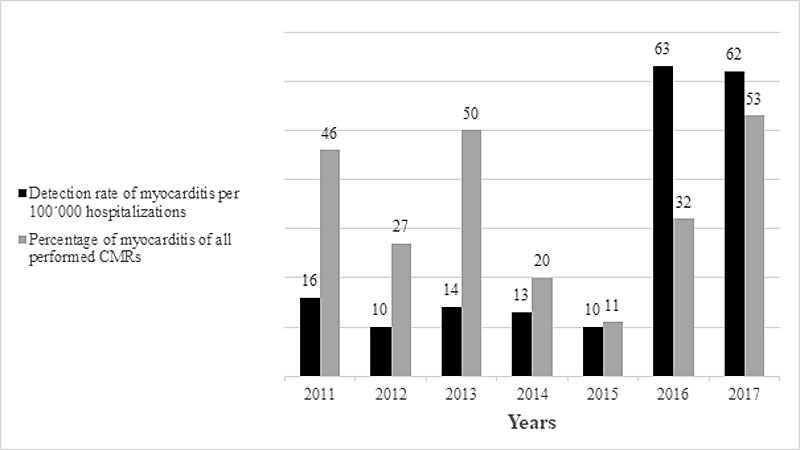
Figure 1 Detection rate of myocarditis diagnosed using cardiac magnetic resonance imaging in the years2011–2017.
DOI: https://doi.org/10.4414/smw.2019.20098
Myocarditis is a heterogeneous inflammatory heart disease which manifests itself through a variety of symptoms [1, 2]. Over time, its clinical development can take an adverse trajectory and result in dilated cardiomyopathy (DCM), impaired left ventricular ejection fraction (LVEF) [3], malignant arrhythmias and sudden cardiac death (SCD) [2, 4–6]. Even though endomyocardial biopsy and cardiac magnetic resonance imaging (CMR) are increasingly used to diagnose myocarditis [2, 7, 8], the true incidence of myocarditis remains unknown [9]. Post mortem studies have identified myocarditis in up to 42% [5, 10] of cases of SCD, suggesting a significant epidemiological impact. Hence, a systematic change of the diagnostic algorithm may be necessary to improve the rate of myocarditis detection in patients [11].
As symptoms of myocarditis can mimic myocardial infarction and lead to elevated troponin levels [2] [12], inflammatory cardiomyopathy is commonly found amongst patients with angina-like symptoms, elevated high-sensitivity troponin-T (TnT-hs) and unobstructed coronaries (i.e. myocardial infarction and non-obstructive coronary artery disease [MINOCA]) [13–15]. This clinically challenging population often does not receive specific treatment [16, 17] and was found to display an increased mortality rate [18]. In patients with MINOCA, multiple underlying mechanisms have been suspected to be the cause of the disease, including coronary spasm, coronary microvascular dysfunction, myocarditis, Takotsubo syndrome (TTS), spontaneous coronary thrombosis and emboli [18]. Interestingly, myocarditis is one of the most common underlying causes within this patient population, with the percentage of cases ranging from 16 to 50% [19, 20].
While endomyocardial biopsy (EMB) remains the gold standard [21] for the diagnosis of myocarditis, CMR has been established as a non-invasive alternative for the diagnosis of myocarditis, when EMB is not indicated [14, 22]. In a previous pilot study, we showed that the introduction of a novel diagnostic algorithm using a lowered threshold for CMR led to a 6.3-fold increase of the incidence of myocarditis in patients with MINOCA. Furthermore, the algorithm increased the percentage of CMRs that tested positive for myocarditis [23]. In order to confirm these first results, we expanded our analysis to the preceding and subsequent years, thereby evaluating a potential temporary incidence peak in 2016. We hypothesised that the detection rate of myocarditis would continue to be higher in 2017, when CMR was systematically applied, compared to the detection rate between 2011 and 2015, when CMR was performed only when there had been moderate to high clinical suspicion of myocarditis.
This was a retrospective single-centre study investigating a new diagnostic algorithm for myocarditis amongst patients with MINOCA. The study was performed at the University Hospital of Zurich, a tertiary health care centre with 980 hospital beds, covering the catchment area of the Canton of Zurich with approximately 1.5 million citizens. The cohort includes patients who were admitted to the University Hospital Zurich between January 2011 and December 2017 with angina-like symptoms, elevated levels of TnT-hs (reference range 0–14 ng/l) and non-obstructive coronary artery disease (CAD) (<50% stenosis) and who underwent CMR for further diagnostic work-up. CAD was excluded using coronary angiography by cardiac catheterization or computed tomography (CT) and CMR if pretest probability of CAD was very low [23]. No patients were excluded. CMR analyses were performed under the supervision of the head of cardiac imaging who performed CMR between early 2011 and late 2018 and who had more than eleven years of experience in CMR.
In the years 2011 to 2015, only patients with classical symptoms of myocarditis, such as chest pain, dyspnoea and palpitations after viral prodrome, underwent CMR. Starting in 2016, the threshold for CMR imaging was lowered to examine all unexplained cases of MINOCA in line with current guidelines [24]. A MRI was performed within 90 days after symptom onset.
The primary goal of this study was to measure the detection rate of myocarditis amongst patients suffering from MINOCA, using systematic CMR work-up at our institution, the University Hospital Zurich, before and after implementation of a new diagnostic work-up algorithm. To evaluate the impact of this use of CMR, we compared the number of detected cases of myocarditis after its introduction in 2016 and 2017 with the preceding five years, from 2011 to 2015.
In addition, we compared baseline characteristics of patients with MINOCA without signs for myocarditis, with the characteristics of patients suffering from myocarditis. We described the presence of other types of cardiomyopathies identified by CMR amongst patients with MINOCA at the University Hospital Zurich between 2011 and 2017. Finally, we evaluated potential differences in our baseline characteristics between patients with myocarditis and MINOCA in order to evaluate potential clinical differences.
Clinical data were generated with the help of the University Hospital Zurich’s electronic medical records database. All patients were anonymised and numbered sequentially. IBM SPSS Statistics version 22 and MedCalc version 18.5 were used to carry out calculations and analysis. The study was approved by the Ethics Committee of Zurich (PB_2016_01152).
CMR images were generated using a 1.5 or 3.0 Tesla scanner (SiemensSkyra, Erlangen, Germany or Phillips Achieva Best, Netherlands) with electrocardiography-gated breath-hold protocol and weight-adapted dose of gadolinium-based contrast agent (Gadovist, Bayer AG, Zurich, Switzerland) (0.1 mmol/kg). Diagnosis of myocarditis was based on cine-CMR, T2-weighted imaging and Late Gadolinium Enhancement (LGE) images [25–27]. Edema was assessed using T2-weighted images. Images were obtained using a surface coil [25–27]. LGE images were acquired 10 minutes after an intravenous injection of a gadolinium-based contrast agent and were used to detect necrosis. LGE was categorised according to its localisation, distribution and pattern using the 17 segment model. Epicardial or midwall distribution of LGE, associated with a patchy pattern and non-coronary distribution, were regarded as potential indicators for myocarditis.
Descriptive statistics were used to examine clinical data. Metric scale parameters were described as averages accompanied by their standard deviation. Nominal scale parameters were reported as total numbers and as percentages of the total number of patients who underwent CMR in a particular year. Diagnoses of rare diseases, such as scleroderma, pericardial effusion and coronary anomalies, were summarised as “others”. Baseline characteristics of patients with myocarditis vs. normal CMR findings were compared using Student’s t-test and chi-square test for equality of proportions. A p-value <0.05 was considered significant for all statistical tests.
In order to compare the detection rate for myocarditis by CMR in 2016 and 2017 with that between 2011 and 2015, percentages of positive CMR findings for myocarditis for each time interval were compared using the chi-square test. Detection rate among the total number of patients who underwent CMR at the University Hospital of Zurich was calculated. The detection ratio compares the total rate between 2011 and 2015 to that between 2016 and 2017.
A total of 556 patients who were admitted to hospital with MINOCA between January 2011 and December 2017, underwent CMR. The mean age of this group was 57 years (range 19–92 years) with 392 (70.5%) male patients and an average LVEF of 51 ± 15% measured by CMR (table 1). The median time between symptom onset and CMR was 10 days (IQR 4–23 days).
Table 1 Baseline parameters of patients undergoing cardiac magnetic resonance imaging in the years 2011–2017.
| Year | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | Total |
|---|---|---|---|---|---|---|---|---|
| Number of patients | n = 35 | n = 27 | n = 28 | n = 62 | n = 88 | n = 199 | n = 117 | n = 556 |
| Male, n (%) | 26 (74.3) | 18 (66.7) | 22 (78.6) | 36 (58.1) | 71 (80.7) | 139 (70) | 80 (68.4) | 392 (71) |
| Age, % ± δ | 54 ± 16 | 56 ± 17 | 55 ± 15 | 52 ± 17 | 66 ± 15 | 60 ± 16 | 49 ± 18 | 57 ± 17 |
| LVEF, % ± δ | 45 ± 19 | 52 ± 18 | 56 ± 15 | 52 ± 15 | 48 ± 15 | 51 ± 13 | 54 ± 14 | 51 ± 15 |
LVEF = left ventricular ejection fraction
Patterns of myocarditis were present in 76 patients (13.7%), DCM in 34 (6.1%), ischaemia during stress in 41 (7.4%), hypertrophic (HCM) in 15 (2.7%), pericarditis in 13 (2.3%), amyloidosis in 8 (1.4%), TTS in 7 (1.3%), sarcoidosis in 5 (0.9%), Fabry’s disease in 3 (0.5%) and “other” diagnoses were present in 22 patients (4%). In total, 332 (59.7%) of the performed CMRs were deemed unremarkable (table 2).
Table 2 Diagnoses based on cardiac magnetic resonance imaging in the years 2011–2017.
| Year | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | Total |
|---|---|---|---|---|---|---|---|---|
| CMRs performed | n = 35 | n = 27 | n = 28 | n = 62 | n = 88 | n = 199 | n = 117 | n = 556 |
| Normal heart, n (%) | 18 (51.4) | 13 (48.1) | 14 (50) | 43 (69.4) | 68 (77.3) | 110 (55) | 66 (56.4) | 332 (60) |
| Myocarditis, n (%) | 6 (17.1) | 4 (14.8) | 5 (17.8) | 5 (8.1) | 4 (4.5) | 26 (13.1) | 26 (22.2) | 76 (13.7) |
| Ischaemia, n (%) | 1 (2.9) | 1 (3.7) | 1 (3.6) | 1 (1.6) | 8 (9.1) | 26 (13.1) | 3 (2.6) | 41 (7.4) |
| Pericarditis, n (%) | 0 (0) | 0 (0) | 1 (3.6) | 2 (3.2) | 0 (0) | 4 (2) | 6 (5.1) | 13 (2.3) |
| Amyloidosis, n (%) | 1 (2.9) | 0 (0) | 0 (0) | 1 (1.6) | 1 (1.1) | 2 (1) | 3 (2.6) | 8 (1.4) |
| Sarcoidosis, n (%) | 1 (2.9) | 0 (0) | 0 (0) | 0 (0) | 1 (1.1) | 1 (0.5) | 2 (1.7) | 5 (0.9) |
| FD, n (%) | 0 (0) | 1 (3.7) | 0 (0) | 0 (0) | 1 (1.1) | 1 (0.5) | 0 (0) | 3 (0.5) |
| TTS, n (%) | 0 (0) | 0 (0) | 1 (3.6) | 0 (0) | 0 (0) | 4 (2) | 2 (1.7) | 7 (1.3) |
| DCM, n (%) | 6 (17.1) | 6 (22.3) | 6 (21.4) | 9 (14.5) | 0 (0) | 1 (0.5) | 6 (5.1) | 34 (6.1) |
| HCM, n (%) | 2 (5.7) | 2 (7.4) | 0 (0) | 0 (0) | 2 (2.3) | 9 (4.5) | 0 (0) | 15 (2.7) |
| Others, n (%) | 0 (0) | 0 (0) | 0 (0) | 1 (1.6) | 3 (3.4) | 15 (7.5) | 3 (2.6) | 22 (4) |
FD = Fabry`s disease; TTS = takotsubo syndrome; DCM = dilated cardiomyopathy; HCM = hypertrophic cardiomyopathy
There was no difference in the length of time between symptom onset and CMR between the two time periods (11 [IQR 5–24] vs 10 [IQR 3–23]). Of the total 556 patients initially idenfied as suffering from MINOCA, 35 (6.3%) cases occured in 2011, 27 (4.9%) in 2012, 28 (5%) in 2013, 62 (11.2%) in 2014, 88 (15.8%) in 2015, 199 (35.8%) in 2016 and 117 (21%) in 2017. This means that 240 (43.2%) patients enrolled during the time period from 2011 to 2015, while 316 (56.8%) did so during the years 2016 and 2017. There was no difference in baseline parameters such as age, gender or LVEF between these two time periods. The average number of CMRs performed annually was 48 from 2011 to 2015. This number increased to 158 during the years 2016 to 2017. There were significantly fewer annual cases of DCM (p = 0.0072) from 2015 to 2016, while the average annual proportion of CMR reports that tested negativeF for any pathology did not change (p = 0.25; table 3).
Table 3 Diagnoses based on cardiac magnetic resonance imaging in in the years 2011–2015 vs 2016–2017.
| Year | 2011–2015 | 2016–2017 | p-value for χ2 equality of proportions |
|---|---|---|---|
| Average number of CMRs per year |
Total 240
µ = 48 |
Total 316
µ = 158 |
|
| Days to CMR, median (IQR) | 11 (5-24) | 10 (3-23) | 0.26 |
| Normal heart, µ (%) | 31.2 (65) | 88 (55.7) | 0.25 |
| Myocarditis, µ (%) | 4.8 (10) | 26 (16.4) | 0.28 |
| Ischaemia, µ (%) | 2.4 (5) | 14.5 (9.2) | 0.35 |
| Pericarditis, µ (%) | 0.6 (1.3) | 5 (3.2) | 0.48 |
| Amyloidosis, µ (%) | 0.6 (1.3) | 2.5 (1.6) | 0.88 |
| Sarcoidosis, µ (%) | 0.4 (0.8) | 1.5 (0.9) | 0.95 |
| FD, µ (%) | 0.4 (0.8) | 0.5 (0.3) | 0.64 |
| TTS, µ (%) | 0.2 (0.4) | 3 (1.9) | 0.46 |
| DCM, µ (%) | 5.4 (11.2) | 3.5 (2.2) | 0.0072 |
| HCM, µ (%) | 1.2 (2.5) | 4.5 (2.9) | 0.97 |
| Others, µ (%) | 0.8 (1.7) | 9 (5.7) | 0.26 |
CMR = cardiac magnetic resonance imaging; FD = Fabry`s disease; TTS = takotsubo syndrome; DCM = dilated cardiomyopathy; HCM = hypertrophic cardiomyopathy
In total, 24 cases of myocarditis were identified between 2011 and 2015 vs. 52 cases between 2016 and 2017, resulting in 4.8 (10%) cases of myocarditis on average between 2011 and 2015 and 26 (16.7%) cases on average between 2016 and 2017.
In total, 272,688 patients were hospitalised at the University Hospital of Zurich from 2011 to 2017 (37,520 in 2011, 36,484 in 2012, 36,941 in 2013, 38,896 in 2014, 39,694 in 2015, 41,121 in 2016, 42,032 in 2017). As a result, the annual detection rate was 12.7 per 100,000 (CI 0.00008–0.00019) hospitalisations in the years 2011 to 2015. This number increased to 62.5 per 100,000 hospitalisations (CI 0.00047–0.00083) in the period of time from 2016 to 2017, resulting in an detection ratio of 4.9 (p <0.0001). The detection rate for each year is shown in figure 1.

Figure 1 Detection rate of myocarditis diagnosed using cardiac magnetic resonance imaging in the years2011–2017.
We identified 76 cases of myocarditis between 2011 and 2017. Patients suffering from myocarditis were significantly younger (47 ± 17 years vs 58 ± 17; p = 0.00000075) and more likely to be male (81.6 vs 68.8%; p = 0.023) than MINOCA patients, in whom no CMR signs of myocarditis could be detected. Average LVEF on CMR was 51 ± 14% and did not differ between the two groups.
During the course of this study, we found a remarkable increase in the detection rate of myocarditis at the University Hospital Zurich over two subsequent years as a result of the implementation of a systematic CMR work-up algorithm for patients suffering from MINOCA.
We recently reported the detection rate of myocarditis in 2015 vs 2016 at the University Hospital Zurich following the implementation of a novel diagnostic work-up algorithm in January 2016 in a pilot study, in which patients with MINOCA were given a systematic work-up through CMR [23]. We were able to detect a 6.3-fold increase in the approximated incidence of myocarditis. Following the success of this algorithm, it was decided to continue with it in 2017. Through this new, additional study we were able to verify our earlier results over a longer time period. We compared the number of cases of myocarditis diagnosed by CMR in patients suffering from MINOCA in 2016–2017, with the number of identified cases of myocarditis in the previous years, 2011–2015, in which the threshold for performing CMR in patients with MINOCA was higher. The finding of a 4.9-fold increase in the total detection rate of myocarditis between 2016 and 2017 based on CMR and clinical findings compared to previous years, suggests that myocarditis continues to be underdetected. This supports the notion that diagnostic work-up tools have to be improved [1, 5, 14, 15, 20, 23, 28, 29]. Nonetheless, the exact incidence of myocarditis cannot be fully determined, as a definitive diagnosis would require myocardial biopsy, which has limitations itself. A previous study by Karjalainen, Heikkilä and colleagues which evaluated the incidence of myocarditis in young military recruits in Finland from 1977 until 1996, based on clinical criteria, found myocarditis to occur in 17 per 100,000 person years [1].
While the detection rate of myocarditis increased between 2016 and 2017, the annual percentage of CMR testing positive for myocarditis did not change significantly over the years. This was despite patients without typical symptoms of myocarditis having been included in 2016 and 2017. This finding suggests that the diagnosis can be challenging if based on medical history and physical examination only. Pathik and colleagues demonstrated that a clinically suspected diagnosis by cardiologists in patients with MINOCA was changed in approximately 50% of cases after applying the CMR technique, demonstrating that clinical identification of the etiology of MINOCA is challenging [30].
Similarly to previous studies, we found myocarditis to be the most common underlying cause in patients who suffer from MINOCA and who undergo CMR [14, 15, 18, 23]. Interestingly, the percentage of CMR scans which tested positive for myocarditis (22.2% in 2017) was lower than previously described in literature. For instance, Pasupathy et al. found myocarditis to be present in 33% of CMRs in MINOCA patients based on 26 publications [18]. Moreover, studies on the diagnostic yield of CMR in MINOCA showed myocarditis to be present in 41.6–59.9% [14, 15]. In addition, they revealed a considerably lower rate of unremarkable CMR scans with only 8–10.3% [14, 15] compared to the rates we found in our study (in total, 59.7%). However, we did include all CMR scans with a clinical suspicion of myocarditis, while the afore-mentioned studies excluded patients suffering from known cardiac diseases. This could have led to higher concentrations of relevant findings in the other publications.
Consistent with previous studies, patients with myocarditis were significantly younger and more frequently male than those who were not diagnosed with myocarditis [14, 31]. LVEF did not vary between cases of myocarditis and non-myocarditis with a globally just mildly impaired LVEF (51 ± 15%). Other underlying cause diagnoses in CMR, with the exception of DCM, did not differ between the periods of time 2011–2015 and 2016–2017.
In summary, systematic work-up of patients with MINOCA implemented in 2016 led to an almost five-fold increase in the diagnosis of myocarditis for two consecutive years compared to the preceding five years. While the lack of tissue diagnosis is a limitation of this study, wide diagnostic work-up with low risk of complications can only be applied through a non-invasive approach. Thus, this non-invasive approach provides an approximation of the true incidence of myocarditis.
Due to the retrospective character of the study, as well as the single centre design, the global applicability of our results is limited. As mentioned above, a limitation of this study was that histopathological confirmation of myocarditis was not performed because we followed the guidelines for endomyocardial biopsy, which restrict the indication for endomyocardial biopsy as a more severe clinical course [25]. Moreover, CMR diagnosis was performed within three months of, rather than immediately upon, symptom onset. This may have affected CMR findings, since edema and LGE are dynamic and, in general, regress during the clinical course. Furthermore, we did not use the most recent T1 and T2 mapping techniques, which could have led to a higher detection rate of myocarditis on CMR [32].
The successful identification of the underlying illness of patients with MINOCA may be challenging without additional diagnostic imaging. We have demonstrated that systematic work-up of patients with angina-like symptoms, elevated troponin and no significant coronary artery disease, lea to an almost five-fold increase in the diagnosis of myocarditis in our tertiary care centre. These data suggest that myocarditis remains underdiagnosed and that lowering the threshold for diagnostic work-up with CMR may help to identify myocarditis early. Timely and accurate diagnosis is crutial as certain subtypes of myocarditis require specific therapy. Furthermore, following diagnosis of myocarditis, strenuous activity should be avoided for three to six weeks, since the risk of exercise-induced arrhythmias may be higher during that period.
We thank our Information and Communication Technology Department for assisting with our retrospective chart review.
This work was supported through funding awarded to Dr. Bettina Beidecker by the Forschungskredit of the University of Zurich, the Holcim Foundation (Holcim Stiftung zur Förderung der wissenschaftlichen Fortbildung, Switzerland), the Hartmann Müller Foundation and the Walter and Gertrud Siegenthaler Foundation.
The authors have no conflict of interest to disclose.
1 Karjalainen J , Heikkilä J . Incidence of three presentations of acute myocarditis in young men in military service. A 20-year experience. Eur Heart J. 1999;20(15):1120–5. doi:.https://doi.org/10.1053/euhj.1998.1444
2 Caforio ALP , Pankuweit S , Arbustini E , Basso C , Gimeno-Blanes J , Felix SB , et al.; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33):2636–48, 2648a–2648d. doi:.https://doi.org/10.1093/eurheartj/eht210
3 Dec GW, Jr , Palacios IF , Fallon JT , Aretz HT , Mills J , Lee DC-S , et al. Active myocarditis in the spectrum of acute dilated cardiomyopathies. Clinical features, histologic correlates, and clinical outcome. N Engl J Med. 1985;312(14):885–90. doi:.https://doi.org/10.1056/NEJM198504043121404
4 Corrado D , Basso C , Thiene G . Sudden cardiac death in young people with apparently normal heart. Cardiovasc Res. 2001;50(2):399–408. doi:.https://doi.org/10.1016/S0008-6363(01)00254-1
5 Phillips M , Robinowitz M , Higgins JR , Boran KJ , Reed T , Virmani R . Sudden cardiac death in Air Force recruits. A 20-year review. JAMA. 1986;256(19):2696–9. doi:.https://doi.org/10.1001/jama.1986.03380190066026
6 Te ALD , Wu T-C , Lin Y-J , Chen Y-Y , Chung F-P , Chang S-L , et al. Increased risk of ventricular tachycardia and cardiovascular death in patients with myocarditis during the long-term follow-up: A national representative cohort from the National Health Insurance Research Database. Medicine (Baltimore). 2017;96(18):e6633. doi:.https://doi.org/10.1097/MD.0000000000006633
7 Baccouche H , Mahrholdt H , Meinhardt G , Merher R , Voehringer M , Hill S , et al. Diagnostic synergy of non-invasive cardiovascular magnetic resonance and invasive endomyocardial biopsy in troponin-positive patients without coronary artery disease. Eur Heart J. 2009;30(23):2869–79. doi:.https://doi.org/10.1093/eurheartj/ehp328
8 Gräni C , Eichhorn C , Bière L , Murthy VL , Agarwal V , Kaneko K , et al. Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients With Suspected Myocarditis. J Am Coll Cardiol. 2017;70(16):1964–76. doi:. Correction in: J Am Coll Cardiol. 2017;70(21):2736. doi:https://doi.org/10.1016/j.jacc.2017.08.050
9 Friman G . The incidence and epidemiology of myocarditis. Eur Heart J. 1999;20(15):1063–6. doi:.https://doi.org/10.1053/euhj.1999.1640
10 Basso C , Calabrese F , Corrado D , Thiene G . Postmortem diagnosis in sudden cardiac death victims: macroscopic, microscopic and molecular findings. Cardiovasc Res. 2001;50(2):290–300. doi:.https://doi.org/10.1016/S0008-6363(01)00261-9
11 Jessup M , Lindenfeld J . Light at the End of the Myocarditis Tunnel. JACC Heart Fail. 2018;6(7):580–2. doi:.https://doi.org/10.1016/j.jchf.2018.04.015
12 Angelini A , Calzolari V , Calabrese F , Boffa GM , Maddalena F , Chioin R , et al. Myocarditis mimicking acute myocardial infarction: role of endomyocardial biopsy in the differential diagnosis. Heart. 2000;84(3):245–50. doi:.https://doi.org/10.1136/heart.84.3.245
13 Pasupathy S , Tavella R , Zeitz C , Worthley M , Sinhal A , Arstall M , et al. Utility of Clinical Assessments in Patients Presenting With Troponin Positive Non Obstructive Coronary Arteries (TP-NOCA). Circulation. 2017;136(Suppl 1):abstract 19443.
14 Assomull RG , Lyne JC , Keenan N , Gulati A , Bunce NH , Davies SW , et al. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J. 2007;28(10):1242–9. doi:.https://doi.org/10.1093/eurheartj/ehm113
15 Leurent G , Langella B , Fougerou C , Lentz P-A , Larralde A , Bedossa M , et al. Diagnostic contributions of cardiac magnetic resonance imaging in patients presenting with elevated troponin, acute chest pain syndrome and unobstructed coronary arteries. Arch Cardiovasc Dis. 2011;104(3):161–70. doi:.https://doi.org/10.1016/j.acvd.2011.01.005
16 Tavella R , Pasupathy S , Zeitz C , Worthley M , Sinhal A , Arstall M , et al. A Comprehensive Comparison Between Myocardial Infarction With Non Obstructive Coronaries (MINOCA) and Myocardial Infarct Patients With Coronary Artery Disease (MICAD). Circulation. 2017;136(Suppl 1):abstract 19361.
17 Maddox TM , Ho PM , Roe M , Dai D , Tsai TT , Rumsfeld JS . Utilization of secondary prevention therapies in patients with nonobstructive coronary artery disease identified during cardiac catheterization: insights from the National Cardiovascular Data Registry Cath-PCI Registry. Circ Cardiovasc Qual Outcomes. 2010;3(6):632–41. doi:.https://doi.org/10.1161/CIRCOUTCOMES.109.906214
18 Pasupathy S , Air T , Dreyer RP , Tavella R , Beltrame JF . Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131(10):861–70. doi:.https://doi.org/10.1161/CIRCULATIONAHA.114.011201
19 Panovský R , Borová J , Pleva M , Feitová V , Novotný P , Kincl V , et al. The unique value of cardiovascular magnetic resonance in patients with suspected acute coronary syndrome and culprit-free coronary angiograms. BMC Cardiovasc Disord. 2017;17(1):170. doi:.https://doi.org/10.1186/s12872-017-0610-6
20 Gerbaud E , Harcaut E , Coste P , Erickson M , Lederlin M , Labèque JN , et al. Cardiac magnetic resonance imaging for the diagnosis of patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Int J Cardiovasc Imaging. 2012;28(4):783–94. doi:.https://doi.org/10.1007/s10554-011-9879-1
21 Hufnagel G , Pankuweit S , Richter A , Schönian U , Maisch B . The European Study of Epidemiology and Treatment of Cardiac Inflammatory Diseases (ESETCID). First epidemiological results. Herz. 2000;25(3):279–85. doi:.https://doi.org/10.1007/s000590050021
22 Caforio ALP , Calabrese F , Angelini A , Tona F , Vinci A , Bottaro S , et al. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J. 2007;28(11):1326–33. doi:.https://doi.org/10.1093/eurheartj/ehm076
23 Patriki D , Gresser E , Manka R , Emmert MY , Lüscher TF , Heidecker B . Approximation of the Incidence of Myocarditis by Systematic Screening With Cardiac Magnetic Resonance Imaging. JACC Heart Fail. 2018;6(7):573–9. doi:.https://doi.org/10.1016/j.jchf.2018.03.002
24 Ibanez B , James S , Agewall S , Antunes MJ , Bucciarelli-Ducci C , Bueno H , et al.; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77. doi:.https://doi.org/10.1093/eurheartj/ehx393
25 Bozkurt B , Colvin M , Cook J , Cooper LT , Deswal A , Fonarow GC , et al.; American Heart Association Committee on Heart Failure and Transplantation of the Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; and Council on Quality of Care and Outcomes Research. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement From the American Heart Association. Circulation. 2016;134(23):e579–646. doi:.https://doi.org/10.1161/CIR.0000000000000455
26 Friedrich MG , Sechtem U , Schulz-Menger J , Holmvang G , Alakija P , Cooper LT , et al.; International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53(17):1475–87. doi:.https://doi.org/10.1016/j.jacc.2009.02.007
27 Hundley WG , Bluemke DA , Finn JP , Flamm SD , Fogel MA , Friedrich MG , et al.; American College of Cardiology Foundation Task Force on Expert Consensus Documents. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;121(22):2462–508. doi:.https://doi.org/10.1161/CIR.0b013e3181d44a8f
28 Basso C , Carturan E , Corrado D , Thiene G . Myocarditis and dilated cardiomyopathy in athletes: diagnosis, management, and recommendations for sport activity. Cardiol Clin. 2007;25(3):423–9, vi. doi:.https://doi.org/10.1016/j.ccl.2007.08.008
29 Mahmoudi M , Harden S , Abid N , Peebles C , Nicholas Z , Jones T , et al. Troponin-positive chest pain with unobstructed coronary arteries: definitive differential diagnosis using cardiac MRI. Br J Radiol. 2012;85(1016):e461–6. doi:.https://doi.org/10.1259/bjr/90663866
30 Pathik B , Raman B , Mohd Amin NH , Mahadavan D , Rajendran S , McGavigan AD , et al. Troponin-positive chest pain with unobstructed coronary arteries: incremental diagnostic value of cardiovascular magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. 2016;17(10):1146–52. doi:.https://doi.org/10.1093/ehjci/jev289
31 Berg J , Kottwitz J , Baltensperger N , Kissel CK , Lovrinovic M , Mehra T , et al. Cardiac Magnetic Resonance Imaging in Myocarditis Reveals Persistent Disease Activity Despite Normalization of Cardiac Enzymes and Inflammatory Parameters at 3-Month Follow-Up. Circ Heart Fail. 2017;10(11):e004262. doi:.https://doi.org/10.1161/CIRCHEARTFAILURE.117.004262
32 Ferreira VM , Schulz-Menger J , Holmvang G , Kramer CM , Carbone I , Sechtem U , et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J Am Coll Cardiol. 2018;72(24):3158–76. doi:.https://doi.org/10.1016/j.jacc.2018.09.072
This work was supported through funding awarded to Dr. Bettina Beidecker by the Forschungskredit of the University of Zurich, the Holcim Foundation (Holcim Stiftung zur Förderung der wissenschaftlichen Fortbildung, Switzerland), the Hartmann Müller Foundation and the Walter and Gertrud Siegenthaler Foundation.
The authors have no conflict of interest to disclose.