klarin I . The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30(10):911–8 .https://doi.org/10.2165/00002018-200730100-00009
13 Knopf H , Du Y . Perceived adverse drug reactions among non-institutionalized children and adolescents in Germany. Br J Clin Pharmacol. 2010;70(3):409–17 .https://doi.org/10.1111/j.1365-2125.2010.03713.x
14 Smyth RMD , Gargon E , Kirkham J , Cresswell L , Golder S , Smyth R , et al. Adverse drug reactions in children--a systematic review. PLoS One. 2012;7(3):e24061 .https://doi.org/10.1371/journal.pone.0024061
15 Magro L , Moretti U , Leone R . Epidemiology and characteristics of adverse drug reactions caused by drug-drug interactions. Expert Opin Drug Saf. 2012;11(1):83–94 .https://doi.org/10.1517/14740338.2012.631910
16 Salem F , Rostami-Hodjegan A , Johnson TN . Do children have the same vulnerability to metabolic drug–drug interactions as adults? A critical analysis of the literature. J Clin Pharmacol. 2013;53(5):559–66 .https://doi.org/10.1002/jcph.13
17 Langerová P , Prokeš M , Konvalinka M , Fürstová J , Urbánek K . Incidence of potential drug interactions in medication prescriptions for children and adolescents in the University Hospital Olomouc, Czech Republic. Eur J Pediatr. 2013;172(5):631–8 .https://doi.org/10.1007/s00431-013-1933-7
18 Dai D , Feinstein JA , Morrison W , Zuppa AF , Feudtner C . Epidemiology of Polypharmacy and Potential Drug-Drug Interactions among Pediatric Patients in Intensive Care Units of U.S. Pediatr Crit Care Med. 2017;17(5):1–18.
19 Feinstein J , Dai D , Zhong W , Freedman J , Feudtner C . Potential drug-drug interactions in infant, child, and adolescent patients in children’s hospitals. Pediatrics. 2015;135(1):e99–108 .https://doi.org/10.1542/peds.2014-2015
20 Glassman PA , Belperio P , Lanto A , Simon B , Valuck R , Sayers J , et al. The utility of adding retrospective medication profiling to computerized provider order entry in an ambulatory care population. J Am Med Inform Assoc. 2007;14(4):424–31 .https://doi.org/10.1197/jamia.M2313
21 Marengoni A , Pasina L , Concoreggi C , Martini G , Brognoli F , Nobili A , et al. Understanding adverse drug reactions in older adults through drug-drug interactions. Eur J Intern Med. 2014;25(9):843–6 .https://doi.org/10.1016/j.ejim.2014.10.001
22 Phansalkar S , Desai AA , Bell D , Yoshida E , Doole J , Czochanski M , et al. High-priority drug-drug interactions for use in electronic health records. J Am Med Inform Assoc. 2012;19(5):735–43 .https://doi.org/10.1136/amiajnl-2011-000612
23 Shah VS , Weber RJ , Nahata MC . Contradictions in contraindications for drug-drug interactions. Ann Pharmacother. 2011;45(3):409–11 .https://doi.org/10.1345/aph.1P792
24 Kannankeril PJ . Understanding drug-induced torsades de pointes: a genetic stance. Expert Opin Drug Saf. 2008;7(3):231–9 .https://doi.org/10.1517/14740338.7.3.231
25 Trinkley KE , Page RL, 2nd , Lien H , Yamanouye K , Tisdale JE . QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin. 2013;29(12):1719–26 .https://doi.org/10.1185/03007995.2013.840568
26 Anderson HN , Bos JM , Haugaa KH , Morlan BW , Tarrell RF , Caraballo PJ , et al. Phenotype of Children with QT Prolongation Identified Using an Institution-Wide QT Alert System. Pediatr Cardiol. 2015;36(7):1350–6 .https://doi.org/10.1007/s00246-015-1164-y
27 Letsas KP , Efremidis M , Kounas SP , Pappas LK , Gavrielatos G , Alexanian IP , et al. Clinical characteristics of patients with drug-induced QT interval prolongation and torsade de pointes: identification of risk factors. Clin Res Cardiol. 2009;98(4):208–12 .https://doi.org/10.1007/s00392-008-0741-y
28CredibleMeds®. Combined list of drugs that prolong QT and/or cause Torsade de Pointes (TdP). 2018 Available at: https://crediblemeds.org/pdftemp/pdf/CombinedList.pdf
29CredibleMeds®. Drugs to be avoided by congenital long QT patients. 2018. Available at: https://crediblemeds.org/pdftemp/pdf/DrugsToAvoidList.pdf
30 Gan TJ , Diemunsch P , Habib AS , Kovac A , Kranke P , Meyer TA , et al.; Society for Ambulatory Anesthesia. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118(1):85–113 .https://doi.org/10.1213/ANE.0000000000000002
31 Chan MTV , Choi KC , Gin T , Chui PT , Short TG , Yuen PM , et al. The additive interactions between ondansetron and droperidol for preventing postoperative nausea and vomiting. Anesth Analg. 2006;103(5):1155–62 .https://doi.org/10.1213/01.ane.0000239223.74552.0a
32Swissmedic. Arzneimittelinformation: Droperidol.
33 White PF , Song D , Abrao J , Klein KW , Navarette B . Effect of low-dose droperidol on the QT interval during and after general anesthesia: a placebo-controlled study. Anesthesiology. 2005;102(6):1101–5 .https://doi.org/10.1097/00000542-200506000-00007
34 Nuttall GA , Malone AM , Michels CA , Trudell LC , Renk TD , Marienau MES , et al. Does low-dose droperidol increase the risk of polymorphic ventricular tachycardia or death in the surgical patient? Anesthesiology. 2013;118(2):382–6 .https://doi.org/10.1097/ALN.0b013e31827dde8d
35 Habib AS , Gan TJ . Pro: The Food and Drug Administration Black box warning on droperidol is not justified. Anesth Analg. 2008;106(5):1414–7 .https://doi.org/10.1213/ane.0b013e31816ba463
36Swissmedic. Arzneimittelinformation: Ondansetron.
37 Mehta D , Sanatani S , Whyte SD . The effects of droperidol and ondansetron on dispersion of myocardial repolarization in children. Paediatr Anaesth. 2010;20(10):905–12 .https://doi.org/10.1111/j.1460-9592.2010.03408.x
38 Charbit B , Alvarez JC , Dasque E , Abe E , Démolis JL , Funck-Brentano C . Droperidol and ondansetron-induced QT interval prolongation: a clinical drug interaction study. Anesthesiology. 2008;109(2):206–12 .https://doi.org/10.1097/ALN.0b013e31817fd8c8
39 Preston KL , Bigelow GE , Liebson IA . Antagonist effects of nalbuphine in opioid-dependent human volunteers. J Pharmacol Exp Ther. 1989;248(3):929–37.
40 Galinkin J , Koh JL ; Committee on Drugs; Section On Anesthesiology and Pain Medicine; American Academy of Pediatrics. Recognition and management of iatrogenically induced opioid dependence and withdrawal in children. Pediatrics. 2014;133(1):152–5 .https://doi.org/10.1542/peds.2013-3398
41 Wang J-J , Ho S-T , Tzeng J-I . Comparison of intravenous nalbuphine infusion versus naloxone in the prevention of epidural morphine-related side effects. Reg Anesth Pain Med. 1998;23(5):479–84.
42 Chen MK , Chau SW , Shen YC , Sun YN , Tseng KY , Long CY , et al. Dose-dependent attenuation of intravenous nalbuphine on epidural morphine-induced pruritus and analgesia after cesarean delivery. Kaohsiung J Med Sci. 2014;30(5):248–53 .https://doi.org/10.1016/j.kjms.2014.01.001
43 Ganesh A , Maxwell LG . Pathophysiology and management of opioid-induced pruritus. Drugs. 2007;67(16):2323–33.
44 Kubica-Cielińska A , Zielińska M . The use of nalbuphine in paediatric anaesthesia. Anaesthesiol Intensive Ther. 2015;47(3):252–6.
45 Yeh YC , Lin TF , Lin FS , Wang YP , Lin CJ , Sun WZ . Combination of opioid agonist and agonist-antagonist: patient-controlled analgesia requirement and adverse events among different-ratio morphine and nalbuphine admixtures for postoperative pain. Br J Anaesth. 2008;101(4):542–8 .https://doi.org/10.1093/bja/aen213
46 van Niel JCG , Schneider J , Tzschentke TM . Efficacy of Full µ-Opioid Receptor Agonists is not Impaired by Concomitant Buprenorphine or Mixed Opioid Agonists/Antagonists - Preclinical and Clinical Evidence. Drug Res (Stuttg). 2016;66(11):562–70 .https://doi.org/10.1055/s-0042-109393
47 Yeh YC , Lin TF , Chang HC , Chan WS , Wang YP , Lin CJ , et al. Combination of low-dose nalbuphine and morphine in patient-controlled analgesia decreases incidence of opioid-related side effects. J Formos Med Assoc. 2009;108(7):548–53 .https://doi.org/10.1016/S0929-6646(09)60372-7
48 Blaser LS , Tramonti A , Egger P , Haschke M , Krähenbühl S , Rätz Bravo AE . Hematological safety of metamizole: retrospective analysis of WHO and Swiss spontaneous safety reports. Eur J Clin Pharmacol. 2015;71(2):209–17 .https://doi.org/10.1007/s00228-014-1781-z
49 Stammschulte T , Ludwig WD , Mühlbauer B , Bronder E , Gundert-Remy U . Metamizole (dipyrone)-associated agranulocytosis. An analysis of German spontaneous reports 1990-2012. Eur J Clin Pharmacol. 2015;71(9):1129–38 .https://doi.org/10.1007/s00228-015-1895-y
50 Ziesenitz VC , Erb TO , Trachsel D , van den Anker JN . Safety of dipyrone (metamizole) in children-What’s the risk of agranulocytosis? Paediatr Anaesth. 2018;28(2):186–7 .https://doi.org/10.1111/pan.13312
51 Mori S , Hidaka M , Kawakita T , Hidaka T , Tsuda H , Yoshitama T , et al. Factors associated with myelosuppression related to low-dose methotrexate therapy for inflammatory rheumatic diseases. PLoS One. 2016;11(4):e0154744 .https://doi.org/10.1371/journal.pone.0154744
52 Messerer B , Grögl G , Stromer W , Jaksch W . [Pediatric perioperative systemic pain therapy: Austrian interdisciplinary recommendations on pediatric perioperative pain management]. Schmerz. 2014;28(1):43–64 .https://doi.org/10.1007/s00482-013-1384-0
53 Gaertner J , Stamer UM , Remi C , Voltz R , Bausewein C , Sabatowski R , et al. Metamizole/dipyrone for the relief of cancer pain: A systematic review and evidence-based recommendations for clinical practice. Palliat Med. 2017;31(1):26–34 .https://doi.org/10.1177/0269216316655746
54 Lancaster EM , Hiatt JR , Zarrinpar A . Acetaminophen hepatotoxicity: an updated review. Arch Toxicol. 2015;89(2):193–9 .https://doi.org/10.1007/s00204-014-1432-2
55 Zapater P , Llanos L , Barquero C , Bellot P , Pascual S , Carnicer F , et al. Acute effects of dipyrone on renal function in patients with cirrhosis: a randomized controlled trial. Basic Clin Pharmacol Toxicol. 2015;116(3):257–63 .https://doi.org/10.1111/bcpt.12312
56 Laporte J-R , Ibáñez L , Vidal X , Vendrell L , Leone R . Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents. Drug Saf. 2004;27(6):411–20 .https://doi.org/10.2165/00002018-200427060-00005
57 Moura CS , Prado NM , Belo NO , Acurcio FA . Evaluation of drug-drug interaction screening software combined with pharmacist intervention. Int J Clin Pharm. 2012;34(4):547–52 .https://doi.org/10.1007/s11096-012-9642-2
58 Halkin H , Katzir I , Kurman I , Jan J , Malkin BBO . Preventing drug interactions by online prescription screening in community pharmacies and medical practices. Clin Pharmacol Ther. 2001;69(4):260–5 .https://doi.org/10.1067/mcp.2001.114228
59 Salili AR , Hammann F , Taegtmeyer AB . [Preventing adverse drug events using clinical decision support systems]. Ther Umsch. 2015;72(11-12):693–700 .https://doi.org/10.1024/0040-5930/a000739
60 Bertsche T , Pfaff J , Schiller P , Kaltschmidt J , Pruszydlo MG , Stremmel W , et al. Prevention of adverse drug reactions in intensive care patients by personal intervention based on an electronic clinical decision support system. Intensive Care Med. 2010;36(4):665–72 .https://doi.org/10.1007/s00134-010-1778-8
61 Bertoli R , Bissig M , Caronzolo D , Odorico M , Pons M , Bernasconi E . Assessment of potential drug-drug interactions at hospital discharge. Swiss Med Wkly. 2010;140:w13043 .https://doi.org/10.4414/smw.2010.13043
62 Oertle M . Frequency and nature of drug-drug interactions in a Swiss primary and secondary acute care hospital. Swiss Med Wkly. 2012;142:w13522 .https://doi.org/10.4414/smw.2012.13522
63 Fernández de Palencia Espinosa MA , Díaz Carrasco MS , Fuster Soler JL , Ruíz Merino G , De la Rubia Nieto MA , Espuny Miró A . Pharmacoepidemiological study of drug-drug interactions in onco-hematological pediatric patients. Int J Clin Pharm. 2014;36(6):1160–9 .https://doi.org/10.1007/s11096-014-0011-1
64 van Leeuwen RWF , Jansman FGA , van den Bemt PMLA , de Man F , Piran F , Vincenten I , et al. Drug-drug interactions in patients treated for cancer: a prospective study on clinical interventions. Ann Oncol. 2015;26(5):992–7 .https://doi.org/10.1093/annonc/mdv029
No financial support and no other potential conflict of interest relevant to this article was reported.