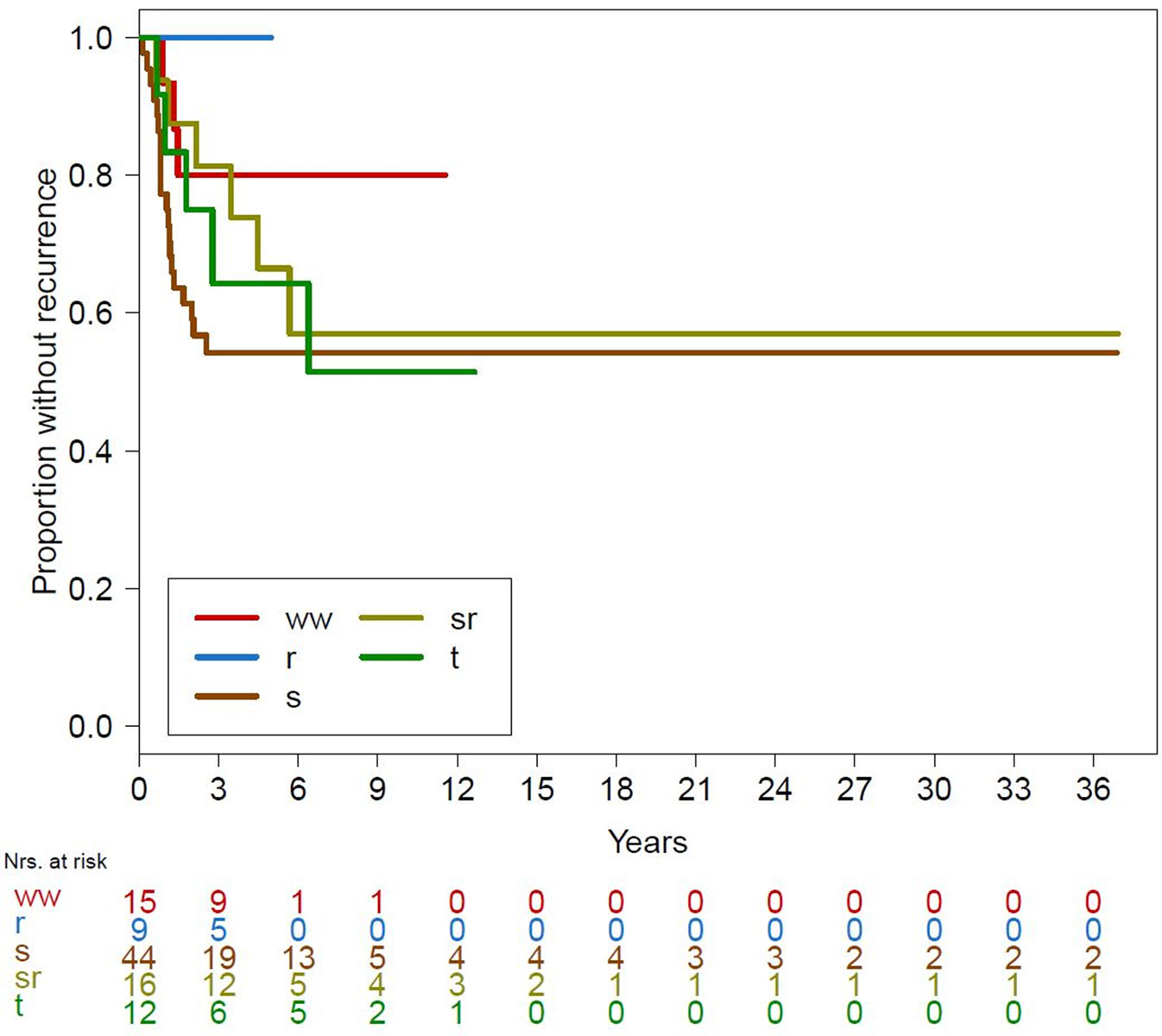
Figure 1 Kaplan-Meier curves for recurrence over all treatment groups.
ww = watchful waiting; r = radiation alone; s = surgery alone; sr = surgery combined with radiation; t = systemic treatment
DOI: https://doi.org/10.4414/smw.2019.20107
Desmoid tumours are rare and histologically benign but locally aggressive neoplasms characterised by infiltrative growth and a tendency towards local recurrence [1].
The pathophysiology of these tumours is not yet fully understood. Genetic, endocrine and physical factors play a role in their development and growth [2]. Growth factors expressed as a result of trauma (including surgery) may contribute to growth and/or recurrence [3].
Desmoid tumours mainly affect individuals between 10 and 40 years of age. Extra-abdominal desmoid tumours arise in the extremities, head, neck and trunk, and the thoracic and abdominal walls [1, 4, 5]. Spontaneous regression may also be observed [4, 5]. Considering the wide variation in clinical presentation and the unpredictable natural history of the disease, an individualised treatment approach and interdisciplinary input from a tumour centre are recommended [6].
Desmoid tumours create a treatment dilemma. In the past, wide or radical local excision was the most common treatment. However, the risk of recurrence was high (>40%) [7–11]. Furthermore, the extent of the correlation between positive margins and recurrence is controversial [6, 12–14]. Finally, there is a paucity of good data on the natural history of the disease with, or especially without treatment [9].
The aim of this study was to estimate the recurrence rates in patients diagnosed with extra-abdominal desmoid tumours according to treatment modality. The primary outcome was the recurrence rate of desmoid tumours. In patients who did not undergo tumour removal, disease progression was assessed.
We retrospectively enrolled 96 patients (61 female, 35 male) diagnosed with extra-abdominal desmoid tumours at the Sarcoma Centres of Basel, Bern, Zurich or Lausanne. The study was approved by the Ethics Committees of Northwest and Central Switzerland (approval 2014/172). The committee waived the need to obtain written informed consent from all patients. Inclusion criteria were follow-up of at least 24 months and age at diagnosis of ≥16 years. Exclusion criteria were intra-abdominal desmoid tumours, retroperitoneal tumours and patients with incomplete data (n = 24 patients).
Initial and subsequent treatment, follow-up status with recurrence rates, and time to an event were recorded for five different treatment modalities. Final follow-up status was defined as recurrence of the desmoid tumour versus no evidence of disease. In patients treated non-surgically, disease progression was considered as “recurrence”. The five treatment modalities were surgery without radiation (n = 44), surgery with radiation (n = 16), radiation without surgery (n = 9), systemic treatment (n = 12), and watchful waiting (n = 15). In the case of watchful waiting, no treatment commenced unless disease progression was observed.
All histological diagnoses were made and/or confirmed by a reference pathologist at one of the sarcoma centres (DB, BB).
Recurrence was analysed using Cox proportional hazards models, implementing Firth’s penalised likelihood bias reduction due to the low number of events in some of the groups [15, 16]. Kaplan-Meier curves were used to show the recurrence over time. Patients without recurrence were censored at their last follow-up. The time to an event for each patient started on the date of the first treatment. In patients with no active treatment (i.e., watchful waiting), the time started on the date of diagnosis. Only the first recurrence was considered as an endpoint in our analysis. Further treatments and recurrences were ignored.
The watchful waiting group was used as the reference group. First, the watchful waiting group was compared to the largest groups, those of surgery or surgery with radiation patients. Secondly, the association between tumour location and recurrence rate was examined in surgery (or surgery with radiation) patients. Finally, in patients who underwent active treatment (i.e., not watchful waiting), rate of recurrence was compared between radiation patients and patients who had received other treatments (surgery, surgery + radiation or systemic).
Descriptive statistics were used to summarise demographics and baseline patient data. Comparisons of baseline values between patients who suffered recurrence and those who did not were based on Wilcoxon’s rank-sum test for continuous variables and Fisher’s exact test for categorical variables.
All analyses were performed using R, version 3.3.1. A p value <0.05 was considered significant.
Baseline characteristics are summarised in table 1. Mean age at diagnosis was 38.9 years and median follow-up was 8.4 years (range 2.0–44.5 years). At the last follow-up, 92 patients (95.8%) were alive and 4 patients (4.2%) had died for unrelated reasons. Age at diagnosis was a significant factor for recurrence: recurrence was more frequently seen in younger patients (p = 0.028). The median follow-up time in patients without recurrence was 4.6 years (range 1.8–37 years). A summary of the follow-up times for groups without recurrence is given in table 2.
Table 1 Summary of patient data.
| Overall | No recurrence | Recurrence | p-value | ||
|---|---|---|---|---|---|
| No. | 96 | 62 | 34 | ||
| Sex = female, n (%) | 61 (63.5) | 36 (58.1) | 25 (73.5) | 0.184 | |
| Age at diagnosis, median (IQR) | 36.5 (27.8–49.0) | 40.00 (29.25–53.50) | 32.00 (25.00–42.50) | 0.028 | |
| Site, n (%) | Abdomen | 16 (16.7) | 13 (21.0) | 3 (8.8) | 0.184 |
| Extremities | 45 (46.9) | 28 (45.2) | 17 (50.0) | ||
| Pelvis | 7 (7.3) | 3 (4.8) | 4 (11.8) | ||
| Thoracic wall | 10 (10.4) | 7 (11.3) | 3 (8.8) | ||
| Trunk | 18 (18.8) | 11 (17.7) | 7 (20.6) | ||
| Diagnosis method, n (%) | Computed tomography | 9 (10.0) | 4 (6.8) | 5 (16.1) | 0.343 |
| Magnetic resonance imaging | 78 (86.7) | 53 (89.8) | 25 (80.6) | ||
| Ultrasound | 3 (3.3) | 2 (3.4) | 1 (3.2) | ||
| Beta-catenin = yes, n (%) | 44 (88.0) | 27 (84.4) | 17 (94.4) | 0.399 | |
| Treatment, n (%) | Watchful waiting | 15 (15.6) | 12 (19.4) | 3 (8.8) | 0.053 |
| Radiation alone | 9 (9.4) | 9 (14.5) | 0 (0.0) | ||
| Surgery alone | 44 (45.8) | 24 (38.7) | 20 (58.8) | ||
| Surgery and radiation | 16 (16.7) | 10 (16.1) | 6 (17.6) | ||
| Systemic treatment | 12 (12.5) | 7 (11.3) | 5 (14.7) | ||
IQR = interquartile range Comparison between patients with and without recurrence using Fisher’s exact test for categorical variables and Wilcoxon’s rank-sum test for continuous variables.
Table 2 Follow-up times (years) in patients with no recurrence, by treatment group.
| Minimum | 1st quartile | Median | Mean | 3rd Quartile | Maximum | |
|---|---|---|---|---|---|---|
| Watchful waiting | 2.43 | 2.89 | 3.37 | 4.29 | 4.74 | 11.55 |
| Radiation alone | 1.88 | 2.21 | 3.25 | 3.29 | 4.60 | 4.96 |
| Surgery alone | 2.00 | 3.19 | 6.30 | 9.25 | 8.27 | 36.88 |
| Surgery and radiation | 2.98 | 4.66 | 6.22 | 10.29 | 11.44 | 36.91 |
| Systemic treatment | 1.98 | 2.98 | 7.60 | 6.66 | 9.20 | 12.66 |
In cases with surgery (with or without radiation, n = 46), resection margins were wide in 16 patients, marginal in 24 patients, and intralesional in 6 patients. Of the 16 patients treated with both surgery and radiation, the dose was unknown in 5 cases; the other 11 (68.8%) received a median dose of 54 Gy (range 25–60 Gy). Patients treated with radiation only received a median dose of 52 Gy (range 46–60 Gy). The dose was unknown in two cases.
Eight out of the 12 patients who received systemic treatment (t) received tamoxifen only. Three patients received tamoxifen in combination with celecoxib and sulindac. In one case, a combination of methotrexate and vinblastine was administered.
Local recurrence occurred in 34 patients (35.4%), and 62 patients (64.6%) showed no recurrence or progression. The time to recurrence varied from 2 months to 6.4 years. In patients with recurrence or progression after non-surgical treatment, 44.1% (15 out of 34) had more than one recurrence. No recurrences (or disease progression) were recorded in the radiation group. Results with regards to recurrence for the different treatment modalities are shown in figure 1. The overall recurrence rate was <50%.

Figure 1 Kaplan-Meier curves for recurrence over all treatment groups.
ww = watchful waiting; r = radiation alone; s = surgery alone; sr = surgery combined with radiation; t = systemic treatment
Hazard ratios (HRs) for recurrence show that with the exception of the radiation group, the HRs for all other groups were higher (>1) than that of the watchful waiting group (table 3). The HR was lowest in the radiation group (no events). None of these differences were statistically significant. The overall clinical observation times differed significantly between treatment groups (p <0.001).
Table 3 Analysis of recurrence rates for different treatment modalities according to treatment group
| Treatment | No recurrence | Recurrence | HR | 95% CI | p-value |
|---|---|---|---|---|---|
| Watchful waiting | 12 (80.0) | 3 (20.0) | Reference | ||
| Radiation alone | 9 (100.0) | 0 (0.0) | 0.23 | 0.00–2.39 | 0.252 |
| Surgery alone | 24 (54.5) | 20 (45.5) | 2.31 | 0.83–8.70 | 0.113 |
| Surgery and radiation | 10 (62.5) | 6 (37.5) | 1.56 | 0.44–6.58 | 0.495 |
| Systemic treatment | 7 (58.3) | 5 (41.7) | 1.93 | 0.51–8.37 | 0.328 |
Overall incidence (%) reported with hazard ratios (HR) and 95% confidence intervals (CI) calculated from a Cox-proportional hazard regression implementing Firth’s penalised likelihood bias reduction due to the low number of events.
The observation time was significantly shorter in the radiation group compared to the surgery and surgery with radiation groups (p = 0.005 and p = 0.078, respectively). Furthermore, the observation time was shorter in the watchful waiting group than in the surgery group (figs 1 and 2 ).
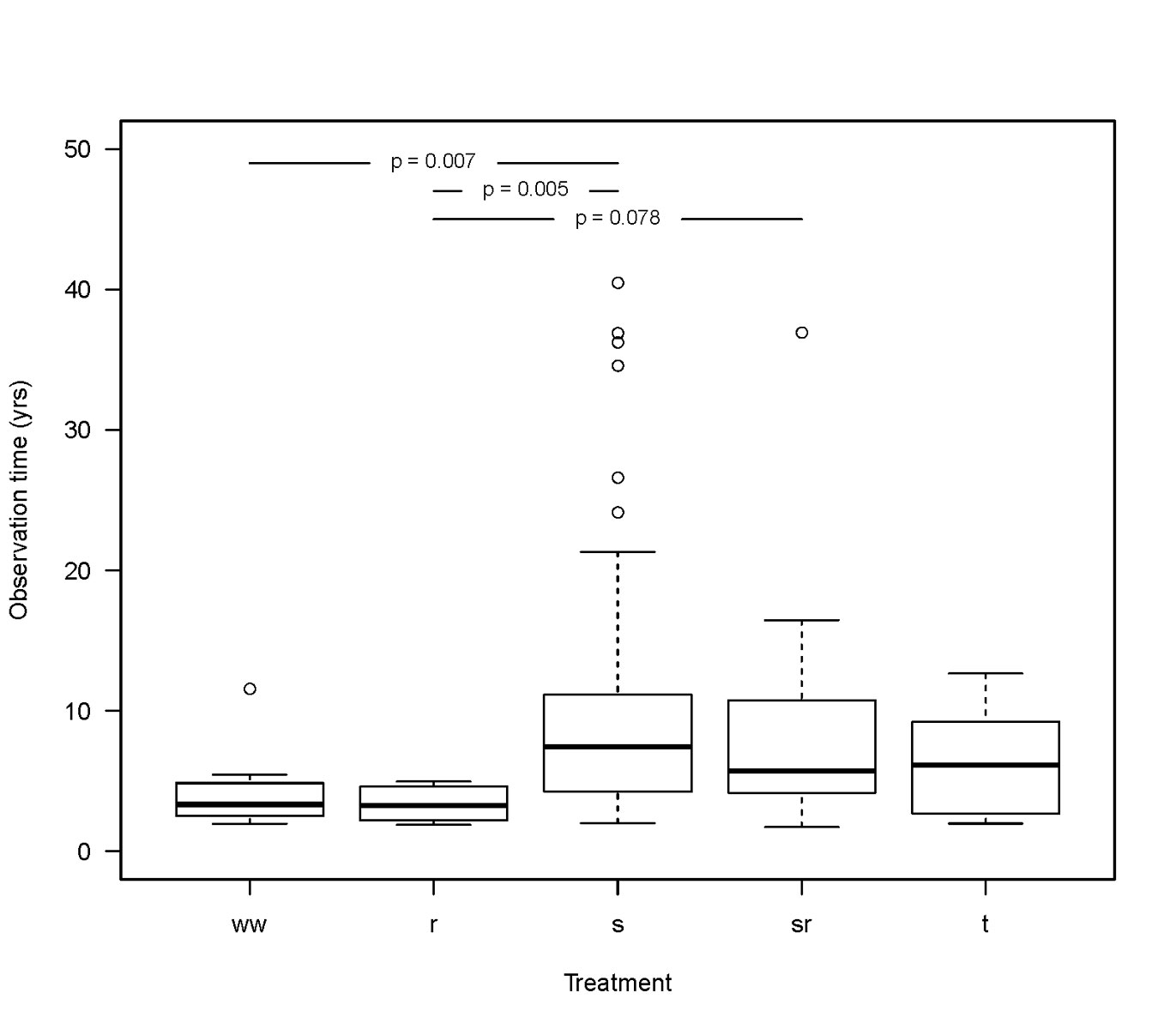
Figure 2 Boxplot shows that the observation time was shorter in the radiation treatment group compared to the surgery and surgery with radiation groups (marginally significant differences). The observation time in the watchful waiting group was shorter than in the surgery group.
ww = watchful waiting; r = radiation alone; s = surgery alone; sr = surgery combined with radiation; t = systemic treatment
Tumour location was not a significant factor for recurrence (p = 0.547). No significant differences in the HR of recurrence were found between different tumour sites (HR 1.27, 95% confidence interval [CI] 0.58–2.75).
After primary surgery (n = 44), 45.4% (n = 20) of patients developed recurrence, with a mean time to recurrence of 1.1 years (range 0.14-2.5). Thirty-seven patients with known resection margins underwent surgery without radiation. Recurrence occurred in 30.8% (n = 4) of patients with wide resection (n = 13), compared to 50.0% (n = 10) of those with marginal resection (n = 20) and 100.0% (n = 4) of those with intralesional resection (n = 4). Resection margins were unknown in seven patients, and the recurrence rate of these cases was 28.6% (n = 2). The recurrence rate was significantly higher in the intralesional resection group (p = 0.021). No significant difference was recorded between patients with marginal and wide resection. The overall likelihood ratio of the effect of surgical margins was not statistically significant (p = 0.086) (fig. 3).
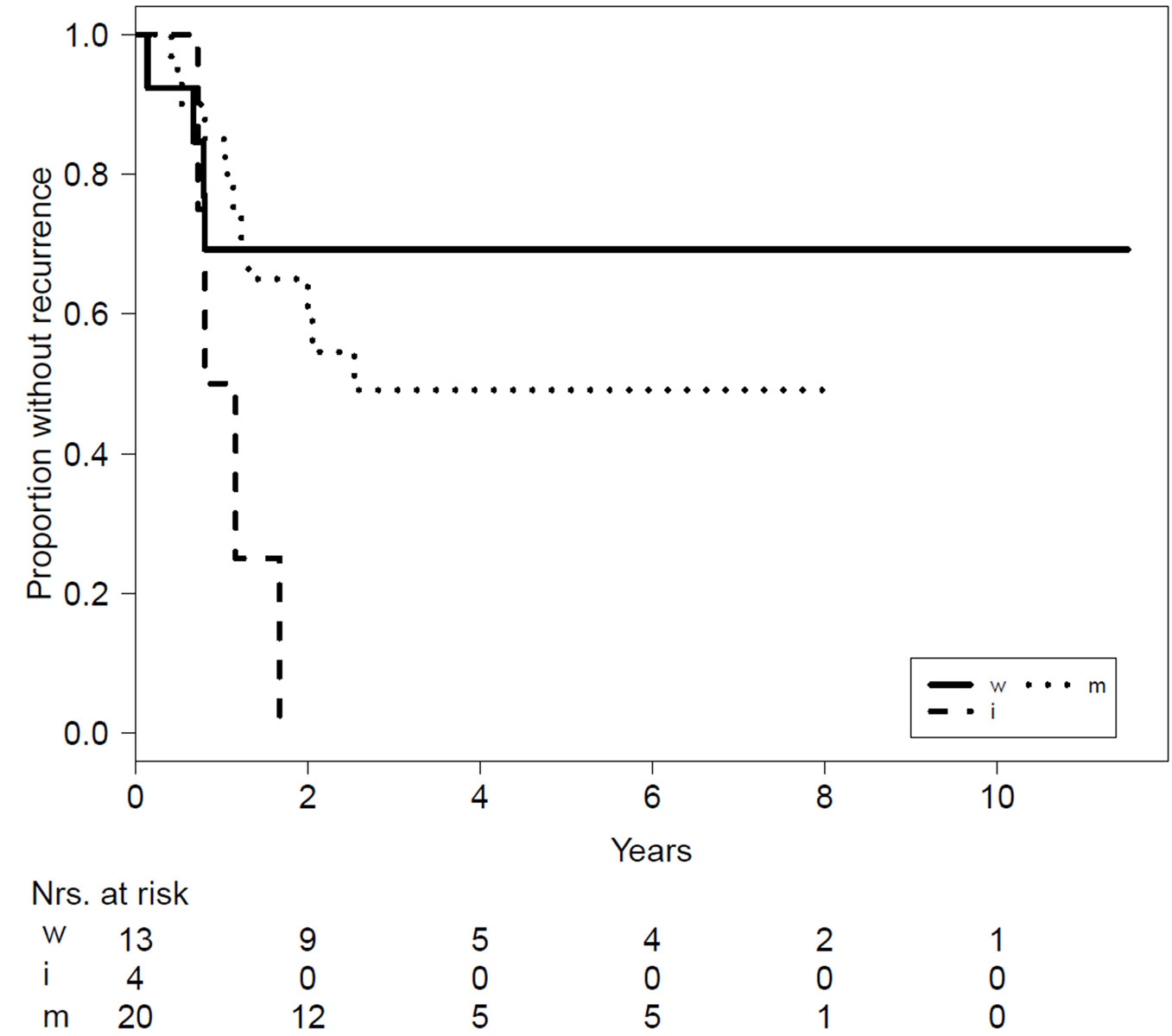
Figure 3 Kaplan-Meier curves for recurrence comparing the different surgical margins (). Table shows the overall incidence (%) reported, with hazard ratios (HR) and 95% confidence intervals (CI) calculated from a Cox-proportional hazard regression.
w = wide; m = marginal; i = intralesional
Patients with recurrence (n = 20) received the following additional treatments, with different re-recurrence rates (RRR): 13 patients received surgery alone (RRR 69.2%, n = 9), three patients received surgery plus radiation (RRR 33.3%, n = 1), three patients received radiation alone (RRR 33.3%, n = 1), and one patient was treated with tamoxifen (RRR 100.0%, n = 1).
In the surgery with radiation group (n = 16), 37.5% (n = 6) had recurrent desmoid tumours, with a mean time to recurrence of 3.1 years (range 0.88–5.8). Recurrence occurred in 33.3% (n = 1) of patients with wide resection (n = 3), compared to 36.4% (n = 4) of patients with marginal resection (n = 11) and 50.0% (n = 1) of patients with intralesional resection (n = 2). One patient with marginal initial resection developed radiation-induced secondary sarcoma after 12 years.
Patients with recurrences (n = 6) received the following additional treatments, with different RRR: three patients received surgery alone (RRR 0.0%, n = 0), two patients underwent systemic treatment (tamoxifen and imatinib plus sulindac, respectively; RRR 100%, n = 2), and one patient had watchful waiting (RRR 100%, n = 1).
All patients treated with radiation alone (n = 9) had regressive (33.3%, n = 3) or stable (66.6%, n = 6) disease at the last follow-up, with no progression reported.
A comparison of the recurrence rates of radiation patients with the recurrence rates with all other treatment methods except watchful waiting showed a significant HR (HR 8.46, 95% CI 1.19–1071.58; p = 0.027).
In surgery patients (n = 12), 41.7% (n = 5) showed progression, 33.3% (n = 4) had stable disease, and 25.0% (n = 3) showed regression. Mean time to progression was 2.5 years (range 0.67-6.4).
Patients with progression (n = 5) received the following additional treatments, with different outcomes: one patient underwent surgery with wide resection (no evidence of disease after 9.2 years), one patient underwent surgery with intralesional resection (stable disease after 1.7 years), two patients received radiation therapy (stable disease after 1.1 years and lost to follow-up), and one patient had systemic treatment (stable disease after 4.2 years).
In watchful waiting patients (n = 15), 60.0% had stable disease (n = 9), 20.0% had spontaneous regression (n = 3; fig. 4), and 20.0% showed progression (n = 3). Mean follow-up was 4.1 years (range 2.0–11.5) and mean time to progression was 1.2 years (range 0.9–1.5).
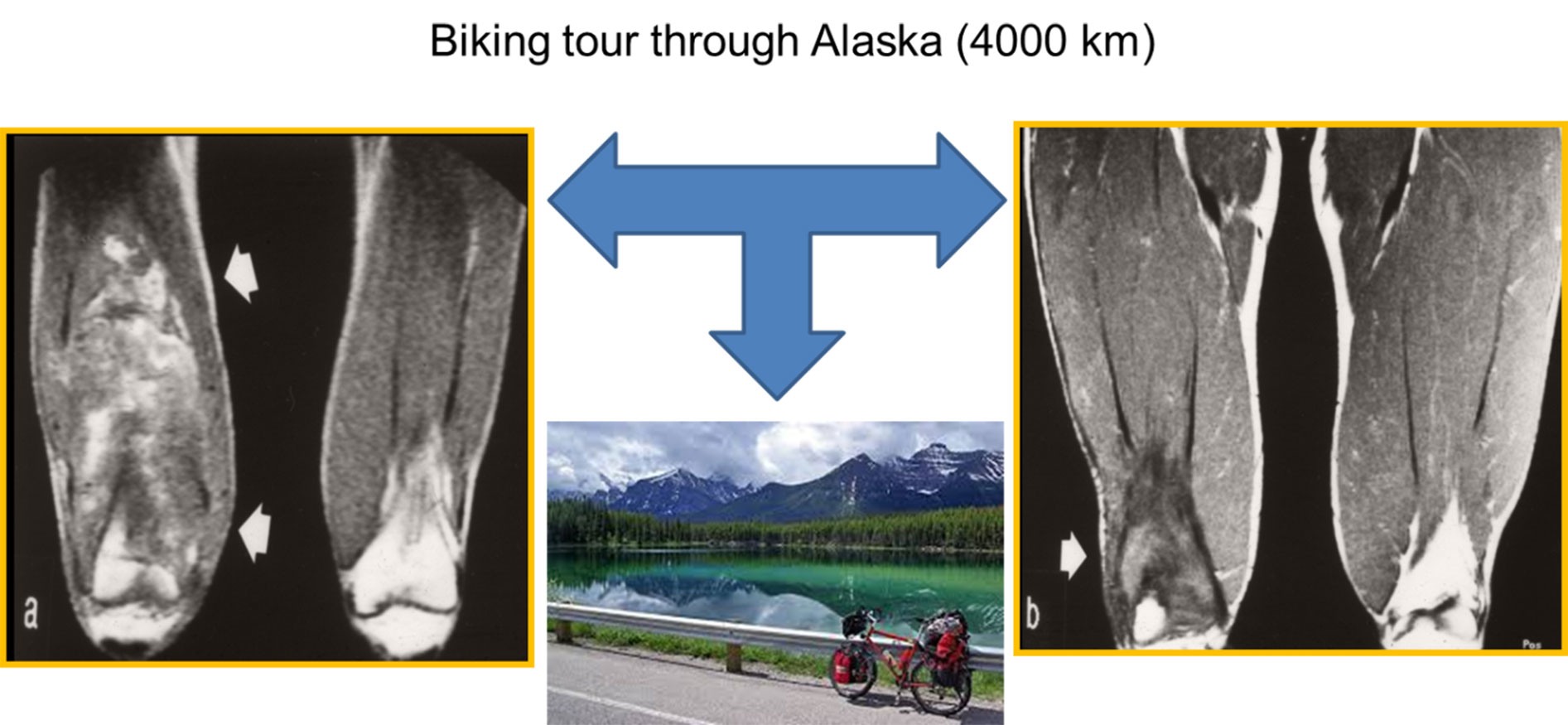
Figure 4 Example of spontaneous regression. An 18-year-old male athlete with a very large desmoid tumour located over the whole quadriceps muscles of the right thigh (a) with severe pain and the request for surgery with significant functional impact. Before surgery, he fulfilled a wish to ride his bike through Alaska, and the follow-up showed a significant decrease in the tumour size (b) and symptoms, removing the necessity for surgery.
Patients with progression (n = 3) received the following further treatments, with different outcomes: one patient underwent surgery plus radiation (no evidence of disease after 4.0 years), and of the two patients for whom subsequent treatment was unknown, one showed stable disease (after 1.6 years) and the other showed progression again (after 0.6 years).
The aim of this study was to estimate the recurrence rates in patients diagnosed with extra-abdominal desmoid tumours according to treatment modality. Data beginning in 1970 were collected and patients were followed for varying time periods, up to >40 years. The primary outcome was the recurrence/disease progression of the desmoid tumour.
The results show an overall recurrence rate of 35.4% (34 of 96), which contrasts with previously reported local recurrence rates of 44-68% [10–14]. The rate of re-recurrence, however, was 44.12% (15 of 34), within this range. The shortest time to recurrence was two months, likely due to incomplete resection.
We found no significant differences in the HR for recurrence between different tumour sites. Although location was not identified as an independent prognostic factor, outcomes did vary between different locations in another study, which could be attributed to the close proximity to vital structures and the difficulty of resection [17]. There was a clear association between site and local recurrence-free survival (LRFS) in a large study, with the best outcome (5-year LRFS of 90%) for abdominal wall tumours, followed by chest wall (72%), upper extremity (60%), lower extremity (47%), and desmoids of the neck (43%) [18].
Regarding the impact of surgical margins on recurrence rates, a significant increase in HR was found for the intralesional treatment group, whereas the difference in HR between the marginal resection and wide resection groups was not significant. This finding agrees with other reports, which show a lack of any correlation between positive margins and recurrence [9, 16–18]. Other studies with more than 100 patients also reported a lack of correlation. Thus, complete surgical clearance does not prevent recurrence, nor does incomplete resection necessarily lead to recurrence. However, other results are contradictory, as the impact of margins depends on a multitude of factors [6, 12–14].
Not all tumours recur. In fact, the observed spontaneous stabilisation or occasional regression corresponds with previous observations [7, 8].
No recurrences were observed in the radiation group (0 out of 9). However, this result could be biased, given the low number of patients. In addition, the follow-up time for radiation patients was considerably shorter than for the patients on other treatment regimens (see fig. 2), and so recurrences could have been missed.
Post-operative radiotherapy is an option for the treatment of desmoid tumours, especially in patients with positive margins after surgery, after recurrence, or for limb preservation. Moreover, the recurrence rate was lower in the surgery with radiation group than in the surgery group. Radiotherapy can be a sufficient adjuvant treatment option in patients with positive margins [15, 16]. The negative side effects (e.g., secondary sarcomas) must be considered, however, especially in young patients [16, 19]. One of our patients developed a radiation-induced sarcoma following treatment of a desmoid tumour.
Although the number of systemic treatment patients was small and the results were not promising, chemotherapy represents an efficient therapeutic option. Potential side effects of the secondary treatment must be considered, however [20].
Results for alternative systemic treatment options such as anti-oestrogen therapy were encouraging, with 10/13 responding to tamoxifen + sulindac in familial adenomatous polyposis-associated desmoid tumours, and 3/3 in sporadic tumours [21].
Although the HR of recurrence / progression were generally higher in all groups other than the radiation and watchful waiting groups, none of the differences were statistically significant. The relatively small sample size and the low event rate limited the study’s power to identify significant effects. These biases could be overcome with a larger cohort, but this might be difficult within Switzerland because of the low prevalence of desmoid tumours. Nevertheless, the tendency towards low progression rates in the watchful waiting group encourages a watchful waiting approach with careful observation [4, 17]. This approach has been recommended by others, who have shown progression-free survival rates of 50% after five years [22–25].
Local recurrence of desmoid tumours is frequent, but has been inconsistently associated with the adequacy of surgical excision. Attempts to achieve tumour-free resection margins may result in significant morbidity. Despite the lack of metastatic potential, desmoid tumours may prove fatal due to the local effects of growth, especially in the head and neck. Because the timing of diagnosis is unknown in relation to disease progression, an initial observation period may be considered in these patients, especially when surgery could result in significant loss of function. Thus, quality of life should be the focus of treatment decisions. Misunderstanding of the watchful waiting approach can be avoided by detailed briefing of patients, as well as by continuous care to avoid loss to follow-up [6]. A stepwise consensus algorithm was proposed recently, based on expertise from the sarcoma community, the European Organisation for Research and Treatment of Cancer’s Soft Tissue and Bone Sarcoma Group, patients’ advocates from Sarcoma Patients EuroNet, and available evidence [6].
This study has several limitations. It was retrospective, and a lower level of evidence was accepted for clinical decision-making owing to the rarity and long natural history of the disease. This leads to reduced statistical power and to reduced estimate precision. Secondly, outcome measures were based on data collected at four different Swiss Sarcoma Centres over the last 40 years, and the quality of recordkeeping and decision-making has changed over time. Moreover, the study cohort and follow-up times for different treatment groups were heterogeneous. However, the prevalence of desmoid tumours was low and – although 24 patients were excluded – a respectable number of patients were included in the analysis. Although limited, our results support existing evidence that watchful waiting should be followed for desmoid tumours.
The study was financially supported by “Tauchen gegen Krebs”, www.tauchengegenkrebs.ch.
All authors declare that they have no conflict of interest.
1Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, eds. WHO Classification of Tumours of Soft Tissue and Bone, Fourth edition. Lyons: IARC Press; 2013.
2 Okuno S . The enigma of desmoid tumors. Curr Treat Options Oncol. 2006;7(6):438–43. doi:.https://doi.org/10.1007/s11864-006-0019-4
3 Escobar C , Munker R , Thomas JO , Li BD , Burton GV . Update on desmoid tumors. Ann Oncol. 2012;23(3):562–9. doi:.https://doi.org/10.1093/annonc/mdr386
4 Kasper B , Ströbel P , Hohenberger P . Desmoid tumors: clinical features and treatment options for advanced disease. Oncologist. 2011;16(5):682–93. doi:.https://doi.org/10.1634/theoncologist.2010-0281
5 Nakayama T , Tsuboyama T , Toguchida J , Hosaka T , Nakamura T . Natural course of desmoid-type fibromatosis. J Orthop Sci. 2008;13(1):51–5. doi:.https://doi.org/10.1007/s00776-007-1187-1
6 Kasper B , Baumgarten C , Bonvalot S , Haas R , Haller F , Hohenberger P , et al.; Desmoid Working Group. Management of sporadic desmoid-type fibromatosis: a European consensus approach based on patients’ and professionals’ expertise - a sarcoma patients EuroNet and European Organisation for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group initiative. Eur J Cancer. 2015;51(2):127–36. doi:.https://doi.org/10.1016/j.ejca.2014.11.005
7 Dalén BP , Bergh PM , Gunterberg BU . Desmoid tumors: a clinical review of 30 patients with more than 20 years’ follow-up. Acta Orthop Scand. 2003;74(4):455–9. doi:.https://doi.org/10.1080/00016470310017785
8 Higaki S , Tateishi A , Ohno T , Abe S , Ogawa K , Iijima T , et al. Surgical treatment of extra-abdominal desmoid tumours (aggressive fibromatoses). Int Orthop. 1995;19(6):383–9. doi:.https://doi.org/10.1007/BF00178355
9 Pignatti G , Barbanti-Bròdano G , Ferrari D , Gherlinzoni F , Bertoni F , Bacchini P , et al. Extraabdominal desmoid tumor. A study of 83 cases. Clin Orthop Relat Res. 2000;(375):207–13. doi:.https://doi.org/10.1097/00003086-200006000-00025
10 Rock MG , Pritchard DJ , Reiman HM , Soule EH , Brewster RC . Extra-abdominal desmoid tumors. J Bone Joint Surg Am. 1984;66(9):1369–74. doi:.https://doi.org/10.2106/00004623-198466090-00007
11 Shido Y , Nishida Y , Nakashima H , Katagiri H , Sugiura H , Yamada Y , et al. Surgical treatment for local control of extremity and trunk desmoid tumors. Arch Orthop Trauma Surg. 2009;129(7):929–33. doi:.https://doi.org/10.1007/s00402-008-0750-3
12 Gronchi A , Casali PG , Mariani L , Lo Vullo S , Colecchia M , Lozza L , et al. Quality of surgery and outcome in extra-abdominal aggressive fibromatosis: a series of patients surgically treated at a single institution. J Clin Oncol. 2003;21(7):1390–7. doi:.https://doi.org/10.1200/JCO.2003.05.150
13 Lev D , Kotilingam D , Wei C , Ballo MT , Zagars GK , Pisters PW , et al. Optimizing treatment of desmoid tumors. J Clin Oncol. 2007;25(13):1785–91. doi:.https://doi.org/10.1200/JCO.2006.10.5015
14 Salas S , Dufresne A , Bui B , Blay JY , Terrier P , Ranchere-Vince D , et al. Prognostic factors influencing progression-free survival determined from a series of sporadic desmoid tumors: a wait-and-see policy according to tumor presentation. J Clin Oncol. 2011;29(26):3553–8. doi:.https://doi.org/10.1200/JCO.2010.33.5489
15 Ballo MT , Zagars GK , Pollack A . Radiation therapy in the management of desmoid tumors. Int J Radiat Oncol Biol Phys. 1998;42(5):1007–14. doi:.https://doi.org/10.1016/S0360-3016(98)00285-5
16 Baumert BG , Spahr MO , Von Hochstetter A , Beauvois S , Landmann C , Fridrich K , et al. The impact of radiotherapy in the treatment of desmoid tumours. An international survey of 110 patients. A study of the Rare Cancer Network. Radiat Oncol. 2007;2(1):12. doi:.https://doi.org/10.1186/1748-717X-2-12
17 Huang K , Wang CM , Chen JG , Du CY , Zhou Y , Shi YQ , et al. Prognostic factors influencing event-free survival and treatments in desmoid-type fibromatosis: analysis from a large institution. Am J Surg. 2014;207(6):847–54. doi:.https://doi.org/10.1016/j.amjsurg.2013.08.007
18 Crago AM , Denton B , Salas S , Dufresne A , Mezhir JJ , Hameed M , et al. A prognostic nomogram for prediction of recurrence in desmoid fibromatosis. Ann Surg. 2013;258(2):347–53. doi:.https://doi.org/10.1097/SLA.0b013e31828c8a30
19 Mavrogenis AF , Angelini A , Pala E , Calabro T , Bianchi G , Casadei R , et al. Radiation-induced sarcomas. J Long Term Eff Med Implants. 2011;21(3):233–40. doi:.https://doi.org/10.1615/JLongTermEffMedImplants.v21.i3.70
20 Garbay D , Le Cesne A , Penel N , Chevreau C , Marec-Berard P , Blay JY , et al. Chemotherapy in patients with desmoid tumors: a study from the French Sarcoma Group (FSG). Ann Oncol. 2012;23(1):182–6. doi:.https://doi.org/10.1093/annonc/mdr051
21 Hansmann A , Adolph C , Vogel T , Unger A , Moeslein G . High-dose tamoxifen and sulindac as first-line treatment for desmoid tumors. Cancer. 2004;100(3):612–20. doi:.https://doi.org/10.1002/cncr.11937
22 Briand S , Barbier O , Biau D , Bertrand-Vasseur A , Larousserie F , Anract P , et al. Wait-and-see policy as a first-line management for extra-abdominal desmoid tumors. J Bone Joint Surg Am. 2014;96(8):631–8. doi:.https://doi.org/10.2106/JBJS.M.00988
23 Fiore M , Rimareix F , Mariani L , Domont J , Collini P , Le Péchoux C , et al. Desmoid-type fibromatosis: a front-line conservative approach to select patients for surgical treatment. Ann Surg Oncol. 2009;16(9):2587–93. doi:.https://doi.org/10.1245/s10434-009-0586-2
24 Bonvalot S , Eldweny H , Haddad V , Rimareix F , Missenard G , Oberlin O , et al. Extra-abdominal primary fibromatosis: Aggressive management could be avoided in a subgroup of patients. Eur J Surg Oncol. 2008;34(4):462–8. doi:.https://doi.org/10.1016/j.ejso.2007.06.006
25 Lewis JJ , Boland PJ , Leung DH , Woodruff JM , Brennan MF . The enigma of desmoid tumors. Ann Surg. 1999;229(6):866–72, discussion 872–3. doi:.https://doi.org/10.1097/00000658-199906000-00014
The study was financially supported by “Tauchen gegen Krebs”, www.tauchengegenkrebs.ch.
All authors declare that they have no conflict of interest.