Patterns of multimorbidity in internal medicine patients in Swiss university hospitals: a multicentre cohort study
DOI: https://doi.org/10.4414/smw.2019.20094
Carole E.
Aubertab, Niklaus
Fankhauserc, Pedro
Marques-Vidald, Jérôme
Stirnemanne, Drahomir
Aujeskya, Andreas
Limacherc, Jacques
Donzéafgh
aDepartment of General Internal Medicine, Bern University Hospital, University of Bern, Switzerland
bInstitute of Primary Health Care (BIHAM), University of Bern, Switzerland
cClinical Trials Unit, University of Bern, Switzerland
dDepartment of Internal Medicine, Lausanne University Hospital, Lausanne, Switzerland
eDepartment of Internal Medicine, Geneva University Hospital, Geneva, Switzerland
fBWH Hospitalist Service, Division of General Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, USA
g Harvard Medical School, Boston, Massachusetts, USA
hDepartment of Internal Medicine, Hôpital neuchâtelois, Neuchâtel, Switzerland
Summary
AIMS OF THE STUDY
Despite the high prevalence of multimorbidity, we lack detailed descriptive data on the most prevalent combinations of chronic comorbidities in Switzerland. We aimed to describe and quantify the most prevalent combinations of comorbidities in internal medicine multimorbid inpatients.
METHODS
We conducted a multicentre retrospective cohort study including all consecutive adults (n = 42,739) discharged from the general internal medicine department of three Swiss tertiary teaching hospitals in 2010–2011. We used the Chronic Condition Indicator and the Clinical Classification Software to classify International Classification of Diseases diagnosis codes into chronic or acute diseases, into body system categories and into categories of chronic comorbidities. We defined multimorbidity as ≥2 chronic diseases. We described the most prevalent combinations of comorbidities and their prevalence.
RESULTS
Seventy-nine percent (n = 33,871) of the patients were multimorbid, with a median of four chronic diseases. Chronic heart disease, chronic kidney disease, solid malignancy and substance-related disorders were the most prevalent comorbidities, with a prevalence of more than 10% for each. All these comorbidities were frequently found in combination with chronic obstructive pulmonary disease and bronchiectasis, pulmonary heart disease, and peripheral and visceral atherosclerosis. Chronic heart disease was identified in 80% of the most prevalent combinations. Half of the combinations occurred more often than it would have been expected if they were independent.
CONCLUSIONS
The vast majority of patients fulfilled the criteria for multimorbidity. Chronic heart disease, chronic kidney disease, solid malignancy and substance-related disorders were each present in at least one tenth of the patients. This in-depth description of the most frequent comorbidities and of their frequent associations in a multicentre population may advise healthcare providers to improve preventive care and develop appropriate guidelines for multimorbid patients.
Introduction
With the progress of medical science and increasing life expectancy, multimorbidity, most often defined as the presence of at least two chronic diseases [1], is affecting a growing proportion of patients and its prevalence is expected to further increase in the coming years [2–6]. Given its association with adverse outcomes such as functional decline, poorer quality of life and higher healthcare costs and mortality, it represents a significant burden for patients and healthcare systems [6–8]. In Switzerland, multimorbidity was mostly studied in ambulatory care settings, with reported prevalence rates of 13 to 77% [7, 9–12]; a small study including 170 patients hospitalised on the internal medicine ward of a university hospital reported a prevalence of 50 to 97%, depending on the definition of multimorbidity [5].
To better describe multimorbidity, a growing interest has developed in studying non-random patterns of diseases [13–24]. However, most studies were conducted in ambulatory care settings, did not use standardised tools to classify the diseases, and included not only diseases, but also risk factors or symptoms in the analyses [13–23, 25]. Doing so, they often revealed expected associations, such as cardiovascular diseases and risk factors. In Switzerland, a recent study described patterns of multimorbidity in patients cared for in general practitioner practices [9], while data for hospitalised patients are lacking.
Therefore, with the use of standardised tools to classify the diseases, the primary aim of our study was to identify and quantify the most prevalent combinations of chronic comorbidities in multimorbid patients hospitalised in internal medicine wards in Switzerland. We hypothesised that besides expected prevalent combinations such as chronic kidney disease (CKD) with chronic heart disease, or cardiovascular diseases and risk factors, we could identify other prevalent but less obvious combinations of comorbidities. We focused on chronic and not on acute comorbidities to study the long-term burden of diseases in multimorbidity. The secondary aim was to describe the distribution of acute and chronic diseases in the same population. We hypothesised that the number of chronic diseases was higher than the number of acute diseases, and that the proportion of acute versus chronic diseases varied according to the total number of diseases.
Materials and methods
Study design, setting and participants
We used a retrospective cohort including all consecutive adult patients discharged home or to a nursing home from the department of general internal medicine of three tertiary teaching hospitals between 2010 and 2011: Bern University Hospital (Inselspital), Lausanne University Hospital (CHUV) and Geneva University Hospitals (HUG). We included only patients with multimorbidity, as defined below, in the analysis. As it was a secondary analysis of fully anonymised retrospective data, the study was waived from ethical approval according to Swissethics regulations. Reporting is in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement [26].
Study variables, definition of multimorbidity and classification of diseases
We retrospectively extracted all data from electronic medical records, including International Classification of Diseases (ICD) codes version 10 at discharge. We defined multimorbidity based on its most usual definition – the presence of two or more chronic diseases [1, 27]. We assessed multimorbidity according to: (1) acute and chronic diseases; (2) chronic comorbidities; (3) number of body systems involved; (4) the Deyo-Charlson Comorbidity Index and Elixhauser-Van Walraven Comorbidity Index [28–32].
We classified all ICD diagnosis codes with two tools developed by the Healthcare Cost and Utilization Project, a partnership between the US federal government and the industry, sponsored by the Agency for Healthcare Research and Quality. First, we used the Chronic Condition Indicator to classify ICD codes into acute or chronic diseases and into 18 body system categories (details in appendix 1) [33]. The Chronic Condition Indicator defines a chronic disease as a health condition lasting minimum 12 months and that causes limitations on self-care, independent living and social interactions, and/or results in the need for ongoing intervention with medical products, services and special equipment. Second, we categorised all chronic diseases into a clinically meaningful smaller number of comorbidities using the Clinical Classification Software, which aggregates ICD codes into 285 mutually exclusive categories (full list available on https://www.hcup-us.ahrq.gov/toolssoftware/ccs/CCSCategoryNames_FullLabels.pdf) [34]. For clinical relevance, we further merged some categories of the Clinical Classification Software in the same comorbidity (details in appendix 2). Moreover, we decided to focus on chronic comorbidities, because the definition of multimorbidity most often includes only chronic conditions [1], and because we wanted to assess the long-term burden of diseases in multimorbidity, i.e. which underlying combinations of chronic comorbidities are present in hospitalised patients. Therefore, we did not include acute diseases, nor the ICD codes for screening strategies, symptoms, complications or risk factors, in this classification of comorbidities.
Statistical analyses
We presented baseline characteristics as numbers with frequency or median with interquartile range (IQR) in the whole cohort and according to the presence of multimorbidity.
For the primary aim, we described the 20 most prevalent combinations of two comorbidities and their observed prevalence. We calculated the expected prevalence of each combination considering that the two diseases were independent (expected prevalence of combination [A, B] = prevalence [A] × prevalence [B]). We then computed the observed/expected (O/E) ratio, which indicated the degree of independence of the two comorbidities. We separately counted different comorbidities occurring in the same patient. We also considered looking at combinations of more than two chronic comorbidities, but because of the low frequency of such combinations and the very high number of possible combinations, the results were not clinically relevant, and therefore not shown. We then identified the comorbidities with a prevalence of more than 10% and presented in a plot their five most prevalent combined comorbidities. Finally, we used latent class analysis to identify five groups of patients with combinations of comorbidities occurring together more often than expected by chance. In order to identify the most prevalent comorbidities, we included in the different groups only comorbidities with a probability of at least 10%, and presented those probabilities. We labelled the groups according to the predominant comorbidities identified in each class. We calculated each patient probability to belong to the different groups, classified the patients in the group with the highest probability and presented the proportion of patients in each group.
For the secondary aim, using a two y-axis bar/line plot, we presented the proportions of acute and chronic diseases, as well as the median number of body systems involved, according to the total number of diseases. We performed all analyses with R version 3.4.4 (R Project for Statistical Computing).
Results
Of the 42,739 patients from the initial cohort, 33,871 (79%) had multimorbidity and 23,843 (56%) were men (table 1). A flow chart of study recruitment and follow-up is shown in supplementary figure S1 in appendix 3. Median age was 68 years (IQR 56–78) in patients with multimorbidity and 57 years (IQR 41–70) in those without. The median number of diseases was 7 (IQR 5–10) for a median number of chronic diseases of 4 (IQR 3–6) in patients with multimorbidity. In those without multimorbidity, the median number of diseases was 2 (IQR 1–4) for a median number of chronic diseases of 1 (IQR 0–1). The number of patients with at least 10 chronic diseases was 1,628 (4.8% of the multimorbid patients). The following comorbidities occurred with a prevalence of more than 10%: chronic heart disease, CKD, solid malignancy, substance-related disorders.
Table 1 Baseline characteristics.
|
Characteristics
|
Total population (n = 42,739)
|
With multimorbidity (n = 33,871)
|
Without multimorbidity (n = 8,868)
|
| Age, years, median (IQR) |
66 (53–77) |
68 (56–78) |
57 (41–70) |
| Men, n (%) |
23,843 (55.8) |
19,170 (56.6) |
4,673 (52.7) |
| Description of multimorbidity |
Number of acute and chronic diseases, median (IQR) |
6 (3–9) |
7 (5–10) |
2 (1–4) |
| Number of chronic diseases, median (IQR) |
2 (1–3) |
4 (3–6) |
1 (0–1) |
| Number of body systems involved, median (IQR) |
2 (1–4) |
3 (2–4) |
1 (1–2) |
| Deyo-Charlson Comorbidity Index, median (IQR) |
1 (0–3) |
2 (1–3) |
0 (0–1) |
| Elixhauser-Van Walraven Comorbidity Index, median (IQR) |
5 (0–11) |
7 (2–12) |
0 (0–5) |
| Most prevalent comorbidities (>10%) |
Chronic heart disease, n (%) |
16,946 (39.7) |
15,717 (46.4) |
1,229 (13.9) |
| Chronic kidney disease, n (%) |
5,745 (13.4) |
5,174 (15.3) |
781 (8.8) |
| Solid malignancy, n (%) |
5,277 (12.4) |
4,964 (14.7) |
103 (1.2) |
| Substance–related disorders, n (%) |
4,296 (10.1) |
4,173 (12.3) |
143 (1.6) |
| Hospitalisation characteristics |
Length of stay, days, median (IQR) |
7 (3–12) |
8 (4–14) |
4 (2–7) |
| Number of admissions in the past year, n (%) |
0 (0–2) |
0 (0–2) |
0 (0–1) |
Most prevalent combinations of comorbidities
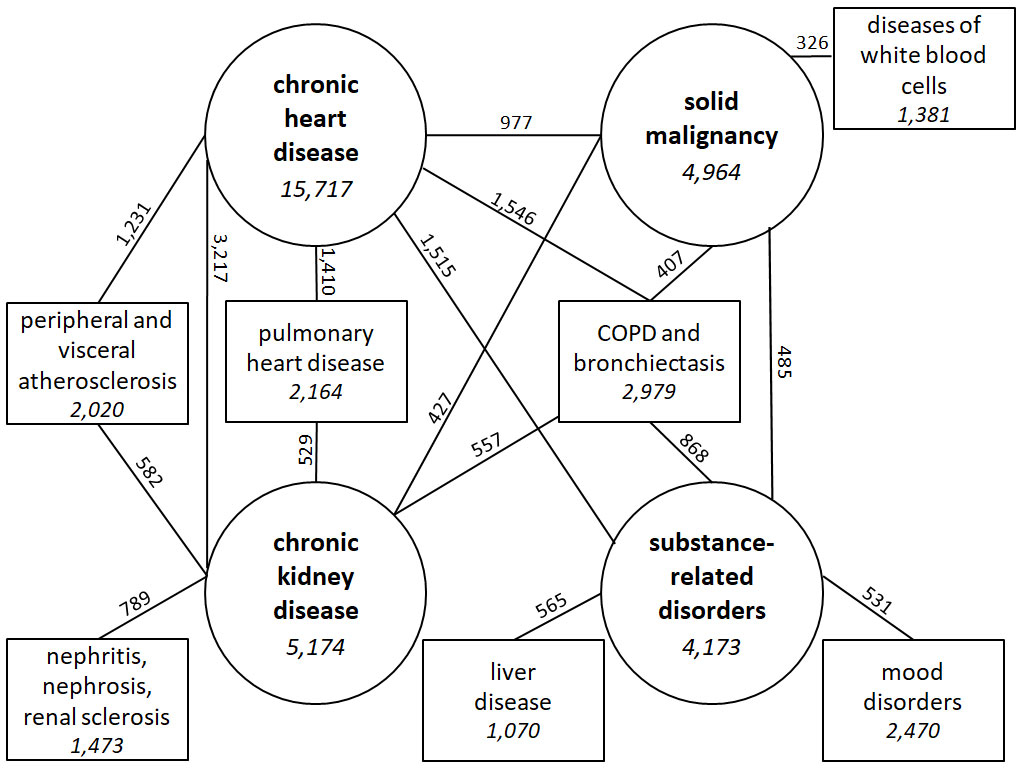
Chronic heart disease was found in 16 of the 20 most prevalent combinations of comorbidities, and part of the five most prevalent combinations (table 2). The overall most prevalent combination was chronic heart disease with CKD (n = 3,217, 9.5%). The four next combinations were found in 3.6 to 4.6% of the patients and included chronic heart disease combined with (in order of decreasing frequency): chronic obstructive pulmonary disease (COPD) and bronchiectasis; substance-related disorders; pulmonary heart disease; peripheral and visceral atherosclerosis. The prevalence of all comorbidities and of the body systems, as well as all combinations of comorbidities with their frequency found in the cohort, are described in the appendix 3 (tables S1–S3).
Table 2 Most prevalent combinations of comorbidities.
|
Combinations of comorbidities
|
Cases, n
|
Prevalence, %
|
Ratio O/E
|
|
Observed
|
Expected
|
| Chronic heart disease |
Chronic kidney disease |
3,217 |
9.5 |
7.1 |
1.3 |
| Chronic heart disease |
COPD and bronchiectasis |
1,546 |
4.6 |
4.1 |
1.1 |
| Chronic heart disease |
Substance-related disorders |
1,515 |
4.5 |
5.7 |
0.8 |
| Chronic heart disease |
Pulmonary heart disease |
1,410 |
4.2 |
3.0 |
1.4 |
| Chronic heart disease |
Peripheral and visceral atherosclerosis |
1,231 |
3.6 |
2.8 |
1.3 |
| Cerebrovascular disease |
Paralysis |
1,157 |
3.4 |
0.5 |
6.8 |
| Chronic heart disease |
Arthropathy and arthritis |
1,046 |
3.1 |
3.4 |
0.9 |
| Chronic heart disease |
Cerebrovascular disease |
1,025 |
3.0 |
3.9 |
0.8 |
| Chronic heart disease |
Acute and unspecified renal failure |
1,009 |
3.0 |
2.8 |
1.1 |
| Chronic heart disease |
Solid malignancy |
977 |
2.9 |
6.8 |
0.4 |
| Chronic heart disease |
Other nervous system disorders |
959 |
2.8 |
3.8 |
0.7 |
| Chronic heart disease |
Other and ill-defined heart disease |
926 |
2.7 |
1.5 |
1.8 |
| Chronic heart disease |
Thyroid disorders |
910 |
2.7 |
2.6 |
1.0 |
| Substance-related disorders |
COPD and bronchiectasis |
868 |
2.6 |
10.8 |
0.2 |
| Chronic heart disease |
Dementia |
817 |
2.4 |
2.4 |
1.0 |
| Chronic kidney disease |
Nephritis, nephrosis and renal sclerosis |
789 |
2.3 |
0.7 |
3.3 |
| Chronic heart disease |
Mood disorders |
769 |
2.3 |
3.4 |
0.7 |
| Chronic heart disease |
Nephritis, nephrosis and renal sclerosis |
763 |
2.3 |
2.0 |
1.2 |
| Haematological malignancy |
Diseases of white blood cells |
675 |
2.0 |
0.2 |
10.0 |
| Chronic heart disease |
Paralysis |
611 |
1.8 |
2.8 |
0.6 |
Half of the combinations occurred more frequently than it would have been expected if they were independent of each other, with a particularly high O/E ratio for haematological malignancy with diseases of white blood cells (O/E ratio 10.0), for cerebrovascular disease with paralysis (O/E ratio 6.8), and for CKD with nephritis, nephrosis and renal sclerosis (O/E ratio 3.3). On the contrary, the expected prevalence was higher than the observed prevalence for eight combinations, with a particularly low O/E ratio for substance-related disorders with COPD and bronchiectasis (O/E ratio 0.2) and for chronic heart disease with solid malignancy (O/E ratio 0.4).
Figure 1 summarises the five most frequent combinations of chronic comorbidities for each of the four most frequent comorbidities. COPD and bronchiectasis was the only comorbidity found in combination with each of the four most prevalent comorbidities. This comorbidity was identified in 868 (20.8%) of the patients with substance-related disorders, 557 (10.8%) of those with CKD, 1,546 (9.8%) of those with chronic heart disease, and 407 (8.2%) of those with solid malignancy. Pulmonary heart disease occurred in 529 (10.2%) of the patients with CKD and in 1,410 (9.0%) of those with chronic heart disease. Peripheral and visceral atherosclerosis was found in 582 (11.2%) of the patients with CKD and in 1,231 (7.8%) of those with chronic heart disease. Other most prevalent comorbidities were identified in combination with only one of those four most prevalent comorbidities, and included: mood disorders; nephritis, nephrosis and renal sclerosis; diseases of white blood cells; liver disease.
Groups of comorbidities
We identified five groups of comorbidities occurring together more often than would have been expected by chance (table 3). Each group included between two and eight different comorbidities. Except for group 4, all groups could be labelled according to their predominant comorbidities. Group 1 (“neurological diseases”) included four neurological diseases with chronic heart disease. Group 2 (“heart/kidney diseases”) included chronic heart disease with CKD. Group 3 (“malignancy”) included solid and haematological malignancies with disorders of white blood cells. Group 5 (“psychiatric diseases”) included psychiatric and neurological disorders, along with chronic heart disease, COPD and bronchiectasis, and arthropathy and arthritis. Group 4 included miscellaneous comorbidities: chronic heart disease, CKD, nephritis, nephrosis and renal sclerosis, peripheral and visceral atherosclerosis, other nervous system disorders, arthropathy and arthritis, COPD and bronchiectasis, and pulmonary heart disease. Ten percent of the patients were in group 1, 31% in group 2, 20% in group 3, 16% in group 4 and 23% in group 5.
Table 3 Latent class analysis.
|
Comorbidities
|
Group 1
neurological
(10%)*
|
Group 2
heart/kidney
(31%)*
|
Group 3
malignancy
(20%)*
|
Group 4
miscellaneous
(16%)*
|
Group 5
psychiatric
(23%)+
|
|
Probability of comorbidity†
|
| Cerebrovascular diseases |
63%
|
2% |
<1% |
3% |
1% |
| Paralysis |
44%
|
<1% |
2% |
2% |
2% |
| Chronic heart disease |
27%
|
100%
|
10%‡
|
55%
|
19%
|
| Other nervous system disorders |
13%
|
<1% |
4% |
16%
|
12%
|
| Epilepsy – convulsions |
11%
|
<1% |
4% |
1% |
7%
|
| Chronic kidney disease |
4% |
13%
|
6% |
51%
|
<1% |
| Solid malignancy |
5% |
4% |
49%
|
5% |
9% |
| Haematological malignancy |
<1% |
1% |
22%
|
3% |
<1% |
| Diseases of white blood cells |
<1% |
<1% |
17%
|
2% |
<1% |
| Nephritis, nephrosis and renal sclerosis |
1% |
<1% |
2% |
20%
|
<1% |
| Peripheral and visceral atherosclerosis |
4% |
<1% |
<1% |
16%
|
3% |
| Arthropathy and arthritis |
5% |
3% |
3% |
14%
|
12%
|
| COPD and bronchiectasis |
2% |
8% |
5% |
12%
|
15%
|
| Pulmonary heart disease |
2% |
9% |
4% |
11%
|
4% |
| Substance-related disorders |
7% |
8% |
7% |
7% |
30%
|
| Mood disorders |
5% |
2% |
4% |
6% |
19%
|
| Anxiety disorders |
2% |
1% |
2% |
3% |
10%
|
Proportions of acute and chronic diseases
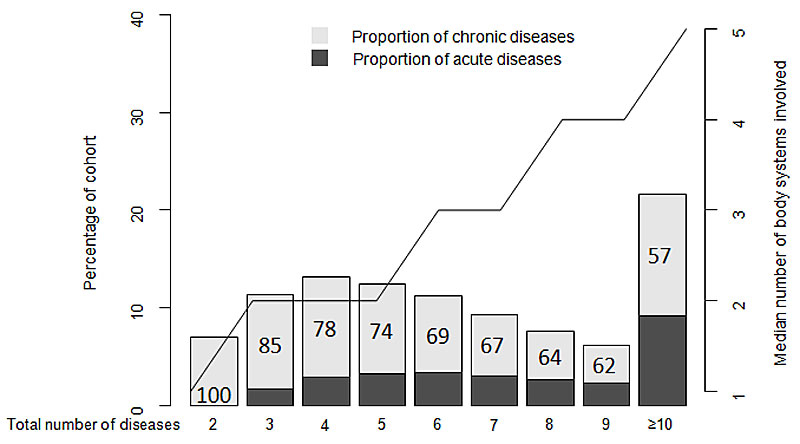
In multimorbid patients, 71% of all coded diseases were chronic diseases. The percentage of chronic versus acute diseases progressively decreased as the number of diseases increased, from 100% in patients with overall two diseases (by definition, as we included only patients with at least two chronic diseases), to 57% in those with 10 or more diseases (fig. 2). The median number of body systems involved increased proportionally with the total number of diseases, with therefore a constant proportion of about 50%.
Discussion
We identified and quantified the most common combinations of chronic comorbidities in a large sample of patients hospitalised in internal medicine in Switzerland. Almost 80% of the patients were multimorbid, with a median of four chronic diseases. Chronic diseases represented 71% of all diseases and their proportion decreased when the total number of diseases increased. Chronic heart disease, CKD, solid malignancy and substance-related disorders were each found in more than 10% of the patients. Chronic heart disease was identified in 80% of all of the most prevalent combinations of comorbidities. Half of the combinations occurred more often than expected. We identified some frequent groups of chronic comorbidities that occurred more often than expected, and that could be grouped into neurological diseases, heart/kidney diseases, malignancy, miscellaneous diseases, and psychiatric diseases. This study fills a previous lack of descriptive and quantitative data on the burden of chronic diseases in multimorbid patients hospitalised in internal medicine in Switzerland.
Prevalence of multimorbidity
Studies in other countries reported prevalence rates of multimorbidity between 23 to 99%, depending on the settings and number of conditions assessed, with the highest rates found in hospital settings (63–99%) [1, 35–39]. Previous studies in Switzerland also reported highly variable prevalence rates, ranging from 13 to 95% [5, 7, 9–12, 27]. A small study in patients hospitalised in internal medicine in Switzerland reported a prevalence of 93% when defining multimorbidity as two or more ICD diagnoses in medical records [5]. There may be several explanations for our slightly lower prevalence rate. First, the previous study considered any health condition active during hospitalisation, whereas we included only chronic diseases, because we were interested in the long-term burden of diseases in multimorbidity. Second, it excluded elective admissions, thus selecting possibly different patients. Finally, its small sample size (n = 170) may have led to chance findings.
Most prevalent comorbidities
Solid malignancy and substance-related disorders were the third and fourth most prevalent comorbidities after chronic heart disease and CKD. A similar prevalence rate was described among hospitalised patients aged 65 years or older [40], whereas prevalence rates were lower in ambulatory care patients [9, 14]. This may reveal fragility of oncological patients, who may be more likely to decompensate other comorbidities, as we did not include oncology departments where patients receive oncology-specific treatment. The absence of malignancy among the most frequent diagnoses in the study by Schneider et al. may be explained by the assessment of all ICD diagnoses separately in their study [5], whereas we grouped all solid malignancies using the Clinical Classification Software. The high prevalence of substance-related disorders may be due to the inclusion in this category of not only substance consummation causing acute problems, but also of uncomplicated consummation, such as nicotine dependence [34]. This is coherent with a study in ambulatory care in Switzerland that found tobacco and alcohol dependencies as one of the four described clusters [9].
Most prevalent combinations of comorbidities
Previous studies mostly described expected combinations of conditions, such as metabolic and cardiovascular diseases and risk factors [18, 20, 22, 36, 37, 40, 41]. A main innovation of our study was to group comorbidities expected to cluster together and to include only chronic comorbidities related to multimorbidity in order to assess the long-term burden of diseases, which revealed fewer expected combinations than previous studies. For example, COPD and bronchiectasis, pulmonary heart disease, and peripheral and visceral atherosclerosis were the most prevalent comorbidities found in combination with the four comorbidities occurring in 10% or more of the patients.
Some combinations occurred at a higher prevalence than expected. The reason could be a shared pathophysiology (e.g. vascular calcification for chronic heart disease and CKD) or risk factor (e.g. smoking for chronic heart disease and COPD and bronchiectasis), or that one of the comorbidities may be the consequence of the other one (e.g. paralysis following cerebrovascular disease, or diseases of white blood cells resulting from haematological malignancy or from its treatment). However, some combinations were found at a surprisingly lower prevalence than expected, such as substance-related disorders with COPD and bronchiectasis. This may be because substance-related disorders include not only ICD codes related to smoking, but also to all other addictive substances, such as alcohol, opioids or sedatives. Although these substance-related disorders are frequent among patients with multimorbidity, they may not be especially more prevalent among patients with COPD in particular. However, we cannot exclude underreporting of smoking-related categories as an alternative explanation for this lower than expected prevalence. The co-occurrence of such combinations among the most prevalent combinations of comorbidities may thus be explained by the high prevalence of both comorbidities, rather than by a common factor.
Groups of comorbidities
Despite growing international interest in assessing patterns of multimorbidity [42], data on this topic in Switzerland were scarce, particularly for hospitalised patients. Assessing chronic conditions using the International Classification of Primary Care 2, a study conducted in ambulatory primary care described four clusters [9]: (1) cardiovascular risk factors and conditions, (2) general age-related and metabolic conditions, (3) tobacco and alcohol dependencies, and (4) pain, musculoskeletal and psychological conditions. The comparability with our study is limited by the different classification of diseases, including risk factors and symptoms, and the ambulatory care setting. In our study, we identified five groups of comorbidities, labelled according to their predominant comorbidities: (1) neurological diseases, (2) heart/kidney diseases, (3) malignancy, (4) miscellaneous diseases, and (5) psychiatric diseases. On the one hand, the combinations of comorbidities found in groups 1, 2 and 3 may be explained by common pathophysiological pathways or risk factors, and/or because one comorbidity may cause or result from the other one. On the other hand, groups 4 and 5 included more various comorbidities, such as arthropathy and arthritis among psychiatric diseases in group 5. Notably, chronic heart disease was identified in all groups except in group 3. This analysis suggests which chronic comorbidities are more likely to be found in patients with particular chronic comorbidities, and may thus have important preventive implications for development and allocation of interventions to reduce the overall burden of diseases in multimorbid patients.
Proportions of acute and chronic diseases
The proportions of chronic versus acute diseases progressively decreased when the total number of diseases increased. This suggests that patients with more diseases may be more severely ill and therefore at higher risk of developing an additional acute disease, underlying the important burden of chronic diseases in patients with multimorbidity. On the other hand, the number of body systems was constantly about half the total number of diseases, suggesting that different diseases affect the same body system. It may be partly explained by an acute decompensation of a chronic disease, both diseases affecting of course the same body system. We found no previous study looking at the proportions of acute and chronic diseases and at the number of body systems involved in multimorbid patients.
Clinical implications
Despite the high prevalence of multimorbidity, little was known about which comorbidities are frequently found in combinations and on the proportion of acute and chronic diseases among patients hospitalised in internal medicine departments in Switzerland. Furthermore, these patients were often excluded from trials [43], so that clinical guidelines may not apply to them. Our detailed and quantitative description of the patterns of multimorbidity outlines the interdisciplinary competences absolutely needed to care for such patients, taking into account the several different comorbidities together, rather than treating a single comorbidity at a time. Furthermore, highlighting the most prevalent combinations of comorbidities and combinations occurring more frequently than expected may help to identify targets for intervention, and thus to prioritise the development of guidelines and treatment protocols specific for multimorbid patients, with the final aim to reduce the burden of diseases in this population. Moreover, the latent class analysis revealed comorbidities that may predispose to other ones. Although we cannot conclude on causal relationships, this information may be useful to improve secondary and tertiary prevention and thus possibly reduce the overall burden of diseases in multimorbid patients.
Limitations and strengths
Our study has several limitations. First, we included only medical patients of tertiary teaching hospitals, so that our results may have limited generalisability to patients admitted to primary and secondary care hospitals due to a possible selection bias of sicker patients in tertiary care hospitals. Second, although ICD codes allow assessment of many diseases, we cannot exclude underreporting due to coding quality, a limitation of any data extracted from electronic medical records. This may have biased the analyses for particular comorbidities, such as mood and anxiety disorders, that are frequent but often remain undetected and not documented. Although review of individual charts may have reduced underreporting, it would have added subjectivity in diagnoses assessment and been hardly feasible for such a large cohort. Third, we used data from calendar years 2010 and 2011, but substantial change is unlikely to have occurred in the last eight years. Fourth, we used an epidemiologically oriented definition of multimorbidity and did not assess complex interactions of diseases, as mentioned in 2018 National Library of Medicine definition of multimorbidity. Fifth, because data were originally collected to study readmission, we did not include patients who died during hospitalisation, which may limit generalisability of the results. Finally, despite the use of standardised classification tools, we included only chronic comorbidities in our analysis, which may have prevented comparison with other studies.
Our study presents several strengths also. First, we assessed multimorbidity in several different and innovative ways and used standardised tools to classify the diseases, allowing a more objective assessment than self-reporting used in up to 75% of previous studies [44]. Second, using ICD codes, we could assess a high number of diseases, unlike most previous analyses that often included a more restricted number of diseases, for example due to the limitation of insurance claims data [7, 41, 44–46]. Finally, we used a large and multicentre cohort.
Conclusion
We found that nearly 80% of the patients were multimorbid. The most prevalent comorbidities were chronic heart disease, chronic kidney disease, solid malignancy and substance-related disorders, and were most frequently found in combination with COPD or bronchiectasis. Our descriptive and quantitative findings provide a better understanding of the various patterns of multimorbidity that physicians may face at hospital. Describing prevalent combinations of comorbidities and combinations occurring more often than expected may facilitate the development of guidelines and treatment protocols appropriate for multimorbid patients with the final aim to reduce the burden of diseases in these patients.
Appendix 1. Eighteen body system categories of the Chronic Condition Indicator
The Chronic Condition Indicator classifies the chronic diseases into following 18 body system categories:
- infectious and parasitic diseases;
- neoplasms;
- endocrine, nutritional and metabolic diseases, and immunity disorders;
- diseases of blood and blood-forming organs;
- mental disorders;
- diseases of the nervous system and sense organs;
- diseases of the circulatory system;
- diseases of the respiratory system;
- diseases of the digestive system;
- 10- diseases of the genitourinary system;
- complications of pregnancy, childbirth, and the puerperium;
- diseases of the skin and subcutaneous tissue;
- diseases of the musculoskeletal system;
- congenital anomalies;
- certain conditions originating in the perinatal period;
- symptoms, signs, and ill-defined conditions;
- injury and poisoning;
- factors influencing health status and contact with health services.
Appendix 2. Categorisation of diseases
For clinical relevance, we further merged some of the 285 mutually exclusive categories of the Clinical Classification Software (CCS) into broader categories:
- chronic heart disease: CCS 105-107 (cardiac dysrhythmias), CCS 100-101 (coronary heart disease), CCS 10 (nonhypertensive congestive heart failure), CCS 96 (heart valve disorder);
- cerebrovascular diseases: CCS 109 and 111-112;
- solid malignancies: CCS 11-36 and 41-42;
- haematological malignancies: CCS 37-40;
- arthropathy and arthritis: CCS 54 (gout and other crystal arthropathies), CCS 201 (infective arthritis and osteomyelitis), CCS 202 (rheumatoid arthritis and related disease), CCS 203 (osteoarthritis), CCS 204 (other non-traumatic joint disorders), CCS 205 (spondylosis, intervertebral disc disorders and other back problems);
- osteoporosis and pathological fractures: CCS 206-207;
- liver disease: CCS 6 and 150-151;
- psychosis and schizophrenic disorders: CCS 70-71 and 659;
- other nutritional, endocrine or metabolic disorder: CCS 51 and 58;
- substance-related disorders: CCS 660-661.
Statistical analyses
All analyses were performed using R version 3.4.4 (R Project for Statistical Computing). We used the R-package 'poLCA' for the latent class analysis, 'icd' for computing Deyo-Charlson Comorbidity Index and Elixhauser-Van Walraven Comorbidity Index from ICD codes, and 'arules' for detecting the most frequent combinations of disease categories.
Appendix 3. Supplementary data
Appendix 3 is available in a separate file at https://doi.org/10.4414/smw.2019.20094
Author contributions
CEA and JD designed the study, analysed and interpreted the data. NF and AL performed the statistical analyses. CEA wrote the manuscript. JD was a major contributor to revise the manuscript. PMV, DA, and JS read and approved the manuscript.
References
1
Johnston
MC
,
Crilly
M
,
Black
C
,
Prescott
GJ
,
Mercer
SW
. Defining and measuring multimorbidity: a systematic review of systematic reviews. Eur J Public Health. 2019;29(1):182–9.
2
Aubert
CE
,
Streit
S
,
Da Costa
BR
,
Collet
TH
,
Cornuz
J
,
Gaspoz
JM
, et al.
Polypharmacy and specific comorbidities in university primary care settings. Eur J Intern Med. 2016;35:35–42. doi:.https://doi.org/10.1016/j.ejim.2016.05.022
3
Barnett
K
,
Mercer
SW
,
Norbury
M
,
Watt
G
,
Wyke
S
,
Guthrie
B
. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi:.https://doi.org/10.1016/S0140-6736(12)60240-2
4
Rizza
A
,
Kaplan
V
,
Senn
O
,
Rosemann
T
,
Bhend
H
,
Tandjung
R
; FIRE study group. Age- and gender-related prevalence of multimorbidity in primary care: the Swiss FIRE project. BMC Fam Pract. 2012;13(1):113. doi:.https://doi.org/10.1186/1471-2296-13-113
5
Schneider
F
,
Kaplan
V
,
Rodak
R
,
Battegay
E
,
Holzer
B
. Prevalence of multimorbidity in medical inpatients. Swiss Med Wkly. 2012;142:w13533. doi:.https://doi.org/10.4414/smw.2012.13533
6
Gijsen
R
,
Hoeymans
N
,
Schellevis
FG
,
Ruwaard
D
,
Satariano
WA
,
van den Bos
GA
. Causes and consequences of comorbidity: a review. J Clin Epidemiol. 2001;54(7):661–74. doi:.https://doi.org/10.1016/S0895-4356(00)00363-2
7
Bähler
C
,
Huber
CA
,
Brüngger
B
,
Reich
O
. Multimorbidity, health care utilization and costs in an elderly community-dwelling population: a claims data based observational study. BMC Health Serv Res. 2015;15(1):23. doi:.https://doi.org/10.1186/s12913-015-0698-2
8
Marengoni
A
,
Angleman
S
,
Melis
R
,
Mangialasche
F
,
Karp
A
,
Garmen
A
, et al.
Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430–9. doi:.https://doi.org/10.1016/j.arr.2011.03.003
9
Déruaz-Luyet
A
,
N’Goran
AA
,
Senn
N
,
Bodenmann
P
,
Pasquier
J
,
Widmer
D
, et al.
Multimorbidity and patterns of chronic conditions in a primary care population in Switzerland: a cross-sectional study. BMJ Open. 2017;7(6):e013664. doi:.https://doi.org/10.1136/bmjopen-2016-013664
10
Pache
B
,
Vollenweider
P
,
Waeber
G
,
Marques-Vidal
P
. Prevalence of measured and reported multimorbidity in a representative sample of the Swiss population. BMC Public Health. 2015;15(1):164. doi:.https://doi.org/10.1186/s12889-015-1515-x
11
Rizza
A
,
Kaplan
V
,
Senn
O
,
Rosemann
T
,
Bhend
H
,
Tandjung
R
; FIRE study group. Age- and gender-related prevalence of multimorbidity in primary care: the Swiss FIRE project. BMC Fam Pract. 2012;13(1):113. doi:.https://doi.org/10.1186/1471-2296-13-113
12
Streit
S
,
da Costa
BR
,
Bauer
DC
,
Collet
TH
,
Weiler
S
,
Zimmerli
L
, et al.
Multimorbidity and quality of preventive care in Swiss university primary care cohorts. PLoS One. 2014;9(4):e96142. doi:.https://doi.org/10.1371/journal.pone.0096142
13
Freund
T
,
Kunz
CU
,
Ose
D
,
Szecsenyi
J
,
Peters-Klimm
F
. Patterns of multimorbidity in primary care patients at high risk of future hospitalization. Popul Health Manag. 2012;15(2):119–24. doi:.https://doi.org/10.1089/pop.2011.0026
14
García-Olmos
L
,
Salvador
CH
,
Alberquilla
Á
,
Lora
D
,
Carmona
M
,
García-Sagredo
P
, et al.
Comorbidity patterns in patients with chronic diseases in general practice. PLoS One. 2012;7(2):e32141. doi:.https://doi.org/10.1371/journal.pone.0032141
15
Cornell
JE
,
Pugh
JA
,
Williams
JW, Jr
,
Kazis
L
,
Lee
AFS
,
Parchman
ML
, et al.. Multimorbidity clusters: clustering binary data from multimorbidity clusters: clustering binary data from a large administrative medical database. App Multiv Res.
2008;12(3):163. doi:.https://doi.org/10.22329/amr.v12i3.658
16
Goldstein
G
,
Luther
JF
,
Jacoby
AM
,
Haas
GL
,
Gordon
AJ
. A Taxonomy of medical comorbidity for veterans who are homeless. J Health Care Poor Underserved. 2008;19(3):991–1005. doi:.https://doi.org/10.1353/hpu.0.0040
17
John
R
,
Kerby
DS
,
Hagan Hennessy
C
. Patterns and impact of comorbidity and multimorbidity among community-resident American Indian elders. Gerontologist. 2003;43(5):649–60. doi:.https://doi.org/10.1093/geront/43.5.649
18
Kirchberger
I
,
Meisinger
C
,
Heier
M
,
Zimmermann
AK
,
Thorand
B
,
Autenrieth
CS
, et al.
Patterns of multimorbidity in the aged population. Results from the KORA-Age study. PLoS One. 2012;7(1):e30556. doi:.https://doi.org/10.1371/journal.pone.0030556
19
Newcomer
SR
,
Steiner
JF
,
Bayliss
EA
. Identifying subgroups of complex patients with cluster analysis. Am J Manag Care. 2011;17(8):e324–32.
20
Marengoni
A
,
Rizzuto
D
,
Wang
HX
,
Winblad
B
,
Fratiglioni
L
. Patterns of chronic multimorbidity in the elderly population. J Am Geriatr Soc. 2009;57(2):225–30. doi:.https://doi.org/10.1111/j.1532-5415.2008.02109.x
21
Schäfer
I
,
von Leitner
EC
,
Schön
G
,
Koller
D
,
Hansen
H
,
Kolonko
T
, et al.
Multimorbidity patterns in the elderly: a new approach of disease clustering identifies complex interrelations between chronic conditions. PLoS One. 2010;5(12):e15941. doi:.https://doi.org/10.1371/journal.pone.0015941
22
van den Bussche
H
,
Koller
D
,
Kolonko
T
,
Hansen
H
,
Wegscheider
K
,
Glaeske
G
, et al.
Which chronic diseases and disease combinations are specific to multimorbidity in the elderly? Results of a claims data based cross-sectional study in Germany. BMC Public Health. 2011;11(1):101. doi:.https://doi.org/10.1186/1471-2458-11-101
23
Holden
L
,
Scuffham
PA
,
Hilton
MF
,
Muspratt
A
,
Ng
SK
,
Whiteford
HA
. Patterns of multimorbidity in working Australians. Popul Health Metr. 2011;9(1):15. doi:.https://doi.org/10.1186/1478-7954-9-15
24
Déruaz-Luyet
A
,
N’Goran
AA
,
Pasquier
J
,
Burnand
B
,
Bodenmann
P
,
Zechmann
S
, et al.
Multimorbidity: can general practitioners identify the health conditions most important to their patients? Results from a national cross-sectional study in Switzerland. BMC Fam Pract. 2018;19(1):66. doi:.https://doi.org/10.1186/s12875-018-0757-y
25
Prados-Torres
A
,
Poblador-Plou
B
,
Calderón-Larrañaga
A
,
Gimeno-Feliu
LA
,
González-Rubio
F
,
Poncel-Falcó
A
, et al.
Multimorbidity patterns in primary care: interactions among chronic diseases using factor analysis. PLoS One. 2012;7(2):e32190. doi:.. Correction in: PLoS One. 2013;8(12): 6e3c77093262. doi:https://doi.org/10.1371/journal.pone.0032190
26
von Elm
E
,
Altman
DG
,
Egger
M
,
Pocock
SJ
,
Gøtzsche
PC
,
Vandenbroucke
JP
; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–9. doi:.https://doi.org/10.1016/j.ijsu.2014.07.013
27
Violan
C
,
Foguet-Boreu
Q
,
Flores-Mateo
G
,
Salisbury
C
,
Blom
J
,
Freitag
M
, et al.
Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS One. 2014;9(7):e102149. doi:.https://doi.org/10.1371/journal.pone.0102149
28
Elixhauser
A
,
Steiner
C
,
Harris
DR
,
Coffey
RM
. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi:.https://doi.org/10.1097/00005650-199801000-00004
29
Charlson
ME
,
Pompei
P
,
Ales
KL
,
MacKenzie
CR
. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi:.https://doi.org/10.1016/0021-9681(87)90171-8
30
Deyo
RA
,
Cherkin
DC
,
Ciol
MA
. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. doi:.https://doi.org/10.1016/0895-4356(92)90133-8
31
van Walraven
C
,
Austin
PC
,
Jennings
A
,
Quan
H
,
Forster
AJ
. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–33. doi:.https://doi.org/10.1097/MLR.0b013e31819432e5
32
Quan
H
,
Sundararajan
V
,
Halfon
P
,
Fong
A
,
Burnand
B
,
Luthi
JC
, et al.
Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9. doi:.https://doi.org/10.1097/01.mlr.0000182534.19832.83
33Agency for Healthcare Research and Quality (AHRQ), Healthcare Cost and Utilization Project (HCUP). Chronic Condition Indicator (CCI) for ICD-9-CM. https://www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp. Accessed April 4 2019.
34Agency for Healthcare Research and Quality (AHRQ). Clinical Classifications Software (CCS) for ICD-9-CM. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed April 4 2019.
35
Clerencia-Sierra
M
,
Calderón-Larrañaga
A
,
Martínez-Velilla
N
,
Vergara-Mitxeltorena
I
,
Aldaz-Herce
P
,
Poblador-Plou
B
, et al.
Multimorbidity Patterns in Hospitalized Older Patients: Associations among Chronic Diseases and Geriatric Syndromes. PLoS One. 2015;10(7):e0132909. doi:.https://doi.org/10.1371/journal.pone.0132909
36
Wong
A
,
Boshuizen
HC
,
Schellevis
FG
,
Kommer
GJ
,
Polder
JJ
. Longitudinal administrative data can be used to examine multimorbidity, provided false discoveries are controlled for. J Clin Epidemiol. 2011;64(10):1109–17. doi:.https://doi.org/10.1016/j.jclinepi.2010.12.011
37
Friedman
B
,
Jiang
HJ
,
Elixhauser
A
,
Segal
A
. Hospital inpatient costs for adults with multiple chronic conditions. Med Care Res Rev. 2006;63(3):327–46. doi:.https://doi.org/10.1177/1077558706287042
38
Schellevis
FG
,
van der Velden
J
,
van de Lisdonk
E
,
van Eijk
JT
,
van Weel
C
. Comorbidity of chronic diseases in general practice. J Clin Epidemiol. 1993;46(5):469–73. doi:.https://doi.org/10.1016/0895-4356(93)90024-U
39
Fortin
M
,
Bravo
G
,
Hudon
C
,
Vanasse
A
,
Lapointe
L
. Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med. 2005;3(3):223–8. doi:.https://doi.org/10.1370/afm.272
40
Marengoni
A
,
Bonometti
F
,
Nobili
A
,
Tettamanti
M
,
Salerno
F
,
Corrao
S
, et al.; Italian Society of Internal Medicine (SIMI) Investigators. In-hospital death and adverse clinical events in elderly patients according to disease clustering: the REPOSI study. Rejuvenation Res. 2010;13(4):469–77. doi:.https://doi.org/10.1089/rej.2009.1002
41
Pati
S
,
Swain
S
,
Metsemakers
J
,
Knottnerus
JA
,
van den Akker
M
. Pattern and severity of multimorbidity among patients attending primary care settings in Odisha, India. PLoS One. 2017;12(9):e0183966. doi:.https://doi.org/10.1371/journal.pone.0183966
42
Prados-Torres
A
,
Calderón-Larrañaga
A
,
Hancco-Saavedra
J
,
Poblador-Plou
B
,
van den Akker
M
. Multimorbidity patterns: a systematic review. J Clin Epidemiol. 2014;67(3):254–66. doi:.https://doi.org/10.1016/j.jclinepi.2013.09.021
43
Jadad
AR
,
To
MJ
,
Emara
M
,
Jones
J
. Consideration of multiple chronic diseases in randomized controlled trials. JAMA. 2011;306(24):2670–2. doi:.https://doi.org/10.1001/jama.2011.1886
44
Diederichs
C
,
Berger
K
,
Bartels
DB
. The measurement of multiple chronic diseases--a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci. 2011;66A(3):301–11. doi:.https://doi.org/10.1093/gerona/glq208
45
Pati
S
,
Swain
S
,
Hussain
MA
,
van den Akker
M
,
Metsemakers
J
,
Knottnerus
JA
, et al.
Prevalence and outcomes of multimorbidity in South Asia: a systematic review. BMJ Open. 2015;5(10):e007235. doi:.https://doi.org/10.1136/bmjopen-2014-007235
46
Lochner
KA
,
Cox
CS
. Prevalence of multiple chronic conditions among Medicare beneficiaries, United States, 2010. Prev Chronic Dis. 2013;10:120137. doi:.https://doi.org/10.5888/pcd10.120137