
Figure 1 Patient selection from the Arteris Group.
DOI: https://doi.org/10.4414/smw.2019.20006
In Switzerland, every second case of acute coronary syndrome occurs in primary care subjects classified as being at low coronary risk according to the Swiss Atherosclerosis Association (Arbeitsgruppe Lipide und Atherosklerose; AGLA) [1]. This was found in a large national Swiss study, where the sensitivity at the 5% SCORE level was only 37% in patients presenting with a first acute coronary syndrome [1] (SCORE = Systematic COronary Risk Evaluation). A “present time” assessment from Copenhagen of the diagnostic accuracy of coronary risk calculators at various risk thresholds, derived from patients admitted for a first myocardial infarction, confirmed the Swiss observations by Selby [2, 3]: a low sensitivity for cardiovascular events was found in the prospective Copenhagen General Population Study, in which 68 fatal and 767 fatal or nonfatal cardiovascular events occurred over an observation time of 7 years. Sensitivity at the 5% SCORE level was 42% and 26% for fatal and fatal or nonfatal events, respectively [2].
For prevention of cardiovascular events in primary care, the trade-off between sensitivity and specificity is important. If sensitivity thresholds are lowered, the risk of treating healthy subjects who will never experience a clinical event increases and cost efficiency decreases. At the current decision thresholds for statin intervention – 10% risk according to AGLA and low density lipoprotein (LDL) >3.0 mmol/l – the specificity of such interventions is high, but sensitivity is low [4].
Atherosclerotic plaques indicate high-risk patients with very high specificity [5] and may therefore improve the poor sensitivity of cardiovascular risk scores without loss in specificity. For the purpose of this study, we defined “old” arteries as a vascular age of 70 years or more (VA70), because this corresponds to a highly specific cut-off for the occurrence of myocardial infarction as evidenced in a large cohort study of 6336 male and female persons [5]. We assessed the diagnostic accuracy of PROCAM/AGLA (PROCAM = Prospective Cardiovascular Münster-Studie) and SCORE to detect VA70 in a group of healthy women and men (age range 40–65 years) in Olten (Switzerland) and the Koblenz area in Germany. Given the known limitations in the sensitivity of PROCAM/SCORE among younger healthy subjects [3, 6], the study further investigated how cardiovascular disease prevention decisions might change if results from carotid atheroma scanning with ultrasound were known and if such an approach is cost efficient.
The outcome measure was vascular age of 70 years or more (VA70) in healthy women and men aged 40–65 years who were not taking statins or antihypertensive drugs.
Subjects were assessed at the practice level as described elsewhere [7]. The selection process is outlined in figure 1. In the imaging centre in Olten, subjects were referred by their primary care physician (58%) or self-referred (42%) to the vascular risk foundation [8]. In the imaging centre in Koblenz, all subjects were referred within a working medicine setting [9]. Subjects had to be free of cardiovascular symptoms or disease and not currently treated for high blood pressure or cholesterol. The time range and the median time of assessment were between 2002 and 2017 in the Olten area (median year 2009) and between 2008 and 2017 in the Koblenz area (median year 2012). Laboratory values, blood pressure and medical history were measured locally and entered into a spread-sheet (Excel, Microsoft, Richmond, USA).

Figure 1 Patient selection from the Arteris Group.
The burden of longitudinal carotid plaque surface was imaged with a high-resolution ultrasound linear transducer probe (7.5–12.0 MHz), which identified plaques with intimal thickening ≥1.0 mm. The longitudinal area of all plaques was summed to give the total plaque area (TPA) in mm2. All TPA measurements were made by M.R. in Olten and by A.A. in Koblenz. Intraobserver reproducibility by M.R. for both carotid arteries in 57 patients showed a correlation coefficient of r2 = 0.964 (left carotid artery: r2 = 0.944, both arteries r2 = 0.976, supplementary fig. S1 in appendix 1). For the cut-offs of TPA 0–9, 10–49, 50–99 and ≥100 mm2, the linear weighted intraobserver kappa value was good at 0.80 (95% confidence interval [CI] 0.69–0.90). Interobserver variability was not formally assessed (no trained staff besides M.R. in Switzerland). Intraobserver reproducibility was tested for both carotid arteries in 56 patients for A.A. with a correlation coefficient of r2 = 0.976 (left carotid artery: r2 = 0.949, both arteries r2 = 0.953). For the cut-offs of TPA 0–9, 10–49, 50–99 and ≥100 mm2, the linear weighted kappa value was very good at 0.97 (95% CI 0.92–1.00).
The mean values of TPA derived from 5-year intervals for 1500 men and women aged 35 to 79 years were plotted against their chronological age, as previously described [7]. An exponential function was added, which connected these 5-year intervals, and the equation of the line was displayed. These exponential equations describing TPA (y) as a function of age (x) were solved for x in order to determine the vascular age for men and women separately [7]. The distribution of VA70 was counted for low, intermediate and high cardiovascular risk for PROCAM/AGLA and SCORE.
SCORE risk was computed using the published risk formulae in an Excel spread sheet for low risk populations [10]. SCORE intermediate risk is defined as between 1.0 and 4.9% [11]. According to European guidelines, more intensive primary prevention activities are needed in subjects with a high SCORE (above 5%).
PROCAM risk was calculated online, since the formula for risk calculations is kept secret [12]. For Switzerland, PROCAM risk was multiplied by the factor 0.7 (according to the Swiss AGLA guidelines 2014 [13]) in order to calculate the AGLA risk. For Switzerland, it is recommended that an AGLA risk below 10% should be viewed as a low coronary risk. We used several sensitivity thresholds in order to calculate the sensitivity and specificity to diagnose VA70.
First, SCORE was recalculated with vascular age instead of chronological age, if the vascular age were higher than the chronological age. Only low risk subjects (SCORE risk <1.0%) were analysed and the numbers shifted to a SCORE risk of 1% or more were counted as subjects reclassified to a higher risk level. This calculation was not available for PROCAM, because the calculations are not publicly available and repeat calculations for vascular age were time consuming.
Second, for better comparability, post-test risk was calculated for SCORE and PROCAM using the Bayes formula, based on the sensitivity and specificity of TPA, which were derived from the Tromsø study [5, 14] and based on personal communication of the raw data. Subjects were counted as reclassified with PROCAM if their risk was changed from <10% to 10% or more, and were reclassified with SCORE if their risk was changed from <1.0% to 1.0% or more.
We used a cost-efficiency analysis developed by the vascular risk foundation (personalised price model, PEP), which is based on an estimate of avoidable direct and indirect costs of a cardiovascular event, and compared the results with the quality-adjusted life year (QALY) model for statins of the Swiss Medical Board [15, 16]. The major input variables of the two models were kept identical and were: (1) average LDL and cardiovascular risk of reclassified subjects, (2) 50% LDL reduction at a cost of 0.68 CHF/day (the effect of 20 mg rosuvastatin per day), (3) cost of imaging of carotid arteries CHF 100, (4) effect of statins: 22%/35% relative risk reduction (RRR) per 1 mmol/l LDL reduction [17, 18]. The PEP model calculates indirect and direct costs of a cardiovascular event as being CHF 200,000 over 10 years as follows [19]: direct and indirect costs were CHF 4.8 billion for myocardial infarction and CHF 3.2 billion for stroke in 2011 in Switzerland [20]. The Swiss death statistics of the Federal Office of Statistics showed 8150 due to ischaemic heart disease; further, an incidence of 150 strokes per 100,000 habitants (n = 11,850) are expected to occur, of which around 20% are fatal [21]. The risk of second and further events after a first event is 34% for myocardial infarction and 24% for stroke in 5 years [22], which leads to average costs of CHF 251,622 per cardiovascular event. For the purpose of this study, we used total costs per event of CHF 200,000, which is therefore a conservative estimate. For further details refer to our calculator online [19]. The calculation of cost efficiency used by the Swiss Medical Board has been described elsewhere [16], as follows. “For one fatal cardiovascular event (myocardial infarction, stroke, coronary revascularisation), 4.5 nonfatal events occur. The cost is CHF 8500 per fatal event and CHF 25,000 per nonfatal event in the first year and CHF 8000 in subsequent years. Loss of QALYs is 1.0 for fatal and 0.2 for nonfatal events. The annual preventive medical cost per individual, including statin costs, is CHF 470, all cardiovascular events occur uniformly after 50% of the total observation time of 5 years. Loss of QALY at 2.5 years was therefore 2 × 2.5 ×1 = 5.0 QALY for fatal events and 9 × 2.5 × 0.2 = 4.5 QALYs for nonfatal events, and thus 5.0 + 4.5 = 9.5 QALYs in 1000 persons or 0.0095 QALYs per person. When this effect model is applied to a 10-year period, then 4 fatal events and 18 nonfatal events can be prevented; therefore, 4 × 5 × 1 = 20 QALYs for fatal and 18 × 5 × 0.2 = 18 QALYs for nonfatal events, or a total of 38 QALY losses, can be prevented in 1000 persons, which is 0.038 QALYs per person. Therefore, the effect model is 4 times higher in 10 years when compared with 5 years.”
Figure 2 shows the measurement of a plaque area through tracing the longitudinal surface of a carotid plaque (23 mm2). A plaque area of 23 mm2 corresponds to a vascular age of 34 years in men and 43 years in women. VA70 corresponds to a total plaque area of 108 mm2 in men and 66 mm2 in women. This represents high risk atherosclerosis: VA70 = 99th percentile for men (sensitivity 9%, specificity 97%) and 99th percentile for women (sensitivity 18%, specificity 95%) in the Tromsø study (n = 6226, with follow up of 6 years, 297 myocardial infarctions during follow up [2]. Bayes post-test risk [23] of VA70: for 4% AGLA 11% in men, 13% in women; for 10% AGLA: 25% in men and 29% in women. Therefore, VA70 transforms low into intermediate and intermediate into high cardiovascular risk.
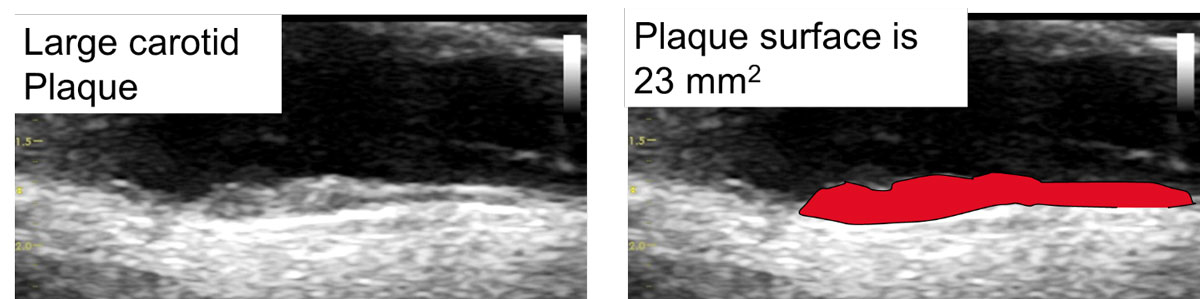
Figure 2 Vendor independent easy to perform measurement of TPA using the surface tool, resulting in an area of 23 mm2, which would correspond to an vascular age of 34 years in men and to an vascular age of 43 years in women. VA70 corresponds to a total plaque area of 108 mm2 in men and 66 mm2 in women, indicating advanced carotid atherosclerosis.
We determined the diagnostic accuracy of AGLA/PROCAM and SCORE to detect VA70 using receiver operating curves (ROCs) and derived the areas under the curves (AUCs) separately for sex and geographical region.
Subjects self-referred to the Vascular Risk Foundation gave written consent. The study protocol was approved by the local ethics committee of Solothurn, Switzerland. Practice-based subjects were entered into an anonymised study registry, for which current legislation in Switzerland and Germany does not require formal ethics committee consent.
The selection of our subjects was not based on a random population sample. Our results pertain to the primary care setting described.
We compared subjects with and without VA70 by means of an independent samples t-test for statistical analysis. For comparisons of cardiovascular risk, we used the rank-sum test because risk was not normally distributed even after log-transformation. MedCalc software (Version 16.8.4) was also used to calculate and compare ROCs [24]. The level of statistical significance was set at p <0.05.
We assessed 5383 healthy German and Swiss subjects aged 20–80 years from the Arteris Group. We excluded 1265 subjects who were outside the pre-specified age range of 40 to 65 years, and we further excluded 870 subjects who were taking statins or antihypertensive treatment at the time of the cross-sectional observation (fig. 1). Therefore, 3248 subjects remained for further evaluation.
Clinical and ultrasound information from carotid plaques for Olten area subjects (n = 1429) was collected and compared with 1819 Koblenz area subjects.
Olten area subjects were older (54, standard deviation [SD] 7 vs 50 [SD 6] years) with comparable results for the numbers of females and current smokers in each group, and for systolic blood pressure, lipids, and global risk scores (table 1).
Table 1 Clinical characteristics of the Arteris Group subjects (age 40–65, primary care, no statin or antihypertensive drug use).
| Olten area group | Koblenz area group | |
|---|---|---|
| Number of subjects (n) | 1429 | 1819 |
| Female, n (%) | 679 (48) | 640 (35) |
| Mean age, years (SD) | 53.9 (6.7) | 49.8 (6.1) |
| Family history of CAD, n (%) | 273 (19) | 408 (22) |
| Current smoker, n (%) | 332 (23) | 435 (24) |
| Blood pressure systolic, mm Hg, mean (SD) | 127 (15) | 123 (15) |
| TPA mm2, mean (SD) | 42 (41) | 39 (49) |
| Individuals with vascular age ≥ 70 | 165 (12) | 229 (13) |
| Total cholesterol, mmol/l, mean (SD) | 6.0 (1.2) | 6.0 (1.1) |
| HDL cholesterol, mmol/l, mean (SD) | 1.5 (0.5) | 1.5 (0.4) |
| LDL cholesterol, mmol/l, mean (SD) | 3.8 (1.0) | 3.9 (0.9) |
| Triglycerides, mmol/l, mean (SD) | 1.5 (0.9) | 1.7 (1.2) |
| SCORE, 10-year risk in % (SD) | 1.6 (1.6) | 1.2 (1.2) |
| PROCAM, 10-year risk in % (SD) | 5.2 (6.3) | 5.0 (6.5) |
| AGLA, 10-year risk in % (SD) | 3.7 (4.4) |
CAD = coronary artery disease; HDL = high density lipoprotein; LDL = low density lipoprotein; SD = standard deviation; TPA = total plaque area
Average 10-year risk among groups was low (SCORE CH 1.6%, SD 1.6%; SCORE DE 1.2%, SD 1.2%). In the Olten area, the prevalence of vascular age ≥70 (VA70) was 12% in women and 11% in men (11 and 14%, respectively, in the Koblenz area) and was one in eight for the entire population.
VA70 was defined as a TPA of 66 mm2 in women and of 108 mm2 in men, corresponding to the 99th percentile for each gender group in the Tromsø cohort (n = 6226, personal communication) and corresponding to the 80th and 86th percentile, respectively, in the Arteris cohort (n = 5383, supplementary table S1 in appendix 1).
Most subjects with VA70 were classified as low risk according to PROCAM/AGLA (Olten area 82%, 70% without calibration factor of 0.7; Koblenz area 57%). For SCORE, 20% of patients with VA70 were in the low risk group (<1% risk), but only 12% were classified as high-risk patients (5% risk or more) (table 2).
Table 2 Frequency distribution of VA70 among risk categories.
| n | % | |
|---|---|---|
| CH-AGLA 0.0–9.9% | 135 | 82 |
| CH-AGLA 10.0–19.9% | 23 | 14 |
| CH-AGLA ≥20.0% | 7 | 4 |
| Sum | 165 | 100 |
| DE-PROCAM 0.0–9.9% | 130 | 57 |
| DE-PROCAM 10.0–19.9% | 62 | 27 |
| DE-PROCAM ≥20.0% | 37 | 16 |
| Sum | 229 | 100 |
| SCORE 0.00–0.99 | 78 | 20 |
| SCORE 1.00–4.99 | 270 | 69 |
| SCORE ≥5.00 | 46 | 12 |
| Sum | 394 | 100 |
Subjects with VA70 (n = 394) were significantly older and were more frequently current smokers (39 vs 22%). Highly significant differences (p <0.0001) for total cholesterol, LDL cholesterol, blood pressure, and SCORE and PROCAM were found (table 3). In patients with VA70, the average vascular age in women was 79 years and 76 years in men.
Table 3 Comparison of Group 1 (VA70 present) and Group 2 (VA70 absent).
| Group 1 | Group 2 | p-value | |
|---|---|---|---|
| Number of subjects (n) | 394 | 2854 | |
| Female, n (%) | 144 (37) | 1175 (41) | 0.0800 |
| Age women, mean (SD) | 57.0 (5.8) | 51.6 (6.7) | <0.0001 |
| Age men, mean (SD) | 55.5 (5.6) | 50.5 (6.4) | <0.0001 |
| Vascular age women, mean (SD) | 79.2 (7.9) | 35.8 (16.4) | <0.0001 |
| Vascular age men, mean (SD) | 76.9 (6.4) | 39.5 (16.6) | <0.0001 |
| Family history of CAD, N (%) | 93 (24) | 588 (21) | <0.0001 |
| Current smoker, N (%) | 153 (39) | 614 (22) | <0.0001 |
| Blood pressure, mm Hg, mean (SD) | 132 (18) | 124 (15) | <0.0001 |
| TPA, mm2, mean (SD) | 131 (51) | 28 (26) | <0.0001 |
| Total cholesterol, mmol/l, mean (SD) | 6.4 (1.6) | 6.0 (1.1) | <0.0001 |
| HDL cholesterol, mmol/l, mean (SD) | 1.4 (0.4) | 1.5 (0.4) | 0.0001 |
| LDL cholesterol, mmol/l, mean (SD) | 4.2 (1.1) | 3.8 (0.9) | <0.0001 |
| Triglycerides, mmol/l, mean (SD) | 1.7 (1.1) | 1.6 (1.1) | 0.0043 |
| PROCAM, 10-year risk in % (SD) | 9.9 (9.6) | 4.4 (5.5) | <0.0001 |
| SCORE, 10-year risk in % (SD) | 2.6 (2.1) | 1.2 (1.3) | <0.0001 |
CAD = coronary artery disease; HDL = high density lipoprotein; LDL = low density lipoprotein; SD = standard deviation; TPA = total plaque area
At current intervention thresholds (AGLA/PROCAM ≥10% 10-year risk, SCORE ≥ 5% 10-year risk), the sensitivity to detect VA70 was 6% (95% CI 2–14%) in Olten area women (higher in men at 30%, 95% CI 21–41%) and was 8% (95% CI 3–18%) in women from the Koblenz area (higher in men at 56%, 95% CI 48–64%); similarly, for SCORE, sensitivity for women from the Olten area was 5% (18% in men) and was 0% for women from the Koblenz area (16% in men) (table 2). For lower-risk decision thresholds, sensitivity increased and specificity decreased: for AGLA 2%, sensitivity was 54% in women (90% in men) in the Olten area and for PROCAM 2% was 47% in women and 96% in men in the Koblenz area. On the other hand, for VA70 specificity was very high in women (99–100%) and in men (83–94%) at the decision thresholds of 10 and 5% (table 4).
Table 4 Sensitivity (Sens) and specificity (Spec) of AGLA, PROCAM, and SCORE to detect VA70 at various decision cut-offs for female and male subjects from the Olten area and the Koblenz area including 95% confidence intervals (Note: AGLA = PROCAM × 0.7).
| Olten area | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male |
|---|---|---|---|---|---|---|---|---|---|---|
| AGLA 2% | AGLA 4% | AGLA 6% | AGLA 8% | AGLA 10% | ||||||
| Sens | 54 (42–65) | 90 (82–96) | 33 (23–44) | 72 (61–82) | 16 (9–26) | 53 (42–64) | 11 (5–20) | 40 (29–51) | 6 (2–14) | 30 (21–41) |
| Spec | 77 (73–80) | 30 (26–33) | 92 (90–94) | 57 (53–61) | 96 (95–98) | 73 (70–77) | 98 (96–99) | 81 (78–84) | 99 (97–99) | 87 (84–89) |
| SCORE 1% | SCORE 2% | SCORE 3% | SCORE 4% | SCORE 5% | ||||||
| Sens | 73 (62–82) | 95 (88–99) | 33 (23–44) | 76 (65–85) | 15 (8–24) | 52 (41–63) | 10 (4–18) | 31 (22–42) | 5 (1–12) | 18 (11–28) |
| Spec | 67 (63–71) | 31 (28–35) | 89 (86–92) | 59 (56–63) | 97 (96–98) | 79 (76–82) | 99 (98–100) | 90 (87–92) | 99 (98–100) | 94 (92–96) |
| Koblenz Area | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male |
| PROCAM 2% | PROCAM 4% | PROCAM 6% | PROCAM 8% | PROCAM 10% | ||||||
| Sens | 47 (34–60) | 96 (92–98) | 21 (12–33) | 86 (80–91) | 11 (5–22) | 75 (68–82) | 8 (3–18) | 63 (68–82) | 8 (3–18) | 56 (48–64) |
| Spec | 84 (81–87) | 24 (22–27) | 94 (92–96) | 50 (47–53) | 97 (95–98) | 66 (63–69) | 98 (97–99) | 78 (75–80) | 99 (98–100) | 83 (81–86) |
| SCORE 1% | SCORE 2% | SCORE 3% | SCORE 4% | SCORE 5% | ||||||
| Sens | 35 (24–49) | 92 (87–96) | 10 (4–20) | 65 (58–73) | 2 (0–9) | 40 (32–47) | 0 (0–6) | 26 (19–33) | 0 (0–6) | 16 (10–22) |
| Spec | 90 (88–93) | 52 (49–56) | 97 (95–98) | 79 (77–82) | 99 (98–100) | 91 (89–93) | 100 (99–100) | 96 (95–97) | 100 (99–100) | 98 (97–99) |
Using SCORE in women, 17% were shifted from low risk to intermediate or high risk on the basis of their vascular age (21% men) (table 5).
Table 5 Reclassification based on carotid atherosclerosis in low risk subjects calculated for sex and risk assessment tool (SCORE/PROCAM) using either vascular age (SCORE only) or Bayes theorem (SCORE and PROCAM).
| Reclassification with SCORE and PROCAM | Female | Male | ||||||
|---|---|---|---|---|---|---|---|---|
| n | % | 10-year risk | LDL | n | % | 10-year risk | LDL | |
| Low risk by SCORE | 983 | 100 | 0.3 | 3.6 | 755 | 100 | 0.6 | 3.7 |
| Reclassification to higher risk by arterial age | 163 | 17 | 3.6 | 3.8 | 155 | 21 | 3.4 | 3.8 |
| Reclassification to higher risk by Bayes theorem | 274 | 28 | 1.7 | 3.9 | 261 | 35 | 1.4 | 3.8 |
| Number needed to image with Bayes Theorem | 3.6 | 2.9 | ||||||
| Low risk by AGLA/PROCAM | 1293 | 100 | 1.4 | 3.7 | 1552 | 100 | 3.8 | 3.7 |
| Reclassification to higher risk by Bayes theorem | 96 | 7 | 15.6 | 4.8 | 305 | 20 | 14.5 | 4.1 |
| Number needed to image with Bayes theorem |
13 | 5 | ||||||
Using PROCAM In women, 7% were shifted from low risk to intermediate or high risk using their vascular age (20% men). The number needed to image was 13.5 in women (5.1 in men). In women using SCORE, 28% were shifted from low risk to intermediate or high risk using their vascular age (35% men). The number needed to image was 3.6 in women (2.9 in men) (table 5).
The inclusion of imaging costs was cost effective for both the PEP and the QALY model, even with a lower RRR of active treatment of 22% per 1 mmol/l LDL reduction. Using SCORE or PROCAM, there was a cost saving of between about CHF 2400 and CHF 21,000 per avoided event. Cost/QALY were within commonly accepted thresholds of 150,000 CHF/QALY or resulted even in a return on investment, such as in women assessed with PROCAM (table 6a). The calculation of different models for a 22% RRR with LDL reduction of 1.0 mmol/l resulted in a cost-efficiency threshold for imaging between 940 and 3200 CHF per imaged patient (table 6b).
Table 6 a: Cost-efficiency analysis in CHF based on the personalised price (PEP) and cost per quality adjusted life-year (QALY) models.
| SCORE | PROCAM | |||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Number needed to image | 3.6 | 2.9 | 13.5 | 5.1 |
| ROI per patient / PEP model 35% RRR | −8784 | −6319 | −21,306 | −16,718 |
| ROI per patient / PEP model 22% RRR | −3982 | −2466 | −11,445 | −8,888 |
| Cost/QALY model 35% RRR per patient | 8588 | 16,789 | −5784 | −4686 |
| Cost/QALY model 22% RRR per patient | 30,427 | 43,539 | 7,452 | 9,207 |
| ROI = return on investment; RRR = relative risk reduction | ||||
Table 6b Maximum threshold for cost efficiency in the personalised price (PEP) and the quality adjusted life-year (QALY) models.
| Relative risk reduction 22% | ||||
|---|---|---|---|---|
| Female | Male | |||
| SCORE | AGLA/PROCAM | SCORE | AGLA/PROCAM | |
| Cost/event threshold of CHF 200,000 | 1200 | 960 | 940 | 1840 |
| Cost/QALY threshold of CHF 150,000 | 2400 | 1600 | 2100 | 3200 |
We assessed the diagnostic accuracy of PROCAM and SCORE to detect women and men with VA70 by geographical region. Per region and sex, there were no significant differences between risk measurement tools (SCORE/PROCAM) (table 7).
Table 7 Area under receiver operating curves by sex, SCORE/PROCAM and region for the detection of VA70.
| SCORE | ||
|---|---|---|
| Female | Male | |
| Olten | 0.757 (95% CI 0.723–0.789) p <0.0001 | 0.750 (95% CI 0.717–0.780) p <0.0001 |
| Koblenz | 0.805 (95% CI 0.773–0.835) p <0.0001 | 0.820 (95% CI 0.797–0.841) p <0.0001 |
| AGLA/PROCAM | ||
| Female | Male | |
| Olten | 0.736 (95% CI 0.701–0.769) p <0.0001 | 0.694 (95% CI 0.660–0.727) p <0.0001 |
| Koblenz | 0.773 (95% CI 0.739–0.805) p <0.0001 | 0.777 (95% CI 0.753–0.801) p <0.0001 |
We found a clinically relevant prevalence of old arteries (VA70), a highly specific marker for future myocardial infarction, in one out of eight of 3238 practice-based healthy subjects aged 40–65 years. In the Olten area, 543 had a low risk of <10%, defined with AGLA, and VA70 was found in 135 out of 165 (82%) of these low risk subjects (table 2). In the Koblenz area, 1543 subjects had a low risk of <10% defined with PROCAM, and VA70 was found in 130 out of 229 (57%). Therefore, high-risk carotid plaque (VA70) shows the highest frequency distribution in subjects classified as low risk by PROCAM/AGLA. Further, only 12% of subjects with VA70 were classified as high-risk patients by SCORE (table 2).
This finding raises several important questions about the allocation of resources in preventive medicine, which is traditionally focused on high-risk patients, despite the fact that most cardiovascular events occur in low-risk subjects, as shown in Switzerland [1] and in Denmark [3, 25]. Therefore, carotid imaging, as well as observational studies, have congruently revealed that a focus on high-risk patients, as proposed by the Swiss Medical Board [15], fails by a large proportion to identify most patients at risk and is therefore unable to reduce the cardiovascular disease burden imposed on individuals and society. Important issues about sensitivity of cardiovascular risk charts to trigger intensified preventive medical efforts and the potential for reclassification of risk with carotid imaging at an affordable cost appear inevitable in order to substantially improve the current situation.
As outlined in table 4, an AGLA risk of 4% instead of 10% is much better suited for the definition of intermediate or higher risk in Olten area women (sensitivity 33%, specificity 92%), and current Swiss guidelines may therefore need further adjustments for women in order to detect at least 33% instead of only 6% of women having VA70. To obtain acceptable sensitivities, decision thresholds for SCORE ought also to be lowered in women to 1–2% and in men to 3–4% instead of 5%.
Alternatively, we show how carotid ultrasound can be used to better detect higher-risk subjects defined as being at low risk by PROCAM or SCORE (table 5). Since in Switzerland up to 50% of acute coronary syndromes occur in healthy persons defined by risk charts to be at low risk [1], it appears appropriate to filter out high-risk subjects from the low-risk segment in order to obtain a reclassification to a higher risk category where more intensive measures of prevention could be applied.
In this study, the Bayes theorem was used to calculate post-test probabilities in those with carotid plaque. For this purpose, the sensitivity and specificity of a given TPA in a patient was entered into the Bayes formula. Using this approach, in our study primary care subjects were shifted in 7–34% of cases (depending on gender and the pretest probability used) from low to a higher risk category. The number of low-risk subjects needed to image ranged between 3 and 15 (table 5).
All low risk subjects defined with either SCORE or PROCAM had a carotid imaging examination. Those shifted from low to intermediate or high cardiovascular risk formed the intervention group, where the effect simulation allowed for a 50% LDL reduction at a cost of 0.68 CHF/d (which is the effect of 20 mg rosuvastatin per day,) and the RRR per 1 mmol/l LDL reduction was 22% (any patient) and 35% (primary care patient with cardiovascular risk <20%) in 10 years [17]. This approach allowed us to calculate several possibilities (table 6a) and the maximum cost per ultrasound examination that would allow cost efficiency according to the defined thresholds (table 6b). Using these assumptions, we found a high cost efficiency when carotid ultrasound was added to the clinical work-up in low-risk patients.
As shown in table 7, the AUC as a measure of diagnostic accuracy showed similar results for the different calculators (0.75–0.82); however, in men from the Olten area there was a trend to lower accuracy (0.69).
Imaging carotid arteries to quantify carotid plaque burden is valid, because carotid TPA accurately predicts cardiovascular events, similar to calcifications in coronary arteries [5, 26, 27]. We previously derived our vascular age risk tool from TPA in 1500 men and women by substitution of chronological age by vascular age in the PROCAM function, and we validated this approach externally in a Canadian cohort of 684 primary care subjects with an observation time of 2.6 years on average and where 13 myocardial infarctions occurred [7]: AUC was significantly improved by 0.13 from 0.65 to 0.78 (p = 0.02). Therefore, we were able to furnish a preliminary validation of our vascular age tool. Our measurement of plaque area is manufacturer independent, encompasses the whole carotid artery from clavicles to lower jaw (not just 6 cm as described in the PESA study [28]), does not need any costly software and the tracings are made in a few minutes. Therefore, TPA has the potential for widespread use in primary care, since the cost of ultrasound machines with included software is below EUR 5000 and measurements are very easy to perform. Others use 3D imaging of atherosclerotic plaque [28, 29], but the clinical use is limited by costs, calcified plaque, vendor dependence (Philips iU22 ultrasound system), limited field of view (imaging length of 3.8 cm with the potential to miss significant amounts of plaque) and need for specialised centres to quantify the total plaque volume [28]. Further, the correlation coefficients for 2D-TPA and 3D plaque imaging are good, whereas the correlation coefficients for carotid intima-media thickness and 3D plaque imaging in carotid arteries are very poor [30].
Vascular age has also been used by others as a surrogate marker for cardiovascular risk, and may be used to replace chronological age in risk equations. The additional information regarding the burden of atherosclerosis derived from coronary calcifications, TPA and intima-media thickness has recently been reviewed extensively [31]. It is acknowledged that the presence of carotid plaque is a high-risk cardiovascular finding [13, 32, 33]. The addition of subclinical markers in cardiovascular risk prediction has been shown to improve the performance of SCORE [34], and US and European guidelines recommend treating atherosclerosis detected by means of imaging [35–37].
The following limitations have to be addressed. Our vascular age tool was used within a clinical setting as part of routine measurements by two different observers and the remarkable congruence of the findings from the two imaging centres may be viewed as a mutual confirmation of our findings. We examined practice-based subjects in order to validate currently recommended risk thresholds, since decisions about the intensity of preventive therapy are made at the practice level. Whether our findings can be generalised to the population level needs to be confirmed; however, they do call for a better validation of risk prediction tools as also discussed recently in a comprehensive overview [38]. Finally, VA70 is a highly specific (≈95%) but poorly sensitive (≈10%) marker for the occurrence of myocardial infarction [5]. However, if VA70 is used in serial testing with PROCAM/SCORE as the first test, then post-test probability increases according to the Bayes theorem by a factor of ≈2 [39, 40]. Therefore, when looking at reclassification of risk, a highly specific marker of risk is helpful.
In our practice-based middle-aged (40–65 years) and predominantly low-risk group of 3248 subjects without cardiovascular disease and without preventive use of statins and antihypertensive drugs, we found that the prevalence of old arteries (VA70) was one in eight and that current decision thresholds using PROCAM, AGLA, or SCORE had a very low sensitivity, especially in women, to detect old arteries. There are two potential solutions to this problem. Either intermediate risk is defined as, for example, 5% risk in 10 years instead of 10% for PROCAM/AGLA, or a reclassification is applied using ultrasound of carotid imaging as shown in this study. This has the potential to cost-effectively allocate preventive resources to those estimated as having low-risk, but have atherosclerosis to an amount that shifts them from low-risk to a higher cardiovascular risk category.
Table S1 Percentile value of VA70 in the Arteris (n = 5383) and Tromsø cohorts (n = 6226).
| Total plaque area (mm2) | Arteris | Tromsø | |
|---|---|---|---|
| Female | 66 | 80th | 99th |
| Male | 108 | 86th | 99th |

Figure S1 Intraobserver variability for total plaque area (M.R.).
Vascular Risk Foundation VARIFO, www.varifo.ch
No conflict of interest relevant to this article was reported.
1 Selby K , Nanchen D , Auer R , Gencer B , Räber L , Klingenberg R , et al. Low statin use in adults hospitalized with acute coronary syndrome. Prev Med. 2015;77:131–6. doi:.https://doi.org/10.1016/j.ypmed.2015.05.012
2 Mortensen MB , Afzal S , Nordestgaard BG , Falk E . The high-density lipoprotein-adjusted SCORE model worsens SCORE-based risk classification in a contemporary population of 30,824 Europeans: the Copenhagen General Population Study. Eur Heart J. 2015;36(36):2446–53. doi:.https://doi.org/10.1093/eurheartj/ehv251
3 Mortensen MB , Falk E . Real-life evaluation of European and American high-risk strategies for primary prevention of cardiovascular disease in patients with first myocardial infarction. BMJ Open. 2014;4(10):e005991. doi:.https://doi.org/10.1136/bmjopen-2014-005991
4 Riesen WF , Darioli R , Noseda G , Bertel O , Buser P . Empfehlungen zur Prävention der Atherosklerose. Schweiz Arzteztg. 2005;86(22):1355–61. doi:.https://doi.org/10.4414/saez.2005.11250
5 Johnsen SH , Mathiesen EB , Joakimsen O , Stensland E , Wilsgaard T , Løchen M-LL , et al. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6-year follow-up study of 6226 persons: the Tromsø Study. Stroke. 2007;38(11):2873–80. doi:.https://doi.org/10.1161/STROKEAHA.107.487264
6 Navar-Boggan AM , Peterson ED , D’Agostino RB, Sr , Pencina MJ , Sniderman AD . Using age- and sex-specific risk thresholds to guide statin therapy: one size may not fit all. J Am Coll Cardiol. 2015;65(16):1633–9. doi:.https://doi.org/10.1016/j.jacc.2015.02.025
7 Romanens M , Ackermann F , Sudano I , Szucs T , Spence JDD . Arterial age as a substitute for chronological age in the AGLA risk function could improve coronary risk prediction. Swiss Med Wkly. 2014;144:w13967. doi:.https://doi.org/10.4414/smw.2014.13967
8Romanens M. Vascular risk foundation (Varifo) [Internet]. Available from: www.varifo.ch
9 Adams A , Bojara W . Vorhersage einer stenosierenden KHK durch Bestimmung von Plaque-Fläche und -Dicke vs. IMT an der A. carotis [Prediction of coronary artery stenosis by measurement of total plaque area and thickness versus intima media thickness of the carotid artery]. Herz. 2015;40(5):817–22. Article in German. doi:.https://doi.org/10.1007/s00059-015-4312-5
10 Descamps OS , Cooney MT , De Backer G , Graham I . A simple multiplier to calculate the impact of HDL cholesterol on cardiovascular risk estimation using SCORE. Atherosclerosis. 2012;222(2):564–6. doi:.https://doi.org/10.1016/j.atherosclerosis.2012.03.035
11 Perk J , De Backer G , Gohlke H , Graham I , Reiner Z , Verschuren M , et al.; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33(13):1635–701. doi:.https://doi.org/10.1093/eurheartj/ehs092
12 Assmann G , Schulte H , Cullen P , Seedorf U . Assessing risk of myocardial infarction and stroke: new data from the Prospective Cardiovascular Münster (PROCAM) study. Eur J Clin Invest. 2007;37(12):925–32. doi:.https://doi.org/10.1111/j.1365-2362.2007.01888.x
13Eckardstein A. AGLA Guidelines [Internet]. 2014 [cited 2016 Aug 1]. Available from: www.agla.ch
14 Romanens M , Ackermann F , Sudano I , Szucs T , Riesen WF , Darioli R , et al. LDL-cholesterol and the potential for coronary risk improvement: evidence from a practice based carotid Imaging study. Cardiovasc Med. 2011;14(12):345–50. doi:.https://doi.org/10.4414/cvm.2011.01628
15Felder S, Jüni P, Meier CA, et al. SMB Statin Recommendation [Internet]. 2014. Available from: http://www.medical-board.ch/fileadmin/docs/public/mb/fachberichte/2014-07-21_bericht_statine_final_anpassung.pdf
16 Romanens M , Sudano I , Szucs T , Adams A . Medical costs per QALY of statins based on Swiss Medical Board assumptions. Cardiovasc Med. 2017;17:96–100. doi:
17 Mihaylova B , Emberson J , Blackwell L , Keech A , Simes J , Barnes EH , et al., Cholesterol Treatment Trialists’ (CTT) Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581–90. doi:.https://doi.org/10.1016/S0140-6736(12)60367-5
18Cholesterol Treatment Trialists’ Ctt Collaborators. CTT Appendix Online 2012. Append online [Internet]. Available from: https://researchonline.lshtm.ac.uk/1649027/1/mmc1.pdf.
19Romanens M. VARIFO Cost Model for direct and indirect costs of cardiovascular events in Switzerland [Internet]. 2018. Available from: www.docfind.ch/CVDCosts2011.xlsx
20Wieser S, Tomonaga Y, Riguzzi M, Fischer B, Telser H, Pletscher M, et al. Die Kosten der nicht übertragbaren Krankheiten in der Schweiz. 2014;195. Available from: http://www.bag.admin.ch/themen/medizin/00683
21Philipp Lyrer. Epidemiologie des Hirnschlages. Schweizerische Aerztezeitung [Internet]. 2000 [cited 2018 Mar 30];81:835–8. Available from: https://saez.ch/de/resource/jf/journal/file/view/article/saez/de/saez.2000.07245/2000-16-224.pdf/
22 Yeo KK , Zheng H , Chow KY , Ahmad A , Chan BPL , Chang HM , et al. Comparative analysis of recurrent events after presentation with an index myocardial infarction or ischaemic stroke. Eur Heart J Qual Care Clin Outcomes. 2017;3(3):234–42.
23 Romanens M , Ackermann F , Spence JD , Darioli R , Rodondi N , Corti R , et al. Improvement of cardiovascular risk prediction: time to review current knowledge, debates, and fundamentals on how to assess test characteristics. Eur J Cardiovasc Prev Rehabil. 2010;17(1):18–23. doi:.https://doi.org/10.1097/HJR.0b013e3283347059
24MedCalc Software bvba, Ostend B. MedCalc Statistical Software version 17.8.1 [Internet]. 2017. Available from: http://www.medcalc.org
25 Mortensen MB , Falk E . Limitations of the SCORE-guided European guidelines on cardiovascular disease prevention. Eur Heart J. 2017;38(29):2259–63 .
26 Baber U , Mehran R , Sartori S , Schoos M , Falk E , Sillesen H , et al. Detection and Impact of Subclinical Coronary and Carotid Atherosclerosis on Cardiovascular Risk Prediction and Reclassification in Asymptomatic Us Adults: Insights From the High Risk Plaque Bioimage Study. J Am Coll Cardiol. 2014;63(12):A998. doi:.https://doi.org/10.1016/S0735-1097(14)60998-0
27 Spence JD , Eliasziw M , DiCicco M , Hackam DG , Galil R , Lohmann T . Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke. 2002;33(12):2916–22. doi:.https://doi.org/10.1161/01.STR.0000042207.16156.B9
28 López-Melgar B , Fernández-Friera L , Oliva B , García-Ruiz JM , Peñalvo JL , Gómez-Talavera S , et al. Subclinical Atherosclerosis Burden by 3D Ultrasound in Mid-Life: The PESA Study. J Am Coll Cardiol. 2017;70(3):301–13. doi:.https://doi.org/10.1016/j.jacc.2017.05.033
29 Fernández-Friera L , Peñalvo JL , Fernández-Ortiz A , Ibañez B , López-Melgar B , Laclaustra M , et al. Prevalence, Vascular Distribution, and Multiterritorial Extent of Subclinical Atherosclerosis in a Middle-Aged Cohort: The PESA (Progression of Early Subclinical Atherosclerosis) Study. Circulation. 2015;131(24):2104–13. doi:.https://doi.org/10.1161/CIRCULATIONAHA.114.014310
30 Lindenmaier TJ , Buchanan DN , Pike D , Hartley T , Reid RD , Spence JD , et al. One, two and three-dimensional ultrasound measurements of carotid atherosclerosis before and after cardiac rehabilitation: preliminary results of a randomized controlled trial. Cardiovasc Ultrasound. 2013;11(1):39. doi:.https://doi.org/10.1186/1476-7120-11-39
31 Groenewegen KA , den Ruijter HM , Pasterkamp G , Polak JF , Bots ML , Peters SA . Vascular age to determine cardiovascular disease risk: A systematic review of its concepts, definitions, and clinical applications. Eur J Prev Cardiol. 2016;23(3):264–74. doi:.https://doi.org/10.1177/2047487314566999
32 Robinson JG , Stone NJ . The 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk: a new paradigm supported by more evidence. Eur Heart J. 2015;36(31):2110–8. doi:.https://doi.org/10.1093/eurheartj/ehv182
33 Piepoli M , Hoes A , Agewall S , Albus C , Brotons C , Catapano A , et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice, update 2016. Eur Heart J. 2016;37(29):2315–81. doi:.https://doi.org/10.1093/eurheartj/ehw106
34 Sehestedt T , Jeppesen J , Hansen TW , Wachtell K , Ibsen H , Torp-Pedersen C , et al. Risk prediction is improved by adding markers of subclinical organ damage to SCORE. Eur Heart J. 2010;31(7):883–91. doi:.https://doi.org/10.1093/eurheartj/ehp546
35 Mansia G , De Backer G , Dominiczak A , Cifkova R , Fagard R , Germano G , et al., European Society of Cardiology. 2007 ESH-ESC Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Blood Press. 2007;16(3):135–232. doi:.https://doi.org/10.1080/08037050701461084
36 De Backer G , Ambrosioni E , Borch-Johnsen K , Brotons C , Cifkova R , Dallongeville J , et al., European Society of Cardiology Committee for Practice Guidelines. European guidelines on cardiovascular disease prevention in clinical practice: third joint task force of European and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of eight societies and by invited experts). Eur J Cardiovasc Prev Rehabil. 2003;10(4):S1–10. doi:.https://doi.org/10.1097/00149831-200308000-00004
37 Stone NJ , Robinson JG , Lichtenstein AH , Bairey Merz CN , Blum CB , Eckel RH , et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–934. doi:.https://doi.org/10.1016/j.jacc.2013.11.002
38 Damen JAAG , Hooft L , Schuit E , Debray TPA , Collins GS , Tzoulaki I , et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416. doi:.https://doi.org/10.1136/bmj.i2416
39 Spitalnic S . Test Properties 2: Likelihood Ratios, Bayes’ Formula, and Receiver Operating Characteristic Curves. Hosp Physician. 2004;(October):53–8.
40 Bayes T , Price M . An essay towards solving a problem in the doctrine of chances. Philos Trans. 1763;53(0):370–418. doi:.https://doi.org/10.1098/rstl.1763.0053
Vascular Risk Foundation VARIFO, www.varifo.ch
No conflict of interest relevant to this article was reported.