Antimicrobial resistance trends in Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis urinary isolates from Switzerland: retrospective analysis of data from a national surveillance network over an 8-year period (2009–2016)
DOI: https://doi.org/10.4414/smw.2019.20110
Veronica
Zanichellia, Angela
Huttnerb, Stephan
Harbarthab, Andreas
Kronenbergc, Benedikt
Huttnerab, , the Swiss Centre for Antibiotic Resistance (ANRESIS)
aInfection Control Programme, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland
bDivision of Infectious Diseases, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland
cSwiss Centre for Antibiotic Resistance, Institute for Infectious Diseases, University of Bern, Switzerland
1The Swiss Centre for Antibiotic Resistance (ANRESIS): Burnens A., Synlab Suisse; Cherkaoui A., Bacteriology Laboratory, Geneva University Hospitals, Switzerland; Corradi C., Federal Office of Public Health, Bern, Switzerland; Dubuis O., Viollier AG, Basel, Switzerland; Egli A., Clinical Microbiology, University Hospital Basel, Switzerland; Gaia V., Department of microbiology, EOLAB, Bellinzona, Switzerland; Koch D., Federal Office of Public Health, Bern, Switzerland; Kronenberg A., Institute for Infectious Diseases, University of Bern, Switzerland; Leib S.L., Institute for Infectious Diseases, University of Bern, Switzerland; Nordmann P., Molecular Microbiology, Department of Medicine, University Fribourg, Switzerland; Perreten V., Institute of Veterinary Bacteriology, University of Bern, Switzerland; Piffaretti J.-C., Interlifescience, Massagno, Switzerland; Prod’hom G., Institute of Microbiology, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland; Schrenzel J., Bacteriology Laboratory, Geneva University Hospitals, Geneva, Switzerland; Widmer A.F., Division of Infectious Diseases and Hospital Epidemiology, University of Basel, Switzerland; Zanetti G., Service of Hospital Preventive Medicine, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland; Zbinden R., Institute of Medical Microbiology, University of Zürich, Switzerland
Summary
OBJECTIVES
Most urinary tract infections (UTIs) are treated empirically with antibiotics, making comprehensive resistance surveillance data essential to guide empiric regimens. We describe trends in the antibiotic resistance of urinary Enterobacteriaceae isolates in Switzerland between 2009 and 2016.
METHODS
We analysed data from routinely collected Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis urinary samples from community and hospital settings in Switzerland. The data were collected by ANRESIS, the national laboratory-based antimicrobial resistance surveillance system. Our analyses focused on resistance to antibiotics commonly prescribed for UTIs for the period 2009–2016. Only the first isolate per patient per year was included.
RESULTS
297,200 urinary samples were included in the analysis, of which 246,656 (83.0%) were E. coli isolates. Overall, E. coli showed high susceptibility to 3rd/4th-generation cephalosporins, nitrofurantoin and fosfomycin, with the annual proportions of resistant isolates <6%, <5% and <2%, respectively, for all study years. Resistance to fluoroquinolones was >14% and increased over time (from 14.5% in 2009 to 19.3% in 2016). Resistance to cotrimoxazole was >20% for the whole study period. K. pneumoniae (n = 32,757; 11.0%) showed low resistance to cotrimoxazole and quinolones (<11% and <12%, respectively), while for P. mirabilis (n = 17,787; 6.0%) the proportion of resistant isolates was <35% for cotrimoxazole and <18% for quinolones. Even though quinolone resistance remained low for both pathogens (<12% for K. pneumoniae and <18% for P. mirabilis), it increased significantly over time. Proportions of isolates resistant to 3rd/4th generation cephalosporins remained low (<5% for K. pneumoniae and <2% for P. mirabilis), but in the case of K. pneumoniae they increased over time.
CONCLUSIONS
Swiss surveillance data confirm that resistance among uropathogenic E. coli isolates to nitrofurantoin and fosfomycin remains low. While resistance to 3rd/4th-generation cephalosporins also remains relatively low, it has been increasing and needs further surveillance. As for K. pneumoniae and P. mirabilis, high levels of susceptibility to 3rd-generation cephalosporins and quinolones were confirmed, while high prevalences of resistance to nitrofurantoin and fosfomycin discourage their use as first-line therapies for these pathogens.
Introduction
Urinary tract infections (UTIs), particularly uncomplicated cystitis in women, are a frequent cause of antibiotic use in the community and hospital settings [1–3]. Enterobacteriaceae, primarily Escherichia coli, are the main pathogens, responsible for >80% of episodes where a pathogen is identified [4]. Until recently, lower UTIs were commonly treated with fluoroquinolones, resulting in a rapidly increasing prevalence of quinolone-resistant E. coli in many countries [5]. In addition, Enterobacteriaceae that produce extended-spectrum beta-lactamases (ESBL) have become endemic in many parts of the world, including Switzerland [6–8]. Recent guidelines (including Swiss guidelines) on UTI now recommend nitrofurantoin and fosfomycin as first-line options for lower UTI in women and discourage the use of quinolones for acute cystitis because of their potential collateral damage in terms of the selection of drug-resistant organisms [9]. Nitrofurantoin and fosfomycin continue to have good activity against most E. coli isolates, including ESBL producers, and have a smaller ecologic impact than the fluoroquinolones [10–12]. We examined antibiotic resistance trends and assessed whether an increase in resistance to the newly recommended agents or a decrease in quinolone resistance had occurred in Switzerland between 2009 and 2016.
Methods
Antibiotic resistance data for urinary isolates from adult patients (>15 years) for E. coli, Klebsiella pneumoniae and Proteus mirabilis were obtained from the ANRESIS database. ANRESIS (the Swiss Centre for Antibiotic Resistance) is the national surveillance system that collects antibiotic resistance data from routinely collected samples from a selection of microbiology laboratories. These laboratories are distributed homogeneously across Switzerland and represent at least 70% of the annual hospitalisation days and at least 30% of Swiss practitioners [13–15].
For the present analysis we only used data collected by the 11 laboratories that provided data on at least 200 urinary samples per year for the entire study period (2009 to 2016). Only the first isolate per patient per year was included, unless the same patient had multiple isolates from the same year but with different resistance patterns. Isolates that grew >1 pathogen were also included. Antimicrobial susceptibility testing was performed according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) or the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [16, 17]. Most laboratories switched from CLSI to EUCAST breakpoints between 2011 and 2013 (Enterobacteriaceae CLSI and EUCAST cut-offs at the time of the switch are reported for selected antibiotics in table 1). Isolates with intermediate susceptibilities were considered resistant. An isolate with intermediate or full resistance to an antibiotic was considered resistant to the antibiotic’s entire class.
Table 1 CLSI and EUCAST minimum inhibitory concentration (MIC) breakpoints for Enterobacteriaceae for selected antibiotics.
|
Antibiotic
|
CLSI MIC breakpoints (mg/l)*
|
EUCAST MIC breakpoints (mg/l)†
|
|
S ≤ |
R ≥ |
S ≤ |
R >
|
| Nitrofurantoin |
32 |
128 |
64 |
64 |
| Oral fosfomycin |
64 |
256 |
32 |
32 |
| Cotrimoxazole |
2 |
4 |
2 |
4 |
| Ciprofloxacin |
1 (0.25) |
4 (1) |
0.5 (0.25) |
1 (0.5) |
| Ceftriaxone |
1 |
4 |
1 |
2 |
| Cefepime |
8 (2) |
32 (16) |
1 |
4 |
Isolates resistant to at least one 3rd/4th-generation cephalosporin are often used as surrogates for ESBL producers [18] (data regarding the confirmation of ESBL production was not available, and the data are therefore most likely an overestimation of ESBL prevalence since other mechanisms of resistance may cause resistance to 3rd/4th-generation cephalosporins) [6].
Results were stratified by setting (“inpatients” versus “outpatients”, i.e. isolates from patients having consulted a general practitioner (GP) versus isolates from patients in emergency departments and outpatient clinics within hospitals), age (<65 or ≥65 years) and sex. All data were analysed using Stata v15 (StataCorp, College Station, TX, USA). We reported the proportions (%) of antibiotic-resistant isolates in the first and last year of the study period and analysed the time trends between 2009 and 2016 with the χ
2 test. We also tested differences between the proportions in various subgroups (e.g. inpatients versus outpatients, males versus females) using the z test. A two-sided p value of <0.05 was considered statistically significant.
Results
The database included 246,656 E. coli (83.0%), 32,757 K. pneumoniae (11.0%) and 17,787 (6.0%) P. mirabilis urinary isolates from 233,746 patients. The basic characteristics of the study population are described in table 2. 238,403 (80.2%) isolates were from female patients. The median age was 65 years (interquartile range [IQR] 40-80), and male patients were older than female patients (mean age 65.7 years versus 57.2 years, p <0.01).
Table 2 Characteristics of the study population.
| |
All
n (%)
|
E. coli
n (%)
|
K. pneumoniae
n (%)
|
P. mirabilis
n (%)
|
| Sex |
Males |
58,797 (19.8) |
43,705 (17.7) |
8840 (27.0) |
11,535 (64.8) |
| Females |
238,403 (80.2) |
202,951 (82.3) |
23,917 (73.0) |
6252 (35.1) |
| Age (years) |
<65 (15–64) |
142,894 (48.1) |
125,703 (51.0) |
11,261 (34.4) |
5930 (33.3) |
| ≥65 |
154,309 (51.9) |
120,955 (49.0) |
21,497 (65.6) |
11,857 (66.7) |
| Setting |
Inpatients |
90,663 (30.5) |
70,493 (28.6) |
12,980 (39.6) |
7190 (40.4) |
| Outpatients (general practitioners) |
141,603 (47.6) |
121,189 (49.1) |
13,291 (40.6) |
7123 (40.0) |
| Outpatients clinics*
|
64,937 (21.8) |
54,976 (22.3) |
6487 (19.8) |
3474 (19.5) |
Most samples were collected in an outpatient setting (GPs 47.6% and outpatient clinics 21.8%). E. coli remained the most frequently identified pathogen in all years (range 82.2-83.9%) in both community (84.7% in isolates from outpatient clinics and 85.6% in those from GPs) and hospital (77.7%) isolates. The key findings are presented in figures 1–3
and tables 3 and 4
.
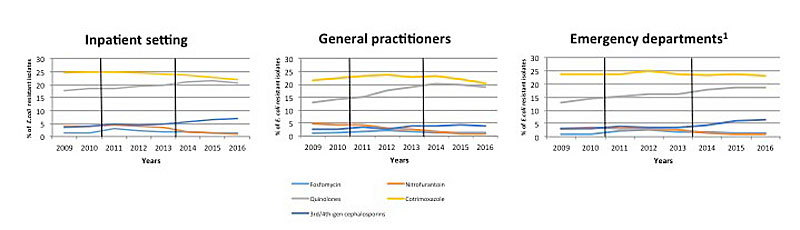
Figure 1 Proportion of resistant E. coli isolates from urine samples collected from inpatients and outpatients between 2009 and 2016 in Switzerland. Data were provided by ANRESIS, the national laboratory-based antimicrobial resistance surveillance system. Vertical black lines indicate the time period of the switch from CLSI to EUCAST breakpoints.
1 Includes outpatient clinics within hospitals
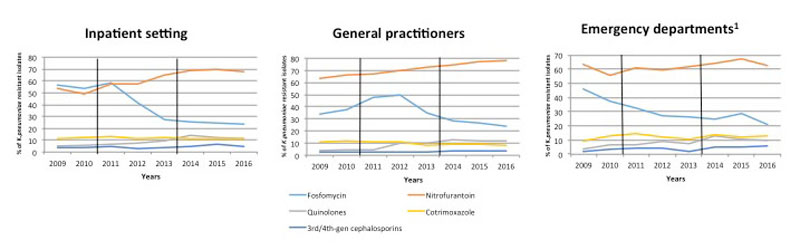
Figure 2 Proportion of resistant K. pneumoniae isolates from urine samples collected from inpatients and outpatients between 2009 and 2016 in Switzerland. Data were provided by ANRESIS, the national laboratory-based antimicrobial resistance surveillance system. Vertical black lines indicate the time period of the switch from CLSI to EUCAST breakpoints.
1 Includes outpatient clinics within hospitals
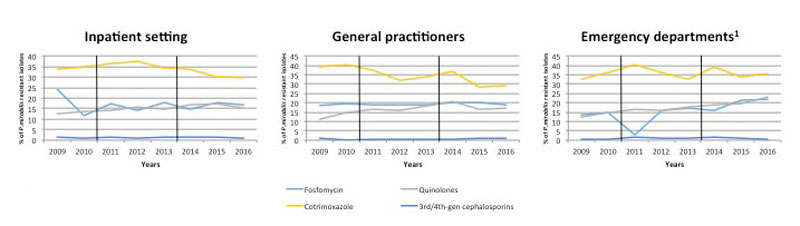
Figure 3 Proportion ofresistant P. mirabilis isolates from urine samples collected from inpatients and outpatients between 2009 and 2016 in Switzerland. Data were provided by ANRESIS, the national laboratory-based antimicrobial resistance surveillance system. Vertical black lines indicate the time period of the switch from CLSI to EUCAST breakpoints. Nitrofurantoin resistance data are not presented since P. mirabilis is considered to be intrinsically resistant.
1 Includes outpatient clinics within hospitals
Table 3 Number and proportion of E. coli, K. pneumoniae and P. mirabilis resistant isolates in Switzerland, 2009 and 2016.
| |
E. coli
|
K. pneumoniae
|
P. mirabilis
|
|
No. resistant isolates / all isolates (%)
|
p-value
|
No. resistant isolates / all isolates (%)
|
p-value
|
No. resistant isolates / all isolates (%)
|
p-value
|
|
2009
|
2016
|
|
2009
|
2016
|
|
2009
|
2016
|
|
| Fosfomycin |
Switzerland all |
140/12,137
(1.1) |
432/34,065
(1.3) |
<0.001*
|
493/1214
(40.6) |
1035/4460
(23.2) |
<0.001*
|
163/827
(19.7) |
426/2282
(18.7) |
0.558*
|
| Setting
†
|
|
<0.001‡
|
|
0.894‡
|
|
0.003‡
|
| |
Inpatients |
26/1844
(1.4) |
136/9090
(1.5) |
0.029*
|
184/ 325
(56.6) |
351/ 1,504
(23.3) |
<0.001*
|
48/197
(24.4) |
141/832
(16.9) |
0.743*
|
| Outpatients |
106/9566
(1.1) |
215/18,583
(1.2) |
0.003*
|
283/833
(34.0) |
509/2124
(24.0) |
<0.001*
|
109/586
(18.6) |
191/1025
(18.6) |
0.775*
|
| Outpatient clinics§
|
8/727
(1.1) |
81/6392
(1.3) |
0.023*
|
26/56
(46.4) |
175/832
(21.0) |
<0.001*
|
6/44
(13.6) |
94/425
(22.1) |
0.002*
|
| Region¶
|
|
<0.001‖
|
|
<0.001‖
|
|
<0.001‖
|
| Sex |
|
0.778‡
|
|
<0.001‡
|
|
0.956‡
|
| |
Female |
119/10,380
(1.1) |
363/27,941
(1.3) |
0.013*
|
394/946
(41.6) |
789/3280
(24.0) |
<0.001*
|
108/555
(19.5) |
272/1431
(19.0) |
0.526*
|
| Male |
21/1757
(1.2) |
69/6124
(1.1) |
0.001*
|
99/268
(36.9) |
246/1180
(20.8) |
<0.001*
|
55/272
(20.2) |
154/851
(18.1) |
0.907*
|
| Age |
≥65 years |
74/5081
(1.5) |
260/16,786
(1.5) |
0.001*
|
303/746
(40.6) |
717/2947
(24.3) |
<0.001*
|
98/506
(19.4) |
305/1530
(19.9) |
0.093*
|
| <65 years |
66/7056
(0.9) |
172/17,279
(1.0) |
0.011*
|
190/468
(40.6) |
318/1513
(21.0) |
<0.001*
|
65/321
(20.2) |
121/752
(16.1) |
0.108*
|
| Nitrofurantoin |
Switzerland all |
997/24,145
(4.1) |
297/36,091 (0.8) |
<0.001*
|
1607/2736
(58.7) |
2612/3592
(72.7) |
<0.001*
|
1742/1765
(98.7) |
2039/2048
(99.6) |
0.001*
|
| Setting
†
|
|
<0.001‡
|
|
<0.001‡
|
|
0.388‡
|
| |
Inpatients |
310/7697
(4.0) |
85/9890
(0.9) |
<0.001*
|
706/1309
(53.9) |
731/1076
(67.9) |
<0.001*
|
781/790
(98.9) |
712/712
(100.0) |
0.001*
|
| Outpatients |
526/11,477
(4.6) |
148/19,097
(0.8) |
<0.001*
|
629/999
(63.0) |
1547/1980
(78.1) |
<0.001*
|
691/705
(98.0) |
970/975
(99.5) |
0.010*
|
| Outpatient clinics§
|
161/4971
(3.2) |
64/7104
(0.9) |
<0.001*
|
272/428
(63.5) |
334/536
(62.3) |
0.009*
|
270/270
(100.0) |
357/361
(98.9) |
0.235*
|
| Region¶
|
|
<0.001‖
|
|
<0.001‖
|
|
<0.001‖
|
| Sex |
|
<0.001‡
|
|
0.035‡
|
|
0.851‡
|
| |
Female |
798/20,142
(4.0) |
223/29,557
(0.7) |
<0.001*
|
1178/2026
(58.1) |
1984/2703
(73.4) |
<0.001*
|
1140/1159
(98.4) |
1301/1306
(99.6) |
<0.001*
|
| Male |
199/4003
(5.0) |
74/6534
(1.1) |
<0.001*
|
429/710
(60.4) |
628/889
(70.6) |
<0.001*
|
602/606
(99.3) |
738/742
(99.5) |
0.777*
|
| Age |
≥65 years |
599/11,289
(5.3) |
196/17,809
(1.1) |
<0.001*
|
994/1766
(56.3) |
1613/2278
(70.8) |
<0.001*
|
1094/1108
(98.7) |
1352/1359
(99.5) |
0.108*
|
| <65 years |
398/12,856
(3.1) |
101/18,282
(0.5) |
<0.001*
|
613/970
(63.2) |
999/1314
(76.0) |
<0.001*
|
648/657
(98.6) |
687/689
(99.7) |
0.001*
|
| Quinolones |
Switzerland all |
3622/24,971
(14.5) |
7032/36,342
(19.3) |
<0.001*
|
129/2872
(4.5) |
591/5186
(11.4) |
<0.001*
|
225/1904
(11.8) |
459/2609
(17.6) |
<0.001*
|
| Setting†
|
|
|
<0.001‡
|
|
|
0.971‡
|
|
|
<0.001‡
|
| |
Inpatients |
1432/8090
(17.7) |
2052/9990
(20.5) |
<0.001*
|
71/1373
(5.2) |
220/1910
(11.5) |
<0.001*
|
108/871
(12.4) |
159/1035
(15.4) |
0.002*
|
| Outpatients |
1497/11,534
(13.0) |
3627/19,097
(19.0) |
<0.001*
|
40/1017
(3.9) |
276/2285
(12.1) |
<0.001*
|
79/725
(10.9) |
189/1096
(17.2) |
<0.001*
|
| Outpatient clinics§
|
693/5347
(13.0) |
1353/7255
(18.6) |
<0.001*
|
18/482
(3.7) |
95/991 (9.6) |
<0.001*
|
38/308
(12.3) |
111/478
(23.2) |
<0.001*
|
| Region¶
|
|
<0.001‖
|
|
<0.001‖
|
|
<0.001‖
|
| Sex |
|
<0.001‡
|
|
<0.001‡
|
|
<0.001‡
|
| |
Female |
2702/20,786
(13.0) |
5222/29,766
(17.5) |
<0.001*
|
78/2127
(3.7) |
363/3794
(9.6) |
<0.001*
|
142/1237
(11.5) |
254/1640
(15.5) |
<0.001*
|
| Male |
920/4185
(22.0) |
1810/6576
(27.5) |
<0.001*
|
51/745
(6.8) |
228/1392
(16.4) |
<0.001*
|
83/667
(12.4) |
205/969
(21.2) |
<0.001*
|
| Age |
≥65 years |
2346/11,766
(19.9) |
4117/17,947
(22.9) |
<0.001*
|
84/1858
(4.5) |
406/3462
(11.7) |
<0.001*
|
167/1212
(13.8) |
360/1766
(20.4) |
<0.001*
|
| <65 years |
1276/13,205
(9.7) |
2915/18,395
(15.8) |
<0.001*
|
45/1014
(4.4) |
185/1724
(10.7) |
<0.001*
|
58/692
(8.4) |
99/843
(11.7) |
<0.001*
|
| Cotrimoxazole |
Switzerland all |
5704 /24,823
(23.0) |
7333/34,423
(21.3) |
<0.001*
|
310/2864
(10.8) |
487/4820
(10.1) |
0.001*
|
671/1889
(35.5) |
745/2446
(30.5) |
<0.001*
|
| Setting†
|
|
<0.001‡
|
|
<0.001‡
|
|
0.184‡
|
| |
Inpatients |
1976/8082
(24.4) |
1881/8607
(21.8) |
<0.001*
|
155/1371
(11.3) |
178/1617
(11.0) |
0.300*
|
293/870
(33.7) |
267/898
(29.7) |
0.003*
|
| Outpatients |
2463/11,395
(21.6) |
3890/19,054
(20.4) |
<0.001*
|
110/1011
(10.9) |
186/2282
(8.1) |
<0.001*
|
278/711
(39.1) |
315/1089
(28.9) |
<0.001*
|
| Outpatient clinics§
|
1265/5346
(23.7) |
1562/6762
(23.1) |
0.364*
|
45/482
(9.3) |
123/921
(13.4) |
0.374*
|
100/308
(32.5) |
163/459
(35.5) |
0.869*
|
| Region¶
|
|
<0.001‖
|
|
<0.001‖
|
|
<0.001‖
|
| Sex |
|
<0.001‡
|
|
<0.001‡
|
|
0.275‡
|
| |
Female |
4684/20,637
(22.7) |
5785/28,241
(20.5) |
<0.001*
|
212/2119
(10.0) |
289/3538
(8.2) |
<0.001*
|
434/1222
(35.5) |
462/1543
(29.9) |
<0.001*
|
| Male |
1020/4186
(24.4) |
1548/6182
(25.0) |
0.542*
|
98/745
(13.1) |
198/1282
(15.4) |
0.908*
|
237/667
(35.5) |
283/903
(31.3) |
0.001*
|
| Age |
≥65 years |
2832/11,762
(24.1) |
3715/16,778
(22.1) |
<0.001*
|
177/1856
(9.5) |
314/3191
(9.8) |
0.092 |
454/1212 (37.5) |
544/1644
(33.1) |
<0.001*
|
| <65 years |
2872/13,061
(22.0) |
3618/17,645
(20.5) |
<0.001*
|
133/1008
(13.2) |
173/1629
(10.6) |
0.002 |
217/677
(32.1) |
201/802
(25.1) |
<0.001*
|
| 3rd/4th-generation cephalosporins |
Switzerland all |
697/23,850
(2.9) |
1942/36,469
(5.3) |
<0.001*
|
79/2732
(2.9) |
229/5195
(4.4) |
<0.001*
|
24/1805
(1.3) |
24/2618
(0.9) |
0.931*
|
| Setting†
|
|
<0.001‡
|
|
<0.001‡
|
|
0.001‡
|
| |
Inpatients |
269/7714
(3.5) |
693/10,038
(6.9) |
<0.001*
|
48/1317
(3.6) |
93/1913
(4.9) |
0.002*
|
13/826
(1.6) |
11/1038
(1.1) |
0.736*
|
| Outpatients |
276/11,144
(2.5) |
777/19,140
(4.1) |
<0.001*
|
24/986
(2.4) |
79/2288
(3.4) |
0.001*
|
9/696
(1.3) |
10/1099
(0.9) |
0.353*
|
| Outpatient clinics§
|
152/4992
(3.0) |
472/7291
(6.5) |
<0.001*
|
7/429
(1.6) |
57/994
(5.7) |
<0.001*
|
2/283
(0.7) |
3/ 481
(0.6) |
0.916*
|
| Region¶
|
|
<0.001‖
|
|
<0.001‖
|
|
<0.001‖
|
| Sex |
|
<0.001‡
|
|
<0.001‡
|
|
0.368‡
|
| |
Female |
507/19,860
(2.5) |
1338/29,860
(4.5) |
<0.001*
|
50/2017
(2.5) |
120/3798
(3.2) |
0.003*
|
15/1169
(1.3) |
16/1646
(1.0) |
0.939*
|
| Male |
190/3990
(4.8) |
604/6609
(9.1) |
<0.001*
|
29/715
(4.1) |
109/1397
(7.8) |
<0.001*
|
9/636
(1.4) |
8/972
(0.8) |
0.989*
|
| Age |
≥65 years |
385/11,201
(3.4) |
1151/18,042
(6.4) |
0.679 *
|
54/1768
(3.0) |
161/3469
(4.6) |
<0.001*
|
12/1136
(1.1) |
18/1773
(1.0) |
0.679*
|
| <65 years |
312/12,649
(2.5) |
791/18,427
(4.3) |
0.612 *
|
25/964
(2.6) |
68/1726
(3.9) |
0.017 *
|
12/669
(1.8) |
6/845
(0.7) |
0.612*
|
Table 4 Resistance of E. coli isolates in 2016 according to age group and setting.
|
Quinolone resistant
n (%)
|
Cotrimoxazole resistant
n (%)
|
3rd/4th-generation cephalosporin resistant
n (%)
|
Nitrofurantoin resistant
n (%)
|
Fosfomycin resistant
n (%)
|
| Patients ≥65 years |
4117/17,947
(22.9) |
3715/16,778
(22.1) |
1151/18,042
(6.4) |
196/17,809
(1.1) |
260/16,786
(1.5) |
| Patients <65 years |
2915/18,395
(15.8) |
3618/17,645
(20.5) |
791/18,427
(4.3) |
101/18,282
(0.5) |
172/17,279
(1.0) |
| Inpatients |
2052/9990
(20.5) |
1881/8607
(21.8) |
693/10,038
(6.9) |
85/9890
(0.9) |
136/9090
(1.5) |
| Outpatients |
3627/19,097
(19.0) |
3890/19,054
(20.4) |
777/19,140
(4.1) |
148/19,097
(0.8) |
215/18,583
(1.2) |
| Outpatient clinics*
|
1353/7255 (18.6) |
1562/6762
(23.1) |
472/729
(6.5) |
64/7104
(0.9) |
81/6392
(1.3) |
Escherichia coli
High susceptibility to nitrofurantoin and fosfomycin were consistently observed, with overall annual proportions of resistant isolates <5% and <2% respectively, across all settings. Nevertheless, a significant increase over time was observed for fosfomycin (from 1.1% in 2009 to 1.3% in 2016; p <0.001) while resistance to nitrofurantoin decreased (4.1% in 2009 to 0.8% in 2016; p <0.001). Resistance to quinolones increased from 14.5% in 2009 to 19.3% in 2016 (p <0.001). Resistance to cotrimoxazole decreased from 23.0% in 2009 to 21.3% in 2016 (p <0.001) but always remained >20%. E. coli resistant to 3rd/4th-generation cephalosporins increased in all settings, from 2.9% in 2009 to 5.3% in 2016 (p <0.001).
In 2016, proportions of resistant isolates were higher among men than women for all antibiotics investigated. Fosfomycin was the only exception (27.5% versus 17.5% for quinolones, 25.0% versus 20.5% for cotrimoxazole, 9.1% versus 4.5% for 3rd/4th generation cephalosporins, 1.1% versus 0.7% for nitrofurantoin). Isolates from patients aged ≥65 years and from inpatients were more resistant for all antibiotic classes investigated (table 4).
Klebsiella pneumoniae
Overall, cotrimoxazole and quinolone resistance remained low, ranging from 10–11% and 4–11% of all K. pneumoniae isolates respectively, although the prevalence of quinolone-resistant isolates more than doubled from 2009 to 2016 (4.5% to 11.4%, p <0.001). The proportions of 3rd/4th-generation cephalosporin-resistant isolates increased overall between 2009 and 2016 (2.9% to 4.4%, p <0.001) but remained low (<5%), with 2016 resistance prevalences of 3.4% and 4.9% in outpatient and hospital isolates, respectively (p <0.001). Resistance to fosfomycin and nitrofurantoin remained high (>20% and >58% of all isolates respectively) in samples from both hospitals and the community. Fosfomycin resistance decreased after 2011, however, from 40.6% to 23.2%.
Proteus mirabilis
In P. mirabilis isolates, the highest prevalence of resistance was to cotrimoxazole (>30%), while quinolone and fosfomycin resistance remained below 20% throughout the study period. For quinolones, however, resistance increased from 11.8% in 2009 to 17.6% in 2016 (p <0.001). Cotrimoxazole resistance decreased over time from 35.5% in 2009 to 30.5% in 2016 (p <0.001). Resistance to 3rd/4th-generation cephalosporins remained low over the study period (1.3% in 2009 to 0.9% in 2016) and the changes in the proportions of resistant isolates were <0.5% per year in all categories.
Discussion
This retrospective study describes resistance patterns for a large number of urinary samples collected from inpatients and outpatients across Switzerland from 2009 to 2016, with a focus on those antibiotic classes commonly used to treat UTI. Although many studies have already described increasing resistance rates in urinary isolates, Swiss data are limited either to single centre studies or to studies focusing on resistance to single antibiotic classes [19]. Moreover, to the best of our knowledge, no data on Swiss samples collected after 2011 have been published. This reflects a lack of information concerning the impact of the most recent IDSA/ESCMID (2010) [9] and Swiss (2014) [20] guidelines, which recommend antibiotics with high efficacy but “minimal resistance and propensity for collateral damage”, such as nitrofurantoin and fosfomycin, for uncomplicated cystitis, reserving quinolones for upper UTI.
Moreover, while cotrimoxazole is discouraged by IDSA/ESCMID guidelines in settings where resistance prevalence exceeds 20% (without stating a clear rationale for this cut-off), it is mentioned together with fosfomycin and nitrofurantoin as a first-choice option in the Swiss guidelines.
In this study we were able to analyse data on resistance to the more frequently prescribed antibiotics for UTI provided by a large national surveillance program. The most important finding of this study was the low resistance (<5%) of E. coli isolates to both nitrofurantoin and fosfomycin during the whole study period. Interestingly, resistance to nitrofurantoin showed a statistically significant decrease over time for E. coli isolates, from 4.1% in 2009 to 0.8% in 2016, although this was likely due to the switch from the CLSI to the EUCAST breakpoints. Resistance to fosfomycin increased slightly from 1.1% in 2009 to 1.3% in 2016.
This may be explained in part by a higher uptake of fosfomycin by GPs. In fact, in Switzerland between 2013 and 2017 (so going beyond the study period), fosfomycin use in the outpatient setting went up from 0.03 to 0.04 defined daily doses (DDD) per 1000 inhabitants per day, while nitrofurantoin use went down from 0.23 to 0.20 DDD per 1000 inhabitants per day (unpublished data). This is despite a recent study proving that nitrofurantoin is superior to fosfomycin for both clinical and bacteriological response in women treated for uncomplicated UTI [4].
Low proportions of isolates resistant to nitrofurantoin and fosfomycin were confirmed in samples from both inpatient and outpatient settings and in all patient age and sex categories. For nitrofurantoin, our results were similar to those previously reported in a study investigating resistance trends in urinary isolates collected at the University Hospital of Basel, Switzerland until 2007 [5]. On the other hand, even though the use of quinolones has been discouraged for the treatment of uncomplicated lower UTI in international guidelines since 2010 (no Swiss guidelines were available before 2014), E. coli resistance to quinolones increased over time and reached 19.3% in 2016. This increase was statistically significant and observed for both inpatient and outpatient isolates. Moreover, it reached 22.9% in patients aged >65 years.
E. coli resistance to 3rd- and 4th-generation cephalosporins increased over the study period, in line with data previously published in Switzerland on urinary and bloodstream infections [7]. Similarly, we observed the highest incidence and yearly increase in the age group of over 65 years and in males.
Interestingly, we described similar proportions of resistant isolates and increases over time between the samples collected at hospitals and those from outpatient clinics, whereas previously in Switzerland a statistically significantly higher increase was observed in inpatients, confirming the concerns over the risk of community-acquired resistance to 3rd-generation cephalosporins and its implications for the empirical treatment of severe infections and infection control, even in a Swiss context [18].
For cotrimoxazole we observed a slight decrease in the proportion of resistant E. coli isolates from 2009 to 2016, from 23% to 21.3%. This probably reflects decreased use of cotrimoxazole because of concerns about the proportions of resistant isolates being >20% (mentioned – albeit somewhat arbitrarily – as the cut-off for recommending against using cotrimoxazole empirically for UTI) [9]. It is interesting to note that before 2009 resistance to cotrimoxazole probably increased, as reported in a single-centre Swiss study that described an increase in cotrimoxazole resistance between 1997 and 2007 [5, 18].
K. pneumoniae and P. mirabilis accounted for less than 20% of all isolates and both had high proportions of isolates resistant to nitrofurantoin and fosfomycin, making them suboptimal options for treating infections caused by these pathogens. Third-generation cephalosporins and quinolones retained high intrinsic activity (>85–90% susceptibility in both pathogens and for both drugs).
The main strength of this study is that it is based on a large database of urinary samples from both the hospital and community settings (even though we acknowledge that the study population only covers 70% of annual hospitalisation days and 30% of Swiss practitioners). However, we also acknowledge its limitations. Firstly, we do not have clinical information linked to the isolates (including previous antibiotic use and the fact that we could not establish if these were asymptomatic bacteriuria, complicated or uncomplicated UTI) and so it is likely that, especially in the outpatient setting, the samples that were sent for testing were those of patients with recurrent infections or treatment failure. This could lead to a possible overestimation of the real incidence of resistance [19]. Secondly, most laboratories in Switzerland transitioned from CLSI to EUCAST, so changes in the proportion of resistant isolates may be in part the consequence of a change in the cut-offs used to distinguish between susceptible and resistant isolates (even though, as shown in Table 1, in all cases where the cut-off was changed, the minimum inhibitory concentration was lowered even though the proportion of resistant isolates increased or decreased, depending on the pathogen). This aspect likely explains the important increase in the susceptibility of K. pneumoniae isolates to fosfomycin between 2009 and 2016. Furthermore, we do not have complete information on the trends of antibiotic use for UTI in Switzerland between 2009 and 2016, making it impossible to draw conclusions about the possible impact of the most recent guidelines on the described proportions of antibiotic-resistant isolates. This represents an important limitation, since it has been proven, for example, that antibiotic use for various types of infections (including UTIs) is associated with the emergence and selection of resistance at the individual level, with consequent possible implications on the ecological level (i.e. community, regional and country levels) [21, 22].
Even though Switzerland has a relatively low antibiotic consumption in both inpatient and outpatient settings compared to other European countries (10.7 DDD/1000 inhabitants in the outpatient setting in 2017 versus 21.8 DDD/1000 inhabitants in the European Union (EU) in the same year, and 60 DDD/100 bed-days in Swiss hospitals), some antibiotic classes (such as quinolones) are probably overused and we believe the findings of this study highlight the potential to reduce inappropriate antibiotic use and to standardise UTI treatment in Switzerland.
For example, in light of these findings, the use of cotrimoxazole as a first-line agent for lower UTI (even though overall resistance for E. coli exceeds 20%) must be reconsidered (and it is indeed suggested as an option in the 2014 Swiss guidelines). As mentioned in the IDSA guidelines [9], the threshold of 20% as the resistance prevalence at which the agent is no longer recommended is based on expert opinion derived from clinical, in vitro and mathematical modelling studies. It is important to remember that cystitis is mostly a self-limited condition and 25–42% of women may resolve spontaneously [23], that surveillance data tend to overestimate resistance (because cultures are often requested for more severe or unresponsive cases) and that urinary concentrations of certain antibiotics (including cotrimoxazole) may be higher than those achieved in the serum with equivalent doses (and susceptibility breakpoints are based on achievable serum and not urine concentrations) [24]. All these arguments support the attitude that cotrimoxazole can be reconsidered.
To conclude we observed an increase over time in resistance in urinary isolates for antibiotic classes such as quinolones (for all three described pathogens, highlighting the need for antibiotic stewardship) and 3rd- and 4th-generation cephalosporins (for E. coli and K. pneumoniae). We confirmed the high levels of susceptibility to nitrofurantoin and fosfomycin among E. coli isolates (but not among K. pneumoniae or P. mirabilis isolates, making them good options for the empiric treatment of uncomplicated lower UTI, since E. coli is by far the most common causative pathogen). The proportion of cotrimoxazole-resistant isolates decreased for all isolates but remained >20% for E. coli and P. mirabilis in all settings. Although we did not comment in our discussion on the impact of antibiotic use in animals on the selection for bacteria resistant to the antibiotics used in humans, we acknowledge that in order to combat antimicrobial resistance in gram-negative bacteria, a holistic and multisectoral approach (referred to as One Health) is important.
The preliminary results of this study were presented as a poster presentation (P11529) at the 26th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID); 9–12 April 2016; Amsterdam, The Netherlands.
Acknowledgements
We thank all participating ANRESIS laboratories: ADMED Microbiology, La Chaux-de-Fonds; Bacteriology Laboratory, Geneva University Hospitals, Geneva; Central Institute, Hôpitaux Valaisans (ICHV), Sitten; Central Laboratory, Cantonal Hospital Graubünden; Central Laboratory, Microbiology Section, Cantonal Hospital Baden; Centre for Laboratory Medicine, Cantonal Hospital Luzern; Centre for Laboratory Medicine, Cantonal Hospital Schaffhausen; Centre for Laboratory Medicine Dr. Risch, Schaan; Centre for Laboratory Medicine St. Gallen; Clinical Microbiology, University Hospital, Basel; Hôpital du Jura, Laboratoire, Institute for Infectious Diseases, University Bern; Institute for Laboratory Medicine, Cantonal Hospital Aarau; Institute for Medical Microbiology, University Hospital Zürich; Institute for Microbiology, Université de Lausanne; Laboratoire et analyses médicales, Etablissements Hospitaliers du Nord Vaudois, eHnv; Laboratory for Infectious Diseases, University Children's Hospital Zürich; Laboratory Medicine EOLAB, Department of Microbiology, Bellinzona; Labormedizin, Kantonsspital Winterthur; Microbiology Laboratory Hôpital Fribourgeois, Fribourg; Microbiology Laboratory, Hospital Thurgau; Microbiology Laboratory, Unilabs, Coppet; Promed SA, Laboratoire medical, Marly; Viollier AG, Basel.
References
1
Tan
C
,
Graves
E
,
Lu
H
,
Chen
A
,
Li
S
,
Schwartz
KL
, et al.
A decade of outpatient antimicrobial use in older adults in Ontario: a descriptive study. CMAJ Open. 2017;5(4):E878–85. doi:.https://doi.org/10.9778/cmajo.20170100
2
Aabenhus
R
,
Hansen
MP
,
Siersma
V
,
Bjerrum
L
. Clinical indications for antibiotic use in Danish general practice: results from a nationwide electronic prescription database. Scand J Prim Health Care. 2017;35(2):162–9. doi:.https://doi.org/10.1080/02813432.2017.1333321
3
Foxman
B
. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113(1, Suppl 1A):5–13. doi:.https://doi.org/10.1016/S0002-9343(02)01054-9
4
Huttner
A
,
Kowalczyk
A
,
Turjeman
A
,
Babich
T
,
Brossier
C
,
Eliakim-Raz
N
, et al.
Effect of 5-day nitrofurantoin vs single-dose fosfomycin on clinical resolution of uncomplicated lower urinary tract infection in women: A randomized clinical trial. JAMA. 2018;319(17):1781–9. doi:.https://doi.org/10.1001/jama.2018.3627
5
Blaettler
L
,
Mertz
D
,
Frei
R
,
Elzi
L
,
Widmer
AF
,
Battegay
M
, et al.
Secular trend and risk factors for antimicrobial resistance in Escherichia coli isolates in Switzerland 1997-2007. Infection. 2009;37(6):534–9. doi:.https://doi.org/10.1007/s15010-009-8457-0
6
Seiffert
SN
,
Hilty
M
,
Kronenberg
A
,
Droz
S
,
Perreten
V
,
Endimiani
A
. Extended-spectrum cephalosporin-resistant Escherichia coli in community, specialized outpatient clinic and hospital settings in Switzerland. J Antimicrob Chemother. 2013;68(10):2249–54. doi:.https://doi.org/10.1093/jac/dkt208
7
Kronenberg
A
,
Hilty
M
,
Endimiani
A
,
Muhlemann
K
. Temporal trends of extended-spectrum cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae isolates in in- and outpatients in Switzerland, 2004 to 2011. Euro Surveill. 2013;18(21):18.
8
Woerther
PL
,
Burdet
C
,
Chachaty
E
,
Andremont
A
. Trends in human fecal carriage of extended-spectrum β-lactamases in the community: toward the globalization of CTX-M. Clin Microbiol Rev. 2013;26(4):744–58. doi:.https://doi.org/10.1128/CMR.00023-13
9
Gupta
K
,
Hooton
TM
,
Naber
KG
,
Wullt
B
,
Colgan
R
,
Miller
LG
, et al.; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103–20. doi:.https://doi.org/10.1093/cid/ciq257
10
Bonkat
G
,
Müller
G
,
Braissant
O
,
Frei
R
,
Tschudin-Suter
S
,
Rieken
M
, et al.
Increasing prevalence of ciprofloxacin resistance in extended-spectrum-β-lactamase-producing Escherichia coli urinary isolates. World J Urol. 2013;31(6):1427–32. doi:.https://doi.org/10.1007/s00345-013-1031-5
11
Sanchez
GV
,
Baird
AM
,
Karlowsky
JA
,
Master
RN
,
Bordon
JM
. Nitrofurantoin retains antimicrobial activity against multidrug-resistant urinary Escherichia coli from US outpatients. J Antimicrob Chemother. 2014;69(12):3259–62. doi:.https://doi.org/10.1093/jac/dku282
12
Stewardson
AJ
,
Vervoort
J
,
Adriaenssens
N
,
Coenen
S
,
Godycki-Cwirko
M
,
Kowalczyk
A
, et al.; SATURN WP1 Study Group; SATURN WP3 Study Group. Effect of outpatient antibiotics for urinary tract infections on antimicrobial resistance among commensal Enterobacteriaceae: a multinational prospective cohort study. Clin Microbiol Infect. 2018;24(9):972–9. doi:.https://doi.org/10.1016/j.cmi.2017.12.026
13
Sommerstein
R
,
Atkinson
A
,
Lo Priore
EF
,
Kronenberg
A
,
Marschall
J
,
Burnens
A
, et al.; Swiss Centre for Antibiotic Resistance (ANRESIS). Characterizing non-linear effects of hospitalisation duration on antimicrobial resistance in respiratory isolates: an analysis of a prospective nationwide surveillance system. Clin Microbiol Infect. 2018;24(1):45–52. doi:.https://doi.org/10.1016/j.cmi.2017.05.018
14
Olearo
F
,
Albrich
WC
,
Vernaz
N
,
Harbarth
S
,
Kronenberg
A
,
Swiss
C
; Swiss Centre For Antibiotic Resistance Anresis. Staphylococcus aureus and methicillin resistance in Switzerland: regional differences and trends from 2004 to 2014. Swiss Med Wkly. 2016;146:w14339. doi:.https://doi.org/10.4414/smw.2016.14339
15Anresis Swiss Center for Antibiotic Resistance. Definitions selection criteria; [cited 2019, February 20]. Available from: http://www.anresis.ch/index.php/definition-der-auswahlkriterien.html/.
16The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of mics and zone diameters; [cited 2019, May 3]. Available from: http://www.eucast.org/clinical_breakpoints/.
17Clinical & Laboratory Standards Institute. Clsi guidelines; [cited 2019, May 3]. Available from: https://clsi.org/.
18
Vernaz
N
,
Huttner
B
,
Muscionico
D
,
Salomon
JL
,
Bonnabry
P
,
López-Lozano
JM
, et al.
Modelling the impact of antibiotic use on antibiotic-resistant Escherichia coli using population-based data from a large hospital and its surrounding community. J Antimicrob Chemother. 2011;66(4):928–35. doi:.https://doi.org/10.1093/jac/dkq525
19
Kronenberg
A
,
Koenig
S
,
Droz
S
,
Mühlemann
K
. Active surveillance of antibiotic resistance prevalence in urinary tract and skin infections in the outpatient setting. Clin Microbiol Infect. 2011;17(12):1845–51. doi:.https://doi.org/10.1111/j.1469-0691.2011.03519.x
20Swiss Society for Infectious Diseases. Guidelines urinary tract infections [cited 2019, February 20]. Available from: http://www.sginf.ch/guidelines/guidelines-overview.html/.
21
Costelloe
C
,
Metcalfe
C
,
Lovering
A
,
Mant
D
,
Hay
AD
. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340(may18 2):c2096. doi:.https://doi.org/10.1136/bmj.c2096
22
Bell
BG
,
Schellevis
F
,
Stobberingh
E
,
Goossens
H
,
Pringle
M
. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 2014;14(1):13. doi:.https://doi.org/10.1186/1471-2334-14-13
23
Falagas
ME
,
Kotsantis
IK
,
Vouloumanou
EK
,
Rafailidis
PI
. Antibiotics versus placebo in the treatment of women with uncomplicated cystitis: a meta-analysis of randomized controlled trials. J Infect. 2009;58(2):91–102. doi:.https://doi.org/10.1016/j.jinf.2008.12.009
24
Chastain
DB
,
King
ST
,
Stover
KR
. Rethinking urinary antibiotic breakpoints: analysis of urinary antibiotic concentrations to treat multidrug resistant organisms. BMC Res Notes. 2018;11(1):497. doi:.https://doi.org/10.1186/s13104-018-3599-8


