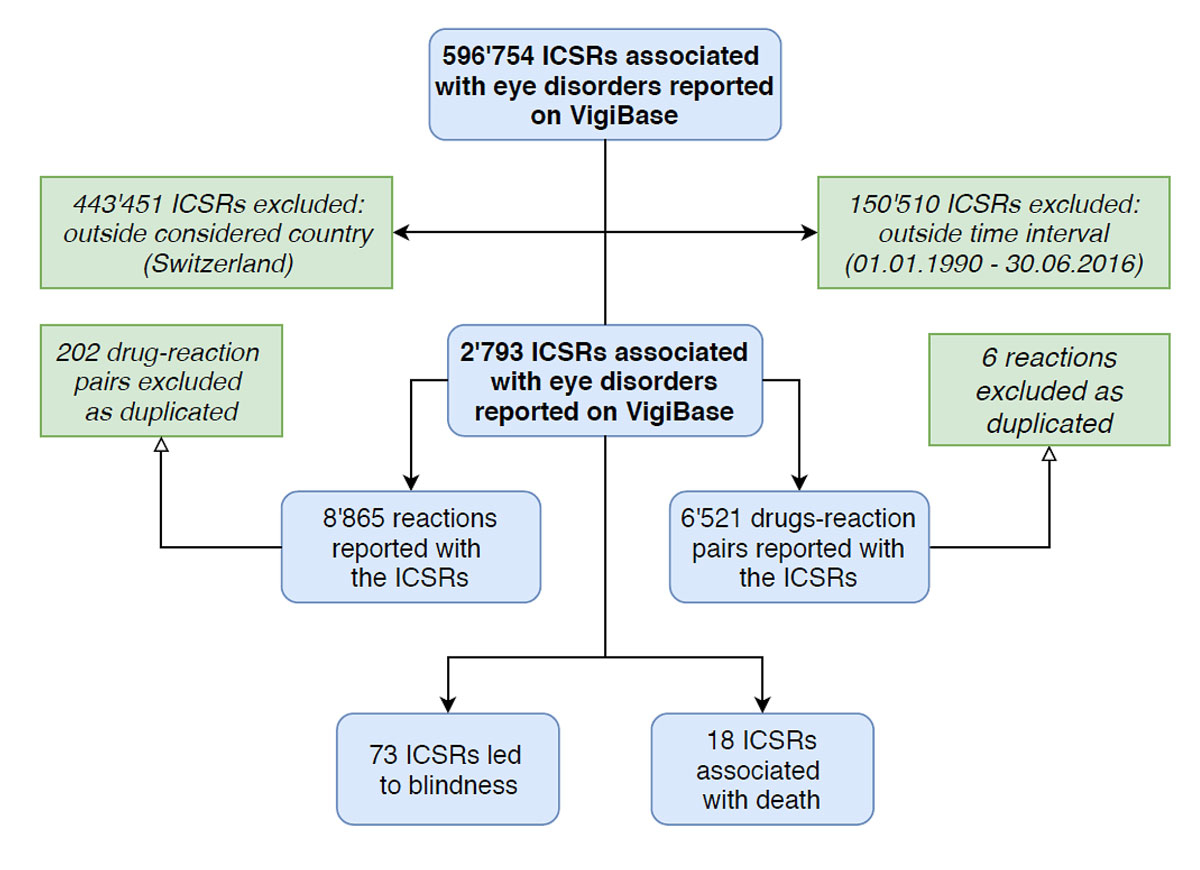
Figure 1 Flow chart showing the systematic processes adopted to achieve the final data sample.
DOI: https://doi.org/10.4414/smw.2019.20085
Ophthalmology is one of the medical specialties for which the fewest assessed adverse drug reactions (ADRs) have been reported, which represents a particular challenge in pharmacovigilance [1]. Numerous systemic drugs produce adverse effects that can involve the eye. For example, anticholinergic drugs such as antihistamines, tricyclic antidepressants, sleep aids or cold preparations can lead, among other adverse reactions, to blurred vision and impaired stability in the upright position; patients under the influence of these side effects thus have a tendency to fall [2]. However, topically applied ocular drugs may be systemically absorbed to a significant extent, resulting in widespread adverse side effects in both the eye and other regions of the body [3]. In particular, new ocular drugs, such as vascular endothelial growth factor (VEGF) inhibitors, which have increasingly been prescribed since their approval in the middle of the last decade, are potentially related to numerous systemic ADRs.
Several reports have indicated an association between ocular ADRs and systemic drugs, but there have been only a few systematic reviews to date [1]. With an increased focus on the safety of these medications, however, more studies are needed to reach further significant conclusions on signals emerging from retrospective studies. Ocular ADRs are frequent and the majority of these events are innocuous for the patients, although detrimental reactions, including fatal ADRs and blindness, have been reported and need to be effectively prevented to safeguard patients and reduce strain on the health system. Visual impairment and blindness are a considerable economic burden, which increases with the degree of visual impairment, for affected persons, their caregivers and society at large [4].
The use of anti-angiogenic therapy imposes potential risks for ocular ADRs. VEGF inhibitors have revolutionised the treatment of retinal disease with an impact on retinal pathologies probably even greater than in oncology, but despite the apparent safety of the small doses used for treating eye disease, these agents are highly potent [5]. Over the past 15 years, a disproportionate increase in cardiovascular and cerebrovascular events has been observed in patients treated with ranibizumab [5]. However, systematic reviews have reported contradictory results, showing low rates of reported serious adverse events after anti-VEGF injections [6]. The incidence of blindness as an adverse event is relevant; increased reporting of endophthalmitis after the topical administration of bevacizumab was observed [5], indicating a risk for this debilitating adverse effect. Although the prognosis for endophthalmitis has improved significantly with advances in antibiotic treatment and vitreoretinal surgery, the number of patients requiring evisceration or enucleation remains significant [7]. Therapeutic approaches using anti-VEGF medications thus require increased attention to safety.
Drugs in this class show diverse pharmacokinetics and pharmacodynamics; for example, bevacizumab is more likely than ranibizumab to induce immune activation and to remain in the systemic circulation. Thus, bevacizumab administration creates a higher risk of systemic adverse events [8].
Current legislation in Switzerland compels any individual who professionally dispenses or administers therapeutic products to humans or animals to notify the Swiss Agency for Therapeutic Products (Swissmedic) of any serious and previously unknown ADRs or quality defects. Spontaneous reporting systems have an important role in the investigation of rare and unpredictable ADRs such as adverse events in ophthalmology. In addition, the collection of these data allows the early detection of ADRs in the post‐marketing phase.
The aim of the present study was to quantify ADR reports in ophthalmology in Switzerland by identifying frequencies in spontaneous reports listed in a global database. Our investigation sought to identify the number of cases in ocular pharmacovigilance and to support the requirement for ongoing surveillance and large population-based studies to investigate these outcomes.
This was a retrospective cohort study using registry data, with a base cohort consisting of all ADRs associated with the system organ class term “eye disorders” [1]. An important focus of our analyses was new ocular drugs licensed in Switzerland in the past 15 years, the majority of which are anti-VEGF agents approved in Switzerland since the middle of the last decade. We reviewed individual case safety reports (ICSRs) submitted in Switzerland between January 1991 and June 2016. ADRs were analysed with respect to reporting rate per year, age and sex of the patient, and reported symptoms. Patients were grouped based on their age by decade and reported symptoms were described according to MedDRA terminology.
ICSRs were obtained using the online software VigiLyze™, which allows registered users to access data from VigiBase™, the world’s largest spontaneous ADR system, with more than 8.4 million reports from 104 countries compiled since the WHO international drug monitoring programme started in 1968 [9]. VigiLyze™ allows users to examine data on ADRs using various filters to facilitate data acquisition and to download additional information on the ICSRs. VigiBase™ is a registry database with no access to clinical or laboratory data. Therefore, the causal relationship between drug and ADR could not be no formally assessed [10].
Many data were adjusted for the statistical analysis, for example by removing redundancy and bringing the variables into alignment – such as by avoiding repeated terms and synonyms – thus promoting an unambiguous interpretation of the available information while retaining the original content of the report. Duplicate records were also systematically removed. A relevant number of ADR reports included only an approximate duration of the event. Where only the month and year of the start or stop of an ADR were reported, we counted the indicated month as a full month. Where only the year was indicated, we excluded the corresponding ADR from the analysis of event duration, indicating it as “unknown”. A similar approach was adopted by Blaser et al. [11] in an analogous study. A similar readjustment was performed in relationship to the individual reporting the single ADR to the authority: when more than one individual was indicated as reporter, only the most competent was considered for the analysis. For example, when a physician and a layman were listed as reporters of the same ADR, only the former was taken into account. While aligning the fields related to the outcomes of the ADRs, only the term pertaining to the most disabling condition – in accordance with the seriousness criteria of the event – was considered.
Polypharmacy is not unequivocally defined in the scientific literature. In our study, we applied the most commonly used definition for polypharmacy, namely the condition of taking five or more medications daily [12]. Missing data or data insufficiently itemised to allow correct categorisation were classified as “unknown” and represented an independent statistical category.
Descriptive statistics were used to summarise and describe the data. The linear correlation between the number of drugs and the rate of serious ADRs was established as the coefficient of determination and was highest when the data were plotted on a simple linear regression model. Reporting odds ratios (RORs), seen as the pharmacovigilance equivalent of the odds ratio (OR), 95% confidence interval (CIs), and p-values were calculated to measure disproportionality for drug-reaction pairs, comparing each of the three anti-VEGF drugs with the other two [13]. Fisher’s exact test was used as a nonparametric statistical test of the null hypothesis that no associations existed between two different RORs as categorical variables against the alternative hypothesis that there was an association between the variables. Values of p <0.05 were considered significant.
All statistical analyses were performed using SPSS Statistics (developed by IBM, Armonk (NY), version 25.0 (2017), software package for Mac) and MATLAB (developed by MathWorks, Natick (MA), version R2017b, software package for Mac).
ICSRs associated with eye disorders reported in Switzerland from January 1991 to June 2016 were selected from the WHO VigiBase. Following data adjustment and the removal of duplicates, we obtained 2793 ICSRs corresponding to 6521 drug-reaction pairs (fig. 1). Ocular adverse reactions constituted a small proportion (3.5%) of all ICSRs reported in Switzerland during the period of interest (total number of reports 80,515). Nevertheless, underreporting could be an important limitation of spontaneous reporting systems for ADRs such as the WHO VigiBase, leading to biased data and possibly false conclusions.

Figure 1 Flow chart showing the systematic processes adopted to achieve the final data sample.
The main Anatomic Therapeutic Chemical / Defined Daily Dose (ATC/DDD) drug group associated with eye disorders comprised medications acting on the nervous system (ATC/DDD class N), with 774 (27.7%) ICSRs attributed to this drug class. This was followed by 563 (20.2%) ICSRs associated with drugs acting on sensory organs (ATC/DDD class S), 503 (18.0%) representing antineoplastic agents (ATC/DDD class L), and 390 (14.0%) representing dermatological medications (ATC/DDD class D).
Table 1 lists two binary variables, polypharmacy and seriousness of the adverse reaction, and shows their association with the medications described in the ICSRs, the gender and age of patients, and the qualification of the individual who reported the adverse reaction. The ten listed medications were the most frequently reported, regardless of the relationship between medication and ADR (suspected, concomitant or interacting). Taken together, these drugs accounted for 773 (27.7%) drug-reaction pairs, among which the most frequently reported ATC/DDD drug group denoted the trend for all reports, with 40% of drugs acting on the nervous system.
Table 1 Number and percentage of cases associated with polypharmacy and serious events, by name of implicated drug, sex and age of the patient, and qualification of the reporting figure.
| Total cases | Polypharmacy | Serious ADR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | Yes | No | Yes | No | ||||||
| n | % | n | % | n | % | n | % | ||||
| Name of the drug | Acetylsalicylic acid | 147 | 5.3% | 46 | 31.3% | 101 | 68.7% | 21 | 14.3% | 17 | 11.6% |
| Paracetamol | 90 | 3.2% | 35 | 38.9% | 55 | 61.1% | 22 | 24.4% | 10 | 11.1% | |
| Lorazepam | 78 | 2.8% | 33 | 42.3% | 45 | 57.7% | 15 | 19.2% | 9 | 11.5% | |
| Venlafaxine | 73 | 2.6% | 52 | 71.2% | 21 | 28.8% | 13 | 17.8% | 21 | 28.8% | |
| Pantoprazole | 72 | 2.6% | 27 | 37.5% | 45 | 62.5% | 13 | 18.1% | 5 | 6.9% | |
| Ranibizumab | 57 | 2.0% | 52 | 91.2% | 5 | 8.8% | 38 | 66.7% | 4 | 7.0% | |
| Fingolimod | 67 | 2.4% | 59 | 88.1% | 8 | 11.9% | 53 | 79.1% | 12 | 17.9% | |
| Atorvastatin | 66 | 2.4% | 29 | 43.9% | 37 | 56.1% | 5 | 7.6% | 8 | 12.1% | |
| Latanoprost | 64 | 2.3% | 58 | 90.6% | 6 | 9.4% | 29 | 45.3% | 20 | 31.3% | |
| Mirtazapine | 59 | 2.1% | 33 | 55.9% | 26 | 44.1% | 8 | 13.6% | 8 | 13.6% | |
| Sex of the patient | Male | 990 | 35.4% | 163 | 16.5% | 827 | 83.5% | 469 | 47.4% | 309 | 31.2% |
| Female | 1651 | 59.1% | 279 | 16.9% | 1372 | 83.1% | 756 | 45.8% | 587 | 35.6% | |
| Unknown | 152 | 5.4% | 5 | 3.3% | 147 | 96.7% | 77 | 50.7% | 62 | 40.8% | |
| Age of the patient (years) | 0–9 | 91 | 3.3% | 24 | 26.4% | 67 | 73.6% | 56 | 61.5% | 23 | 25.3% |
| 10–19 | 138 | 4.9% | 11 | 8.0% | 127 | 92.0% | 76 | 55.1% | 33 | 23.9% | |
| 20–29 | 197 | 7.1% | 17 | 8.6% | 180 | 91.4% | 74 | 37.6% | 68 | 34.5% | |
| 30–39 | 260 | 9.3% | 27 | 10.4% | 233 | 89.6% | 109 | 41.9% | 84 | 32.3% | |
| 40–49 | 365 | 13.1% | 53 | 14.5% | 312 | 85.5% | 175 | 47.9% | 112 | 30.7% | |
| 50–59 | 336 | 12.0% | 54 | 16.1% | 282 | 83.9% | 154 | 45.8% | 121 | 36.0% | |
| 60–69 | 366 | 13.1% | 91 | 24.9% | 275 | 75.1% | 157 | 42.9% | 130 | 35.5% | |
| 70–79 | 310 | 11.1% | 91 | 29.4% | 219 | 70.6% | 168 | 54.2% | 90 | 29.0% | |
| 80–89 | 132 | 4.7% | 42 | 31.8% | 90 | 68.2% | 80 | 60.6% | 32 | 24.2% | |
| 90–99 | 6 | 0.2% | 3 | 50.0% | 3 | 50.0% | 4 | 66.7% | 2 | 33.3% | |
| 100+ | 1 | 0.04% | 0 | 0.0% | 1 | 100.0% | 0 | 0.0% | 1 | 100.0% | |
| Unknown | 591 | 21.2% | 189 | 32.0% | 402 | 68.0% | 242 | 40.9% | 258 | 43.7% | |
| Reporter qualification | Consumer | 245 | 8.8% | 25 | 10.2% | 220 | 89.8% | 105 | 42.9% | 140 | 57.1% |
| Physician | 1692 | 60.6% | 326 | 19.3% | 1366 | 80.7% | 973 | 57.5% | 526 | 31.1% | |
| Pharmacist | 217 | 7.8% | 37 | 17.1% | 180 | 82.9% | 107 | 49.3% | 110 | 50.7% | |
| Lawyer | 2 | 0.1% | 0 | 0.0% | 2 | 100.0% | 2 | 100.0% | 0 | 0.0% | |
| Other health professional | 162 | 5.8% | 16 | 9.9% | 146 | 90.1% | 63 | 38.9% | 98 | 60.5% | |
| Other | 203 | 7.3% | 0 | 0.0% | 190 | 93.6% | 0 | 0.0% | 0 | 0.0% | |
| Unknown | 272 | 9.7% | 30 | 11.0% | 242 | 89.0% | 105 | 38.6% | 84 | 30.9% | |
The majority of cases involved adult patients (70.6%): patients aged over 60 years accounted for 815 (29.2%) ICSRs whereas reactions reported in children were significantly less frequent (8.2%). An analysis of patient age revealed an interesting U-shaped correlation with the seriousness of the adverse drug reaction: children and older patients were more likely to experience serious events than adult patients aged 20–70 years. A similar trend was not observed for the frequency of multidrug therapy: in general, older patients appeared to be exposed to a larger number of medications than younger patients. The only exception to this observation was small children aged under 10 years, who had similar values to patients aged 60–80 years (26.4%).
Health professionals were the main reporters, associated with three out of four ICRSs (75.2%). Overall, physicians had higher reporting rates for ADRs evaluated as serious (57.5%) compared with lay consumers (42.9%).
Given the prevalence of patients receiving several drugs simultaneously, we performed a statistical analysis to investigate the correlation between the number of concurrently ingested drugs and the seriousness of ADRs in the affected patients (fig. 2). The majority of ICSRs pertained to patients who had taken a single drug (1326 ICSRs). In this group of patients, only 576 (43.4%) ICSRs were assessed as serious. This percentage increased linearly: for five concurrently ingested drugs – the definition of polypharmacy – it reached 49.6% of serious ICSRs and for nine concurrently ingested drugs, it reached a maximum, with three out of four ICSRs assessed as serious (75.0%). Our statistical analysis included up to 10 concurrently ingested drugs, as beyond this number the proportion of ICSRs in each group was insufficient to allow a consistent analysis, showing a reverse trend. This may be due to statistical bias caused by the small number of patients included and the non-representative population sample.
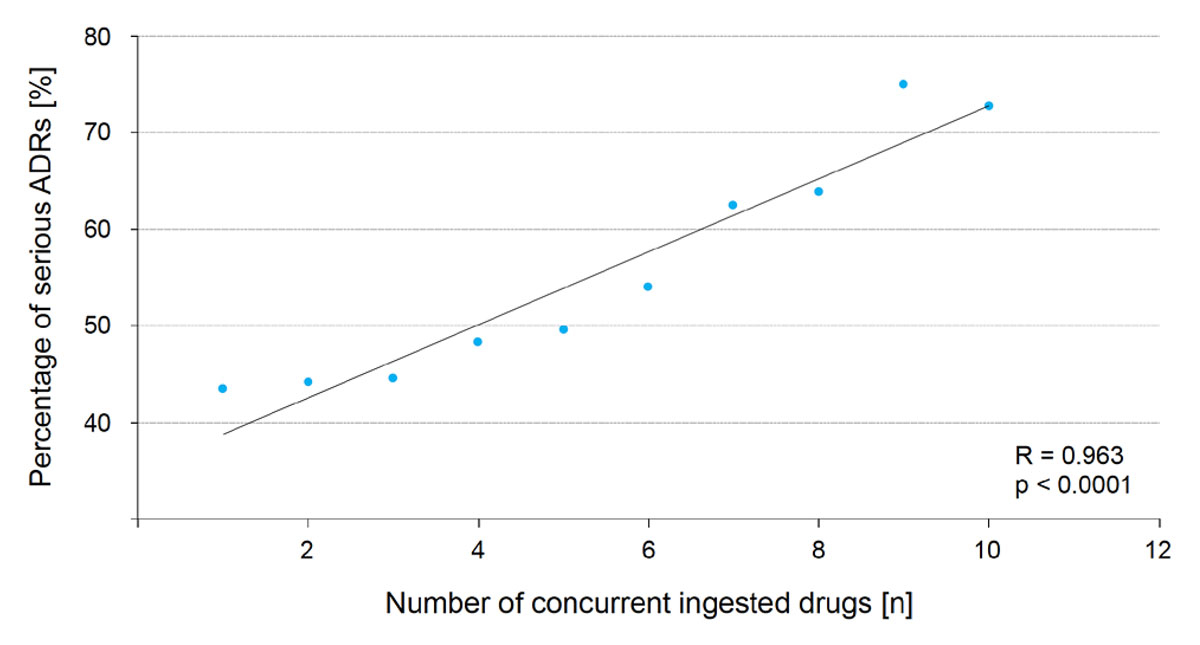
Figure 2 Linear correlation between number of concurrent ingested medications and rate of serious ADRs
Table 2 displays the analysis of causal relationships between drugs and adverse reactions using data derived from the ICSRs as variables. A suspected relationship between drug and event was identified for half of the drug-reaction pairs (51.1%). Of the remaining pairs, most were assessed as concomitant (43.3%), with only a few classified as interacting (2.6%). The duration of ADRs was not normally distributed, shorter ADRs being significantly more prevalent than longer reactions. The range between the shortest and the longest ADR was from 1 day to several years, with a median duration of about 8 days. These data have to be interpreted with caution as only 27.8% of the drug-reaction pairs provided relevant data.
Table 2 Characterisation of the causal relationship between drug and reaction for the different ICSRs sorted by involved medication, duration of the ADR, drug’s route of administration and drug’s indication.
| Total cases | Characterisation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | Suspected | Concomitant | Interacting | |||||
| n | % | n | % | n | % | ||||
| Name of the drug | Acetylsalicylic acid | 147 | 2.3% | 33 | 22.4% | 109 | 74.1% | 5 | 3.4% |
| Paracetamol | 92 | 1.4% | 27 | 29.3% | 61 | 66.3% | 4 | 4.3% | |
| Lorazepam | 79 | 1.2% | 33 | 41.8% | 44 | 55.7% | 2 | 2.5% | |
| Venlafaxine | 73 | 1.1% | 45 | 61.6% | 27 | 37.0% | 1 | 1.4% | |
| Pantoprazole | 72 | 1.1% | 28 | 38.9% | 43 | 59.7% | 1 | 1.4% | |
| Fingolimod | 67 | 1.0% | 51 | 76.1% | 14 | 20.9% | 2 | 3.0% | |
| Atorvastatin | 66 | 1.0% | 30 | 45.5% | 35 | 53.0% | 1 | 1.5% | |
| Latanoprost | 65 | 1.0% | 48 | 73.8% | 16 | 24.6% | 1 | 1.5% | |
| Mirtazapine | 61 | 0.9% | 20 | 32.8% | 40 | 65.6% | 1 | 1.6% | |
| Esomeprazole | 59 | 0.9% | 35 | 59.3% | 24 | 40.7% | 0 | 0.0% | |
| Duration of the ADR | 1 day | 528 | 7.9% | 438 | 83.0% | 77 | 14.6% | 13 | 2.5% |
| 2 days | 119 | 1.8% | 88 | 73.9% | 25 | 21.0% | 6 | 5.0% | |
| 3 days | 79 | 1.2% | 59 | 74.7% | 16 | 20.3% | 4 | 5.1% | |
| 4 days | 49 | 0.7% | 35 | 71.4% | 11 | 22.4% | 3 | 6.1% | |
| 5 days | 38 | 0.6% | 33 | 86.8% | 5 | 13.2% | 0 | 0.0% | |
| 6 days | 48 | 0.7% | 36 | 75.0% | 10 | 20.8% | 2 | 4.2% | |
| 7 days | 41 | 0.6% | 35 | 85.4% | 6 | 14.6% | 0 | 0.0% | |
| 8 days | 43 | 0.6% | 33 | 76.7% | 10 | 23.3% | 0 | 0.0% | |
| Route of drug administration | Enteral | 3170 | 48.6% | 1669 | 52.6% | 1351 | 42.6% | 150 | 4.7% |
| Parenteral | 798 | 12.2% | 635 | 79.6% | 152 | 19.0% | 11 | 1.4% | |
| Topical ocular | 373 | 5.7% | 336 | 90.1% | 36 | 9.7% | 1 | 0.3% | |
| Respiratory | 60 | 0.9% | 23 | 38.3% | 37 | 61.7% | 0 | 0.0% | |
| Topical (not on the eye) | 57 | 0.9% | 46 | 80.7% | 11 | 19.3% | 0 | 0.0% | |
| Unknown | 481 | 7.4% | 287 | 59.7% | 193 | 40.1% | 1 | 0.2% | |
| No data | 1582 | 24.3% | 442 | 27.9% | 1126 | 71.2% | 14 | 0.9% | |
| Drug indication | Depressive episode or disorder | 119 | 1.8% | 82 | 68.9% | 18 | 15.1% | 19 | 16.0% |
| Essential hypertension | 113 | 1.7% | 53 | 46.9% | 59 | 52.2% | 1 | 0.9% | |
| Glaucoma | 92 | 1.4% | 86 | 93.5% | 6 | 6.5% | 0 | 0.0% | |
| Osteoporosis | 52 | 0.8% | 37 | 71.2% | 15 | 28.8% | 0 | 0.0% | |
| Epilepsy | 57 | 0.8% | 35 | 61.4% | 15 | 26.3% | 7 | 12.3% | |
| Diabetes | 43 | 0.6% | 19 | 44.2% | 24 | 55.8% | 0 | 0.0% | |
| Multiple sclerosis | 69 | 1.0% | 67 | 97.1% | 2 | 2.9% | 0 | 0.0% | |
| Migraine | 39 | 0.6% | 28 | 71.8% | 11 | 28.2% | 0 | 0.0% | |
| Asthma | 30 | 0.4% | 12 | 40.0% | 17 | 56.7% | 1 | 3.3% | |
| Disorder of lipid metabolism | 28 | 0.4% | 18 | 64.3% | 10 | 35.7% | 0 | 0.0% | |
| Unknown | 226 | 3.4% | 98 | 43.4% | 128 | 56.6% | 0 | 0.0% | |
| No data | 3830 | 57.0% | 1586 | 41.4% | 2165 | 56.5% | 79 | 2.1% | |
In addition to evaluating the most frequently implicated medications and their relationship with the ICSRs, we assessed the most common types of ADR. From a total of 8865 different reported reactions and 2793 ICSRs notified, each patient presented with an average of 3.17 symptoms. A total of 635 (22.7%) patients experienced “visual impairment” after medication; this was the symptom most commonly reported and constituted 7.2% of all reported symptoms. This was followed by “eyelid oedema” in 206 patients (7.4%), “vision blurred” in 201 patients (7.2%), “diplopia” in 158 patients (5.7%), “eye irritation” in 128 patients (4.6%), “mydriasis” in 121 patients (4.3%) “eye pain” in 112 patients (4.0%), “ocular hyperaemia” in 91 patients (3.3%), “periorbital oedema” in 78 patients (2.8%), and “dry eye” in 63 patients (2.3%). Less frequent but highly relevant ADRs included fatal reactions, with death reported for 18 (0.6%) patients, and “blindness” for 73 (2.6%) patients.
The majority of reactions were temporary, as 4173 (47.1%) resolved completely. One in every three resolved reactions did not resolve completely: 1321 (14.9%) reactions were permanent and 106 (1.2%) led to long-term sequelae. For 669 (7.5%) reactions, resolution was ongoing, and for more than one in four reactions the outcome could not be determined because of missing data (28.7%).
The best prognosis was observed for mydriasis, as 58 (71.1%) reactions were completely resolved. Other common reactions with particularly positive outcomes were periorbital oedema (62.8%), eyelid oedema (62.1%) and diplopia (53.1%). However, we also identified recurring reactions with outcomes that were frequently negative. For example, one out of five reactions leading to visual impairment did not recover (21.1%), and for dry eye 18 (28.6%) reactions were permanent.
VEGF inhibitors are approved in Switzerland for the treatment of diseases affecting the eye that have neovascularisation as the main pathophysiological mechanism. At present, three VEGF inhibitors account for the majority of ocular disorders notified in Switzerland since their approval: of a total of 99 (3.5%) ICSRs reported, 57 (57.6%) represented patients treated with ranibizumab, 33 (33.3%) with aflibercept and 10 (10.1%) with bevacizumab. The first ICSRs pertaining to VEGF inhibitors were reported in 2006, but most ADRs were reported between 2012 and 2016 (76 ICSRs corresponding to 76.8% of patients).
An increasing trend was observed in relation to patient age: for every ICSR reported for a child aged under 2 years, 45 were reported for patients older than 65 years. Female patients were more likely to experience adverse events involving anti-neovascularisation agents: 55.6% of ICSRs concerned women and only 17.2% involved male patients.
Table 3 lists the most common reactions reported in association with each of the three cited VEGF inhibitors. A total of 159 reactions were notified, comprising 106 (66.7%) related to ranibizumab, 42 (26.4%) to aflibercept and 11 (6.9%) to bevacizumab. For bevacizumab, all reactions are listed; for the other two medications, only the 15 most frequently recurring terms are reported.
Table 3 Number and percentage of the first 15 most reported ADRs for ranibizumab, aflibercept und bevacizumab.
| Ranibizumab (n = 106) | Aflibercept (n = 42) | Bevacizumab (n = 11) | ||||||
|---|---|---|---|---|---|---|---|---|
| ADR | n | % | ADR | n | % | ADR | n | % |
| Eye pain | 10 | 9.4% | Retinal haemorrhage | 9 | 21.4% | Visual impairment | 3 | 27.3% |
| Visual impairment | 10 | 9.4% | Blindness | 6 | 14.3% | Blepharitis | 1 | 9.1% |
| Visual acuity reduced | 7 | 6.6% | Uveitis | 6 | 14.3% | Blindness transient | 1 | 9.1% |
| Eye inflammation | 5 | 4.7% | Retinal disorder | 4 | 9.5% | Iridocyclitis | 1 | 9.1% |
| Retinal disorder | 5 | 4.7% | Retinal pigment epithelial tear | 3 | 7.1% | Keratitis | 1 | 9.1% |
| Blindness | 4 | 3.8% | Non-infectious endophthalmitis | 2 | 4.8% | Retinal artery embolism | 1 | 9.1% |
| Retinal vein thrombosis | 4 | 3.8% | Anterior chamber disorder | 1 | 2.4% | Uveitis | 1 | 9.1% |
| Vitreous disorder | 4 | 3.8% | Cataract | 1 | 2.4% | Vision blurred | 1 | 9.1% |
| Vitreous floaters | 4 | 3.8% | Eye irritation | 1 | 2.4% | Vitreous detachment | 1 | 9.1% |
| Vitreous haemorrhage | 4 | 3.8% | Eye pain | 1 | 2.4% | |||
| Eye oedema | 3 | 2.8% | Iridocyclitis | 1 | 2.4% | |||
| Ocular hyperaemia | 3 | 2.8% | Photophobia | 1 | 2.4% | |||
| Ocular hypertension | 3 | 2.8% | Pseudoendophthalmitis | 1 | 2.4% | |||
| Retinal haemorrhage | 3 | 2.8% | Visual acuity reduced | 1 | 2.4% | |||
| Eye irritation | 2 | 1.9% | Visual impairment | 1 | 2.4% | |||
| Total | 71 | 67.0% | Total | 39 | 92.9% | Total | 11 | 100.0% |
Table 4 shows a disproportionality analysis for the intravitreal application of ranibizumab, aflibercept, and bevacizumab. Reporting odds ratio (ROR), confidence interval (CI), and p-value were calculated for each single drug compared with the other two medications considered as a unique category. For ranibizumab, only an inverse significant disproportionality (ROR <1.00) was identified in connection with retinal haemorrhage (ROR 0.14, 95% CI 0.04–0.55; p = 0.005) and uveitis (ROR 0.13, 95% CI 0.03–0.63; p = 0.012), meaning that ranibizumab probably confers less risk for these reactions as compared with aflibercept and bevacizumab. Bevacizumab showed a significant disproportionality for visual impairment (ROR 4.67, 95% CI 1.08–20.16; p = 0.04), and no other ROR showed a significant correlation with the respective data. Aflibercept showed the greatest number of significantly disproportional RORs: retinal haemorrhage (ROR 10.36, 95% CI 2.65–40.50; p <0.001), blindness (ROR 3.73, 95% CI 1.08–12.96; p = 0.04), and uveitis (ROR 6.91, 95% CI 1.64–29.13; p = 0.01) were significantly more frequently reported in association with aflibercept than with the other two VEGF inhibitors.
Table 4 Main ADRs for ranibizumab, bevacizumab, and aflibercept, with reporting odds ratio, 95% confidence intervals, and p-value against the other two drugs.
| Comparison | ADR | ROR | 95% CI | p-value |
|---|---|---|---|---|
| Ranibizumab versus aflibercept and bevacizumab | Eye pain | 5.42 | 0.67–43.50 | 0.11 |
| Visual impairment | 1.28 | 0.38–4.28 | 0.69 | |
| Visual acuity reduced | 3.68 | 0.44–30.69 | 0.23 | |
| Retinal disorder | 0.61 | 0.16–2.36 | 0.47 | |
| Blindness | 0.45 | 0.15–1.36 | 0.16 | |
| Vitreous floaters | 2.04 | 0.22–18.71 | 0.53 | |
| Vitreous haemorrhage | 2.04 | 0.22–18.71 | 0.53 | |
| Retinal haemorrhage | 0.14 | 0.04–0.55 | 0.005 | |
| Uveitis | 0.13 | 0.03–0.63 | 0.012 | |
| Eye irritation | 1.00 | 0.09–11.29 | 1.00 | |
| Anterior chamber disorder | 0.50 | 0.03–8.08 | 0.61 | |
| Photofobia | 0.50 | 0.03–8.08 | 0.61 | |
| Pseudoendophthalmitis | 0.50 | 0.03–8.08 | 0.61 | |
| Vitritis | 0.50 | 0.03–8.08 | 0.61 | |
| Iridocyclitis | 0.16 | 0.00–4.10 | 0.27 | |
| Vision blurred | 0.16 | 0.00–4.10 | 0.27 | |
| Aflibercept versus ranibizumab and bevacizumab | Retinal haemorrhage | 10.36 | 2.65–40.50 | <0.001 |
| Blindness | 3.73 | 1.08–12.96 | 0.04 | |
| Uveitis | 6.91 | 1.64–29.13 | 0.01 | |
| Retinal disorder | 2.36 | 0.60–9.24 | 0.22 | |
| Anterior chamber disorder | 2.83 | 0.17–46.28 | 0.47 | |
| Eye pain | 0.26 | 0.03–2.10 | 0.21 | |
| Iridocyclitis | 2.83 | 0.17–46.28 | 0.47 | |
| Photophobia | 2.83 | 0.17–46.28 | 0.47 | |
| Pseudoendophthalmitis | 2.83 | 0.17–46.28 | 0.47 | |
| Visual acuity reduced | 0.38 | 0.05–3.21 | 0.38 | |
| Vitreous floaters | 0.69 | 0.07–6.35 | 0.74 | |
| Vitreous haemorrhage | 0.69 | 0.07–6.35 | 0.74 | |
| Vitritis | 2.83 | 0.17–46.28 | 0.47 | |
| Visual impairment | 0.20 | 0.02–1.54 | 0.12 | |
| Vision blurred | 0.54 | 0.03–11.55 | 0.70 | |
| Eye irritation | 0.54 | 0.03–11.55 | 0.70 | |
| Bevacizumab versus ranibizumab and aflibercept | Visual impairment | 4.67 | 1.08–20.16 | 0.04 |
| Blindness | 1.38 | 0.16–11.89 | 0.77 | |
| Iridocyclitis | 7.30 | 0.61–87.57 | 0.12 | |
| Uveitis | 1.75 | 0.20–15.41 | 0.61 | |
| Vision blurred | 14.70 | 0.85–252.84 | 0.06 | |
| Eye pain | 0.52 | 0.03–9.40 | 0.66 | |
| Visual acuity reduced | 0.72 | 0.04–13.26 | 0.82 | |
| Retinal disorder | 0.64 | 0.03–11.68 | 0.76 | |
| Vitreous floaters | 1.13 | 0.06–21.82 | 0.93 | |
| Vitreous haemorrhage | 1.13 | 0.06–21.82 | 0.93 | |
| Retinal haemorrhage | 0.47 | 0.03–8.54 | 0.61 | |
| Eye irritation | 1.81 | 0.09–37.17 | 0.70 | |
| Anterior chamber disorder | 2.55 | 0.12–56.28 | 0.55 | |
| Photofobia | 2.55 | 0.13–56.28 | 0.55 | |
| Pseudoendophthalmitis | 2.55 | 0.13–56.28 | 0.55 | |
| Vitritis | 2.55 | 0.13–56.28 | 0.55 |
A total of 8865 different reactions affecting the eye were reported within the period under consideration. The majority of cases involved adult patients and the main class of drugs associated with ocular ADRs were those acting on the nervous system. Health professionals were the main reporters of the ADRs and the majority of ICSRs pertained to patients who had ingested a single drug. A suspected relationship between drug and event was identified for half of the drug-reaction pairs (51.1%). The range between the shortest and the longest ADR was from 1 day to several years, with a median duration of about 8 days. The majority of reactions were temporary, as 4173 (47.1%) resolved completely. Visual impairment was the most commonly reported reaction and constituted 7.2% of all reported symptoms. Analysis of the association of VEGF inhibitors as a single class of drugs with ocular ADRs indicated that aflibercept was positively associated with serious ocular ADRs such as blindness, retinal haemorrhage and uveitis.
Although ADRs affecting the eye appear only in 3 of 100 patients reporting any adverse event, drug-induced ocular events are the second most common reason for official complaints against ophthalmologists [14]. Ocular ADRs primarily manifest as visual impairment, which, as an adverse side-effect of medication, is rare but can lead to considerable individual and societal burdens, and the acquisition of robust data to identify and confirm the relationship between a medication and an uncommon adverse side-effect is challenging [15]. As serious ADRs, blindness and lethal reactions constituted only a small part of the total reported ICSRs, although the real incidence of such cases may be greater as ADRs in ophthalmology are a heterogeneous group that are not commonly detected [5]. This assumption underscores the importance of ophthalmologists’ awareness of specific ocular ADRs related to systemic medication and how to detect and adequately treat these ADRs as part of good clinical practice [5].
Certain sub-populations appear to be more susceptible to ocular adverse reactions. Frail patients are more likely to be exposed to polypharmacy [16], which may increase the risk of inappropriate drug use and ADRs [17]. Our analysis supports these findings, as the percentage of serious ADRs was significantly correlated with the number of ingested medications and with patient age. A previous meta-analysis of the relationship between systemic fluoroquinolones and retinal detachment did not show any significant association [18]. In our data we found 26 cases of retinal detachment and only one case (3.85%) was associated with the intake of fluoroquinolones, supporting the conclusion of the meta-analysis.
Interesting differences were observed with respect to drugs used to prevent angiogenesis. Our data indicate that aflibercept was positively associated with serious ocular ADRs such as blindness, retinal haemorrhage and uveitis. These results were based on very small numbers: six cases of blindness with aflibercept and four with ranibizumab (see table 3). In contrast, bevacizumab showed a significant inverse association with both retinal haemorrhage and uveitis. An Australian case report on uveitis following the use of intravitreal aflibercept also cautions clinicians to be aware of late-onset sterile uveitis and vitritis after aflibercept injection [19]. Conversely, a double-blind randomised controlled trial showed that aflibercept was associated with similar visual and anatomic outcomes as ranibizumab when dosed monthly, as well as similar safety and tolerability [20].
Further research is required to investigate the culpability of VEGF inhibitors in serious eye disorders. The limited literature on this issue may be the main ascribable circumstance, but also the erroneous conduct and technique of administering intravitreal drugs may play a crucial role. One study showed that vision loss was not the result of the drug itself or the injection technique, but rather of the procedures used to prepare the syringes containing bevacizumab [21].
Pharmacovigilance studies based on spontaneous reporting have several limitations, including under- and selective reporting [22], and our findings should be interpreted in this context. Many ICSRs had missing data or data that were only partially specified; these circumstances, when applied to large datasets, can contribute to increased uncertainty surrounding the available information. Another limitation is the multiple comparison issue (the propensity to generate false positive results as the number of comparisons increases) considered endemic in the analysis of spontaneous reporting databases [23]. The voluntary nature of the reports also creates inherent limitations in data interpretation, and population differences across the participating countries have not been accounted for in the present study. In addition, clinical information contained in a spontaneous reporting database such as the WHO VigiBase is necessarily limited and cases can be reported more than once. However, our method allowed us to eliminate as many potential duplicate records as possible [8]. Finally, causality assessment was frequently not reported in the retrieved records [24].
Our findings highlight the importance of the routine detection of adverse reactions by physicians and other health professionals. Failure to recognise an ADR may result in a prescribing cascade whereby a new drug is administered to treat the adverse effect of the culprit drug, thus exposing the patient to a continued risk of an ADR from the culprit drug and additional risks from the newly prescribed drug [25]. To promote reporting of ocular ADRs, the awareness of both ophthalmologists and other practitioners of the risk of such ADRs could be increased by introducing routine pharmacovigilance activities into daily practice or by enhancing education in pharmacovigilance at both the under- and postgraduate level.
In conclusion, ocular ADRs in Switzerland may be more common than the number reported by health professionals suggests, implying that a more meticulous approach is required to detect serious ADRs. Serious adverse events are frequently overlooked because they are more common when polypharmacy is present, increasing the effort needed to determine whether a single drug is more likely to cause a specific ADR than the other medications. Although our data indicate that aflibercept was more significantly associated with serious ocular ADRs than other VEGF inhibitors, care is required in the use of this class of drugs because of the lack of relevant literature and risk of improper drug administration, leading to biased results.
Clare Cox, PhD, from Edanz Group is acknowledged for language editing a draft of this manuscript.
The authors declare no conflicts of interest in connection with the contents of this article. The Department of Clinical Pharmacology and Toxicology is a regional pharmacovigilance centre that reports to the national competent agency, Swissmedic. SW is a member of the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA). Any conclusions drawn in this article are the authors’ personal views based on the data presented, and may not be understood or quoted as being made on behalf of the PRAC or reflecting the position of the PRAC. The data for this work were obtained from VigiLyze™, the software of the WHO Collaborating Centre for International Drug Monitoring, Uppsala, Sweden. Data from spontaneous reporting are inhomogeneous as a result of different reporting policies worldwide and are subject to underreporting and reporting bias. The information contained in this work is therefore not homogeneous, at least with respect to origin and also to the likelihood that the pharmaceutical product caused the adverse reaction. The conclusions drawn based on these data do not necessarily represent the opinion of the World Health Organization.
1 Miguel A , Henriques F , Azevedo LF , Pereira AC . Ophthalmic adverse drug reactions to systemic drugs: a systematic review. Pharmacoepidemiol Drug Saf. 2014;23(3):221–33. doi:.https://doi.org/10.1002/pds.3566
2 Yayla EM , Yavuz E , Bilge U , Keskin A , Binen E . Drugs with anticholinergic side-effects in primary care. Niger J Clin Pract. 2015;18(1):18–21.
3 Selvin BL . Systemic effects of topical ophthalmic medications. South Med J. 1983;76(3):349–58. doi:.https://doi.org/10.1097/00007611-198303000-00020
4 Köberlein J , Beifus K , Schaffert C , Finger RP . The economic burden of visual impairment and blindness: a systematic review. BMJ Open. 2013;3(11):e003471. doi:.https://doi.org/10.1136/bmjopen-2013-003471
5 Biagi C , Conti V , Montanaro N , Melis M , Buccellato E , Donati M , et al. Comparative safety profiles of intravitreal bevacizumab, ranibizumab and pegaptanib: the analysis of the WHO database of adverse drug reactions. Eur J Clin Pharmacol. 2014;70(12):1505–12. doi:.https://doi.org/10.1007/s00228-014-1755-1
6 van der Reis MI , La Heij EC , De Jong-Hesse Y , Ringens PJ , Hendrikse F , Schouten JS . A systematic review of the adverse events of intravitreal anti-vascular endothelial growth factor injections. Retina. 2011;31(8):1449–69. doi:.https://doi.org/10.1097/IAE.0b013e3182278ab4
7 Lu X , Ng DS , Zheng K , Peng K , Jin C , Xia H , et al. Risk factors for endophthalmitis requiring evisceration or enucleation. Sci Rep. 2016;6(1):28100. doi:.https://doi.org/10.1038/srep28100
8 Wang W , Zhang X . Systemic adverse events after intravitreal bevacizumab versus ranibizumab for age-related macular degeneration: a meta-analysis. PLoS One. 2014;9(10):e109744. doi:.https://doi.org/10.1371/journal.pone.0109744
9 Caster O , Conforti A , Viola E , Edwards IR . Methylprednisolone-induced hepatotoxicity: experiences from global adverse drug reaction surveillance. Eur J Clin Pharmacol. 2014;70(4):501–3. doi:.https://doi.org/10.1007/s00228-013-1632-3
10 Petronijevic M , Ilic K . Associations of gender and age with the reporting of drug-induced hepatic failure: data from the VigiBase™. J Clin Pharmacol. 2013;53(4):435–43. doi:.https://doi.org/10.1002/jcph.3
11 Blaser LS , Tramonti A , Egger P , Haschke M , Krähenbühl S , Rätz Bravo AE . Hematological safety of metamizole: retrospective analysis of WHO and Swiss spontaneous safety reports. Eur J Clin Pharmacol. 2015;71(2):209–17. doi:.https://doi.org/10.1007/s00228-014-1781-z
12 Masnoon N , Shakib S , Kalisch-Ellett L , Caughey GE . What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. doi:.https://doi.org/10.1186/s12877-017-0621-2
13 van Puijenbroek E , Diemont W , van Grootheest K . Application of quantitative signal detection in the Dutch spontaneous reporting system for adverse drug reactions. Drug Saf. 2003;26(5):293–301. doi:.https://doi.org/10.2165/00002018-200326050-00001
14 McGee HT , Fraunfelder FW . Toxicities of topical ophthalmic anesthetics. Expert Opin Drug Saf. 2007;6(6):637–40. doi:.https://doi.org/10.1517/14740338.6.6.637
15 Cumberland PM , Russell-Eggitt I , Rahi JS . Active surveillance of visual impairment due to adverse drug reactions: findings from a national study in the United Kingdom. Pharmacol Res Perspect. 2015;3(1):e00107. doi:.https://doi.org/10.1002/prp2.107
16 Saum KU , Schöttker B , Meid AD , Holleczek B , Haefeli WE , Hauer K , et al. Is polypharmacy associated with frailty in older people? Results from the ESTHER cohort study. J Am Geriatr Soc. 2017;65(2):e27–32. doi:.https://doi.org/10.1111/jgs.14718
17 Davies EC , Green CF , Taylor S , Williamson PR , Mottram DR , Pirmohamed M . Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLoS One. 2009;4(2):e4439. doi:.https://doi.org/10.1371/journal.pone.0004439
18 Alves C , Penedones A , Mendes D , Batel Marques F . A systematic review and meta-analysis of the association between systemic fluoroquinolones and retinal detachment. Acta Ophthalmol. 2016;94(5):e251–9. doi:.https://doi.org/10.1111/aos.12931
19 Glading JA , Lake SR , Craig JE , Supramaniam D . Delayed onset panuveitis following intravitreal aflibercept injection. BMJ Case Rep. 2014;2014(jun11 1):bcr2013202515. doi:.https://doi.org/10.1136/bcr-2013-202515
20 Heier JS , Brown DM , Chong V , Korobelnik JF , Kaiser PK , Nguyen QD , et al.; VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–48. doi:.https://doi.org/10.1016/j.ophtha.2012.09.006
21 Gonzalez S , Rosenfeld PJ , Stewart MW , Brown J , Murphy SP . Avastin doesn’t blind people, people blind people. Am J Ophthalmol. 2012;153(2):196–203.e1. doi:.https://doi.org/10.1016/j.ajo.2011.11.023
22 Biagi C , Montanaro N , Buccellato E , Roberto G , Vaccheri A , Motola D . Underreporting in pharmacovigilance: an intervention for Italian GPs (Emilia-Romagna region). Eur J Clin Pharmacol. 2013;69(2):237–44. doi:.https://doi.org/10.1007/s00228-012-1321-7
23 Huang L , Guo T , Zalkikar JN , Tiwari RC . A review of statistical methods for safety surveillance. Ther Innov Regul Sci. 2014;48(1):98–108. doi:.https://doi.org/10.1177/2168479013514236
24 Motola D , Donati M , Biagi C , Calamelli E , Cipriani F , Melis M , et al. Safety profile of H1-antihistamines in pediatrics: an analysis based on data from VigiBase. Pharmacoepidemiol Drug Saf. 2017;26(10):1164–71. doi:.https://doi.org/10.1002/pds.4246
25 Rochon PA , Gurwitz JH . Optimising drug treatment for elderly people: the prescribing cascade. BMJ. 1997;315(7115):1096–9. doi:.https://doi.org/10.1136/bmj.315.7115.1096
The authors declare no conflicts of interest in connection with the contents of this article. The Department of Clinical Pharmacology and Toxicology is a regional pharmacovigilance centre that reports to the national competent agency, Swissmedic. SW is a member of the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA). Any conclusions drawn in this article are the authors’ personal views based on the data presented, and may not be understood or quoted as being made on behalf of the PRAC or reflecting the position of the PRAC. The data for this work were obtained from VigiLyze™, the software of the WHO Collaborating Centre for International Drug Monitoring, Uppsala, Sweden. Data from spontaneous reporting are inhomogeneous as a result of different reporting policies worldwide and are subject to underreporting and reporting bias. The information contained in this work is therefore not homogeneous, at least with respect to origin and also to the likelihood that the pharmaceutical product caused the adverse reaction. The conclusions drawn based on these data do not necessarily represent the opinion of the World Health Organization.